Published online Nov 28, 2018. doi: 10.4329/wjr.v10.i11.162
Peer-review started: August 5, 2018
First decision: August 24, 2018
Revised: September 22, 2018
Accepted: October 6, 2018
Article in press: October 6, 2018
Published online: November 28, 2018
Processing time: 128 Days and 14.6 Hours
To investigate the reliability of the established and new scoring methods for Hoffa’s fat pad synovitis using magnetic resonance imaging (MRI).
A total of 139 knees of 115 patients who underwent MRI of the knee with and without gadolinium contrast were enrolled in this study. Proton density (PD)-weighted, PD-weighted fat-suppressed (PD-FS), and postcontrast T1-weighted fat-suppressed (T1CE) images were used for evaluation. Using contrast and non-contrast images, our grading method for synovitis was performed to measure synovial thickness and signal intensity changes of the fat pad [Synovial membrane (SM) score], which was compared with the established methods, including MRI Osteoarthritis Knee Score (MOAKS), parapatellar synovitis score, Whole-Organ Magnetic Resonance Imaging Score (WORMS), and suprapatellar effusion diameter. Intraclass correlation coefficients (ICC) for intra and interobserver reproducibility and Spearman correlation coefficients (r) were calculated for the parapatellar synovitis score and each scoring method.
All of the scores presented substantial to almost perfect intrareliability. Among three readers, effusion diameter had substantial to almost perfect interreliability (ICC = 0.68-0.81) and WORMS had substantial interreliability (ICC = 0.61-0.70). For two out of three readers, there was substantial interreliability for the thickness score in T1CE (ICC = 0.55-0.69), SM scores in T1CE (ICC = 0.56-0.78) and PD-FS (ICC = 0.51-0.79), and parapatellar synovitis score in T1CE (ICC = 0.53-0.72). The parapatellar synovitis score was significantly correlated with the thickness score in T1CE (r = 0.70) and the SM score in T1CE (r = 0.81) and PD-FS (r = 0.65).
The newly proposed quantitative thickness score on T1CE and the semi-quantitative SM score on T1CE and PD-FS can be useful for Hoffa’s fat pad synovitis.
Core tip: We proposed a new grading method for Hoffa’s fat pad synovitis and compared it with the other established methods. Our method showed substantial to almost perfect reproducibility and significant correlations with the established methods for both non-contrast and contrast images. Our newly proposed scoring system method can be useful for Hoffa’s fat pad synovitis.
- Citation: Hagiwara S, Yang A, Takao S, Kaneko Y, Nozaki T, Yoshioka H. New scoring system in assessment of Hoffa’s fat pad synovitis: A comparative study with established scoring systems. World J Radiol 2018; 10(11): 162-171
- URL: https://www.wjgnet.com/1949-8470/full/v10/i11/162.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i11.162
Osteoarthritis (OA) of the knee is one of the most common chronic disorders that result in pain, deformity, and loss of function. OA has long been considered a wear and tear disease that leads to loss of cartilage because of mechanical stress. Recent experimental data have shown that OA is a complex disease with inflammatory mediators, which are released by cartilage, bone, and synovial fat pad[1-3].
The Hoffa’s fat pad is located in the knee between the patellar tendon, femoral condyle, and tibial plateau. It is adjacent to the synovial layers and the cartilage surface of the femur[4]. Similar to subcutaneous tissue, the Hoffa’s fat pad contains a framework of fibrous cords interspersed among adipose tissue[5] and is thought to distribute the synovial fluid and absorb forces through the knee joint[6]. Several studies have revealed that the fat pad produces growth factors and proinflammatory cytokines, which may contribute to the pathologic development of OA[7-9].
Several semiquantitative methods using magnetic resonance imaging (MRI) for the assessment of knee OA have been developed and used in various observational studies and clinical trials; by dividing the knee into subregions for assessment, these methods enable visualization of structures, such as the synovium, joint effusion, and cartilage[10,11]. Although each method have been reported to be clinically useful, the reliability was not perfect[12-14], and the scoring for Hoffa’s fat pad synovitis based on non-enhanced sequences has not been sufficient, compared with that for the other subregions of the knee[10,11]. Although enhanced sequences allow for better characterization of synovial inflammation and for differentiation between the synovium and effusion, few scoring methods have been reported to be specific to Hoffa’s fat pad synovitis[15,16]. Also, a routine knee MRI is usually obtained without contrast administration.
The aim of this study was to evaluate reliability of the established and new scoring methods, including non-enhanced MRI, for Hoffa’s fat pad synovitis.
The subjects enrolled in this study were all patients who underwent knee MRI with and without Gadolinium (Gd) contrast at our institute from January 2012 to July 2015. During this period, MRI of the knee with and without Gd contrast was performed on 205 knees (102 on the right, 103 on the left) of 168 patients. The exclusion criteria of this study were as follows: (1) under 18 years old; (2) postoperation with an implant around the knee; (3) inflammatory arthritis, such as rheumatoid arthritis and infection; (4) acute trauma with hemarthrosis; (5) intraarticular tumor; (6) difficult evaluation due to severe deformity from OA or amputation; and (7) difficult evaluation due to severe artifact. After exclusion of 66 knees of 53 patients, a total of 139 knees (69 on the right, 70 on the left) of 115 patients available for analyses. The subjects had an average age of 54 years.
The research protocol of this retrospective study was in compliance with the Helsinki Declaration, was approved by the institutional review board, and was registered with the University of California Irvine Medical Center.
Each MRI examination in this study was performed according to a standardized institutional protocol using the 1.5T (Avanto, Siemens Healthcare, Erlangen, Germany) or the 3T MRI system (Achieva or dStream Achieva, Philips Healthcare, Best, Netherland and TtioTim, Siemens Healthcare); 42 knees underwent MRI with 1.5T and 97 knees with 3T. A non-enhanced proton density (PD)-weighted fast spin-echo sequence was performed in the sagittal plane, followed by non-enhanced PD-weighted fat-suppressed (PD-FS) fast spin-echo sequence in the sagittal and axial planes. After injection of 10 mL of Gd contrast (Multihance, Bracco) into a peripheral vein, T1-weighted fat-suppressed fast spin-echo sequence (T1CE) was performed in the sagittal and axial planes. The imaging parameters of all sequences are summarized in Table 1.
| Imaging parameter | Tesla | Sequence | ||||
| Sag PD | Sag PD-FS | Sag T1CE | Ax PD-FS | Ax T1CE | ||
| Repetition time (ms) | 1.5T | 2000 | 3000 | 438 | 3400 | 714 |
| 3T | 2054-3940 | 3000-4150 | 530-782 | 3000-5300 | 530-782 | |
| Echo time (ms) | 1.5T | 46 | 46 | 12 | 43 | 13 |
| 3T | 22-30 | 30-43 | 12-20 | 13-45 | 12-20 | |
| Matrix resolution (mm) | 1.5T | 0.7 × 0.7 | 0.7 × 0.7 | 0.7 × 0.7 | 0.6 × 0.6 | 0.6 × 0.6 |
| 3T | 0.3 × 3.3 | 0.3 × 0.3 | 0.3 × 0.3 | 0.3 × 0.3 | 0.3 × 0.3 | |
| Field of view (mm) | 1.5T | 150 | 150 | 150 | 150 | 150 |
| 3T | 150 | 150 | 150 | 150 | 150 | |
| Slice thickness (mm) | 1.5T | 3.5 | 3.5 | 3.5 | 5 | 5 |
| 3T | 2.5 | 2.5 | 2.5 | 3 | 3 | |
The MRI evaluation of synovitis and joint effusion was performed independently by a board-certified orthopedic surgeon (A), who had 10 years of experience, and two radiology residents (B, C), who had 2 years of experience each. All readers were blinded to the clinical information but not to the MR sequences, because the imaging characteristics were readily apparent to the observer. To evaluate intraobserver reliability, a second trial was performed by Hagiwara S four weeks later.
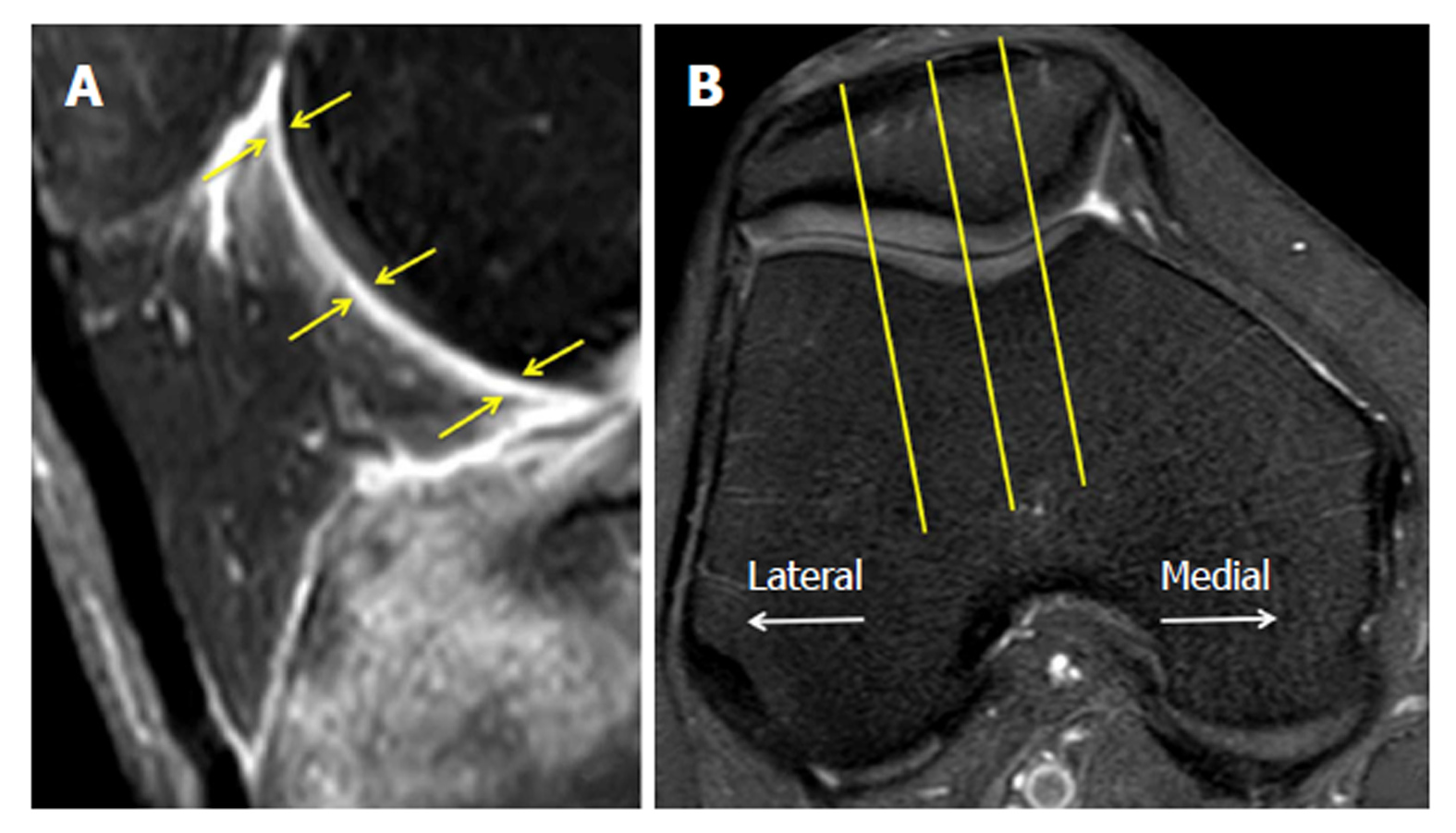
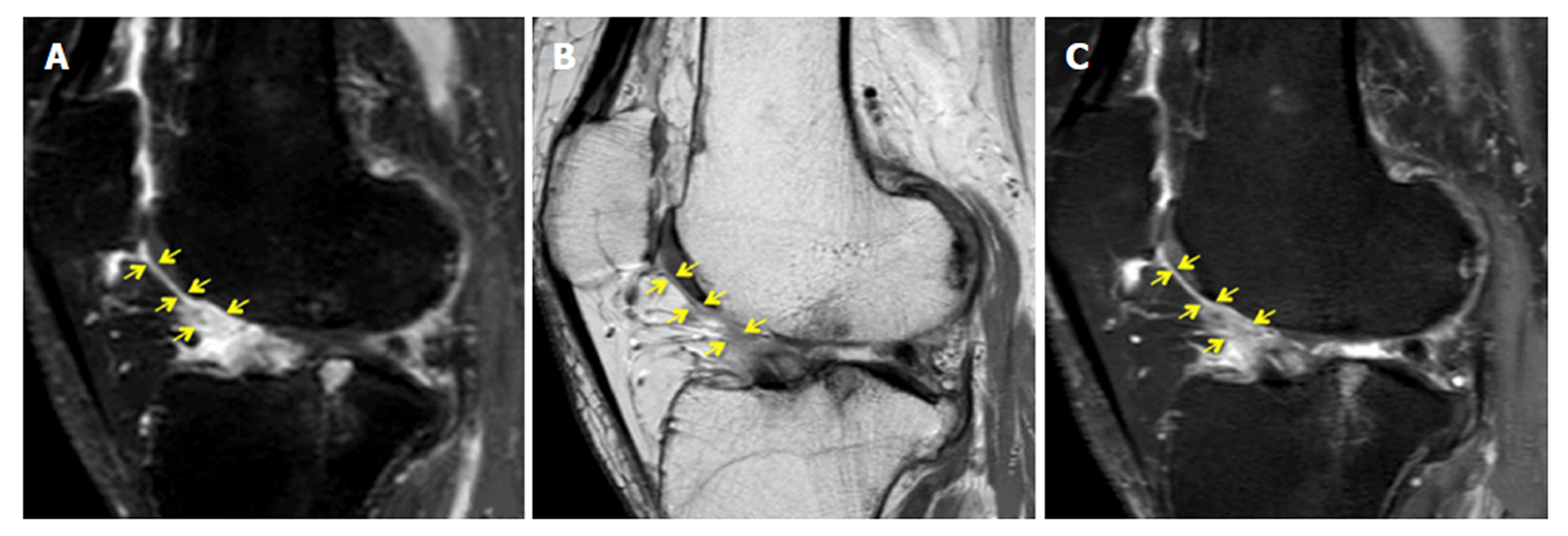
Thickness score: For the new quantitative scoring system, the thickness of the inflamed synovium was determined as the area of enhancement along the posterior Hoffa’s fat pad on sagittal T1CE, as low-signal regions on non-enhanced sagittal PD, and as high-signal regions on non-enhanced sagittal PD-FS. Three sagittal slices, including the medial and lateral aspects of the Hoffa’s fat pad and the central patellofemoral groove, were chosen. In each slice, three points (i.e., proximal, middle, and distal) along the posterior surface of Hoffa’s fat pad were selected. The average thickness of the synovium from the nine points in each sequence (Figure 1) was graded on a three-point scale: grade 1 ≤ 0.8 mm; grade 2 = 0.8 mm to 1.6 mm; and grade 3 ≥ 1.6 mm.
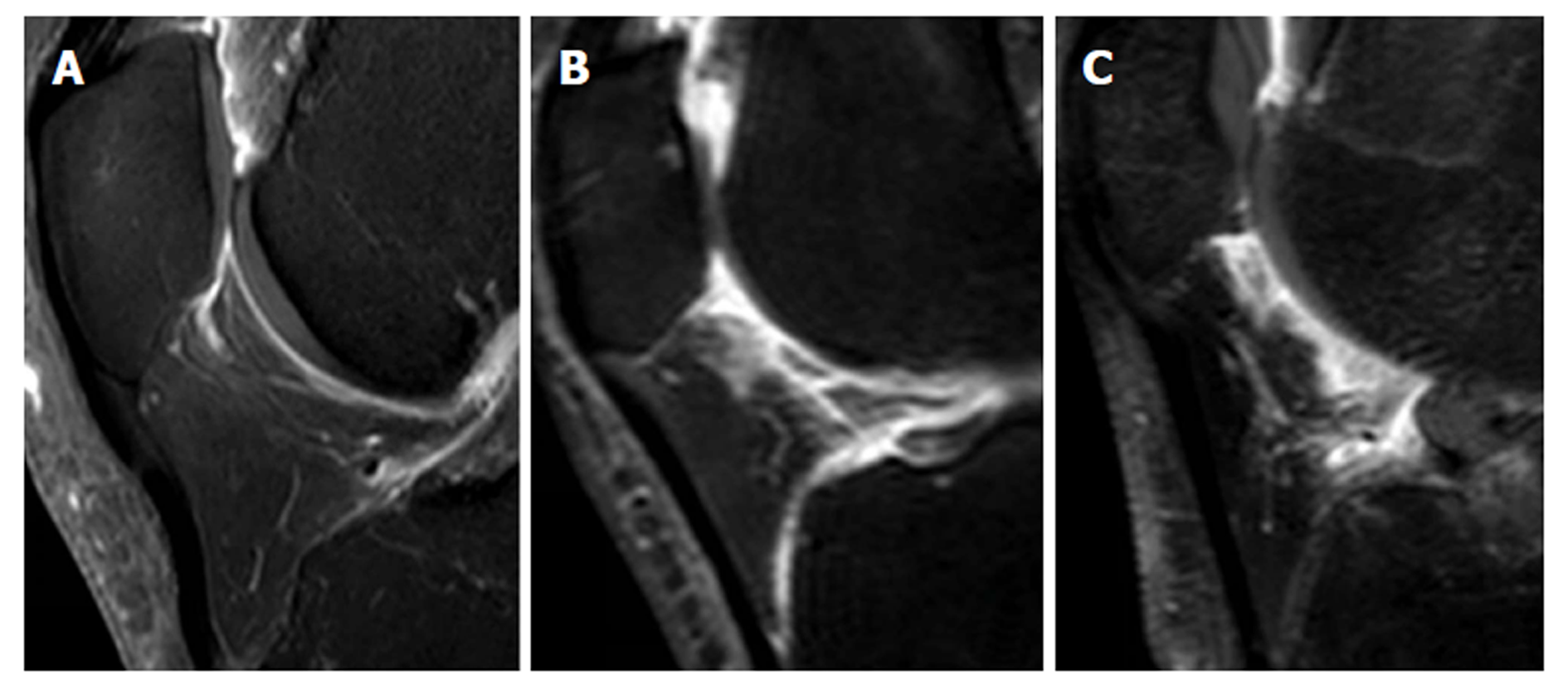
Synovial membrane score (SM score): The contrast effects and signal intensity changes along the posterior surface of the Hoffa’s fat pad on a single sagittal slice through the central patellofemoral groove were used to semiquantitatively grade the synovitis. The score was based on the contrast effects and signal intensity changes[17] on sagittal T1CE; low-signal areas on sagittal PD; and high-signal areas on sagittal PD-FS. The score was graded on a four-point scale as grade 0 for lack of enhancement or signal change; grade 1 for the presence of a linear contrast effect or signal change on the posterior fat pad synovium; grade 2 for the presence of a nodular contrast effect or signal change on the posterior fat pad and/or mild exudation of the fat pad on T1CE; and grade 3 for gross a nodular contrast effect or signal change on the posterior fat pad and/or severe exudation of the fat pad on T1CE, as seen on the sagittal plane at the center of the patella (Figure 2).
MRI Osteoarthritis Knee Score (MOAKS) for fat pad: Hoffa’s synovitis was scored with the MOAKS system[11] by grading the size of the diffuse hyperintense signal in the Hoffa’s fat pad on T1CE and PD-FS on a four-point scale, as follows: 0 = normal; 1 = mild, 2 = moderate, 3 = severe.
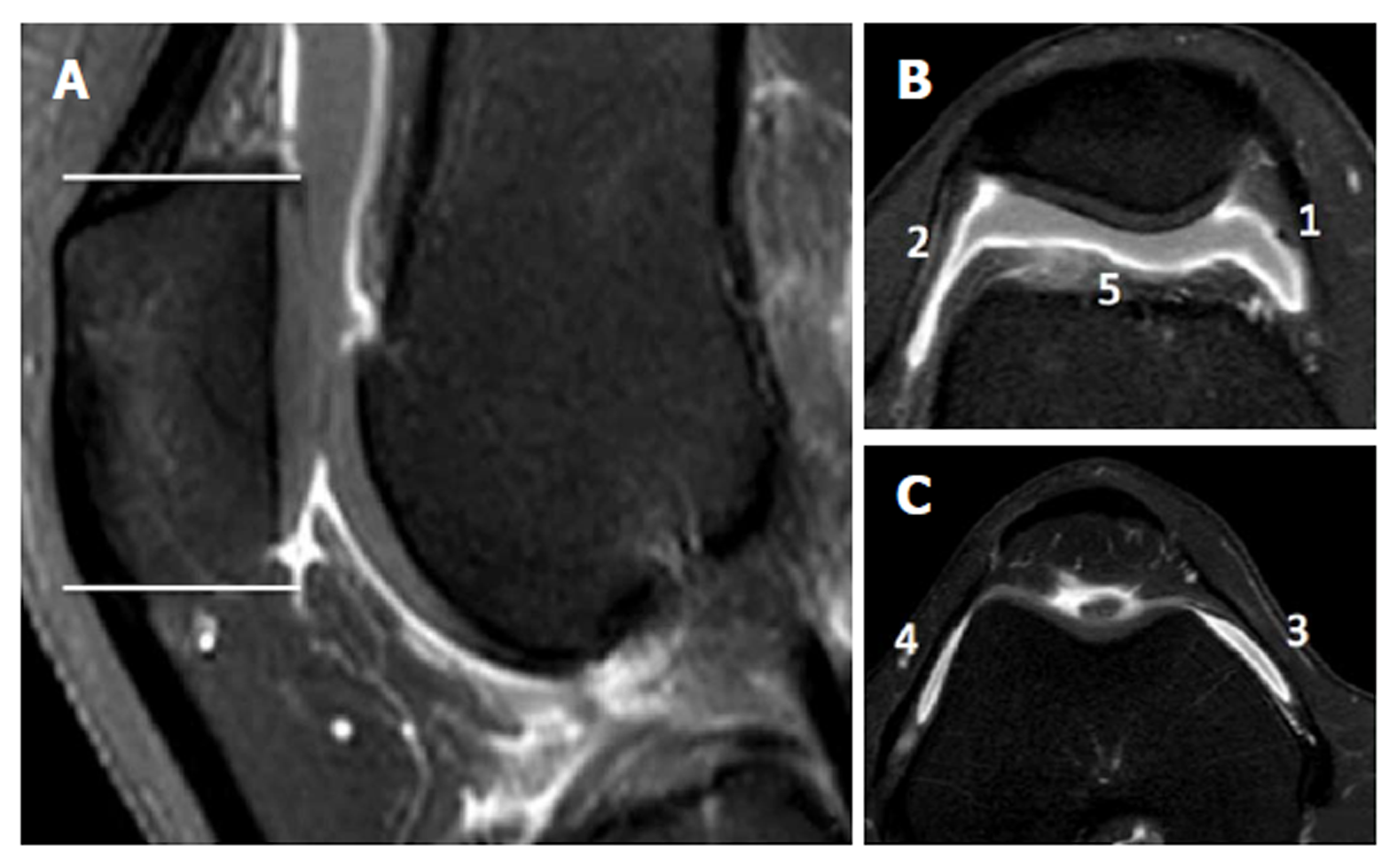
Parapatellar synovitis score: Synovial inflammation in the entire knee was scored, as previously reported[18]. These parapatellar sites included three in the suprapatellar recesses (i.e., lateral, medial, and just above the trochlear groove), as well as the medial and lateral femoral gutters. Thickening of the inflamed synovium was determined in each site and was scored on a four-point scale, according to the thickness, as grade 0 for lack of enhancement of the synovial tissue; grade 1 for < 2-mm thickening of the synovial tissue; grade 2 for 2-4-mm thickening of the synovial tissue; and grade 3 for > 4-mm thickening or nodular pattern of the synovial tissue (Figure 3).
Effusion diameter: Joint effusion was assessed based on the greatest diameter of the fluid accumulation, perpendicular to the long axis of the leg on non-enhanced PD-FS, as follows; grade 0, < 5 mm; grade 1, between 5 mm and 10 mm; grade 2, between 10 mm and 20 mm; and grade 3, > 20 mm[19].
Whole-Organ Magnetic Resonance Imaging Score (WORMS) for synovial effusion: Synovial effusion was evaluated to describe the synovitis according to the WORMS system[10]. Joint effusion was graded collectively from 0 to 3 in terms of the percentage of the estimated maximal distention of the synovial cavity, as follows: grade 0, normal; grade 1, < 33%; grade 2, 33% to 66%; and grade 3, > 66%.
Statistical analysis was performed by the biostatistics service of our institute using MedCalc software (Ver.16, MedCalc Software, Ostend, Belgium). The inter and intraobserver reliabilities of each score were assessed using intraclass correlation coefficient (ICC) analysis. An ICC of 0.2-0.4 was considered as fair, 0.4-0.6 as moderate, 0.6-0.8 as substantial, and 0.8-1 as almost perfect[20]. Spearman’s rank correlation (r) was computed to analyze the correlation between each scoring system and the MOAKS score, which was the most popular MRI semiquantitative scoring system for OA[14]. Because an adequate correlation between the parapatellar synovitis score and arthroscopic and microscopic scoring has been reported[21,22], the correlations of the parapatellar synovitis score with each scoring system were computed on 1.5T and 3T, respectively. Correlation was considered negligible for a r value < 0.2, low for a r value of 0.2-0.4, moderate for a r value of 0.4-0.7, strong for a r value of 0.7-0.9, and very strong for a r value > 0.9[23].
The ICCs for inter and intraobserver reliabilities of each score are shown in Table 2. All of the scores had substantial to almost perfect intrareliability. Among the three readers, interreliability was substantial to almost perfect for effusion diameter (ICC = 0.68-0.81) and substantial for WORMS (ICC = 0.61-0.70). For two out of three readers, there was substantial interreliability for the thickness score in T1CE (ICC = 0.55-0.69), SM scores in T1CE (ICC = 0.56-0.78) and PD-FS (ICC = 0.51-0.79), and parapatellar synovitis score in T1CE (ICC = 0.53-0.72).
| Intra-observer | Inter-observer | ||||
| A1-A2 | A1-B | A1-C | B-C | ||
| Thickness | T1CE | 0.74 (0.65-0.81) | 0.69 (0.60-0.77) | 0.67 (0.56-0.75) | 0.55 (0.42-0.65) |
| PD | 0.68 (0.58-0.76) | 0.45 (0.31-0.57) | 0.49 (0.35-0.61) | 0.54 (0.41-0.65) | |
| PD-FS | 0.71 (0.61-0.78) | 0.59 (0.48-0.69) | 0.49 (0.35-0.60) | 0.63 (0.52-0.72) | |
| SM score | T1CE | 0.88 (0.83-0.91) | 0.67 (0.56-0.75) | 0.78 (0.70-0.83) | 0.56 (0.43-0.66) |
| PD | 0.88 (0.83-0.91) | 0.49 (0.35-0.60) | 0.72 (0.63-0.79) | 0.50 (0.36-0.61) | |
| PD-FS | 0.89 (0.85-0.92) | 0.51 (0.38-0.63) | 0.79 (0.71-0.84) | 0.62 (0.50-0.71) | |
| MOAKS | T1CE | 0.76 (0.68-0.82) | 0.64 (0.53-0.73) | 0.54 (0.41-0.65) | 0.49 (0.35-0.60) |
| Synovitis-score | T1CE | 0.93 (0.91-0.95) | 0.65 (0.54-0.74) | 0.72 (0.62-0.79) | 0.53 (0.40-0.64) |
| WORMS (effusion synovitis) | PD-FS | 0.77 (0.70-0.83) | 0.70 (0.61-0.78) | 0.61 (0.50-0.71) | 0.68 (0.58-0.76) |
| Effusion-diameter | PD-FS | 0.89 (0.85-0.92) | 0.77 (0.69-0.83) | 0.81 (0.75-0.86) | 0.68 (0.58-0.76) |
The Spearman’s rank correlation results for the MOAKS are shown in Table 3. On the average, the MOAKS had moderate to strong correlations with each scoring system (r = 0.44-0.71). The correlations of each scoring system with the parapatellar synovitis score are shown in Table 4. The parapatellar synovitis score had nearly strong correlations with the thickness score in T1CE (r = 0.70), SM score in T1CE (r = 0.81), and SM score in PD-FS (r = 0.65). There were no significant differences between the 1.5T and 3T sequences.
| 1.5T | 3T | Total | ||
| Thickness | T1CE | 0.45 | 0.49 | 0.47 |
| PD | 0.32 | 0.49 | 0.44 | |
| PD-FS | 0.42 | 0.41 | 0.41 | |
| SM score | T1CE | 0.55 | 0.68 | 0.64 |
| PD | 0.61 | 0.64 | 0.63 | |
| PD-FS | 0.64 | 0.74 | 0.71 | |
| Synovitis-score | T1CE | 0.68 | 0.51 | 0.56 |
| WORMS (effusion synovitis) | PD-FS | 0.48 | 0.55 | 0.53 |
| Effusion-diameter | PD-FS | 0.58 | 0.48 | 0.51 |
| 1.5T | 3T | Total | ||
| Thickness | T1CE | 0.63 | 0.73 | 0.70 |
| PD | 0.52 | 0.45 | 0.47 | |
| PD-FS | 0.44 | 0.51 | 0.49 | |
| SM score | T1CE | 0.85 | 0.79 | 0.81 |
| PD | 0.42 | 0.76 | 0.66 | |
| PD-FS | 0.79 | 0.59 | 0.65 | |
| MOAKS | T1CE | 0.66 | 0.56 | 0.59 |
| PD-FS | 0.65 | 0.52 | 0.56 | |
| WORMS (effusion synovitis) | PD-FS | 0.64 | 0.81 | 0.76 |
| Effusion-diameter | PD-FS | 0.52 | 0.62 | 0.59 |
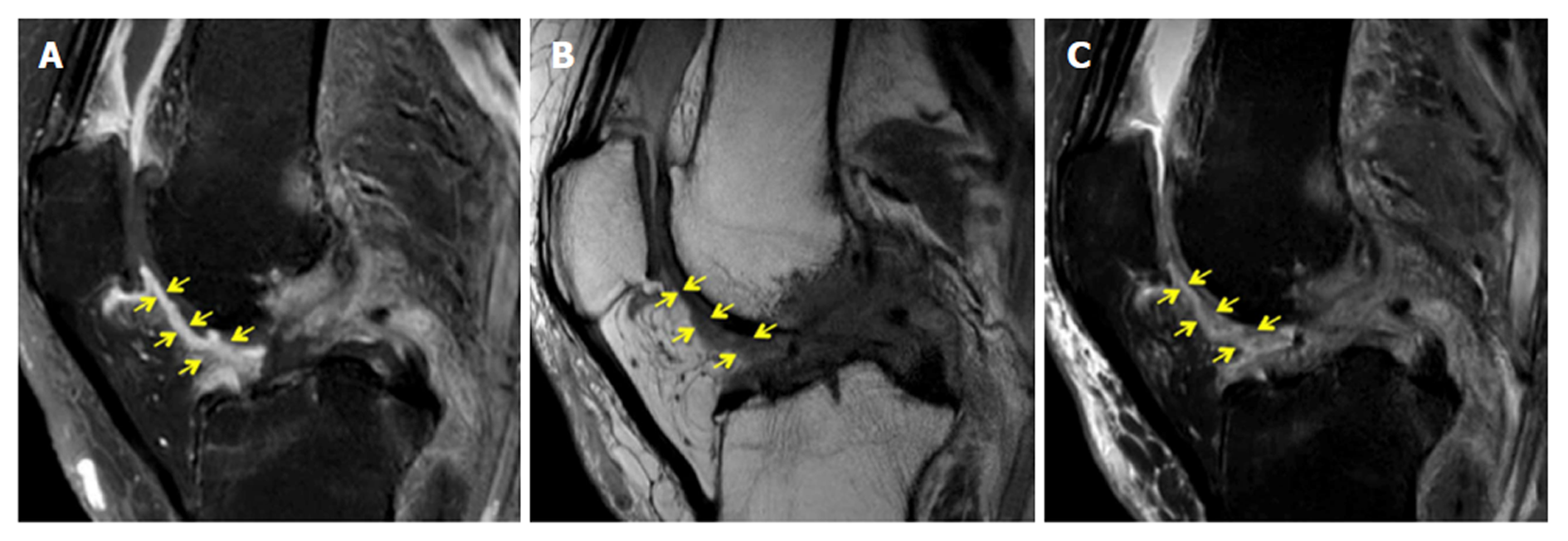
Figure 4 demonstrates a case with similar scores for Hoffa’s synovitis among the PD-FS, PD, and T1CE, whereas Figure 5 demonstrates a case with different PD-FS, PD, and T1CE scores.
This study compared the established scoring methods, including the MOAKS score for fat pad, parapatellar synovitis score, WORMS scoring for synovial effusion, and effusion diameter, and the new scoring methods, including the thickness score and SM score for Hoffa’s fat pad synovitis. The thickness score and the SM score demonstrated almost equal reproducibility with that of the other scoring systems and were superior to the MOAKS. The associations of the thickness score and the SM score with the MOAKS score were equal with that of the other scoring systems. Furthermore, the parapatellar synovitis score had strong associations with the thickness score in T1CE and the SM scores in T1CE and PD-FS.
Although the MOAKS score is based on the size and degree of hyperintensity on T2/ intermediate-weighted imaging or on the PD-FS within the Hoffa’s fat pad[11], it does not clearly define the size and the slice delineation. This may have contributed to the relatively low interreliability for the MOAKS sore in this study. On the other hand, in the WORMS study, interobserver reliability was substantial despite the subjective analysis of the synovial cavity[10]. By evaluating both synovial tissue and fluid, even without clearly distinguishing between the two entities[10], the WORMS system can reliably evaluate the severity of the synovitis. In this study, there was substantial to almost perfect inter and intrareliability for the effusion diameter scoring, likely because measurement of the anterior to posterior dimensions of the joint fluid was straightforward and experience on orthopedic surgery or radiology was not always necessary.
The reliability of the thickness score in T1CE was better than in PD and PD-FS. An advantage of contrast-enhanced MRI is the ability to distinguish synovial inflammation from synovial fluid and fat tissues[20,24]. Guermazi et al[25] reported excellent reproducibility using a contrast-enhanced whole-knee synovitis scoring system. In non-contrast sequences, the border of the intensity change of synovitis is difficult to accurately recognize; this can be one of the reasons for the relatively low inter and intrareliability for the non-contrast analyses of the thickness score. The additional problems of the thickness score in T1CE were fragmentation and contrast exudation of the fat pad, which might have significantly increased the variability in measurement and, in severe cases, overestimated synovitis. The SM score is based on a well-known scoring method for synovitis of the wrist[17,26]. In this method, the high-intensity change seems easy to recognize in both T1CE and PD-FS.
Because the MOAKS enables visualization of the signal intensity change within the Hoffa’s fat pad in asymptomatic patients[27], it was thought to be a sensitive but non-specific finding for synovitis[24]. This was a possible reason for the relatively low correlations between the MOAKS and the other scoring methods. Although the parapatellar synovitis score evaluates a region of interest that is different from the Hoffa’s fat pad, it can be the standard of this study, considering its correlation with the arthroscopic and microscopic scoring of the knee and its previously reported correlation with the WORMS score of the fat pad[21,22]. The thickness score in T1CE and the SM scores in T1CE and PD-FS had strong associations with the parapatellar synovitis score. This suggested that evaluation of synovitis using the SM score on PD-FS had a similar reliability with that of a contrast-enhanced study. Although most contrast studies for synovitis were based on synovial thickness[15,22], this semiquantitative method can simply evaluate both the intensity change on the posterior fat pad and exudation to the fat pad. Due to concerns of increased cost and risk from contrast injection[11], the SM score in PD-FS may provide a viable alternative to contrast-enhanced examinations for the evaluation of Hoffa’s fat pad synovitis.
Several limitations of this study should be acknowledged. First, we did not compare the scores with arthroscopic and microscopic examinations as the standard, because the current study was retrospective and most subjects were not in preoperative status. Second, the parapatellar synovitis score was not a specific scoring method for Hoffa’s fat pad. Despite this, ample correlation of the parapatellar synovitis score with the arthroscopic and microscopic scoring had been demonstrated[19,20]. Third, there was variability in experience among the readers who interpreted the knee MRIs. Although two junior radiology residents were included, there was no significant difference in the scoring results among the readers. This suggested that our methods would be available for many physicians who may not be familiar with knee MRI interpretation. Finally, the use of both 1.5T and 3T MRI systems in this study may have produced variability in the tissue contrast on the PD or PD-FS images and in the degree of contrast enhancement on T1CE images, thereby, affecting the scoring for Hoffa’s synovitis.
In conclusion, the newly proposed quantitative thickness score on T1CE and the semiquantitative SM scores on T1CE and PD-FS can be useful for Hoffa’s fat pad synovitis. Semiquantiative scoring on PD-FS sequences may be a reliable surrogate to contrast-enhanced assessment of Hoffa’s fat pad synovitis.
Osteoarthritis (OA) of the knee is one of the most common chronic disorders resulting in pain, deformity, and loss of function. Several semiquantitative methods using magnetic resonance imaging (MRI) for assessment of knee OA have been developed and used in various observational studies and clinical trials.
Although all assessment methods had been reported to be clinical useful, their reliability was not perfect, and the scoring for Hoffa’s fat pad synovitis based on non-enhanced sequence has not been sufficient, compared with that for the other subregions of the knee.
The aim of this study was to evaluate the reliability of the established and new scoring methods, including non-enhanced MRI, for Hoffa’s fat pad synovitis.
This study enrolled 139 knees of 115 patients who underwent MRI of the knee with and without Gadolinium contrast. Proton density (PD)-weighted, proton density-weighted fat-suppressed (PD-FS), and postcontrast T1-weighted fat-suppressed (T1CE) images were used for evaluation. Our grading method for synovitis was performed using non-contrast and contrast images to measure synovial thickness and signal intensity changes of the fat pad (SM score). Intraclass correlation coefficients (ICC) for intra and interobserver reproducibility and the Spearman correlation coefficients (r) with the parapatellar synovitis score were calculated for each scoring method.
The thickness score in T1CE and the SM scores in T1CE and PD-FS showed substantial to almost perfect reproducibility. The parapatellar synovitis score statistically significant correlation with the thickness score in T1CE (r = 0.68) and thee SM scores in T1CE (r = 0.71) and PD-FS (r = 0.66).
The newly proposed quantitative thickness score on T1CE and the semiquantitative SM scores on T1CE and PD-FS can be useful for Hoffa’s fat pad synovitis.
Our findings indicated that the established scoring systems for Hoffa’s fat pad synovitis could be further improved. Future research may propose more reliable methods for Hoffa’s fat pad synovitis.
We would like to thank Maryam Soltanolkotabi, for helping us make the grading system, and all the staff of the Department of Radiological Sciences at the University of California Irvine, for helping in collecting the data and obtaining the MRI.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gheita TA, Jennane R S- Editor: Wang JL L- Editor: A E- Editor: Huang Y
| 1. | Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 2070] [Article Influence: 129.4] [Reference Citation Analysis (1)] |
| 2. | Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1550] [Cited by in RCA: 2199] [Article Influence: 157.1] [Reference Citation Analysis (2)] |
| 3. | Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1085] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 4. | Hoffa A. Influence of adipose tissue with regard to the pathology of the knee joint. JAMA. 1904;43:795-796. |
| 5. | Vahlensieck M, Linneborn G, Schild H, Schmidt HM. Hoffa’s recess: incidence, morphology and differential diagnosis of the globular-shaped cleft in the infrapatellar fat pad of the knee on MRI and cadaver dissections. Eur Radiol. 2002;12:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Saddik D, McNally EG, Richardson M. MRI of Hoffa’s fat pad. Skeletal Radiol. 2004;33:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Distel E, Cadoudal T, Durant S, Poignard A, Chevalier X, Benelli C. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009;60:3374-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1196] [Article Influence: 54.4] [Reference Citation Analysis (5)] |
| 11. | Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19:990-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 789] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 12. | Lynch JA, Roemer FW, Nevitt MC, Felson DT, Niu J, Eaton CB, Guermazi A. Comparison of BLOKS and WORMS scoring systems part I. Cross sectional comparison of methods to assess cartilage morphology, meniscal damage and bone marrow lesions on knee MRI: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Felson DT, Lynch J, Guermazi A, Roemer FW, Niu J, McAlindon T, Nevitt MC. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2010;18:1402-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Roemer FW, Hunter DJ, Crema MD, Kwoh CK, Ochoa-Albiztegui E, Guermazi A. An illustrative overview of semi-quantitative MRI scoring of knee osteoarthritis: lessons learned from longitudinal observational studies. Osteoarthritis Cartilage. 2016;24:274-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, Bohndorf K. Hoffa’s Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009;192:1696-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Ballegaard C, Riis RG, Bliddal H, Christensen R, Henriksen M, Bartels EM, Lohmander LS, Hunter DJ, Bouert R, Boesen M. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage. 2014;22:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, Lorenzen I. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:918-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, Goebel JC, Mainard D, Blum A, Pourel J. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492-3501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Meredith DS, Losina E, Neumann G, Yoshioka H, Lang PK, Katz JN. Empirical evaluation of the inter-relationship of articular elements involved in the pathoanatomy of knee osteoarthritis using magnetic resonance imaging. BMC Musculoskelet Disord. 2009;10:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 21. | Loeuille D, Rat AC, Goebel JC, Champigneulle J, Blum A, Netter P, Gillet P, Chary-Valckenaere I. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2009;17:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Loeuille D, Sauliere N, Champigneulle J, Rat AC, Blum A, Chary-Valckenaere I. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19:1433-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Guilford JP. Fundamental statistics in psychology and education. New York: McGraw Hill 1956; . |
| 24. | Crema MD, Felson DT, Roemer FW, Niu J, Marra MD, Zhang Y, Lynch JA, El-Khoury GY, Lewis CE, Guermazi A. Peripatellar synovitis: comparison between non-contrast-enhanced and contrast-enhanced MRI and association with pain. The MOST study. Osteoarthritis Cartilage. 2013;21:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, Marra MD, Katur A, Lynch JA, El-Khoury GY. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. 2011;70:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Stomp W, Krabben A, van der Heijde D, Huizinga TW, Bloem JL, Østergaard M, van der Helm-van Mil AH, Reijnierse M. Aiming for a simpler early arthritis MRI protocol: can Gd contrast administration be eliminated? Eur Radiol. 2015;25:1520-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | De Smet AA, Davis KW, Dahab KS, Blankenbaker DG, del Rio AM, Bernhardt DT. Is there an association between superolateral Hoffa fat pad edema on MRI and clinical evidence of fat pad impingement? AJR Am J Roentgenol. 2012;199:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |