Published online Sep 28, 2018. doi: 10.4329/wjr.v10.i9.100
Peer-review started: April 26, 2018
First decision: May 22, 2018
Revised: July 12, 2018
Accepted: July 14, 2018
Article in press: July 16, 2018
Published online: September 28, 2018
Processing time: 155 Days and 20 Hours
Late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) is the gold standard for imaging myocardial viability. An important application of LGE CMR is the assessment of the location and extent of the myocardial scar in patients with ventricular tachycardia (VT), which allows for more accurate identification of the ablation targets. However, a large percentage of patients with VT have cardiac implantable electronic devices (CIEDs), which is a relative contraindication for cardiac magnetic resonance imaging due to safety and image artifact concerns. Previous studies showed that these patients can be safely scanned on 1.5 T scanners provided that an adequate imaging protocol is adopted. Nevertheless, imaging patients with a CIED result in metal artifacts due to the strong frequency off-resonance effects near the device; therefore, the spins in the surrounding myocardium are not completely inverted, and thus give rise to hyperintensity artifacts. These artifacts obscure the myocardial scar tissue and limit the ability to study the correlation between the myocardial scar structure and the electro-anatomical map during catheter ablation. In this study, we developed a modified inversion recovery technique to alleviate the CIED-induced metal artifacts and improve the diagnostic image quality of LGE images in patients with CIEDs without increasing scan time or requiring additional hardware. The developed technique was tested in phantom experiments and in vivo scans, which showed its capability for suppressing the hyperintensity artifacts without compromising myocardium nulling in the resulting LGE images.
Core tip: Late gadolinium-enhancement magnetic resonance imaging is the gold standard for imaging myocardial viability, especially for assessing location and extent of myocardial scar in patients with ventricular-tachycardia, which allows for more identification of the ablation targets. However, large percentages of these patients have cardiac-implantable electronic devices, which results in hyperintensity artifacts that obscure the myocardial scar. In this study, we developed a modified technique to alleviate the metal-induced image artifacts without increasing scan time or requiring additional hardware. The developed technique was tested in phantom and in-vivo scans, which showed its capability for suppressing the hyperintensity artifacts and improving diagnostic image quality.
- Citation: Ibrahim ESH, Runge M, Stojanovska J, Agarwal P, Ghadimi-Mahani M, Attili A, Chenevert T, den Harder C, Bogun F. Optimized cardiac magnetic resonance imaging inversion recovery sequence for metal artifact reduction and accurate myocardial scar assessment in patients with cardiac implantable electronic devices. World J Radiol 2018; 10(9): 100-107
- URL: https://www.wjgnet.com/1949-8470/full/v10/i9/100.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i9.100
Late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) is the gold standard for imaging myocardial viability, scars, and focal fibrosis[1-5]. LGE CMR is based on an inversion recovery (IR) sequence to null myocardial signal and improve the visibility of contrast enhanced tissue[6-10]. An important application of LGE CMR is the assessment of the location and extent of the myocardial scar in patients with ventricular tachycardia (VT), which allows for more accurate identification of the arrhythmogenic substrate and ablation targets[11-13]. However, up to 75% of patients with VT who may benefit from LGE CMR have cardiac implantable electronic devices (CIEDs)[14], which is a relative contraindication for CMR due to safety and image artifact concerns[15,16]. The risks of imaging patients with CIEDs include tissue heating, generation of mechanical forces, and alteration of device function. Nevertheless, studies have shown that these patients can be safely scanned on 1.5 T scanners provided that a protocol with adequate programming of the CIED and monitoring of the patient during the CMR is performed[17,18].
The CIED-induced artifacts are mainly caused by the metallic composition of the device, which results in strong frequency off-resonances near the device, including around cardiac anatomical locations, such as the left ventricular apex, the lateral wall, and the outflow tract. It has been shown that a cardiac pulse generator that is 5-10 cm away from the heart results in a 2-6 kHz resonance frequency offset in the myocardium[19]. Because the frequency band of the inversion radiofrequency (RF) pulse commonly used in LGE MRI is limited, the spins in the affected myocardium regions are not completely inverted, and thus give rise to hyperintensity artifacts[20]. These artifacts obscure the myocardial scar tissue and limit the ability to study the correlation between the myocardial scar structure provided by the LGE image and the electro-anatomical map generated during catheter ablation.
A number of metal artifact reduction techniques have been previously reported, e.g., multi-acquisition variable-resonance image combination (MAVRIC)[21,22] and slice encoding for metal artifact compensation (SEMAC)[23,24], which greatly reduce the metal artifacts. Nevertheless, these techniques require extended scan time because either the image acquisition is repeated several times (in different spectral bands) or additional phase-encoding is applied, which makes them impractical for cardiac imaging of patients with CIEDs.
In this study, we developed a modified IR technique to alleviate the CIED-induced metal artifacts and improve the diagnostic image quality of LGE images in patients with CIEDs without increasing scan time or requiring additional hardware.
The IR pulse sequence was modified to include an adiabatic wideband IR RF pulse with adjustable frequency offset and bandwidth (BW), which allows for optimal myocardial signal nulling even in the presence of off-resonance effects from the CIEDs.
To mitigate the effect of the B1+ variation in CMR, which could be as high as 25% at 1.5 T and even higher at 3 T, adiabatic inversion pulses are often used to reduce the sensitivity to B1+ inhomogeneity[25]. In this study, we used hyperbolic secant (HS) adiabatic pulses, which provide excellent homogeneous and flat inversion profile across their BW[26].
The HS pulse is composed of the following amplitude and frequency modulation functions:
A(t) = A0 sech (αt) (1)
Δω(t) = -cαtanh(αt) (2)
where A0 is the peak B1 amplitude, c is a phase parameter (dimensionless), and α is a frequency modulation parameter (in units of rad/s). The BW of the HS pulse can be obtained from the product of the amplitude modulation and phase modulation parameters: cα. By modifying either or both of these parameters, the BW of the HS pulse can be altered.
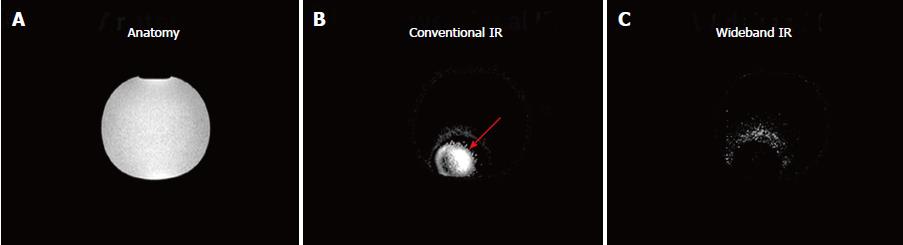
A phantom experiment was conducted on a 1.5 T Achieva scanner (Philips, Best, Netherlands), where a CIED was placed one-inch away from a water-filled bottle doped with 0.15 mmol/kg of gadolinium (Gd) contrast material. A Look-Locker inversion time scout sequence[3] was used to identify the appropriate inversion time that nulls the MRI signal (TI = 250 ms), and cross-sectional images of the bottle were acquired using conventional and wideband IR (BW = 3000 Hz) sequences. The imaging parameters of the IR sequence were: repetition time (TR) = 6.7 ms, echo time (TE) = 3.2 ms, resolution = 1.4 × 2.2 mm2, slice thickness = 8 mm, and specific absorption rate (SAR) limit = 2 W/kg.
The study was approved by our institutional review board (IRB) and written informed consent was obtained from all participants. Twelve patients (10 males, age = 60 ± 18 y.o.) with structural heart disease and a history of ventricular tachycardia (VT) and previously implanted CIEDs [7 patients with a biventricular implantable cardioverter defibrillator (ICD), 4 patients with a dual chamber ICD, and 1 patient with a single chamber ICD; manufacturer: Boston Scientific (n = 5), Medtronic (n = 5), and St Jude (n = 2)], who were referred for a pre-ablation CMR, were enrolled in this study.
The patients were imaged on the same scanner used in the phantom experiment using cine short-axis and long-axis images (steady-state free precession sequence, TR = 4.2 ms, TE = 1.8 ms, resolution = 1.4 × 1.4 mm2, slice thickness = 8 mm) and the conventional and optimized wideband IR sequences for assessment of myocardial scar prior to an ablation procedure to treat VT. The patients received an intravenous injection of 0.15 mmol/kg of gadolinium contrast material (MultiHance, Bracco, Milan, Italy) approximately 15 min before LGE imaging.
The imaging parameters of the LGE sequences were similar to those used in the phantom scan, while the frequency BW and frequency shift parameters of the wideband LGE sequence were optimized for each patient to improve myocardial nulling while minimizing metal artifacts. Frequency bandwidth and frequency offset adjustments were in the ranges of 2000–4000 Hz and -1500–1500 Hz, respectively. The frequency bands that resulted in an artifact-free image were chosen and used for the rest of the LGE scan for each patient. The SAR of the IR sequences was limited to less than 2 W/kg to ensure imaging safety.
The protocol followed at our institution for CMR imaging of CIED patients has been described previously[27]; in brief it consists of: (1) Demonstration of an imaging necessity in the absence of an alternative imaging modality; (2) absence of device-related contraindications, including epicardial ICD defibrillation patches, and the elapsed time from lead implantation < 6 wk; (3) presence of a provider with CIED management expertise who programs the device to the appropriate settings for the MRI; and (4) re-interrogation and reprogramming of the device after completion of the scan, as well as at 1 wk and 3 mo post imaging.
The patients’ vital signs were monitored during the scan by means of an electrocardiogram (ECG), pulse oximetry, and blood pressure measurement by an advanced cardiac life support-certified nurse practitioner, who was present during the entire scan.
All clinical images were reviewed by a fellowship-trained cardiovascular imaging physician with more than 10 years of experience, where the presence and location of artifact-containing segments were reported for each patient. A cardiac segment was considered to be affected by hyperintensity artifact if more than half of the segment was obscured by the artifact.
Contrast-to-noise ratio (CNR) between the blood-pool and myocardium was calculated as the difference between the mean signal intensity in the two regions divided by standard deviation of the background noise in a mid-ventricular slice of the heart. A statistical t-test was used to measure the CNR difference significance between the conventional and modified IR techniques.
The conventional IR sequence resulted in a hyperintensity artifact as shown in Figure 1. After using the wideband IR sequence with 3000-Hz bandwidth, the hyperintensity artifact was suppressed.
All patients were at sinus rhythm during the CMR scan, and were successfully imaged without any adverse events, where the device parameters remained the same immediately, 1 wk, and 3 mo after the scans.
When the conventional IR sequence was utilized, all twelve studied subjects showed varying degrees of hyperintensity artifacts depending on the implanted CIED and its location, including artifacts in the anterior (10 subjects), anteroseptal (3 subjects), anterolateral (4 subjects), and inferior (2 subjects) walls of the left ventricle (LV; especially at the apical and mid-ventricular levels), and in the inferior wall of the right ventricle (RV; 2 subjects).
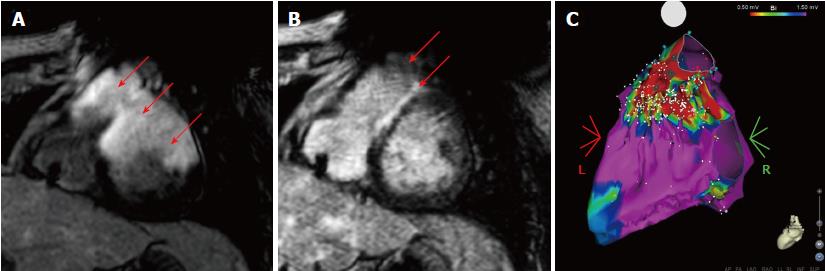
The wideband IR sequence removed the hyperintensity artifacts, and did not introduce additional image artifacts when compared to the conventional IR sequence. This improved image quality allowed for anatomical details to be seen and scar assessment could be confidently performed, as shown in Figure 2.
After the hyperintensity artifacts, which mimicked or obscured the scar tissue, were removed with the wideband IR sequence, eight of the twelve patients showed varying degrees of LGE hyperenhancement in the LV and in the RV (inferior wall; 1 subject), while four patients did not show any signs of LGE hyperenhancement. Table 1 shows the distribution of the LV cardiac segments that showed hyperenhancement artifacts after using the wideband IR sequence.
| Level | Basal | Mid-ventricular | Apical | Apex Cap | ||||||||||||
| Segment | Ant-Lat | Ant-Sept | Inf | Inf-Lat | Inf-Sept | Ant | Ant-Lat | Ant-Sept | Inf | Inf-Lat | Inf-Sept | Ant | Lat | Inf | Sept | |
| Subjects | 1 | 2 | 4 | 4 | 3 | 2 | 1 | 3 | 4 | 2 | 5 | 2 | 4 | 3 | 4 | 2 |
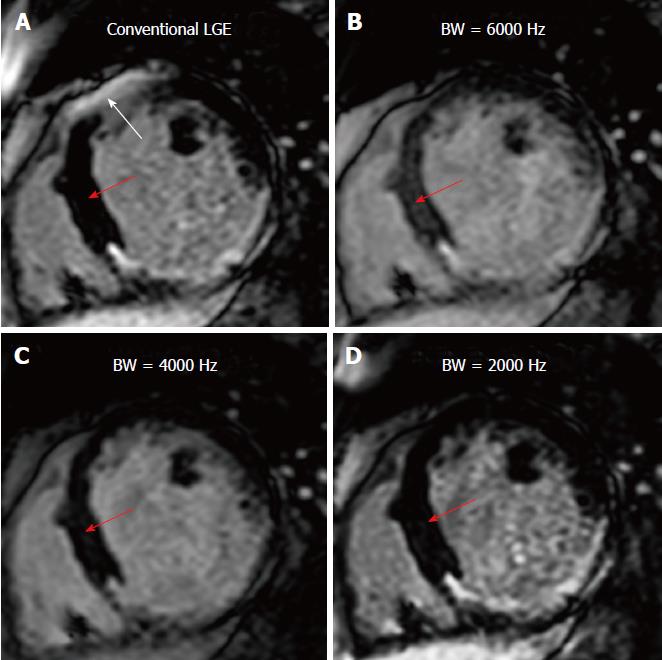
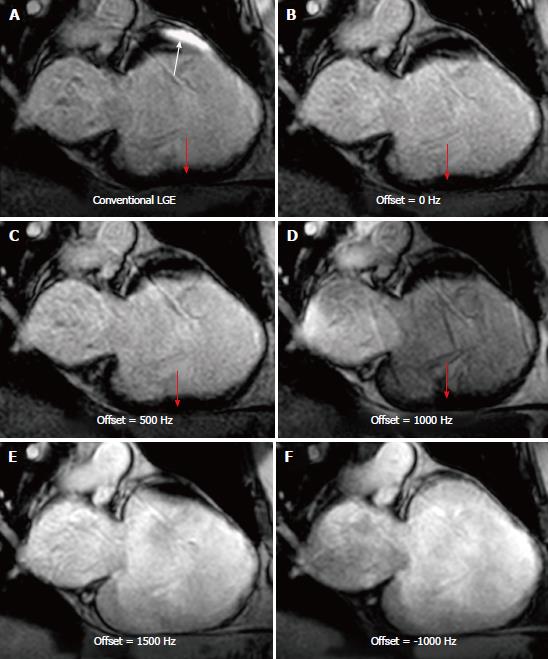
Increasing the IR frequency BW resulted in improved artifact reduction, although this came at the cost of incomplete myocardial nulling, i.e., reduced blood-to-myocardium CNR, as shown in Figure 3 (where the blood-myocardium CNR was 20.8, 13.2, 17.1, and 20.1 for conventional IR, and wideband IR with 6000 Hz, 4000 Hz, and 2000 Hz BW, respectively). Therefore, the BW was set to the minimum value that eliminated the artifact for each patient. Similarly, the frequency shift of the IR pulse affected the artifact appearance, as shown in Figure 4; therefore, proper setting of the frequency shift allows for removing the hyperintensity artifact without the need to increase the frequency BW; thus resulting in optimal myocardium nulling. Based on the studied cases, the optimal BW was in the range of 2000-3000 Hz and the optimal frequency shift was up to 1000 Hz.
In the studied patients, the blood-myocardium CNR was 23.5+/-11 for the wideband IR sequence, which was not significantly different (P = 0.03) from the CNR for conventional IR sequence (25.5+/-13). In the implemented CIED patient scans, the average SAR was 0.20 W/kg for both conventional and wideband IR scans, which is well below the 2 W/kg SAR limit.
The developed wideband IR sequence minimizes the CIED-generated hyperintensity artifacts through simple adjustment of the IR pulse’s BW and offset frequency without increasing scan time or requiring additional hardware, which allows for accurate identification of arrhythmogenic substrate and improved ablation target localization in VT patients, as illustrated by the results of the phantom and in vivo scans.
The implemented wideband adiabatic IR scan could be performed within the same scan-time and produced similar SAR compared to the conventional IR scan, which is well below the 2 W/kg limit. Compared to previous efforts to reduce hyperintensity artifacts[19], the developed method provides the flexibility to adjust the IR pulse BW and frequency offset for optimal artifact suppression and myocardial nulling at the same time.
It should be noted that although the wideband IR sequence removed the hyperintensity artifacts, it did not correct for geometric distortions or signal voids caused by off-resonance, which were typically resolved using localized shimming. Otherwise, a spin-echo or an ultrashort echo time (UTE) sequence[26] could be used to correct for strong intra-voxel dephasing signal voids, although this is more of a problem in musculoskeletal applications due to metal implants. It should be noted that while such techniques minimize the metal artifacts effect on surrounding tissues, the place of the CIED will still show signal void or geometric artifacts, which become worse at higher field strengths. However, for safety purposes, imaging patients with CIEDs is typically limited to 1.5 T scanners; therefore, accentuated metal artifacts at higher field strengths is not a concern in this group of patients.
Finally, compared to 3D LGE sequences, extended signal voids and ripple artifacts did not occur in 2D LGE imaging because of the much thinner slice thickness in 2D imaging compared to the large slab thickness in 3D imaging; therefore, through-plane distortion in 2D LGE is typically negligible, as previously reported[20,28].
One limitation of our study is the small number of studied subjects. However, the intent of this study was to illustrate the feasibility of the developed technique and show its importance for revealing myocardial scar in patients with CIEDs, where the presence of hyperintensity artifacts resulted in images with non-diagnostic image quality. Future work includes testing the developed technique on a large number of VT patients with inter- and intra-observer variability analysis using an image quality scoring system.
In conclusion, we presented a modified wideband IR technique that alleviates the CIED-generated metal artifacts and improves the diagnostic image quality of the LGE images to reveal scar myocardial tissue in patients with CIEDs without increasing scan time or requiring additional hardware.
Late gadolinium enhancement magnetic resonance imaging is typically used for myocardial viability imaging. An important application of the late gadolinium enhancement (LGE) technique is the assessment of myocardial scar in patients with ventricular tachycardia (VT) before the ablation procedure.
LGE imaging is challenging in patients with cardiac implantable electronic devices (CIEDs) due to device-generated metal hyperintensity artifacts, which compromise the effect of the IR pulse and obscure the region of interest.
To develop a modified inversion recovery (IR) technique that eliminates the LGE hyperintensity artifacts and improves diagnostic image quality.
The modified pulse sequence developed in this study includes a wideband IR RF pulse with adjustable frequency offset and bandwidth, which allows for optimal myocardial signal nulling even in the presence of CIEDs. A phantom experiment was performed and twelve in vivo scans were conducted on patients with CIEDs. The imaging parameters were optimized to improve myocardial nulling and minimize metal artifacts.
The developed wideband IR sequence significantly minimized the hyperintensity artifacts, such that scar assessment could be confidently performed. Increasing the IR frequency BW results in better artifact reduction, although this improvement is achieved at the cost of incomplete myocardial nulling.
The developed wideband IR technique minimizes the CIED-generated hyperintensity artifacts without increasing scan time, and allows for accurate identification of ablation targets in VT patients. The RF pulse BW should be set to the minimum value that eliminates the artifact. Further, proper setting of the frequency offset could allow for removing the artifact without the need to increase the frequency BW. Based on the studied cases, optimal BW is in the range of 2000-3000 Hz with optimal frequency shift up to 1000 Hz.
The developed optimized IR technique allows MRI to play a larger role in treatment planning in VT patients with CIEDs. Future studies should investigate the clinical usefulness of the developed technique by implementing it on a large number of VT patients with different disease stages and CIED types.
| 1. | Judd RM, Kim RJ. Imaging time after Gd-DTPA injection is critical in using delayed enhancement to determine infarct size accurately with magnetic resonance imaging. Circulation. 2002;106:e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Judd RM, Wagner A, Rehwald WG, Albert T, Kim RJ. Technology insight: assessment of myocardial viability by delayed-enhancement magnetic resonance imaging. Nat Clin Pract Cardiovasc Med. 2005;2:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson. 2003;5:505-514. [PubMed] [DOI] [Full Text] |
| 4. | Kim HW, Rehwald WG, Jenista ER, Wendell DC, Filev P, van Assche L, Jensen CJ, Parker MA, Chen EL, Crowley ALC. Dark-Blood Delayed Enhancement Cardiac Magnetic Resonance of Myocardial Infarction. JACC Cardiovasc Imaging. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Weinsaft JW, Klem I, Judd RM. MRI for the assessment of myocardial viability. Magn Reson Imaging Clin N Am. 2007;15:505-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 993] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 7. | Shah DJ, Judd RM, Kim RJ. Technology insight: MRI of the myocardium. Nat Clin Pract Cardiovasc Med. 2005;2:597-605; quiz 606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Wagner A, Mahrholdt H, Kim RJ, Judd RM. Use of cardiac magnetic resonance to assess viability. Curr Cardiol Rep. 2005;7:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM; Gadoversetamide Myocardial Infarction Imaging Investigators. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008;117:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Wagner A, Mahrholdt H, Thomson L, Hager S, Meinhardt G, Rehwald W, Parker M, Shah D, Sechtem U, Kim RJ. Effects of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J Am Coll Cardiol. 2006;47:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Bogun FM, Desjardins B, Good E, Gupta S, Crawford T, Oral H, Ebinger M, Pelosi F, Chugh A, Jongnarangsin K. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009;53:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Tian J, Ahmad G, Mesubi O, Jeudy J, Dickfeld T. Three-dimensional delayed-enhanced cardiac MRI reconstructions to guide ventricular tachycardia ablations and assess ablation lesions. Circ Arrhythm Electrophysiol. 2012;5:e31-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol. 2005;28:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 274] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Russo RJ. Determining the risks of clinically indicated nonthoracic magnetic resonance imaging at 1.5 T for patients with pacemakers and implantable cardioverter-defibrillators: rationale and design of the MagnaSafe Registry. Am Heart J. 2013;165:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, Boyle NG, Frabizzio JV, Birgersdotter-Green U, Higgins SL. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med. 2017;376:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 17. | Nazarian S, Hansford R, Roguin A, Goldsher D, Zviman MM, Lardo AC, Caffo BS, Frick KD, Kraut MA, Kamel IR. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, Weiss RG, Berger RD, Bluemke DA, Halperin HR. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, Hu P. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Rashid S, Rapacchi S, Shivkumar K, Plotnik A, Finn JP, Hu P. Modified wideband three-dimensional late gadolinium enhancement MRI for patients with implantable cardiac devices. Magn Reson Med. 2016;75:572-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Koch KM, Hargreaves BA, Pauly KB, Chen W, Gold GE, King KF. Magnetic resonance imaging near metal implants. J Magn Reson Imaging. 2010;32:773-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med. 2009;61:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M, McKinnon GC, Potter HG, King KF. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011;65:71-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn Reson Med. 2009;62:66-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med. 2014;71:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Bernstein MA, King KF, Zhou ZJ. Handbook of MRI pulse sequences. United States, Boston: Academic Press 2004; . |
| 27. | Horwood L, Attili A, Luba F, Ibrahim EH, Parmar H, Stojanovska J, Gadoth-Goodman S, Fette C, Oral H, Bogun F. Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace. 2017;19:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | den Harder JC, van Yperen GH, Blume UA, Bos C. Ripple artifact reduction using slice overlap in slice encoding for metal artifact correction. Magn Reson Med. 2015;73:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mani V, Sobanski T, Xie Qi S- Editor: Cui LJ L- Editor: A E- Editor: Song H