Published online Feb 26, 2026. doi: 10.4330/wjc.v18.i2.114636
Revised: October 30, 2025
Accepted: December 15, 2025
Published online: February 26, 2026
Processing time: 132 Days and 14.2 Hours
Patients suffering from coronary artery disease (CAD) are experiencing sig
To investigate long-term safety and effectiveness of NeoHexa sirolimus-eluting stent (SES) in managing CAD among real-world all-comer patient population.
740 individuals with CAD who had undergone percutaneous coronary in
At a mean follow-up period of 62.17 ± 9.86 months, 32 subjects (4.32%) experienced the key safety endpoint of MACE. This included 13 patients (1.76%) with TLR/target vessel revascularization and 19 patients (2.57%) with cardiac mortality. Additionally, non-cardiac mortality occurred in 35 cases (4.73%). In terms of effectiveness, 673 patients (90.95%) did not require revascularization and remained clinically stable in New York Heart Association functional class I-II.
The study suggests favourable long-term safety and efficacy of NeoHexa SES in a real-world setting with varied clinical presentations.
Core Tip: This retrospective study provides valuable long-term real-world evidence on the safety and efficacy of the NeoHexa drug-eluting stent in an all-comer, real world patient population. Over an average follow-up of more than five years, the study observed low rates of major adverse cardiac events (4.32%), including minimal target lesion/target vessel revascularization. The findings highlight the stent’s durable performance, even in high-risk and complex patients across routine and pandemic-era clinical settings.
- Citation: Trehan VK, Safal S, Trehan S, Kathuria S, Vani PS. Five-year mean follow-up of NeoHexa sirolimus eluting coronary stent: A retrospective evaluation of long-term safety and efficacy. World J Cardiol 2026; 18(2): 114636
- URL: https://www.wjgnet.com/1949-8462/full/v18/i2/114636.htm
- DOI: https://dx.doi.org/10.4330/wjc.v18.i2.114636
Coronary artery disease (CAD) is one of the most prevalent and significant causes of morbidity and mortality worldwide, and presents as a range of conditions, including acute coronary syndromes [encompassing unstable angina, Non-ST segment elevation myocardial infarction (MI) and ST-elevation MI], stable angina, and asymptomatic atherosclerosis. Percutaneous coronary intervention (PCI) has become a widely adopted revascularization technique for the treatment of CAD[1-5]. The evolution of PCI began with Andreas Gruentzig’s first balloon angioplasty in 1977, which reduced artery stenosis but had a high restenosis rate due to elastic recoil and thrombosis. The introduction of bare-metal stents (BMS) in 1987 helped mitigate these issues but brought increased risks of stent thrombosis and restenosis. Drug-eluting stents (DES), first introduced in 2002/2003, reduced restenosis by releasing drugs but faced challenges like impaired vessel healing and late stent thrombosis. Second-generation DES in 2008 improved on these issues, though some hypersensitivity and late/very late stent thrombosis concerns remained, leading to third-generation DES in 2011 with further advancements in safety and efficacy. The implantation of DES during PCI has become a standard treatment for CAD, providing a minimally invasive alternative to coronary artery bypass grafting[6-9]. The NeoHexa sirolimus-eluting stent (SES), developed by Sahajanand Laser Technology Limited (Gujarat, India), is a third generation DES that combines a biodegradable polymer with sirolimus (1.0 μg/mm2) as the active pharmaceutical ingredient. Key innovations in the NeoHexa stent improve its functionality and versatility. Its minimal recoil reduces the risk of stent displacement, ensuring stability and precise positioning, particularly in challenging cases such as ostial lesions. Additionally, its design minimizes foreshortening, making it suitable for procedures requiring exact placement. Previous research by Jambunathan et al[10] in 2019 reported favorable short-term safety and efficacy outcomes for the NeoHexa SES. However, evidence regarding its long-term clinical performance and durability of outcomes, especially within diverse real-world populations, remains scarce. The present study was therefore designed to bridge this knowledge gap by evaluating the long-term safety and effectiveness of the NeoHexa SES across a broad spectrum of patients treated in routine clinical practice[10]. This retrospective study was performed in a real-world, all-comer patient population to assess the long-term safety and efficacy of the NeoHexa SES in real world scenario. The study analyses key outcomes, including rates of major adverse cardiac events (MACEs) consisting of cardiac death, MI, and target lesion revascularization (TLR) over a mean follow-up of 62.17 months. Additionally, patient demographics, procedural details, and trends over time are assessed using data from health records and procedural databases.
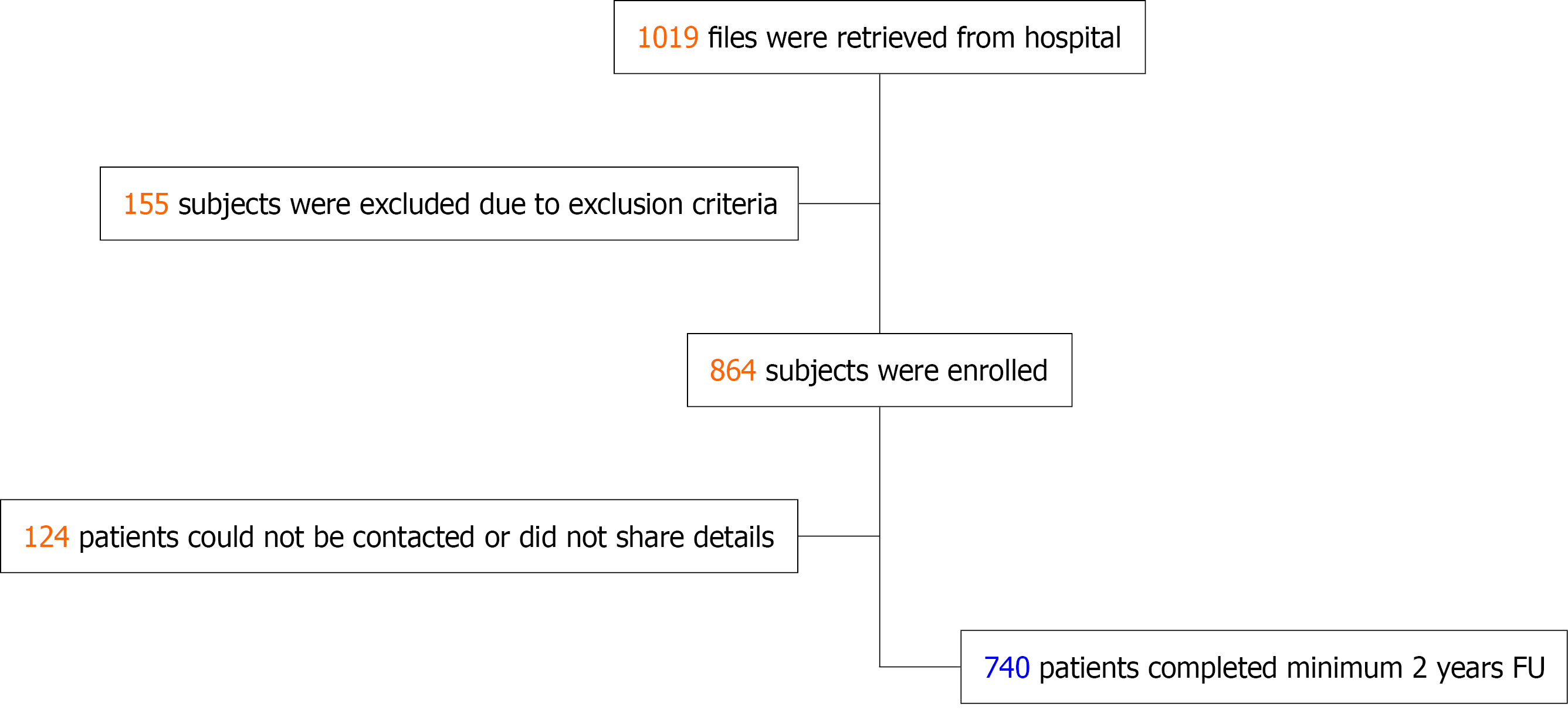
This single center retrospective study evaluates the clinical outcomes with NeoHexa SES in an all-comer, real-world population in routine clinical cardiology practice. The eligibility criteria included patients aged over 18 years who were implanted with NeoHexa SES during PCI, and a minimum of two years had passed post-implantation. Patient screening was conducted using hospital records and data from subjects meeting the inclusion and exclusion criteria was collected after informed consent. Patients implanted with any additional non-study stent in the same vessel as the NeoHexa stent were excluded as it would be difficult to interpret which implant caused an adverse event. Data quality assurance was maintained through systematic verification of hospital records and registry entries, with consistency checks performed for key clinical variables; missing data were addressed using predefined imputation methods and sensitivity analyses to minimize potential bias. Pre-designed study proforma was filled for each included patient either telephonically or during physical visits, depending on subject convenience. Date of last follow-up was taken as the date on which information was recorded in the proforma; in case of mortality the date of death was taken as date of last follow-up. Where additional information was required, including details of subsequent admissions or death certificate in case of mortality, this was procured through WhatsApp or physically. As the angiographic compact disc was not available for some patients, all target vessel revascularization (TVR) was considered as TLR based on patient records. The data from individual proforma was entered into an Excel sheet and further analyzed as per study end-points. The disposition of patients is depicted in the flowchart shown in Figure 1.
The NeoHexa Coronary Stent System is a balloon-expandable SES built on a Cobalt Chromium Alloy (L605) platform, specially designed for coronary interventions. It is distinguished by its ultra-thin 60 μm strut thickness, offering a unique combination of strength and flexibility. The stent’s design incorporates flexible, United States-patented “S-link” and “C-link” structures, minimizing recoil and reducing foreshortening for improved performance. Its hybrid cell architecture, combining open and closed cells, enhances flexibility and structural stability. The open cells conform to complex vascular anatomy, while the closed cells provide scaffolding and radial strength. The NeoHexa stent’s peak-to-valley strut alignment minimizes plaque prolapse and maintains an optimal metal-to-artery ratio. Its biodegradable polymer and sirolimus (1.0 μg/mm2) coating, with a thickness of 4-5 microns, ensures controlled drug release. Sirolimus is released in two phases: 60% within the first seven days and the remaining 40% over the following twenty-one days. The biode
The primary safety endpoint included cumulative MACE at time of follow up (≥ 2 years after implantation). MACE was defined as a composite endpoint consisting of cardiac death, MI, and TLR, providing a comprehensive evaluation of cardiovascular outcomes in patients implanted with the NeoHexa stent. The primary efficacy endpoint involved sustained relief of angina or angina-equivalent symptoms at two years post-implantation, without the need for TLR. The secondary endpoints included individual components of MACE, all-cause mortality, TVR and stent thrombosis[11].
A total of 1019 files of patients deployed with the study stent were retrieved from the hospital records. Of these, 155 participants were eliminated from the study due to the exclusion criteria. Another 124 patients could either not be contacted or were not willing to participate in the study. Hence, 740 individuals (who had received 1317 study implants) were included in the final analysis (Figure 1).
Demographic and treatment characteristics were summarized using descriptive statistics. Continuous variables, such as age and body mass index, were reported as mean ± SD. Categorical variables, including gender, risk factors, and functional class New York Heart Association, were presented as n (%), with percentages calculated based on the number of patients with available data. Missing data were excluded from all analyses. The primary and secondary endpoints of the study were summarized using counts and percentages. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS v.20). Continuous variables are presented as mean ± SD or median (interquartile range) and were compared using t-test. Categorical variables are expressed as counts and percentages and were compared using the χ2. Time-to-event outcomes were analyzed using Kaplan-Meier survival curves, with differences between groups assessed using the log-rank test. Effect sizes and 95% confidence intervals (CI) were reported alongside P values for all comparisons. Additionally, an exploratory time-to-event analysis for MACE, based on all available follow-up data, was conducted using Kaplan-Meier estimates. The χ2 test was used to compare the frequency of continuous variables. Continuous variables are presented as mean ± SD. Statistical significance was set at P < 0.05.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee (approval No. F.1/IEC/MAMC/87/05/2021/No 89).
A total of 740 patients were included in the study and mean duration of follow-up was 62.17 ± 9.86 months. The baseline patient characteristics are summarized in Table 1. The average age was 55.25 ± 10.00 years and majority of patients were male (82.70%). The average heart rate was 80.20 ± 10.57 bpm, while the mean systolic and diastolic blood pressure were 121.8 ± 15.29 mmHg and 77.12 ± 8.57 mmHg, respectively. The estimated glomerular filtration rate was an average of 86.36 ± 20.60 mL/minute/1.73 m2, and the mean serum creatinine level was 1 ± 0.25 mg/dL. The mean hemoglobin concentration was 13.19 ± 1.66 g/dL. 134 (18.1%) patients had chronic stable angina while 606 (81.9%) patients had acute coronary syndrome. The mean left ventricular ejection fraction was 46.10 ± 11.00.
| Variables | n = 740 patients |
| Age in years | 55.25 ± 10.00 |
| Gender | |
| Male | 612 (82.70) |
| Female | 128 (17.29) |
| Heart rate (bpm) | 80.20 ± 10.57 |
| Blood pressure (mmHg) | |
| Diastolic | 77.12 ± 8.57 |
| Systolic | 121.8 ± 15.29 |
| Sr creatinine (mg/dL) | 1 ± 0.25 |
| eGFR (mL/minute/1.73 m2) | 86.36 ± 20.60 |
| Hb (g/dL) | 13.19 ± 1.66 |
| LVEF (%) | 46.10 ± 11.00 |
| Chronic stable angina | 134 (18.10) |
| Acute coronary syndrome | 606 (81.89) |
| STEMI | 361 (48.78) |
| NSTEMI | 80 (10.81) |
| Unstable angina | 165 (22.29) |
| Hypertension | 203 (27.43) |
| Diabetes mellitus | 182 (24.59) |
| Smoking current | 205 (27.70) |
| Previous stroke | 7 (0.94) |
| Previous MI | 48 (6.48) |
| Previous PCI | 64 (8.64) |
| Previous CABG | 7 (0.94) |
| Patients with renal dysfunction (eGFR < 60) | 63 (8.51) |
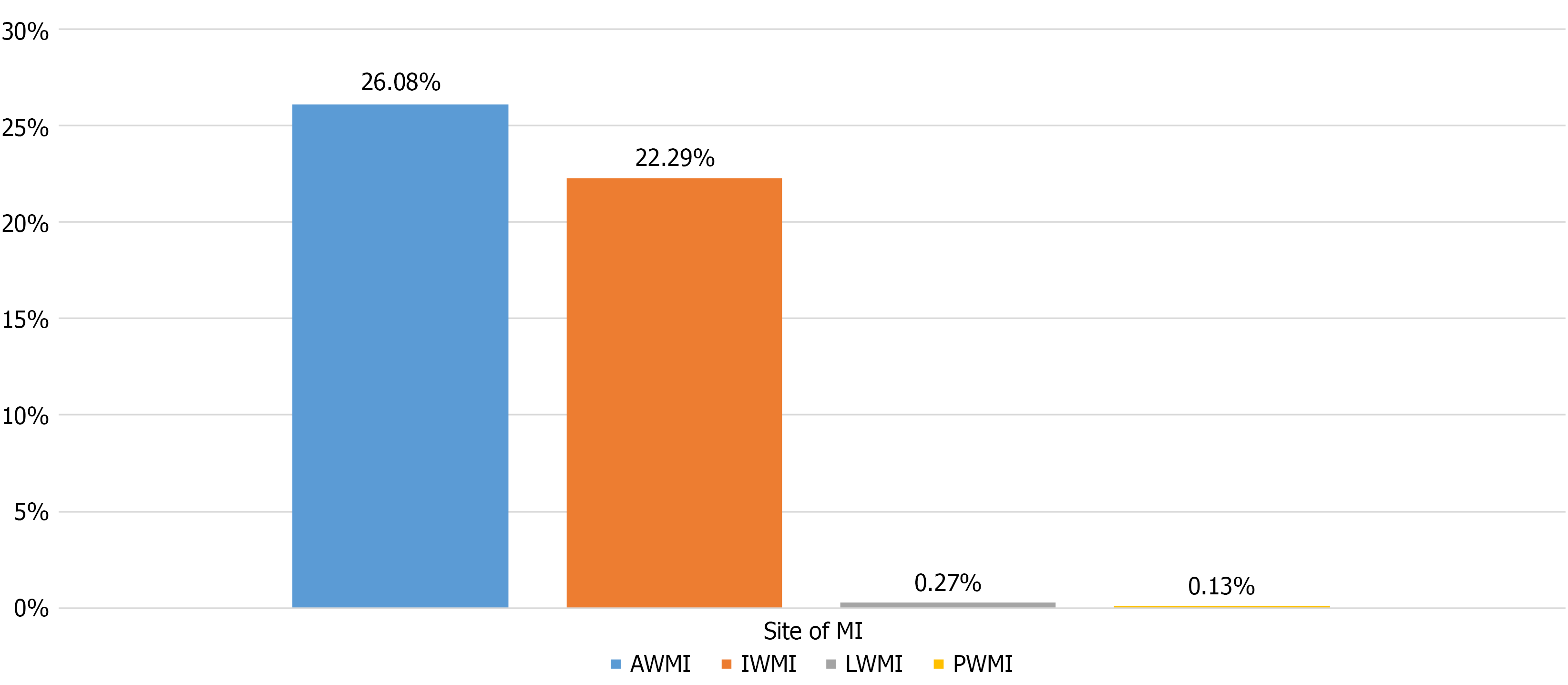
The distribution of MI sites among the ST segment elevation myocardial infarction. Subgroup is illustrated in Figure 2. Anterior wall MI was the most common (26.08% of study population), followed by inferior wall MI (22.29%). While 0.27% patients presented with lateral wall MI, 0.13% suffered isolated posterior wall MI.
The angiographic and procedural characteristics are shown in Table 2. All cases were done through the femoral route and 12.16% patients had undergone primary PCI. Out of 740 patients, 310 patients had single-vessel disease, 276 had double-vessel disease, and 154 had triple-vessel disease. Five percent patients had left main coronary artery (LMCA) disease; for purpose of deciding the number of diseased vessels, isolated LMCA involvement was considered as double vessel disease and ramus intermedius was considered equivalent to left circumflex coronary artery. A total of 1372 lesions causing over 50% coronary stenosis were identified in the study population (mean of 1.85 lesions per patient). Of these, 911 were treated with NeoHexa stent while 343 lesions were managed with medications alone. Additionally, 118 lesions were treated with other stents. A total of 1317 NeoHexa and 176 other stents were deployed, averaging 1.77 stents per patient. Among the 911 lesions treated with NeoHexa stent, the lesion locations included 385 in the left anterior descending artery, 228 in the left circumflex artery, 274 in the right coronary artery, and 24 in other locations including LMCA. Following the procedure, 738 patients had successfully achieved a thrombolysis in myocardial infarction flow grade of 3 in respective coronary artery. The average length and diameter of the study device were 21.90 ± 9.27 mm and 2.90 ± 0.42 mm, respectively.
| Angiographic feature/procedural characteristic | n (%) |
| Number of diseased vessels | |
| Single vessel | 310 (41.89) |
| Double vessel | 276 (37.29) |
| Triple vessel | 154 (20.81) |
| Patients having LMCA disease | 37 (5.0) |
| Primary PCI | 90 (12.16) |
| Post-procedure TIMI 3 flow | 738 (99.7) |
| Lesion characteristics | |
| Total number of lesions | 1372 |
| Total number of lesions treated with NeoHexa | 911 |
| Total number of lesions treated with other stents | 118 |
| Total number of lesions managed medically | 343 |
| Lesion location (for lesions treated with NeoHexa stent) | |
| LAD | 385 (42.26) |
| LCX | 228 (25.03) |
| RCA | 274 (30.07) |
| Others | 24 (2.63) |
| Procedural characteristics | |
| Total number of NeoHexa deployed | 1317 |
| Total number of other stents deployed | 176 |
| Lesion per patient | 1.85 ± 1.06 |
| Study stents deployed per patient | 1.77 ± 1.1 |
| Average length of study device, mm | 21.90 ± 9.27 |
| Average diameter of study device, mm | 2.90 ± 0.42 |
The clinical outcomes of the patients, representing the essential measures for evaluating the efficacy and overall safety profile of the stent system, are depicted in Table 3. The primary safety endpoint of MACE, defined as cumulative of cardiac death, MI and TLR/TVR occurred in 32 (4.32%) patients during the entire period of follow-up (62.17 ± 9.86 months). This included cardiac death in 19 patients (2.57%), and TLR/TVR in 13 (1.76%) patients. Pertinently, if a patient had an event that would qualify for more than one MACE component (e.g., stent thrombosis leading to cardiac death), the harder end-point was considered for purpose of statistical analysis. There was no isolated MI or stent thrombosis that did not fall in the category of TLR/TVR or cardiac death. Additionally, 35 patients (4.73%) experienced non-cardiac death. Regarding efficacy end-point, 673 (90.95%), were clinically in New York Heart Association I to II without the need for TLR/TVR.
| Clinical events | n (%) |
| MACE | 32 (4.32) |
| Cardiac death | 19 (2.57) |
| TLR/TVR | 13 (1.76) |
| Target vessel MI (without TLR/TVR or mortality) | 0 (0.00) |
| Non-cardiac death | 35 (4.73) |
| NYHA I/II without TLR/TVR | 673 (90.95) |
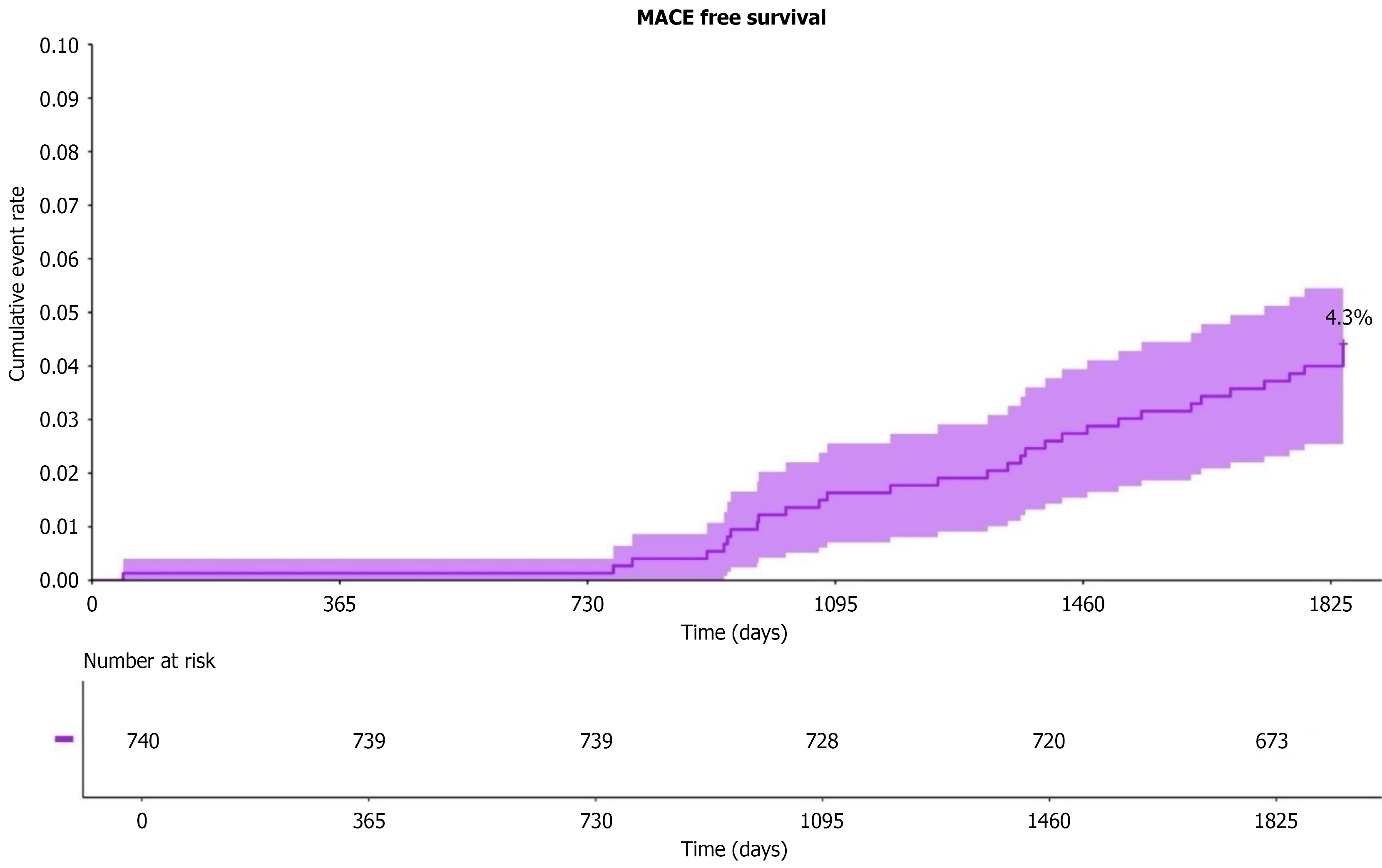
Figure 3 presents Kaplan-Meier curves illustrating the proportion of patients remaining MACE-free over time. By the study’s conclusion (at day 1825), 4.3% of patients experienced MACE. The accompanying “number at risk” table shows the number of patients under follow-up at each time point; for example, 740 patients were enrolled at baseline, with 673 remaining by day 1825. The event rate is low in the early phase (up to approximately 1 year) but increases progressively over time.
The study’s findings offer an extensive assessment of the NeoHexa SES’s overall beneficial impact, safety, and effectiveness in actual patients with CAD. In all, 740 patients were included in this investigation; they were followed up over a minimum two-year period (mean follow-up duration 62.17 ± 9.86 months). The mean age at time of implantation was 55.25 ± 10.00 years, and majority of patients were male (82.70%). Notably, a sizeable proportion of patients had a background of acute coronary syndrome (ACS), with 12.16% undergoing primary PCI. The diverse patient population, combined with the long follow-up duration, offers comforting evidence of the NeoHexa stent’s clinical effectiveness in standard practice.
A total of 1372 lesions were identified, with an average of 1.85 lesions per patient. Among the study population, 41.89% of patients had single-vessel disease, 37.29% had double-vessel disease, and 20.81% had triple-vessel disease, reflecting the distribution of vascular involvement across an all-comer symptomatic CAD population. Also, this distribution suggests that the study implant has been effectively employed across a range of vascular involvement.
The stent’s safety profile has been demonstrated in this five-year mean follow-up, which showed a low MACE rate of 4.32%. Additionally, non-cardiac fatalities occurred in 4.73% of patients, indicating the existence of complicated comorbidities and competing mortality risks in this diversified patient group.
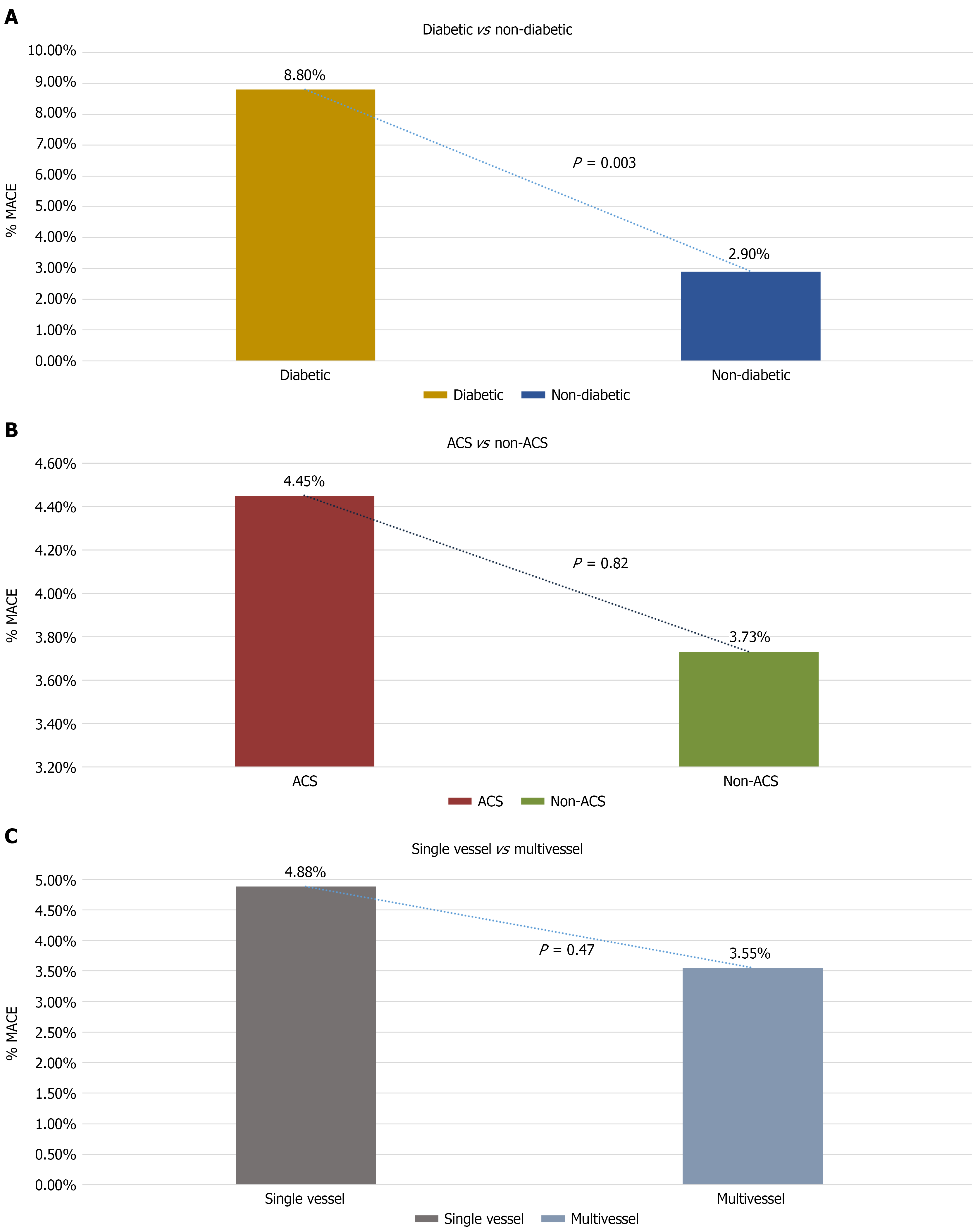
Of the total 740 patients analyzed, 182 (24.6%) had diabetes mellitus. When stratified according to diabetic status, MACE occurred in 16 diabetic patients (8.8%) and 16 non-diabetic patients (2.9%). The incidence of MACE was significantly higher among patients with diabetes compared to those without diabetes (P = 0.003, χ2 test; Figure 4A). The calculated relative risk for MACE in diabetic patients was 3.03 (95%CI: 1.55-5.91). This trend remained consistent across all follow-up time points (Figure 4A). In contrast, MACE rates did not differ significantly between ACS vs non-ACS subgroups (Figure 4B). ACS patients had a relative risk of MACE of 1.19 (95%CI: 0.47-3.04), with an absolute risk difference of 0.72 percentage points (95%CI: -2.88 to 4.33). The difference was not statistically significant (Fisher’s exact test, P = 0.82).
Within the 740 patients, 430 (58.1%) had multivessel CAD, and 310 (41.9%) had single-vessel disease. 21 (4.88%) patients in the multivessel group and 11 (3.55%) patients in the single-vessel group experienced MACE. Although numerically higher in the multivessel group, the difference was statistically insignificant (P = 0.47; Figure 4C).
In a comparative analysis of the NeoHexa stent’s performance against other leading stents in the market, the findings were encouraging. The Scandinavian Organization for Randomized Trials with Clinical Outcome trial, for instance, showed a MACE (cardiac death, MI, definite stent thrombosis, or TVR) of 8.3% and 8.7% after 2 years of everolimus and SES implantation respectively[12]. As expected, MACE rates were lower than in studies with first generation DES, like the SES-SMART clinical trial (4.32% at 2 years in our study vs 12.6% in the SES group of SES-SMART study)[13]. However, the markedly lower MACE rates vs SES-SMART study can also partly be attributed to SES-SMART enrolling patients with small coronary arteries only; the mean diameter of implant in our study was 2.90 mm. In another study of contemporary SES, Nathani et al[14] have reported MACE in 4.92% patients at one year.
The study implant also achieved an impressive TVR/TLR of just 1.76%, representing a significant improvement compared to the 2-year TVR and TLR rates of 7.2% and 3.8% respectively with early generation DES[15]. In a purely diabetic population, Ortolani et al[16] have reported TVR rates of 11.6% and 15.0% for early generation DES and BMS respectively; in our study 24.59% patients were diabetic and MACE occurred in 8.8% of this subgroup. Jensen et al[12] reported TVR rates of 5.5% for everolimus eluting stents 6.2% for SESs at 2 years. As discussed earlier, all TVR was considered as TLR for purpose of this study.
At a mean follow-up duration of 62 months, 90.95% of patients who received treatment with the study implant experienced notable relief from symptoms without need for TVR/TLR, emphasizing long-term efficacy of the NeoHexa SES. This result aligns with findings from other research, which indicated a decrease in the necessity for TLR over a two-year period with the use of SES[17,18]. The clinical efficacy of the NeoHexa SES is on par with other DES available in the market, demonstrating comparable safety and effectiveness. Its formulation, which includes sirolimus and a hybrid design, successfully minimizes neointimal proliferation and restenosis. The stent’s thinner struts enhance biocompatibility, lower inflammation, and promote faster endothelialization, contributing to improved long-term patient outcomes. Furthermore, the biodegradable polymer coating of the NeoHexa stent appears to significantly decrease the risk of late stent thrombosis and long-term complications; notably, no definite late stent thrombosis was noted in this study. This research also corroborates previous findings, demonstrating that current DES surpass both BMS and first-generation DES in performance.
However, the retrospective nature of the study and its dependence on hospital records restrict the ability to make conclusive statements about the NeoHexa stent’s effectiveness relative to other current-generation DES. The inability to contact or refusal to share details by 124 patients (Figure 1) may have introduced a selection bias, where those with early adverse events could not be included in the study. The low adverse events over the first 730 days also point towards this possibility. Nevertheless, while the shortfalls of a retrospective study cannot be nullified, this study also has the strength of enrolling a truly all-comer patient population in a real-world setting - a quality that most prospective studies lack. Additionally, over 90 percent patients being symptomatically comfortable without the need for TLR/TVR at the end of five years, is a strong pointer towards long-term efficacy of the implant. Further studies examining the stent’s perfor
Being a retrospective analysis, it lacked routine angiographic follow-up, and stent thrombosis was not independently confirmed by imaging, which may underestimate adverse events. Additionally, the single-center design limits generalizability. Future research should focus on prospective, multicenter, randomized evaluations of the NeoHexa SES to more comprehensively assess safety and long-term efficacy.
The results of the present retrospective study demonstrate that the NeoHexa SES exhibits favourable safety and performance profiles in patients with CAD, in real-world setting. Notably, the stent showed a low incidence of adverse events over a mean follow-up of 5 years, suggesting favorable long-term performance. Also, over 90 percent patients remained symptom free without the need for repeat TLR/TVR, demonstrating good long-term efficacy of the study implant. Future prospective, multicenter studies are warranted to further validate the safety and efficacy of NeoHexa SES.
| 1. | Canfield J, Totary-Jain H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J Pers Med. 2018;8:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1377] [Cited by in RCA: 1183] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 3. | Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381:510-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L Jr, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2907] [Cited by in RCA: 2745] [Article Influence: 305.0] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Global status report on noncommunicable diseases 2010. [cited 3 August 2025]. Available from: https://iris.who.int/server/api/core/bitstreams/625932a8-bb29-425b-9ce2-f10cb8cd8292/content. |
| 6. | Kuramitsu S, Sonoda S, Ando K, Otake H, Natsuaki M, Anai R, Honda Y, Kadota K, Kobayashi Y, Kimura T. Drug-eluting stent thrombosis: current and future perspectives. Cardiovasc Interv Ther. 2021;36:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnàr F, Falotico R; RAVEL Study Group. Randomized Study with the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with de Novo Native Coronary Artery Lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3050] [Cited by in RCA: 2899] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 8. | Pelliccia F, Zimarino M, Niccoli G, Morrone D, De Luca G, Miraldi F, De Caterina R. In-stent restenosis after percutaneous coronary intervention: emerging knowledge on biological pathways. Eur Heart J Open. 2023;3:oead083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (17)] |
| 9. | Lee DH, de la Torre Hernandez JM. The Newest Generation of Drug-eluting Stents and Beyond. Eur Cardiol. 2018;13:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Jambunathan R, Basavanna D, Vani P, Neuss M, Janbandhu P. One-year outcomes of a NeoHexa sirolimus-eluting coronary stent system with a biodegradable polymer in all-comers coronary artery disease patients: Results from NeoRegistry in India. World J Cardiol. 2019;11:200-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW; Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 612] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 12. | Jensen LO, Thayssen P, Christiansen EH, Tilsted HH, Maeng M, Hansen KN, Kaltoft A, Hansen HS, Bøtker HE, Krusell LR, Ravkilde J, Madsen M, Thuesen L, Lassen JF; SORT OUT IV Investigators. 2-year patient-related versus stent-related outcomes: the SORT OUT IV (Scandinavian Organization for Randomized Trials With Clinical Outcome IV) Trial. J Am Coll Cardiol. 2012;60:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Menozzi A, Solinas E, Ortolani P, Repetto A, Saia F, Piovaccari G, Manari A, Magagnini E, Vignali L, Bonizzoni E, Merlini PA, Cavallini C, Ardissino D; SES-SMART Investigators. Twenty-four months clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur Heart J. 2009;30:2095-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Nathani S, Raheem A, Sanadhya H, Purohit PC, Patel R, Alane PK, Agarwal D, Sinha R. Twelve-month clinical outcomes of sirolimus-eluting stent in coronary artery disease: An experience in real-world Indian patients. Anatol J Cardiol. 2020;24:364-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Al Muradi H, Mehra A, Okolo J, Vlachos H, Selzer F, Marroquin OC, Skelding K, Holper EM, Williams DO, Abbott JD. Clinical presentation and predictors of target vessel revascularization after drug-eluting stent implantation. Cardiovasc Revasc Med. 2012;13:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Ortolani P, Balducelli M, Marzaroli P, Piovaccari G, Menozzi A, Guiducci V, Sangiorgio P, Tarantino F, Geraci G, Castriota F, Tondi S, Saia F, Cooke RM, Guastaroba P, Grilli R, Marzocchi A, Maresta A. Two-year clinical outcomes with drug-eluting stents for diabetic patients with de novo coronary lesions: results from a real-world multicenter registry. Circulation. 2008;117:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Jiménez-Quevedo P, Sabaté M, Angiolillo DJ, Alfonso F, Hernández-Antolín R, SanMartín M, Gómez-Hospital JA, Bañuelos C, Escaned J, Moreno R, Fernández C, Fernández-Avilés F, Macaya C; DIABETES Investigators. Long-term clinical benefit of sirolimus-eluting stent implantation in diabetic patients with de novo coronary stenoses: long-term results of the DIABETES trial. Eur Heart J. 2007;28:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Kim U, Lee SH, Hong GR, Park JS, Shin DG, Kim YJ, Jang JS, Yang TH, Kim DK, Kim DS, Kim DK, Seol SH, Kim DI, Cho YK, Kim HS, Nam CW, Hur SH, Kim KB. Two-year clinical outcomes of patients with long segments drug-eluting stents: comparison of sirolimus-eluting stent with paclitaxel-eluting stent. J Korean Med Sci. 2011;26:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |