Published online Nov 28, 2018. doi: 10.4329/wjr.v10.i11.172
Peer-review started: July 17, 2018
First decision: August 8, 2018
Revised: September 22, 2018
Accepted: October 7, 2018
Article in press: October 7, 2018
Published online: November 28, 2018
Processing time: 146 Days and 14.7 Hours
To compare high-resolution computed tomography (HRCT) findings between humoral primary immunodeficiencies (hPIDs) subtypes; to correlate these findings to pulmonary function tests (PFTs).
We retrospectively identified 52 consecutive adult patients with hPIDs who underwent 64-row HRCT and PFTs at the time of diagnosis. On a per-patient basis, an experienced radiologist recorded airway abnormalities (bronchiectasis, airway wall thickening, mucus plugging, tree-in-bud, and air-trapping) and parenchymal-interstitial abnormalities (consolidations, ground-glass opacities, linear and/or irregular opacities, nodules, and bullae/cysts) found on HRCT. The chi-square test was performed to compare the prevalence of each abnormality among patients with different subtypes of hPIDs. Overall logistic regression analysis was performed to assess whether HRCT findings predicted obstructive and/or restrictive PFTs results (absent-to-mild vs moderate-to-severe).
Thirty-eight of the 52 patients with hPIDs showed common variable immunodeficiency disorders (CVID), while the remaining 14 had CVID-like conditions (i.e., 11 had isolated IgG subclass deficiencies and 3 had selective IgA deficiencies). The prevalence of most HRCT abnormalities was not significantly different between CVID and CVID-like patients (P > 0.05), except for linear and/or irregular opacities (prevalence of 31.6% in the CVID group and 0 in the CVID-like group; P = 0.0427). Airway wall thickening was the most frequent HRCT abnormality found in both CVID and CVID-like patients (71% of cases in both groups). The presence of tree-in-bud abnormalities was an independent predictor of moderate-to-severe obstructive defects at PFTs (Odds Ratio, OR, of 18.75, P < 0.05), while the presence of linear and/or irregular opacities was an independent predictor of restrictive defects at PFTs (OR = 13.00; P < 0.05).
CVID and CVID-like patients showed similar HRCT findings. Tree-in-bud and linear and/or irregular opacities predicted higher risks of, respectively, obstructive and restrictive defects at PFTs.
Core tip: Humoral primary immunodeficiencies (hPIDs) are a group of conditions characterized by impaired antibody production and presenting with recurrent respiratory infections, autoimmune diseases, and malignancy. Chest high-resolution computed tomography (HRCT) is the imaging technique of choice for detecting, characterizing, and quantifying lung complications in these patients. The aims of this study were to compare HRCT findings in 52 patients with hPIDs subtypes (common variable immunodeficiency disorders - CVID vs CVID-like), and evaluate whether these findings may predict pulmonary function tests results. CVID vs CVID-like patients showed comparable HRCT findings. The presence of tree-in-bud and linear and/or irregular opacities were independent predictors of, respectively, significant obstructive and restrictive defects.
- Citation: Cereser L, De Carli M, d’Angelo P, Zanelli E, Zuiani C, Girometti R. High-resolution computed tomography findings in humoral primary immunodeficiencies and correlation with pulmonary function tests. World J Radiol 2018; 10(11): 172-183
- URL: https://www.wjgnet.com/1949-8470/full/v10/i11/172.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i11.172
Humoral primary immunodeficiencies (hPIDs), also known as primary predominantly antibody deficiencies, constitute the most common subgroup of primary immunodeficiency disorders (about 50% of diagnoses)[1]. hPIDs encompass a spectrum of conditions characterized by impaired antibody production, manifesting with recurrent respiratory tract infections, increased susceptibility to autoimmune diseases, and malignancy[1,2]. Common variable immunodeficiency disorders (CVID) are the most clinically significant group of hPIDs (with a prevalence of about 1:25000-1:50000 subjects). These disorders show distinct clinical and laboratory phenotypes associated with low levels of IgG and IgA and/or IgM[3,4]. CVID onset is during adult life in 70% of the cases, generally occurring between 20 and 40 years of age[5,6]. Other hPIDs are often referred as “CVID-like” conditions: these conditions are in most cases asymptomatic and include selective IgA deficiency (the most frequent hPID, with a prevalence of 1/600 in white people) and isolated IgG subclass deficiency[2,7,8].
Overall, thoracic complications develop in 60% of patients with hPIDs, representing the leading cause of morbidity and mortality. Chest high-resolution computed tomography (HRCT) is the imaging technique of choice for detecting, characterizing, and quantifying lung complications, as well as for evaluating the response to therapy[1,9,10]. It is generally accepted that the initial evaluation of newly diagnosed patients should include HRCT and pulmonary function tests (PFTs). Early identification of respiratory complications provides a baseline assessment of lung involvement, allows prompt treatment to reduce the number of pulmonary infections, and impacts on quality of life and mortality, the latter being influenced by both structural and functional pulmonary impairment[5,11,12].
Several studies have reported HRCT findings in hPIDs; these findings include non-infective airway disorders (i.e., bronchiectasis, airway wall thickening, and air trapping), pulmonary infections, diffuse lung parenchymal diseases [e.g., Granulomatous and Lymphocytic Interstitial Lung Disease (GLILD), and organising pneumonia], and thoracic neoplasms (e.g., lymphoma)[10,13-16]. However, most papers refer only to CVID patients, and report that HRCT abnormalities are present in more than 90% of those patients[9,17]. While some Authors reported that the severity of HRCT abnormalities was not significantly different between CVID and CVID-like paediatric patients[18,19], to the best of our knowledge no studies have addressed this issue in adult patients. Demonstrating a difference between CVID and CVID-like patients may influence the time intervals between HRCT examinations during follow-up in these 2 subgroups of hPIDs. In addition to this, previous studies assessing a correlation between HRCT findings and PFTs results demonstrated contradictory results[9,17,18,20].
The purpose of this study was twofold: (1) to compare HRCT pulmonary findings in adult patients among different subgroups of hPIDs (i.e., CVID and CVID-like); and (2) to assess whether HRCT findings predict PFTs results.
Our referring Ethical Committee approved this study. The need for informed consent was waived due to the retrospective design of the study. By performing a computerized search, we identified 56 adult patients who received a definite diagnosis of hPIDs, in accordance with the European Society for Immunodeficiencies criteria[21], in our tertiary referral centre and between 2012 and 2016. Diagnosis was performed after a history of previously undefined respiratory disease ranging from 1 to 5 years in duration. All patients underwent HRCT and PFTs within one month from diagnosis as a part of the diagnostic workflow performed at our institute. Accordingly, hereinafter we are going to refer to the HRCT performed at the time of diagnosis as baseline HRCT. Four patients were excluded from the study due to infectious respiratory disease at the time of HRCT (clinically unstable disease) or unavailability of the PFTs results. Therefore, the final population included 52 patients and had the following distribution of disease subtypes: 38 CVID, 11 isolated IgG subclass deficiency, and 3 selective IgA deficiency cases.
Lung function was evaluated according to the criteria of the European Respiratory Society/American Thoracic Society task force[22]. The following parameters were measured with a spirometer (Vmax 29c; Sensor Medics, Yorba Linda, CA, United States): Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), vital capacity (VC), peak expiratory flow (PEF), and total lung capacity (TLC). Obstructive ventilatory defects were diagnosed when the reduced FEV1/VC ratio was below the 5th percentile of the predicted value; restrictive ventilatory disorders were diagnosed when a reduction in TLC below the 5th percentile of the predicted value was detected in the presence of a normal FEV1/VC ratio. The severity of ventilatory defects was assessed using a six-point scale (absent, mild, moderate, moderately severe, severe, and very severe)[23]. For the purposes of analysis, patients with obstructive or restrictive defects were classified in two groups: (1) patients with absent-to-mild defects (i.e., ≥ 70% of predicted values); and (2) patients with moderate-to-severe defects (i.e., < 70% of predicted values).
HRCT examinations were performed with a 64-row MDCT scanner (Discovery HD 750, GE Healthcare, Milwaukee, WI, United States), with the patient in the supine position. The whole thorax was scanned volumetrically at suspended full inspiration using the following acquisition parameters: tube potential, 120 kV; tube current modulation range, 150-400 mA (based on a Noise Index set at 18.4); rotation time, 0.8 s; detector configuration, 64 mm × 0.625 mm; reconstructed section thickness and reconstructed interval, 1.25 mm; field of view according to patient size. In 32/52 patients an additional end-expiratory volumetric scan with the same parameters was also acquired.
Images were reconstructed using a high-spatial-frequency algorithm, and displayed with lung parenchyma (level, -500 HU; width, 1500 HU) and mediastinum windowing (level, 50 HU; width, 350 HU).
A radiologist with 8 years of experience in pulmonary imaging, blinded to patient history and lung function, reviewed the HRCT examinations on a picture archiving and communication system workstation (Suitestensa Ebit srl, Esaote Group Company, Genoa, Italy). Post-processing techniques, including Multiplanar Reconstruction (MPR), Maximum Intensity Projection (MIP) and Minimum Intensity Projection (MinIP), were available to complement the analysis of thin source images.
For each patient, the reader recorded two classes of abnormalities: airway abnormalities (i.e., airway wall thickening, tree-in-bud, bronchiectasis, mucus plugging, and air trapping), and parenchymal-interstitial abnormalities (i.e., linear and/or irregular opacities, nodules, consolidations, ground-glass opacities, and bullae/cysts). Radiological features were evaluated according to the Fleischner Society glossary[24]. In particular, “linear and/or irregular opacities” describe any linear opacity of irregular thickness that does not respect the lung architecture[9] and has been reported as being a key feature of lung disease in CVID patients.
Any individual abnormality was scored using a double three-point scale (with a total score ranging from 2 to 6), for coding two aspects simultaneously: (1) extension, with 1 = involvement of a single pulmonary lobe, 2 = two-to-three lobes involved, 3 = more than three lobes involved; and (2) conspicuity in the most involved lobe, with 1 = mild, 2 = moderate, and 3 = severe. As to include in the analysis only findings that are reasonably related to hPIDs, the minimum required total score for an abnormality was 3.
We calculated the per-patient prevalence of each of the aforementioned HRCT abnormalities in the overall population and in different subgroups of hPIDs patients, i.e., the CVID group vs CVID-like group (the latter including both isolated IgG subclass deficiencies and selective IgA deficiencies). Main clinical features (age, duration of symptoms before definite diagnosis, and ventilatory defects) and prevalence of HRCT abnormalities were compared between the two groups using, respectively, the u-Mann-Whitney and χ2 tests. A logistic regression analysis (stepwise approach) was performed to assess whether the HRCT findings could predict a significant obstructive or restrictive defect at PFTs on the overall study population. As variables we used each of the aforementioned airway abnormalities and parenchymal-interstitial abnormalities. Obstructive and restrictive defects were defined as relevant only if they were of moderate-to-severe nature, and not if they were absent-to-mild. Air trapping was excluded from the model since HRCT additional expiratory scan was not available for all patients. Analysis was performed with a commercially available software (MedCalc version 12.5.0.0, MariaKerke, Belgium). The α level was set to 0.05.
Of the 52 hPIDs patients 37 were females and 15 were males, with a mean age of 53.9 ± 12.7 years. Thirty-eight of the 52 patients (73%) were included in the CVID group, and 14/52 patients (27%) were in the CVID-like group. No significant differences between the two groups were found in terms of age (mean 54.9 ± 12.9 years in the CVID group vs 51 ± 11.9 years in the CVID-like group) and average duration of symptoms before definite diagnosis of hPIDs (2 years, range 1-3, in the CVID group vs 4 years, range 1-5, in the CVID-like group, P > 0.05). None of the patients underwent HRCT before hPIDs diagnosis. Standard CTs were available for 15 of the 52 patients (one examination each) and had been performed 2-4 years before the HRCT at the time of hPIDs diagnosis.
The results of the PFTs show that almost half of the patients (25/52, 48.1%) had ventilatory defects (Table 1). Three patients in the CVID group had concomitant moderate-to-severe obstructive defects and restrictive defects. None of the patients in the CVID-like group showed restrictive defects. No statistically significant differences were found between the two subgroups of hPIDs in terms of prevalence of obstructive (CVID: 44.7% vs CVID-like: 42.9%, P = 0.8474) and restrictive defects (CVID: 13.1% vs CVID-like: 0%, P = 0.2052).
| Obstructive defect | Restrictive defect | |||||||
| Absent | Mild | Relevant1 | Any | Absent | Mild | Relevant1 | Any | |
| CVID (n = 38) | 21 (55.3) | 10 (26.3) | 7 (18.4) | 17 (44.7) | 33 (86.9) | 1 (2.6) | 4 (10.5) | 5 (13.1) |
| CVID-like (n = 14) | 8 (57.1) | 2 (14.3) | 4 (28.6) | 6 (42.9) | 14 (100) | 0 (0) | 0 (0) | 0 (0) |
| All patients (n = 52) | 29 (55.8) | 12 (23.1) | 11 (21.2) | 23 (44.2) | 47 (90.4) | 1 (1.9) | 4 (7.7) | 5 (9.6) |
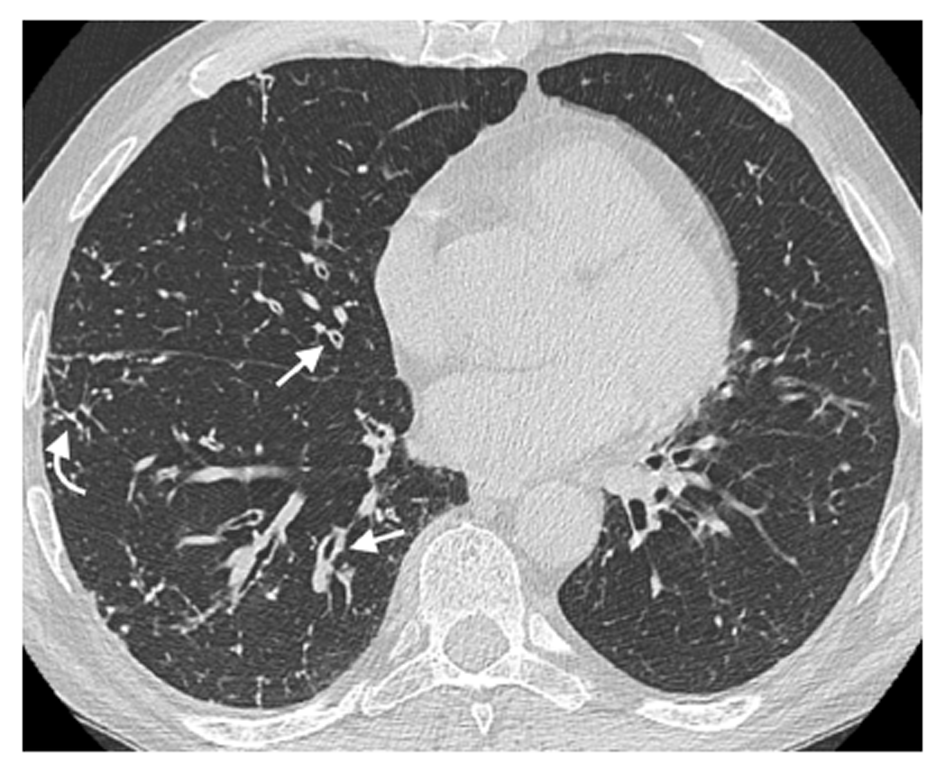
We recorded a high prevalence of HRCT findings, with one or more abnormalities detected in 47/52 hPIDs patients (90.4%). The prevalence of specific airway abnormalities was not significantly different between the CVID and CVID-like groups (P > 0.05, Table 2). Airway wall thickening was the most commonly found abnormality and showed a comparable prevalence in both groups (71.0% in the CVID group and 71.4% in the CVID-like group, Figure 1).
| Abnormality | All patients n = 52 | CVID n = 38 | CVID-like n = 14 | P-value (CVID vs CVID-like) |
| Airway abnormalities | ||||
| Bronchiectasis | 36 (69.2) | 27 (71) | 9 (64.3) | 0.8964 |
| Airway wall thickening | 37 (71.2) | 27 (71) | 10 (71.4) | 0.7501 |
| Tree-in-bud | 10 (19.2) | 6 (15.8) | 4 (28.6) | 0.8464 |
| Mucus plugging | 23 (44.2) | 16 (42.1) | 7 (50) | 0.8888 |
| Air trapping1 | 16 (50) | 12 (52.2) | 4 (44.4) | 0.8557 |
| Parenchymal-interstitial abnormalities | ||||
| Consolidation | 14 (26.9) | 11 (28.9) | 3 (21.4) | 0.8495 |
| Ground-glass opacity | 13 (25) | 12 (31.6) | 1 (7.1) | 0.1487 |
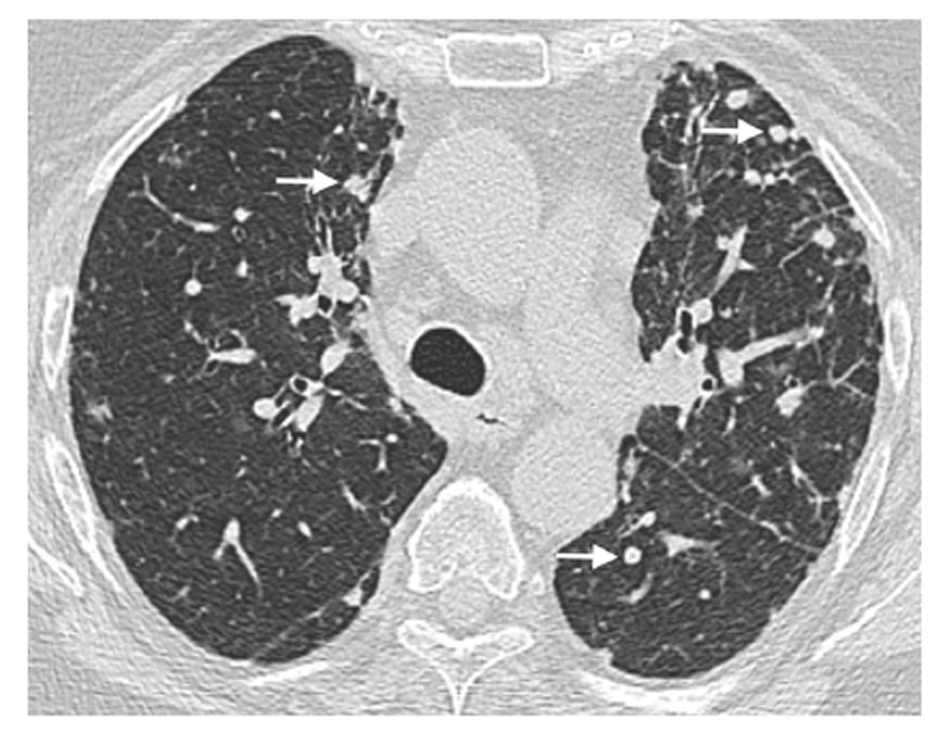
| Nodules | 26 (50) | 19 (50) | 7 (50) | 1 |
| Linear and/or irregular opacities | 12 (23.1) | 12 (31.6) | 0 (0)a | 0.0427 |
| Bullae/cysts | 5 (9.6) | 5 (13.2) | 0 (0) | 0.3695 |
Regarding HRCT parenchymal-interstitial abnormalities, linear and/or irregular opacities were present in 31.6% of the patients in the CVID group and in none of those in the CVID-like group, with borderline significance (P = 0.0427, Table 2). Nodules were the most frequent finding in both groups, with a prevalence of 50.0% (Figure 2).
The distributions of HRCT-detected airway and parenchymal-interstitial abnormalities according to severity of ventilatory defects assessed at PFTs are reported in Tables 3 and 4.
| Abnormality | Absent | Mild | Moderate | Severe | Univariate analysis | Multivariate analysis |
| n = 29 | n = 12 | n = 9 | n = 2 | P-value | P-value (odds ratio) | |
| Bronchiectasis | 19 (66.5) | 9 (75) | 7 (77.8) | 1 (50) | NS | NS |
| Airway wall thickening | 20 (69) | 9 (75) | 6 (66.7) | 2 (100) | NS | NS |
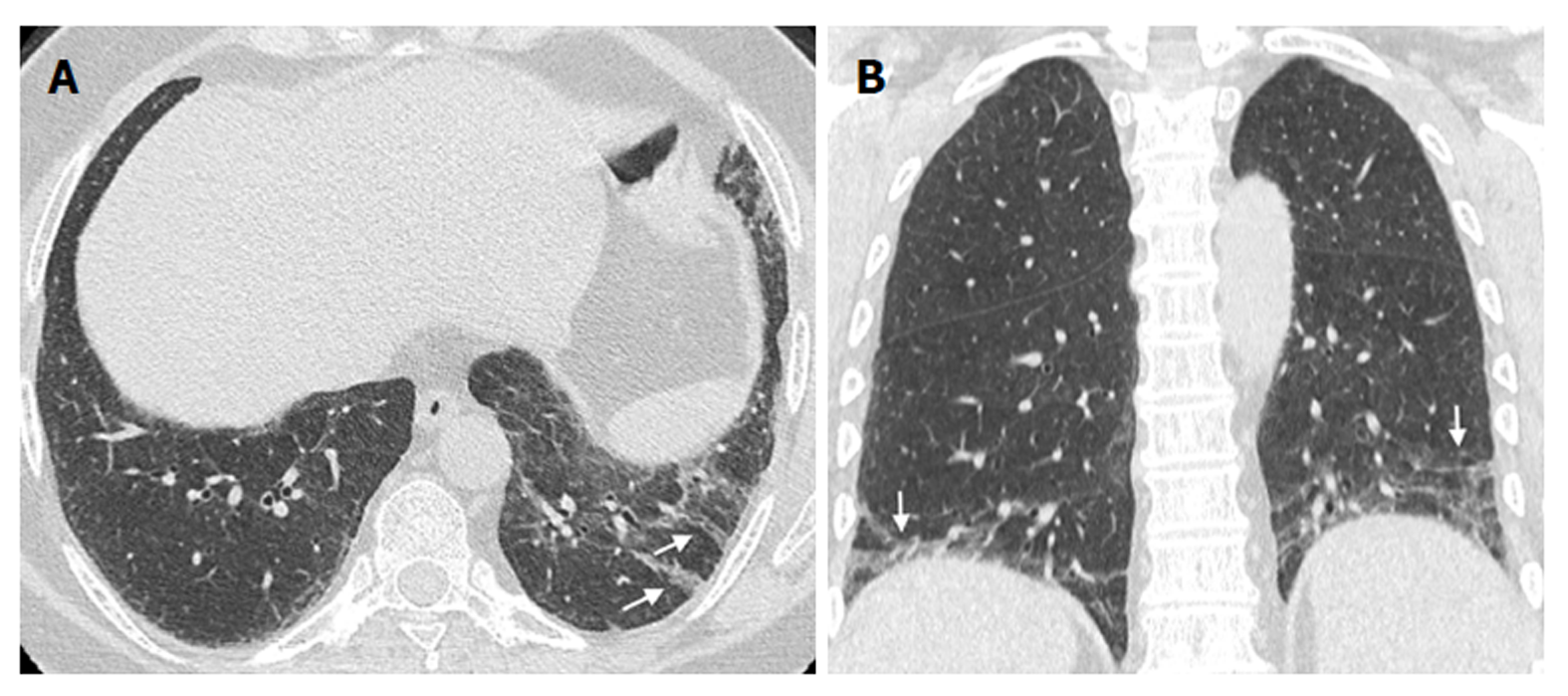
| Tree-in-bud | 2 (6.9) | 2 (16.7) | 5 (55.6) | 1 (50) | 0.0014 | 0.0027 (18.75) |
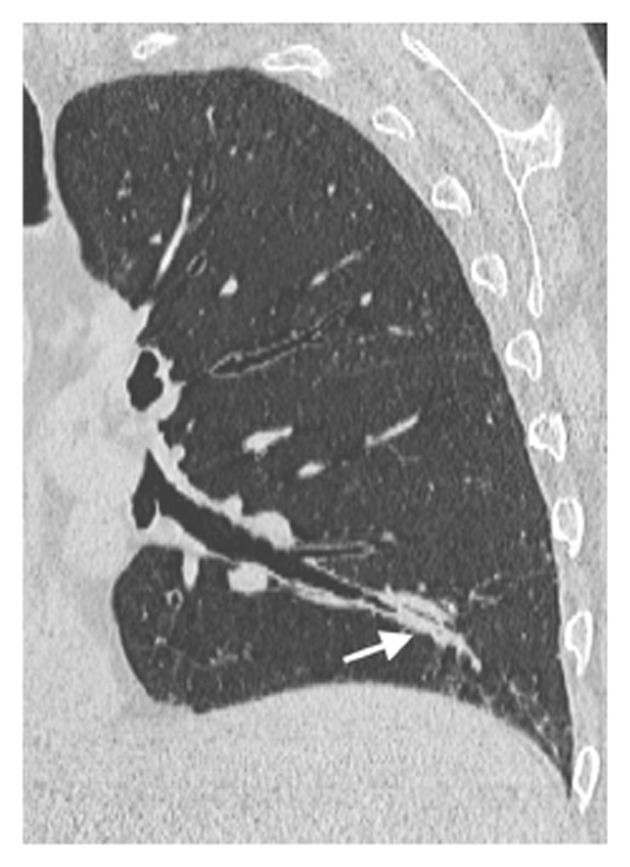
| Mucus plugging | 8 (27.6) | 8 (66.7) | 5 (55.6) | 2 (100) | 0.0112 | NS |
| Abnormality | Absent | Mild | Moderate | Severe | Univariate analysis | Multivariate analysis |
| n = 47 | n = 1 | n = 4 | n = 0 | P-value | P-value (odds ratio) | |
| Consolidation | 11 (23.4) | 1 (100) | 2 (50) | 0 (0) | NS | NS |
| Ground-glass opacity | 10 (21.3) | 1 (100) | 2 (50) | 0 (0) | NS | NS |
| Nodules | 23 (48.9) | 1 (100) | 2 (50) | 0 (0) | NS | NS |
| Linear and/or irregular opacities | 8 (17) | 1 (100) | 3 (75) | 0 (0) | 0.0029 | 0.0344 (13.00) |
| Bullae/cysts | 5 (10.6) | 0 (0) | 0 (0) | 0 (0) | NS | NS |
Regarding HRCT-detected airway abnormalities, both mucus plugging (P = 0.0112, Figure 3) and tree-in-bud (P = 0.0014, Figure 4) were found to be significantly associated with a relevant obstructive defect assessed at PFTs. However, on multivariate analysis, tree-in-bud was the only independent predictor (P = 0.0027) of relevant obstructive defect, with an odds ratio (OR) of 18.75 (95%CI: 2.76-127.52).
Additionally, regarding HRCT-detected parenchymal-interstitial abnormalities, the presence of linear and/or irregular opacities (Figure 5) was the only predictor of relevant restrictive defect both at univariate (P = 0.0029) and multivariate analysis (P = 0.0344; OR, 13.00; 95%CI: 1.21-139.97).
Pulmonary abnormalities in hPIDs adult patients were common in our series: Only 9.6% of our patients had negative HRCTs at the time of diagnosis, a finding in agreement with the literature (range 4%-17%)[9,17,25]. The prevalence of most of airways and parenchymal-interstitial findings was not significantly different between the CVID and the CVID-like groups. Similarly, PFTs results were comparable between the two subtypes of hPIDs, showing a larger prevalence of obstructive defects (45% in the CVID group and 43% in the CVID-like group) over restrictive defects (13% in the CVID group and 0 in the CVID-like group). These findings are in agreement with the previously reported prevalences in CVID patients (9%-53% for obstructive defects and 5%-34% for restrictive defects)[9,17,26,27]. Conversely, to the best of our knowledge, this is the first study reporting prevalences in adult CVID-like patients. Overall, our findings support the hypothesis that neither imaging nor PFTs can reliably differentiate between the two groups of CVID and CVID-like patients. One might argue that the relevance of this result is impaired by the relatively small population included in the present study. However, our series reflects the rarity of both conditions, and is one of the largest published reports on HRCT as far as we know.
Of note, linear and/or irregular opacities were more frequently found in CVID patients (32%) than in CVID-like patients (0), although statistical significance was borderline (P = 0.0427). Gregersen et al[9] highlighted the importance of this abnormality in CVID adult patients, both because of its frequency (about 52% of cases) and its close association with impaired gas diffusion. In line with the relevant literature[11,28], we found that only one HRCT abnormality, linear and/or irregular opacities, was a predictor of significant restrictive defects. This result emphasizes the importance of prompt identification of this abnormality at baseline evaluation. Overall, our results support the assumption that CVID-like patients are clinically and immunologically comparable to CVID patients[18,29], although the latter show more frequently radiological abnormalities associated to interstitial disease (i.e., linear and/or irregular opacities).
It is likely that the prevalence of different HRCT findings might reflect the effects of the specific disease on the lungs in CVID and CVID-like patients. The most frequent findings in our cohort were bronchiectasis (69.2%) and airway wall thickening (71.2%, Figure 2), and are in line with those previously reported in adult patients (range 40%-70% for bronchiectasis and 68%-75% for airway wall thickening)[9,10,17,25,26]. Such a high prevalence can be explained by the cumulative effect of respiratory tract infections[17,30,31]. Other common findings in our study were nodules (found in 50% of patients vs 29%-55% reported in literature)[9,17,25] and mucus plugging (in 44.2% of patients). We did not differentiate among various types of nodules, since their characteristics reflect a wide spectrum of conditions (including infectious diseases, previous infections or lymphoproliferative and/or granulomatous conditions) that cannot be histologically confirmed in most patients[14,25]. We believe that the high prevalence of nodules should be considered as an epiphenomenon of other coexisting pulmonary abnormalities rather than a definite hallmark of CVID or CVID-like hPIDs. However, the finding of mucus plugging mirrors more specifically the inflammation of large airways[9], with a reported prevalence in hPIDs ranging between 25% and 35%[9,17]. Last, air trapping was frequent (50%) in the subgroup of patients for whom an expiratory scan was available. Although incomplete, the prevalence we describe is in the range of those previously reported in CVID patients (45%-63%)[9,17,25].
Concerning the less frequent HRCT findings, we detected tree-in-bud abnormalities in 19% of the patients, similarly to what reported by Tanaka et al[25] (20%) in a study assessing a CVID population. This sign reflects a spectrum of both endobronchiolar and peribronchiolar disorders[24]. The main causes of tree-in-bud findings in the general population are reported to be acute or chronic infections mainly from nontuberculous mycobacteria and bacteria (e.g., Staphylococcus aureus and Pseudomonas aeruginosa)[32,33]. Previous studies demonstrated the presence of potentially pathogenic bacteria (and viruses) in the lungs of patients with clinically stable hPIDs[34]. In our series, most of the tree-in-bud cases were of infectious origin, albeit asymptomatic, at the time of HRCT. The majority of these patients (80%) showed coexisting bronchiectasis, a major predisposing factor for infections[32]. Follicular bronchiolitis (FB) is another cause of tree-in-bud sign detected at HRCT[35,36]. FB is a reactive pulmonary lymphoid disorder reported in CVID patients, is presumably related to recurrent pneumonia, and is characterized by the development of lymphoid follicles and germinal centres with peribronchial/peribronchiolar distribution[37]. In our population, 2 of the 10 cases with tree-in-bud abnormalities were CVID patients who developed GLILD, a condition in which FB is a typical finding[38,39]. In addition, consolidation was found in 27% of our study population (vs 17%-64% reported in the literature) and ground-glass opacities in 25% of the patients (vs 12%-34% in the literature)[9,10,17,25]. Although the nature of most of these abnormalities remained undetermined, it is likely that they represent the effects of subclinical or previous infections, thus not requiring specific radiological work-up in the absence of clinical suspicion of lymphoma or cancer.
In patients with CVID, HRCT was proven to detect silent progression earlier than PFTs, because of its capability to assess even subtle structural abnormalities[34,40]. Not surprisingly, alterations detected by PFTs are less frequent than those detected by HRCT[40], an observation confirmed in our study that also includes CVID-like patients (prevalence was 48.1% by PFTs vs 90.4% by HRCT). This observation raises some questions on how to follow up the patient after baseline evaluation. Current recommendations suggest PFTs intervals of 6-12 mo, and HRCT intervals ranging from 1 to 5 years[11,17]. By multivariate analysis, we found that detection of tree-in-bud abnormalities predicts obstructive defects (OR 18.75), and detection of linear and/or irregular opacities predicts restrictive defects (OR 13.00). By contrast, none of the other, more frequent findings were independent predictors of obstructive or restrictive defects. This observation supports previous data on CVID showing a moderate correlation between PFTs and HRCT results, as well as the idea that these two examinations assess different aspects of the disease[40]. Of note, Maarschalk-Ellerbroek et al[17] found a poor correlation between detection of tree-in-bud abnormalities and PFTs. However, these authors evaluated tree-in-bud abnormalities in a combined score with mucus plugging, which impairs a direct comparison with our findings. Our results suggest that morphological assessment with HCRT might be postponed as much as possible to maximize cost-effectiveness and reduce radiation exposure. A possible exception to this might be the case of patients showing tree-in-bud or linear and/or irregular opacities: Scheduling HRCTs at shorter intervals for these patients might provide a reliable morphological counterpart of pulmonary function. Our hypothesis is extrapolated from the observation of baseline examinations in our population; therefore, further studies with a more specific purpose and a prospective design should be performed to confirm this statement.
We acknowledge that our study has some limitations. First, an HRCT supplementary expiratory scan was not available in 38% of the patients because of the retrospective nature of the study. Hence, we were not able to include air trapping as a variable in the logistic regression analysis to predict PFTs results. Previous studies found a significant correlation between air trapping and airway obstruction both in children and in adult patients with CVID[9,20]. While we acknowledge that we should have included air trapping to study both CVID and CVID-like groups, we believe that most of the relevant HRCT findings support our conclusions. Second, we did not consider the extent and/or distribution of individual HRCT findings in the prediction analysis of PFTs results. The use of dedicated HRCT scoring systems, which were originally developed for CVID patients and/or for paediatric populations only, could help studying this issue also in adult patients with CVID and CVID-like conditions[9,17,18,20]. Third, we were not able to assess the radiologic evolution of the disease over time because of the lack of previous HRCTs, which could have been used for a comparison with the baseline HRCT. However, our study actually reflects the clinical reality, in which a delay between the onset of respiratory infections-related symptoms and the definite diagnosis of hPIDs is common (median of 8 years in adults aged over 30 years old, according to The United Kingdom Primary Immunodeficiency Registry)[41]. In this scenario, the first HRCT is frequently performed only at the time of diagnosis.
In conclusion, we found no significant difference in the prevalence of most of HRCT findings or PFTs abnormalities between CVID and CVID-like conditions. Our results support the hypothesis that these two conditions are comparable hPIDs subtypes and candidate to similar management. The detection of tree-in-bud abnormalities was found to be an independent predictor of obstructive defects assessed at PFTs, while the detection of linear and/or irregular opacities was an independent predictor of restrictive defects assessed at PFTs. Our observations suggest that patients showing these findings might benefit from more frequent HRCTs during follow-up as to evaluate the morphological abnormalities associated with their function impairment.
The authors thank Viviana Moroso of MV Medical Writing (Lulea, Sweden) for copyediting the manuscript, a service that was funded by Department of Medicine, University of Udine (Udine, Italy) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Common variable immunodeficiency disorders (CVID) are the most clinically significant group of humoral primary immunodeficiency diseases (hPIDs), manifesting with recurrent respiratory tract infections and increased susceptibility to autoimmune diseases and malignancy. Other hPIDs are often termed “CVID-like” conditions, and include selective IgA deficiency and isolated IgG subclass deficiency. The initial evaluation of patients newly diagnosed with hPIDs should include high-resolution computed tomography (HRCT) and pulmonary function tests (PFTs).
To the best of our knowledge no previous studies assessed whether HRCT findings differ in terms of prevalence among the two subtypes of hPIDs in adult patients. Demonstrating a difference between CVID and CVID-like patients may influence the time intervals between HRCT examinations during follow-up in these 2 subgroups of hPIDs. Moreover, previous studies assessing a possible correlation between HRCT findings and PFTs results demonstrated contradictory results.
The purpose of this study was twofold: (1) to compare HRCT pulmonary findings in adult patients with different subgroups of hPIDs (i.e., CVID vs CVID-like); and (2) to assess whether HRCT findings predict PFTs results.
We included 52 adult patients (38 CVID and 14 CVID-like) who received a definite diagnosis of hPIDs and underwent HRCT and PFTs within one month from the time of diagnosis. One pulmonary radiologist, blinded to patient history and lung function: (1) reviewed the HRCT examinations; (2) recorded two classes of abnormalities, namely airway abnormalities and/or parenchymal-interstitial abnormalities; and (3) scored all abnormalities according to their extension and conspicuity. We calculated the per-patient prevalence of each HRCT abnormality in the overall population and in both subgroups of hPIDs patients, (CVID and CVID-like groups). We performed a logistic regression analysis to assess whether HRCT findings were predictive of a relevant obstructive or restrictive defect at PFTs on the overall study population.
Of the 52 hPIDs patients, 37 were females and 15 were males, with a mean age of 53.9 ± 12.7 years. We found a high prevalence of HRCT findings (90.4% patients had one or more abnormalities). The prevalence of each of the airway abnormalities considered was not significantly different between the CVID and CVID-like group. Regarding HRCT-detected parenchymal-interstitial abnormalities, the only relevant result was the finding of linear and/or irregular opacities, showing a prevalence of 31.6% in the CVID group and 0 in the CVID-like group, with borderline significance. The presence of tree-in-bud abnormalities was an independent predictor of obstructive defects at PFTs (Odds Ratio, OR, of 18.75, P < 0.05), while the presence of linear and/or irregular opacities was an independent predictor of restrictive defects at PFTs (OR = 13.00; P < 0.05).
No previous research compared the prevalence of HRCT findings in different subtypes of hPIDs adult patients. After dividing hPIDs patients in CVID vs CVID-like groups, we observed no significant difference in the prevalence of most of airways and parenchymal-interstitial findings between the two groups. This observation supports the hypothesis that these two groups represent comparable hPIDs subtypes, and are candidate to similar management. Tree-in-bud and linear and/or irregular opacities were found to be independent predictors of, respectively, obstructive and restrictive defects on PFTs.
Our results suggest that morphological assessment with HRCT might be delayed as much as possible to maximize cost-effectiveness and reduce radiation exposure. A possible exception to this might be the case of patients showing tree-in-bud or linear and/or irregular opacities: Scheduling HRCTs at shorter intervals for these patients might provide a reliable morphological counterpart of pulmonary function. Further prospective studies with a proper design are needed to confirm this hypothesis in the follow-up period.
| 1. | Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, Keller M, Kobrynski LJ, Komarow HD, Mazer B. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186-1205.e1-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 493] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 2. | Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125:S182-S194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. International Union of Immunological Societies. Clin Exp Immunol. 1999;118 Suppl 1:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. 2018;38:96-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 580] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 5. | Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 619] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 6. | Tam JS, Routes JM. Common variable immunodeficiency. Am J Rhinol Allergy. 2013;27:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Janzi M, Kull I, Sjöberg R, Wan J, Melén E, Bayat N, Ostblom E, Pan-Hammarström Q, Nilsson P, Hammarström L. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Palmer DS, O’Toole J, Montreuil T, Scalia V, Yi QL, Goldman M, Towns D. Screening of Canadian Blood Services donors for severe immunoglobulin A deficiency. Transfusion. 2010;50:1524-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Gregersen S, Aaløkken TM, Mynarek G, Kongerud J, Aukrust P, Frøland SS, Johansen B. High resolution computed tomography and pulmonary function in common variable immunodeficiency. Respir Med. 2009;103:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Bondioni MP, Duse M, Plebani A, Soresina A, Notarangelo LD, Berlucchi M, Grazioli L. Pulmonary and sinusal changes in 45 patients with primary immunodeficiencies: computed tomography evaluation. J Comput Assist Tomogr. 2007;31:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Verma N, Grimbacher B, Hurst JR. Lung disease in primary antibody deficiency. Lancet Respir Med. 2015;3:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Hurst JR, Workman S, Garcha DS, Seneviratne SL, Haddock JA, Grimbacher B. Activity, severity and impact of respiratory disease in primary antibody deficiency syndromes. J Clin Immunol. 2014;34:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Bierry G, Boileau J, Barnig C, Gasser B, Korganow AS, Buy X, Jeung MY, Roy C, Gangi A. Thoracic manifestations of primary humoral immunodeficiency: a comprehensive review. Radiographics. 2009;29:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Hampson FA, Chandra A, Screaton NJ, Condliffe A, Kumararatne DS, Exley AR, Babar JL. Respiratory disease in common variable immunodeficiency and other primary immunodeficiency disorders. Clin Radiol. 2012;67:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Cereser L, Girometti R, d’Angelo P, De Carli M, De Pellegrin A, Zuiani C. Humoral primary immunodeficiency diseases: clinical overview and chest high-resolution computed tomography (HRCT) features in the adult population. Clin Radiol. 2017;72:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bang TJ, Richards JC, Olson AL, Groshong SD, Gelfand EW, Lynch DA. Pulmonary Manifestations of Common Variable Immunodeficiency. J Thorac Imaging. 2018;33:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Maarschalk-Ellerbroek LJ, de Jong PA, van Montfrans JM, Lammers JW, Bloem AC, Hoepelman AI, Ellerbroek PM. CT screening for pulmonary pathology in common variable immunodeficiency disorders and the correlation with clinical and immunological parameters. J Clin Immunol. 2014;34:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | van de Ven AA, van Montfrans JM, Terheggen-Lagro SW, Beek FJ, Hoytema van Konijnenburg DP, Kessels OA, de Jong PA. A CT scan score for the assessment of lung disease in children with common variable immunodeficiency disorders. Chest. 2010;138:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | van de Ven AA, de Jong PA, Hoytema van Konijnenburg DP, Kessels OA, Boes M, Sanders EA, Terheggen-Lagro SW, van Montfrans JM. Airway and interstitial lung disease are distinct entities in paediatric common variable immunodeficiency. Clin Exp Immunol. 2011;165:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | van Zeggeren L, van de Ven AA, Terheggen-Lagro SW, Mets OM, Beek FJ, van Montfrans JM, de Jong PA. High-resolution computed tomography and pulmonary function in children with common variable immunodeficiency. Eur Respir J. 2011;38:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | European Society for Immunodeficiencies Registry Working Party. Working definitions for clinical diagnosis of PID. Available from: http://esid.org/Working-Parties/Registry/Diagnosis-criteria. |
| 22. | Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P. Standardisation of spirometry. Eur Respir J. 2005;26:319-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9568] [Cited by in RCA: 11650] [Article Influence: 582.5] [Reference Citation Analysis (0)] |
| 23. | Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3399] [Cited by in RCA: 3933] [Article Influence: 196.7] [Reference Citation Analysis (0)] |
| 24. | Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2674] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 25. | Tanaka N, Kim JS, Bates CA, Brown KK, Cool CD, Newell JD, Lynch DA. Lung diseases in patients with common variable immunodeficiency: chest radiographic, and computed tomographic findings. J Comput Assist Tomogr. 2006;30:828-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Thickett KM, Kumararatne DS, Banerjee AK, Dudley R, Stableforth DE. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM. 2002;95:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Martínez García MA, de Rojas MD, Nauffal Manzur MD, Muñoz Pamplona MP, Compte Torrero L, Macián V, Perpiñá Tordera M. Respiratory disorders in common variable immunodeficiency. Respir Med. 2001;95:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Aghamohammadi A, Allahverdi A, Abolhassani H, Moazzami K, Alizadeh H, Gharagozlou M, Kalantari N, Sajedi V, Shafiei A, Parvaneh N. Comparison of pulmonary diseases in common variable immunodeficiency and X-linked agammaglobulinaemia. Respirology. 2010;15:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | van de Ven AA, van de Corput L, van Tilburg CM, Tesselaar K, van Gent R, Sanders EA, Boes M, Bloem AC, van Montfrans JM. Lymphocyte characteristics in children with common variable immunodeficiency. Clin Immunol. 2010;135:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Sweinberg SK, Wodell RA, Grodofsky MP, Greene JM, Conley ME. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinemia. J Allergy Clin Immunol. 1991;88:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Berger M, Geng B, Cameron DW, Murphy LM, Schulman ES. Primary immune deficiency diseases as unrecognized causes of chronic respiratory disease. Respir Med. 2017;132:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Miller WT Jr, Panosian JS. Causes and imaging patterns of tree-in-bud opacities. Chest. 2013;144:1883-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Rossi SE, Franquet T, Volpacchio M, Giménez A, Aguilar G. Tree-in-bud pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2005;25:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Kainulainen L, Nikoskelainen J, Vuorinen T, Tevola K, Liippo K, Ruuskanen O. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Respir Crit Care Med. 1999;159:1199-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Gosset N, Bankier AA, Eisenberg RL. Tree-in-bud pattern. AJR Am J Roentgenol. 2009;193:W472-W477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Tashtoush B, Okafor NC, Ramirez JF, Smolley L. Follicular Bronchiolitis: A Literature Review. J Clin Diagn Res. 2015;9:OE01-OE05. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Camarasa Escrig A, Amat Humaran B, Sapia S, León Ramírez JM. Follicular bronchiolitis associated with common variable immunodeficiency. Arch Bronconeumol. 2013;49:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Tashtoush B, Memarpour R, Ramirez J, Bejarano P, Mehta J. Granulomatous-lymphocytic interstitial lung disease as the first manifestation of common variable immunodeficiency. Clin Respir J. 2018;12:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Touw CM, van de Ven AA, de Jong PA, Terheggen-Lagro S, Beek E, Sanders EA, van Montfrans JM. Detection of pulmonary complications in common variable immunodeficiency. Pediatr Allergy Immunol. 2010;21:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Edgar JD, Buckland M, Guzman D, Conlon NP, Knerr V, Bangs C, Reiser V, Panahloo Z, Workman S, Slatter M. The United Kingdom Primary Immune Deficiency (UKPID) Registry: report of the first 4 years’ activity 2008-2012. Clin Exp Immunol. 2014;175:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bazeed MF, Chow J, Gao BL, Lacalzada-Almeida J, Tang GH S- Editor: Ji FF L- Editor: A E- Editor: Huang Y