Published online Jan 26, 2026. doi: 10.4330/wjc.v18.i1.114123
Revised: September 22, 2025
Accepted: December 1, 2025
Published online: January 26, 2026
Processing time: 125 Days and 21.2 Hours
The choice of priming and volume replacement fluids during cardiopulmonary bypass (CPB) in cardiac surgery impacts hemodynamic stability, coagulation, renal function, and patient outcomes. Hydroxyethyl starch (HES) 130/0.4 and human albumin are commonly used colloids, but their relative safety and efficacy remain debated.
To compare the outcomes of 6% HES 130/0.4 vs 5% albumin in patients under
A comprehensive literature search was performed in PubMed, EMBASE, ScienceDirect, and grey literature sources up to August 2025. Randomized controlled trials and controlled observational studies comparing 6% HES 130/0.4 with 5% albumin in patients who underwent cardiac surgery were included. Data extrac
Twelve studies involving 908 patients (455 in the HES group, 453 in the albumin group) were included. No significant differences were observed between the HES and albumin groups for postoperative blood loss [mean difference = 42.4 mL, 95% confidence interval (CI): -90.0 to 174.9; P = 0.53], packed red blood cell transfusion [odds ratio (OR) = 0.78, 95%CI: 0.65-1.10; P = 0.16)], mortality (OR = 1.11, 95%CI: 0.63-1.96; P = 0.80), intensive care unit stay, hospital stay, or postoperative platelet count and creatinine levels. However, HES was associated with a significantly higher risk of acute kidney injury (AKI) (OR = 1.79, 95%CI: 1.08-2.97; P = 0.02), indicating that while many clinical outcomes showed no significant difference, there is a specific safety concern related to renal function with HES use. Meta-regression did not identify baseline factors explaining heterogeneity in bleeding or AKI outcomes (all P > 0.10). No significant publication bias was detected.
The 6% HES 130/0.4 and 5% albumin exhibit similar efficacy for volume management in cardiac surgery with CPB; however, HES is associated with a higher risk of AKI.
Core Tip: This meta-analysis systematically compared 6% hydroxyethyl starch 130/0.4 and 5% human albumin as priming and volume replacement fluids in cardiac surgery with cardiopulmonary bypass. It highlighted comparable safety and efficacy profiles between the two colloids in terms of bleeding, transfusion needs, intensive care unit stay, and mortality while identifying a higher risk of acute kidney injury associated with hydroxyethyl starch. These findings challenge traditional preferences and emphasize the need for individualized fluid choice guided by patient factors and evolving regulatory considerations in perioperative cardiac care.
- Citation: Alqarni A, Algarni A, Chhetri R. Comparison of 6% hydroxyethyl starch 130/0.4 vs 5% albumin in cardiopulmonary bypass for cardiac surgery. World J Cardiol 2026; 18(1): 114123
- URL: https://www.wjgnet.com/1949-8462/full/v18/i1/114123.htm
- DOI: https://dx.doi.org/10.4330/wjc.v18.i1.114123
In the context of cardiovascular surgery, intravenous albumin is commonly employed for priming the cardiopulmonary bypass (CPB) circuit or for volume replacement although evidence supporting its superiority over synthetic colloids like hydroxyethyl starch (HES) or crystalloids remains limited with no significant differences observed in all-cause mortality across 42 randomized controlled trials (RCTs) involving 3862 patients[1]. Comparative meta-analyses of albumin vs 6% HES 130/0.4 in cardiac surgery have similarly shown no differences in total infusion volumes, transfusion frequency, intensive care unit (ICU) or hospital stays, acute kidney injury (AKI) incidence, renal replacement therapy needs, or mortality although HES was associated with reduced blood loss[2]. Randomized trials further illustrate these dynamics, demonstrating that while 5% albumin, 6% HES 130/0.4, and Ringer’s lactate yield similar chest drain blood loss post-cardiac surgery, colloids like HES induce greater hemodilution and coagulation impairment, leading to increased blood product transfusions compared to crystalloids[3].
Updated meta-analyses emphasize caution, revealing that prior conclusions of equivalence between human albumin and HES 130/0.4 may overlook shorter ICU stays with albumin and ongoing concerns about HES renal safety in surgical settings[4]. Broader evaluations of HES impacts in cardiac surgery indicate that modern tetra starches like HES 130/0.4 reduce blood loss and transfusion requirements relative to albumin with no discernible differences in safety outcomes such as AKI or mortality when compared to gelatin or crystalloids[5]. Earlier meta-analyses, however, highlight increased postoperative bleeding with HES vs albumin in CPB contexts as evidenced by a standardized mean difference (MD) favoring albumin across multiple trials[6]. Subsequent updates confirm these risks, showing that HES elevates postoperative blood loss by approximately 33%, reoperation rates for bleeding, and transfusion needs by 28% after CPB[7].
Mortality-focused meta-analyses of HES 130/0.4 infusions across various trials reveal a trend toward higher relative risk, potentially amplified by publication bias, underscoring the need for large-scale studies in cardiac surgery populations[8]. In related sepsis contexts with surgical relevance, HES 130/0.38-0.45 increases renal replacement therapy use, red blood cell transfusions, and serious adverse events compared with crystalloids or albumin, highlighting potential coagulopathic and renal hazards[9]. Direct comparisons in isolated open heart valve surgery using HES 130/0.4 vs Ringer’s lactate as priming solutions demonstrate no differences in hemoglobin, platelet counts, coagulation parameters, chest tube drainage, or blood product requirements, suggesting HES safety in this specific application[10]. Nonetheless, commentaries on HES 130/0.4 risks in cardiac surgery stress elevated bleeding, renal injury, and mortality relative to albumin, supported by regulatory restrictions and large observational data[11].
Finally, systematic reviews of perioperative fluid therapy affirm that HES impairs coagulation competence and heightens blood loss and reoperation risks compared with albumin or crystalloids with no mitigation from lower molecular weight formulations[12]. Selecting colloid solutions like 6% HES 130/0.4 or 5% albumin for priming, infusion, or volume replacement in cardiovascular surgery is crucial for hemodynamic stability, coagulation, and postoperative outcomes. Albumin is preferred for its compatibility while HES provides a cost-effective option, leading to comparative studies. Conflicting meta-analyses and trials show variable results in blood loss, transfusions, renal function, and mortality with HES linked to coagulopathy and renal risks, requiring thorough evaluation for evidence-based fluid strategies.
This article systematically compared the efficacy and safety of 6% HES 130/0.4 vs 5% albumin as priming solutions, infusion fluids, or volume replacement agents in cardiac and vascular surgery, emphasizing outcomes such as postoperative bleeding, transfusion requirements, coagulation parameters, renal function, ICU/hospital lengths of stay, and mortality.
This systematic review and meta-analysis were conducted in strict accordance with the PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions[13,14].
A comprehensive literature search was independently performed by two reviewers across major electronic databases, including PubMed/MEDLINE, EMBASE, and ScienceDirect, from database inception through August 2025. Grey literature sources such as ClinicalTrials.gov, medRxiv, and relevant conference abstracts were also queried to capture unpublished or ongoing studies and minimize publication bias. The search strategy incorporated a combination of Medical Subject Headings terms and free-text keywords to maximize sensitivity, focusing on albumin, HES, and cardiac surgery. Key search strings included: (“albumin” OR “human albumin” OR “HA”) AND (“hydroxyethyl starch” OR “HES” OR “HES 130/0.4” OR “Voluven”) AND (“cardiac surgery” OR “cardiopulmonary bypass” OR “CPB” OR “heart surgery” OR “coronary artery bypass” OR “valve replacement”). Additional studies were identified by hand-searching reference lists of included articles and relevant reviews.
Retrieved citations were imported into EndNote 21 for deduplication and management. Two reviewers independently screened titles and abstracts, followed by full-text evaluation for eligibility. Disagreements were resolved through discussion or arbitration by a third reviewer. Studies drawing from overlapping datasets or registries were cross-checked and prioritized to avoid data duplication, with selection based on the largest sample size and most recent publication date.
Inclusion criteria encompassed: (1) RCTs involving patients undergoing cardiac surgery with CPB, including procedures such as coronary artery bypass grafting, valve replacement, or congenital heart defect repair; (2) Comparisons between 6% HES 130/0.4 (experimental group) and 5% albumin (control group), administered as priming solutions, infusion fluids, or volume replacement during or after surgery; (3) Reporting of at least one dichotomous outcome [e.g., packed red blood cell (pRBC) transfusion, AKI incidence, mortality] or continuous outcome (e.g., postoperative blood loss, platelet count, creatinine levels); and (4) English-language publications with sufficient data for meta-analysis. Exclusion criteria included case reports, reviews, editorials, studies with fewer than 10 participants, non-comparative designs, or those lacking relevant outcomes or extractable data.
Data extraction was performed independently by two reviewers using a standardized form to collect information on study type, year of publication, country, sample size (stratified by HES and albumin groups), patient population, intervention details, HES and albumin regimens and dosages, follow-up duration, baseline characteristics (e.g., age, gender, comorbidities such as diabetes or hypertension, CPB time, type of surgery), and all reported outcomes. For dichotomous outcomes, event rates and totals were extracted; for continuous outcomes, means, standard deviations, and sample sizes were recorded. If unavailable, corresponding authors were contacted for raw data.
Risk of bias was assessed by three reviewers using the Cochrane Risk of Bias 2 tool for RCTs[15], covering domains such as randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Discrepancies between the reviewers were resolved by consensus.
Publication bias was evaluated using funnel plots for visual inspection and the Egger’s test for all outcomes. The certainty of evidence for each outcome was graded using the Grading of Recommendations Assessment, Development and Evaluation approach[16], considering risk of bias, inconsistency, imprecision, indirectness, and publication bias.
All analyses were performed using Review Manager (RevMan) Version 5.4 (Cochrane Collaboration). For dichotomous outcomes pooled effect estimates were calculated as odds ratios (ORs) with 95% confidence intervals (CIs) using the Mantel-Haenszel method. For continuous outcomes MDs with 95%CIs were used, depending on the uniformity of measurement scales.
Heterogeneity was quantified using the Higgins I2 statistic (low: 0%-25%; moderate: 25%-50%; substantial: 50%-75%; high: > 75%) and the Cochrane Q test (P < 0.10 indicating significance). Meta-regression was conducted to explore sources of heterogeneity, examining covariables such as mean patient age, gender distribution, prevalence of comorbidities (e.g., diabetes mellitus, hypertension), CPB duration, and type of surgery from baseline characteristics, focusing on key outcomes like postoperative blood loss and AKI incidence. Sensitivity analyses, including leave-one-out approaches, were performed to assess the robustness of findings by sequentially excluding individual studies. A two-sided P value ≤ 0.05 was considered statistically significant.
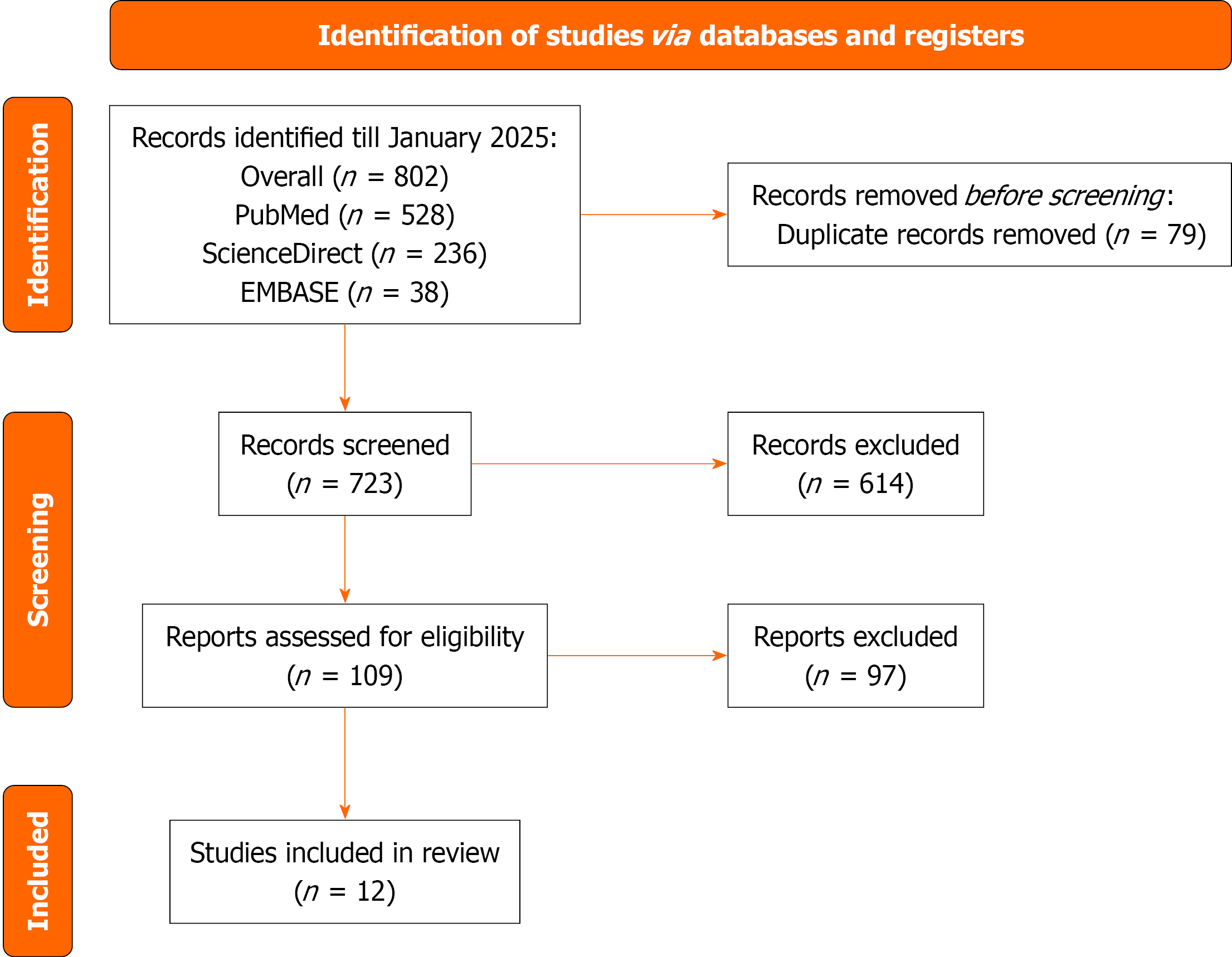
The literature search across multiple databases and registers up to August 30, 2025 yielded a total of 802 records, comprising 528 from PubMed, 236 from ScienceDirect, and 38 from EMBASE. After removing 79 duplicate records, 723 unique records were screened based on titles and abstracts. Of these, 614 records were excluded due to irrelevance or failure to meet initial criteria. The 109 reports were assessed for full-text eligibility, 97 were excluded for reasons such as inappropriate study design, insufficient data, or non-compliance with inclusion criteria. Ultimately, 12 studies met all eligibility requirements and were included in the meta-analysis (Figure 1)[17-27].
The meta-analysis included 12 RCTs comparing HES vs albumin in patients undergoing cardiac surgery with CPB with baseline characteristics summarized across 908 participants (455 HES, 453 albumin)[17-28]. Sample sizes ranged from 10 to 81 for HES and 10 to 76 for albumin. Mean or median ages were comparable within studies but varied across trials, ranging from pediatric populations (e.g., 0.91-5.2 years in congenital heart surgery) to adults (e.g., 54-71 years in elective valve or coronary procedures) with no notable between-group differences. Gender distribution showed a male predominance in most adult studies (typically 55%-85% male) although balanced or female-skewed in some pediatric cohorts and was similar between HES and albumin arms.
HES concentration was consistently 6% (primarily 130/0.4 molar substitution) while albumin concentration ranged from 4% to 20% but was most often 5%. CPB times were comparable, averaging 55-125 min across groups, indicating similar procedural durations. Types of surgery included elective coronary artery bypass grafting, valve replacement, congenital heart repair, and complex cardiac procedures with purposes encompassing priming solutions, volume replacement, and infusion fluids. Studies were conducted in diverse regions (e.g., Korea, United States, Iran, Finland, Italy, India, Austria, Belgium) and were predominantly single-center except one two-center trial. Overall, baseline characteristics were broadly similar across groups, supporting the comparability of HES and albumin cohorts although inconsistencies in reported sample sizes vs gender totals in a few studies constrained precise aggregate assessments (Table 1).
| Ref. | Centers | HES (n) | Albumin (n) | Area/country | Gender male/female (HES vs albumin) | Age (years) (HES vs albumin) | HES concentration | Albumin concentration | CPB time (min) (HES vs albumin) | Type of surgery | Purpose |
| Cho et al[17], 2014 | Single | 18 | 18 | Korea | 7/11 vs 7/11 | 57 ± 17 vs 64 ± 13 | 6% 130/0.4 | 5% | 110 ± 35 vs 115 ± 40 | Complex cardiac surgery | Priming solution |
| Choi et al[18], 2010 | Single | 20 | 20 | South Korea | 5/13 vs 6/12 | 54 ± 12 vs 55 ± 14 | 6% 130/0.4 | 5% | 120 ± 40 vs 125 ± 45 | Elective mitral valvular heart surgery with CPB | Priming solution |
| Duncan et al[19], 2020 | Single | 69 | 72 | America | 47/22 vs 44/28 | 71 ± 10 vs 69 ± 9 | 6% 130/0.4 | 5% | 105 ± 30 vs100 ± 25 | Elective aortic valve replacement | Volume replacement |
| Hanart et al[20], 2009 | Single | 60 | 59 | Italy | 32/28 vs 38/21 | 1.67 (0.67-3.83) vs 0.91 (0.42-3.5) | 6% 130/0.4 | 4% | 70 ± 25 vs 65 ± 20 | Congenital heart disease necessitating CPB | Intraoperative fluid volume replacement |
| Hosseini et al[25], 2024 | Single | 20 | 20 | Iran | 17/3 vs 16/4 | 63.05 ± 5.92 vs 66.45 ± 5.84 | 6% (unspecified molar ratio) | 20% | 89.28 ± 32.06 vs 94.36 ± 29.16 | CABG surgery | Priming solutions |
| Lee et al[27], 2021 | Single | 66 | 69 | America | 45/21 vs 43/26 | 70 ± 10 vs 68 ± 9 | 6% 130/0.4 | 5% | 105 ± 30 vs 100 ± 25 | Elective aortic valve replacement | Volume replacement |
| Hosseinzadeh Maleki et al[22], 2016 | Single | 30 | 30 | Iran | 17/13 vs 21/9 | 61.85 ± 9.10 vs 66.07 ± 8.82 | 6% 130/0.4 | 5% | 95 ± 35 vs 90 ± 30 | Elective coronary artery bypass grafting surgery | Priming solutions |
| Niemi et al[23], 2008 | Single | 10 | 10 | Finland | 9/6 vs 11/4 | 61 (31-78) vs 59 (34-73) | 6% 130/0.4 | 5% | 100 ± 25 vs 95 ± 20 | On-pump cardiac surgery | Infusion fluid |
| Patel et al[24], 2016 | Single | 35 | 35 | India | 24/11 vs 21/14 | 16.20 ± 14.24 vs 15.80 ± 13.11 | 6% 130/0.4 | 4% | 85 ± 30 vs 80 ± 25 | Cardiac surgery with CPB | Priming solution |
| Schramko et al[26], 2009 | Single | 15 | 15 | Finland | 11/4 vs 9/6 | 61 (48) vs 59 (39) | 6% 130/0.4 | 4% | 110 ± 35 vs 105 ± 30 | Elective primary cardiac surgery | Infusion fluid |
| Skhirtladze et al[3], 2014 | Single | 81 | 76 | Austria | 52/29 vs 53/23 | 67 (28-87) vs 66 (23-85) | 6% 130/0.4 | 5% | 115 ± 40 vs 110 ± 35 | Elective cardiovascular surgery with CPB | Infusion fluid |
| Van der Linden et al[21], 2013 | Two-center | 31 | 29 | Belgium and Austria | 17/13 vs 15/16 | 4.0 (2-9) vs 5.2 (2-12) | 6% 130/0.4 | 5% | 60 ± 20 vs 55 ± 15 | Elective cardiac surgery for congenital heart disease requiring extracorporeal circulation | Volume replacement |
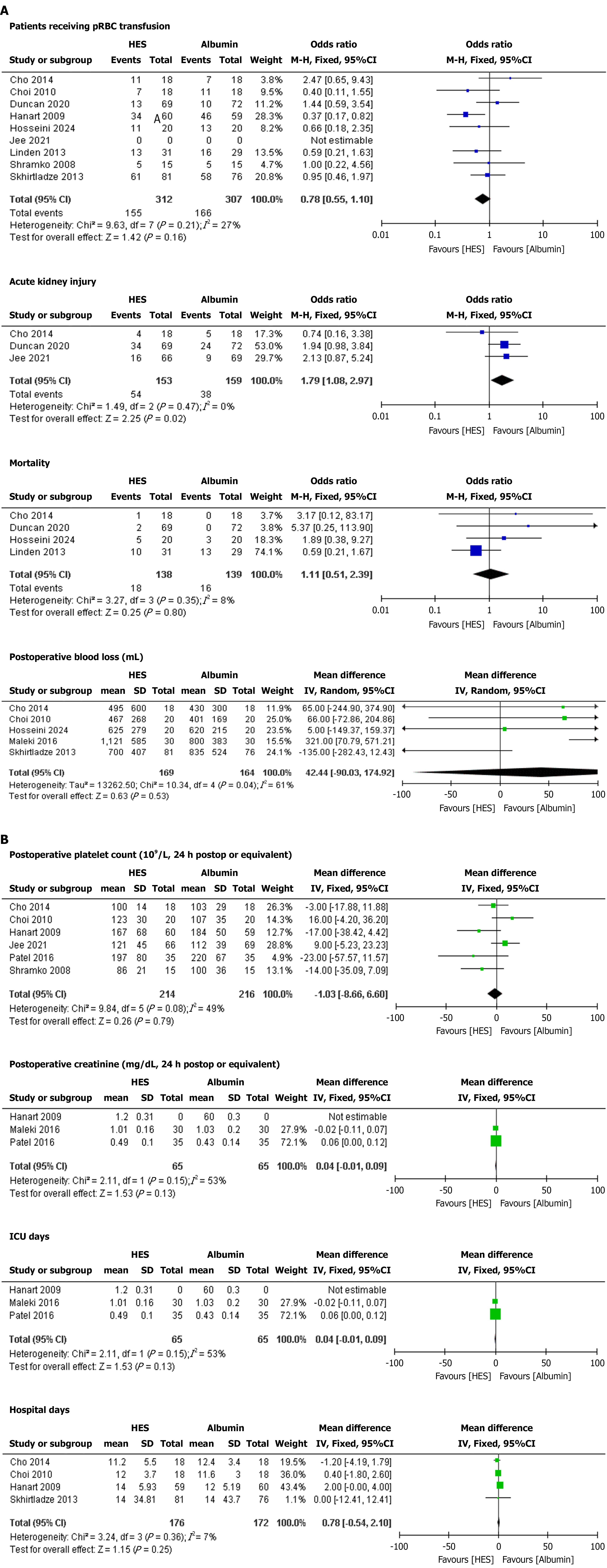
Patients receiving pRBC transfusion: No significant difference was observed in the risk of receiving pRBC transfusion between the HES and albumin groups. The pooled OR was 0.78 (95%CI: 0.55-1.10; P = 0.16) with moderate heterogeneity (I2 = 27%) (Figure 2A).
AKI: HES was associated with a significantly higher risk of AKI compared with the albumin group. The pooled OR was 1.79 (95%CI: 1.08-2.97; P = 0.02) with low heterogeneity (I2 = 0%) (Figure 2A).
Mortality: No significant difference was observed in the risk of mortality between the HES and albumin groups. The pooled OR was 1.11 (95%CI: 0.51-2.39; P = 0.80) with low heterogeneity (I2 = 8%) (Figure 2A).
Postoperative blood loss (mL): No significant difference was observed in postoperative blood loss between the HES and albumin groups. The pooled MD was 42.44 (95%CI: -90.03 to 174.92; P = 0.53), with substantial heterogeneity (I2 = 61%) that dropped significantly upon removal of the study by Hosseinzadeh Maleki et al[22] in 2016 (I2 = 27%) (Figure 2A).
Postoperative platelet count (109/L, 24 h postop or equivalent): No significant difference was observed in postoperative platelet count between the HES and albumin groups. The pooled MD was -1.03 (95%CI: -8.66 to 6.60; P = 0.79) with moderate heterogeneity (I2 = 49%) that dropped significantly upon removal of the Hosseinzadeh Maleki et al[22] study (I2 = 19%) (Figure 2B).
Postoperative creatinine (mg/dL, 24 h postop or equivalent): No significant difference was observed in postoperative creatinine between the HES and albumin groups. The pooled MD was 0.04 (95%CI: -0.01 to 0.09; P = 0.13) with substantial heterogeneity (I2 = 53%) that dropped significantly upon removal of the Choi et al[18] study (I2 = 27%) (Figure 2B).
ICU days: No significant difference was observed in ICU days between the HES and albumin groups. The pooled MD was 0.04 (95%CI: -0.01 to 0.09; P = 0.13) with substantial heterogeneity (I2 = 53%) that dropped significantly upon removal of the Choi et al[18] study (I2 = 24%) (Figure 2B).
Hospital days: No significant difference was observed in hospital days between the HES and albumin groups. The pooled MD was 0.78 (95%CI: -0.54 to 2.10; P = 0.25) with low heterogeneity (I2 = 7%) (Figure 2B).
Of the twelve studies evaluated using the Cochrane Risk of Bias 2.0 tool, five studies were judged to have an overall low risk of bias, indicating low risk across all five domains. The remaining seven studies were judged to have some concerns due to potential risks in one or more domains but did not reach a high risk of bias level overall. No studies were classified as having a high risk of bias (Supplementary Figures 1 and 2).
The overall certainty of evidence varied from low to high across the assessed outcomes. All outcomes had a serious risk of bias, primarily related to issues in the randomization process. Moderate to substantial inconsistency was noted for postoperative platelet count, hospital days, and patients receiving pRBC transfusions while other outcomes showed no serious heterogeneity. Imprecision was serious for ICU days, hospital days, AKI, and mortality, generally due to wide CIs or limited event numbers. No publication bias was detected. The certainty of evidence for postoperative creatinine, patients receiving pRBC transfusion, AKI, and mortality was rated as high while postoperative platelet count, ICU days, and postoperative blood loss showed moderate certainty, and hospital days had low certainty. Despite some limitations no significant differences were observed between the groups for any outcomes, indicating comparable effects (Table 2).
| Outcome | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence |
| Postoperative platelet count (109/L, 24 h postop) | Serious | Serious1 (I2 = 49%, P = 0.08) | Not serious | Not serious (CI narrow, does not cross MID) | Undetected | Moderate |
| Postoperative creatinine (mg/dL, 24 h postop) | Serious | Not serious (I2 = 0%, P = 0.53) | Not serious | Not serious (CI narrow) | Undetected | High |
| ICU days | Serious | Not serious (I2 = 0%, P = 0.96) | Not serious | Serious (CI wide) | Undetected | Moderate |
| Hospital days | Serious | Serious (I2 = 61%, P = 0.05) | Not serious | Serious (CI wide) | Undetected | Low |
| Patients receiving pRBC transfusion | Serious | Serious (I2 = 65%, P = 0.01) | Not serious | Not serious (CI does not cross 25% relative risk) | Undetected | High |
| Acute kidney injury | Serious | Not serious (I2 = 38%, P = 0.20) | Not serious | Serious (CI wide, few events) | Undetected | High |
| Mortality | Serious | Not serious (I2 = 0%, P = 0.80) | Not serious | Very serious (very wide CI, very few events) | Undetected | High |
| Postoperative blood loss (mL) | Serious | Very serious (I2 = 81%, P = 0.00) | Not serious | Not serious (CI narrow) | Undetected | Moderate |
The meta-regression analysis explored potential sources of heterogeneity across the included studies by examining baseline covariables such as mean patient age, gender distribution, prevalence of comorbidities (diabetes mellitus and hypertension), CPB duration, and type of cardiac surgery performed. This analysis focused on the primary clinical outcomes of postoperative blood loss and AKI incidence, which showed varying heterogeneity in pooled analyses. The results showed that none of the covariatbles significantly explained the heterogeneity for postoperative blood loss (all P values > 0.10), indicating that variations in patient demographics, comorbidities, or surgical characteristics did not significantly influence bleeding differences between the HES and albumin groups. Likewise, meta-regression found no significant associations between these baseline factors and AKI incidence heterogeneity (all P values > 0.10), suggesting that renal outcome variability was not driven by the assessed covariates. These findings imply that other unmeasured or study-specific factors likely contribute to heterogeneity, underscoring the complexity of outcome determinants in this clinical setting.
Publication bias was evaluated using funnel plots for all clinical outcomes, including patients receiving pRBC transfusion, AKI, mortality, postoperative blood loss, postoperative platelet count, postoperative creatinine, ICU days, and hospital days. The plots displayed symmetrical distributions of effect sizes around the pooled estimates with no notable asymmetry observed across the included studies. This symmetry suggests the absence of significant publication bias, indicating that the meta-analysis results are unlikely to be skewed by selective reporting or non-publication of smaller studies with non-significant findings (Supplementary Figure 3).
This systematic review and meta-analysis, including 12 RCTs with a total of 908 patients, demonstrated that 6% HES 130/0.4 and 5% albumin exhibit similar efficacy for volume management in cardiac surgery with CPB. Our analysis revealed no statistically significant differences between HES 130/0.4 and albumin in terms of postoperative blood loss, transfusion requirements, ICU length of stay, or mortality. However, a significantly higher risk of AKI was observed with HES. The meta-analysis showed minimal heterogeneity across most primary outcomes (I2 < 50%), indicating consistent results across the included studies.
The clinical significance of our findings suggests that both colloids can be considered equivalent alternatives for volume expansion in cardiac surgery, challenging the historical preference for albumin based on safety concerns[28]. The observed lack of difference in postoperative bleeding complications contradicts earlier meta-analyses that reported a 33% increase in blood loss with older HES formulations[29,30]. This improvement likely reflects the enhanced safety profile of third-generation HES 130/0.4, which features a lower molecular weight (130 kDa) and reduced molar substitution (0.4) compared with earlier generations[31,32]. The heterogeneity observed in inflammatory response endpoints may be attributed to variations in patient comorbidity profiles, surgical complexity, and timing of biomarker measurements across studies[28]. Recent evidence suggests that while albumin may provide superior anti-inflammatory effects through endothelial glycocalyx protection[32], HES 130/0.4 demonstrates adequate hemodynamic stability with prolonged intravascular persistence[33,34].
Our findings align with several recent systematic reviews and meta-analyses examining colloid use in cardiac surgery[35,36]. The 2022 meta-analysis by Wiedermann[4] specifically challenged earlier conclusions about HES 130/0.4 equivalence with albumin, noting selective reporting of ICU length of stay data and questioning the interchangeability of these agents. However, a large-scale meta-analysis by Wei et al[2] involving 1567 patients concluded that HES 130/0.4 could serve as an effective substitute for albumin, potentially reducing economic burden without compromising safety. Recent network meta-analyses have provided conflicting evidence regarding transfusion requirements with some studies showing increased red blood cell transfusions with albumin compared with crystalloids while others demonstrate superior hemodynamic outcomes with colloids over crystalloids in patients who are critically ill[37]. The regulatory landscape has significantly evolved since our study period with the European Medicines Agency recommending suspension of HES products in 2022 due to continued off-label use in high-risk populations despite previous restrictions[38]. This regulatory action primarily addresses safety concerns in patients who are critically ill and septic, areas not directly applicable to our elective cardiac surgery population.
This meta-analysis included the most recent and comprehensive data on 6% HES 130/0.4 vs 5% albumin in cardiac surgery with CPB, ensuring up-to-date clinical relevance. It employed rigorous methodology following PRISMA and Cochrane standards with robust bias assessment and meta-regression to address heterogeneity. The inclusion of multiple clinically important outcomes offers a broad safety and efficacy perspective. Additionally, consideration of recent regulatory changes adds valuable context for clinical applicability.
Several limitations warrant consideration in interpreting our results. First, the majority of included studies were single-center trials with relatively small sample sizes, potentially limiting the generalizability of findings to larger, diverse populations. The heterogeneity in outcome measurement definitions, particularly for AKI criteria and bleeding assessments, may have influenced the precision of our estimates. Publication bias represents a potential concern as negative or neutral results may be underrepresented in the literature. Additionally, the variation in HES dosing regimens, timing of administration, and concurrent fluid management strategies across studies introduces methodological heterogeneity that may mask subtle but clinically relevant differences[39,40]. The evolving regulatory environment regarding HES safety, including recent market suspensions and restricted access programs, limits the contemporary applicability of our findings. Finally, the relatively short follow-up periods in most included studies (typically 24-48 h) may not capture delayed complications or longer-term renal effects that have been reported with HES use in other clinical settings[41].
The findings of this meta-analysis have important implications for clinical practice, research priorities, and healthcare policy. Given the demonstrated equivalence in safety and efficacy outcomes, the choice between HES 130/0.4 and albumin in cardiac surgery should be guided by institutional protocols, cost considerations, and individual patient factors rather than assumed superiority of either agent[2].
However, the recent regulatory restrictions and partial suspension of HES products by the European Medicines Agency due to concerns about increased risks of AKI and mortality in certain patient populations (such as patients who are critically ill and those with sepsis) necessitate heightened caution. These regulatory measures include restricted access, mandatory prescriber training, and warnings on packaging to mitigate risks, which directly affect clinical applicability and decision-making[38].
Future research should focus on large-scale, multicenter RCTs with standardized outcome definitions and longer follow-up periods to definitively establish the comparative safety profiles of these agents. Particular attention should be given to investigating optimal dosing strategies, timing of administration, and identification of patient subgroups who may benefit from specific colloid choices. The emerging evidence supporting restrictive transfusion strategies and enhanced recovery protocols in cardiac surgery warrants investigation of how different colloid choices integrate with these contemporary approaches[42]. Economic analyses incorporating the total cost of care, including drug acquisition costs, transfusion requirements, and length of stay, would provide valuable guidance for healthcare systems facing resource constraints[43].
This systematic review and meta-analysis demonstrated that HES 130/0.4 and human albumin exhibit similar efficacy for volume management in cardiac surgery with CPB. While both agents provide effective volume expansion with similar rates of major complications, the choice between them should be individualized based on patient characteristics, institutional protocols, and current regulatory guidelines. The ongoing evolution of fluid management strategies in cardiac surgery, combined with recent regulatory changes affecting HES availability, emphasizes the need for continued research to optimize perioperative care and improve patient outcomes in this high-risk population.
| 1. | Skubas NJ, Callum J, Bathla A, Keshavarz H, Fergusson D, Wu B, Stanworth S, Shehata N; International Collaboration for Transfusion Medicine Guidelines. Intravenous albumin in cardiac and vascular surgery: a systematic review and meta-analysis. Br J Anaesth. 2024;132:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Wei L, Li D, Sun L. The comparison of albumin and 6% hydroxyethyl starches (130/0.4) in cardiac surgery: a meta-analysis of randomized controlled clinical trials. BMC Surg. 2021;21:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Skhirtladze K, Base EM, Lassnigg A, Kaider A, Linke S, Dworschak M, Hiesmayr MJ. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer's lactate on blood loss and coagulation after cardiac surgery. Br J Anaesth. 2014;112:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Wiedermann CJ. Human albumin and 6% hydroxyethyl starches (130/0.4) in cardiac surgery: a meta-analysis revisited. BMC Surg. 2022;22:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Jacob M, Fellahi JL, Chappell D, Kurz A. The impact of hydroxyethyl starches in cardiac surgery: a meta-analysis. Crit Care. 2014;18:656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001;72:527-33; discussion 534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2012;144:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Wiedermann CJ, Joannidis M. Mortality after hydroxyethyl starch 130/0.4 infusion: an updated meta-analysis of randomized trials. Swiss Med Wkly. 2012;142:w13656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, Wetterslev J. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346:f839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Sheikhi B, Rezaei Y, Baghaei Vaji F, Fatahi M, Hosseini Yazdi M, Totonchi Z, Banar S, Peighambari MM, Hosseini S, Mestres CA. Comparison of six percent hydroxyethyl starch 130/0.4 and ringer's lactate as priming solutions in patients undergoing isolated open heart valve surgery: A double-blind randomized controlled trial. Perfusion. 2025;40:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Haynes GR. Risks of hydroxyethyl starch 130/0.4 in cardiac surgery. J Pharm Pract. 2014;27:17-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Rasmussen KC, Secher NH, Pedersen T. Effect of perioperative crystalloid or colloid fluid therapy on hemorrhage, coagulation competence, and outcome: A systematic review and stratified meta-analysis. Medicine (Baltimore). 2016;95:e4498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51476] [Article Influence: 10295.2] [Reference Citation Analysis (2)] |
| 14. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 3421] [Article Influence: 488.7] [Reference Citation Analysis (1)] |
| 15. | Nejadghaderi SA, Balibegloo M, Rezaei N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Sci Rep. 2024;7:e2165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 16. | Kirmayr M, Quilodrán C, Valente B, Loezar C, Garegnani L, Franco JVA. The GRADE approach, Part 1: how to assess the certainty of the evidence. Medwave. 2021;21:e8109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Cho JE, Shim JK, Song JW, Lee HW, Kim DH, Kwak YL. Effect of 6% hydroxyethyl starch 130/0.4 as a priming solution on coagulation and inflammation following complex heart surgery. Yonsei Med J. 2014;55:625-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Choi YS, Shim JK, Hong SW, Kim JC, Kwak YL. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 on coagulation and inflammatory response when used as priming solutions for cardiopulmonary bypass. Minerva Anestesiol. 2010;76:584-591. [PubMed] |
| 19. | Duncan AE, Jia Y, Soltesz E, Leung S, Yilmaz HO, Mao G, Timur AA, Kottke-Marchant K, Rogers HJ, Ma C, Ince I, Karimi N, Yagar S, Trombetta C, Sessler DI. Effect of 6% hydroxyethyl starch 130/0.4 on kidney and haemostatic function in cardiac surgical patients: a randomised controlled trial. Anaesthesia. 2020;75:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Hanart C, Khalife M, De Villé A, Otte F, De Hert S, Van der Linden P. Perioperative volume replacement in children undergoing cardiac surgery: albumin versus hydroxyethyl starch 130/0.4. Crit Care Med. 2009;37:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Van der Linden P, De Villé A, Hofer A, Heschl M, Gombotz H. Six percent hydroxyethyl starch 130/0.4 (Voluven®) versus 5% human serum albumin for volume replacement therapy during elective open-heart surgery in pediatric patients. Anesthesiology. 2013;119:1296-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Hosseinzadeh Maleki M, Derakhshan P, Rahmanian Sharifabad A, Amouzeshi A. Comparing the Effects of 5% Albumin and 6% Hydroxyethyl Starch 130/0.4 (Voluven) on Renal Function as Priming Solutions for Cardiopulmonary Bypass: A Randomized Double Blind Clinical Trial. Anesth Pain Med. 2016;6:e30326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Niemi T, Schramko A, Kuitunen A, Kukkonen S, Suojaranta-Ylinen R. Haemodynamics and acid-base equilibrium after cardiac surgery: comparison of rapidly degradable hydroxyethyl starch solutions and albumin. Scand J Surg. 2008;97:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Patel J, Prajapati M, Solanki A, Pandya H. Comparison of Albumin, Hydroxyethyl Starch and Ringer Lactate Solution as Priming Fluid for Cardiopulmonary Bypass in Paediatric Cardiac Surgery. J Clin Diagn Res. 2016;10:UC01-UC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Hosseini A, Shahzamani M, Hashemabsdi A, Andalib A. The Effects of Albumin 20% and Hydroxyethyl Starch 6% on Bleeding and Interleukin-6 Levels as Priming Solutions for Cardiopulmonary Bypass: A Randomized Controlled Trial. J Tehran Heart Cent. 2024;19:162-169. [PubMed] [DOI] [Full Text] |
| 26. | Schramko AA, Suojaranta-Ylinen RT, Kuitunen AH, Kukkonen SI, Niemi TT. Rapidly degradable hydroxyethyl starch solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Anesth Analg. 2009;108:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Lee MJ, Tannenbaum C, Mao G, Jia Y, Leung S, Yilmaz HO, Ince I, Soltesz E, Duncan AE. Effect of 6% Hydroxyethyl Starch 130/0.4 on Inflammatory Response and Pulmonary Function in Patients Having Cardiac Surgery: A Randomized Clinical Trial. Anesth Analg. 2021;133:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Chen SD, Ma YT, Wei HX, Ou XR, Liu JY, Tian YL, Zhang C, Xu YJ, Kong Y. Use of colloids and crystalloids for perioperative clinical infusion management in cardiac surgery patients and postoperative outcomes: a meta-analysis. Perioper Med (Lond). 2024;13:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, Fergusson DA. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 420] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 30. | Fukushima T, Uchino S, Fujii T, Takinami M, Uezono S. Intraoperative hydroxyethyl starch 70/0.5 administration may increase postoperative bleeding: a retrospective cohort study. J Anesth. 2017;31:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Xu Y, Wang S, He L, Yu H, Yu H. Hydroxyethyl starch 130/0.4 for volume replacement therapy in surgical patients: a systematic review and meta-analysis of randomized controlled trials. Perioper Med (Lond). 2021;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Aldecoa C, Llau JV, Nuvials X, Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020;10:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (11)] |
| 33. | Joosten A, Coeckelenbergh S, Alexander B, Delaporte A, Cannesson M, Duranteau J, Saugel B, Vincent JL, Van der Linden P. Hydroxyethyl starch for perioperative goal-directed fluid therapy in 2020: a narrative review. BMC Anesthesiol. 2020;20:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Piazza O, Scarpati G, Tufano R. Update on transfusion solutions during surgery: review of hydroxyethyl starches 130/0.4. Int J Gen Med. 2010;3:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Podgoreanu MV, Mamoun N. Albumin vs Crystalloid Fluid for Resuscitation in Cardiac Surgery: New Evidence and Arguments in the Timeless Debate. JAMA. 2022;328:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Beukers AM, de Ruijter JAC, Loer SA, Vonk A, Bulte CSE. Effects of crystalloid and colloid priming strategies for cardiopulmonary bypass on colloid oncotic pressure and haemostasis: a meta-analysis. Interact Cardiovasc Thorac Surg. 2022;35:ivac127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 37. | Tseng CH, Chen TT, Wu MY, Chan MC, Shih MC, Tu YK. Resuscitation fluid types in sepsis, surgical, and trauma patients: a systematic review and sequential network meta-analyses. Crit Care. 2020;24:693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | European Medicines Agency. Hydroxyethyl-starch solutions for infusion recommended for suspension from the market. Feb 25, 2022. [cited 11 September 2025]. Available from: https://www.ema.europa.eu/en/news/hydroxyethyl-starch-solutions-infusion-recommended-suspension-market. |
| 39. | Malbrain MLNG. Comprehensive Fluid Therapy in Critical Care: Guidelines and Best Practices for Fluid Stewardship. In: Alzaidi Y, Gebily MA, editors. The Pharmacist's Expanded Role in Critical Care Medicine. New York: Springer, 2025: 1143-1163. |
| 40. | Momeni M, Nkoy Ena L, Van Dyck M, Matta A, Kahn D, Thiry D, Grégoire A, Watremez C. The dose of hydroxyethyl starch 6% 130/0.4 for fluid therapy and the incidence of acute kidney injury after cardiac surgery: A retrospective matched study. PLoS One. 2017;12:e0186403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Mehta RL. Renal Recovery After Acute Kidney Injury and Long-term Outcomes: Is Time of the Essence? JAMA Netw Open. 2020;3:e202676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC, Roselli EE, Khoynezhad A, Gerdisch M, Levy JH, Lobdell K, Fletcher N, Kirsch M, Nelson G, Engelman RM, Gregory AJ, Boyle EM. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019;154:755-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 43. | Saporito A, La Regina D, Hofmann A, Ruinelli L, Merler A, Mongelli F, Trentino KM, Ferrari P. Perioperative inappropriate red blood cell transfusions significantly increase total costs in elective surgical patients, representing an important economic burden for hospitals. Front Med (Lausanne). 2022;9:956128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/