Published online Sep 26, 2025. doi: 10.4330/wjc.v17.i9.110838
Revised: June 25, 2025
Accepted: August 15, 2025
Published online: September 26, 2025
Processing time: 92 Days and 22.5 Hours
Obese patients (body mass index ≥ 30 kg/m²) undergoing isolated aortic valve replacement (AVR) face increased surgical risks due to comorbidities. Partial upper sternotomy (PUS), a minimally invasive approach, may reduce complications compared to full median sternotomy (FMS). We hypothesize that PUS improves outcomes over FMS in obese patients undergoing AVR.
To compare the efficacy and safety of PUS vs FMS in obese patients undergoing isolated AVR.
This systematic review and meta-analysis followed PRISMA guidelines, searching PubMed, EMBASE, and Cochrane databases for observational studies comparing PUS vs FMS in obese patients undergoing AVR. Outcomes were analyzed using odds ratios (OR), mean differences (MD), 95% confidence intervals (CI), I² statistic, and Newcastle-Ottawa Scale was used for quality assessment.
Four observational studies involving 677 patients were analyzed. PUS reduced intensive care unit stay (MD -2.67 days, 95%CI: -4.43 to -0.90, P = 0.003, I² = 78%) but increased cardiopulmonary bypass time (MD 5.62 minutes, 95%CI: -0.36 to 11.59, I² = 55%). No differences were observed in renal failure (OR 1.13, 95%CI: 0.63-2.94, I² = 0%), atrial fibrillation (OR 0.81, 95%CI: 0.43-1.54, I² = 30%), reexploration (OR 1.09, 95%CI: 0.48-2.47, I² = 0%), postoperative bleeding (OR 1.48, 95%CI: 0.53-4.15, I² = 60%), wound infection (OR 1.23, 95%CI: 0.70-2.14, I² = 0%), hospital stay (MD 0.51 days, 95%CI: -4.13 to 5.15, I² = 90%), or cross-clamp time (MD 4.03 minutes, 95%CI: -0.75 to 8.80, I² = 50%).
PUS is safe and effective for obese patients undergoing AVR, reducing intensive care unit stay and enhancing recovery, provided surgical expertise is available.
Core Tip: This meta-analysis innovatively demonstrates that partial upper sternotomy (PUS) is a safe and effective alternative to full median sternotomy for obese patients (body mass index ≥ 30 kg/m²) undergoing aortic valve replacement, uniquely reducing intensive care unit stay by approximately 2.67 days. Despite a trend toward longer cardiopulmonary bypass times, PUS maintains equivalent safety profiles across major complications, offering a compelling minimally invasive option to enhance recovery in this high-risk population.
- Citation: Gupta A, Chikhradze T, Arshad A, Sakrani RA, Khan Z, Getahun M, Shaikh SRA, Syed W, Baweja T, Remesan A, Lewis C, Doshi J, Khawar M, Hussain A, Khawar MM. Partial upper sternotomy vs full median sternotomy in obese patients undergoing aortic valve replacement: A meta-analysis. World J Cardiol 2025; 17(9): 110838
- URL: https://www.wjgnet.com/1949-8462/full/v17/i9/110838.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i9.110838
Aortic valve disease, including aortic stenosis and regurgitation, significantly contributes to cardiovascular morbidity and mortality, often impairing left ventricular function and leading to heart failure[1]. Aortic valve replacement (AVR) is the definitive treatment for severe cases, with demand rising due to an aging population, where approximately 3% of indi
Full median sternotomy (FMS) is the standard approach for AVR, offering comprehensive cardiac access but posing challenges in obese patients, including higher risks of sternal wound infections and prolonged ventilation[7]. Since the 1990s, minimally invasive partial upper sternotomy (PUS) has emerged as an alternative, utilizing a smaller incision to reduce surgical trauma, blood loss, and recovery time, which may particularly benefit obese patients prone to compli
This meta-analysis addresses the gap in understanding the optimal surgical strategy for obese patients undergoing AVR by comparing the efficacy, safety, and postoperative outcomes of PUS vs FMS. By synthesizing existing evidence, this study seeks to clarify the benefits and limitations of each approach to inform clinical decision-making and guide future research.
This systematic review and meta-analysis adhered to the PRISMA statement and Cochrane Collaboration guidelines[15,16]. Institutional Review Board approval was not required, as data was publicly available from electronic databases.
Inclusion criteria: This review included observational studies (e.g., cohort or case-control studies) of adult patients (aged ≥ 18 years) with obesity (defined as BMI ≥ 30 kg/m²) undergoing isolated AVR via PUS compared to FMS. No randomized controlled trials (RCTs) were identified. Eligible studies reported at least one of the following outcomes of interest: Operative time, cardiopulmonary bypass time, cross-clamp time, intraoperative blood loss, intensive care unit (ICU) stay duration, hospital stay duration, complication rates (e.g., renal failure, atrial fibrillation, reexploration for bleeding, postoperative bleeding, wound infection), and mortality. Studies were limited to English-language, full-text publications from database inception to April 2025, to reflect contemporary surgical techniques.
Exclusion criteria: Excluded studies included case reports, reviews, editorials, letters, and those involving pediatric populations (< 18 years), non-clinical outcomes, or procedures other than isolated AVR (e.g., coronary artery bypass grafting, combined procedures, or non-sternotomy approaches like thoracotomy). Studies lacking comparative data between PUS and FMS, sufficient details on clinical outcomes, or outcomes specific to obese patients were also excluded.
A comprehensive literature search was conducted across PubMed, EMBASE, and Cochrane databases, covering studies published from database inception to April 2025. The key terms included "aortic valve replacement", "AVR", "partial upper sternotomy", "PUS", "full median sternotomy", "FMS", "minimally invasive", "obesity", "obese", and "BMI". These terms were combined with outcome-specific keywords, such as "renal failure", "atrial fibrillation", "reexploration", "postoperative bleeding", "wound infection", "hospital stay", "ICU stay", "cardiopulmonary bypass time", and "cross-clamp time". Search terms were carefully selected to maximize study retrieval and ensure comprehensive capture of relevant literature on PUS vs FMS in obese AVR patients. Limitations of the search strategy included restriction to English-language publications and exclusion of gray literature (e.g., conference abstracts, theses), which may introduce language bias and miss unpublished data.
A comprehensive literature search was conducted across PubMed, EMBASE, and Scopus to identify studies comparing PUS with FMS in obese adults. References were managed using Zotero for duplicate removal. Two independent reviewers screened titles and abstracts, followed by full-text assessments, based on predefined criteria. Discrepancies were resolved through discussion or consultation with a third reviewer. Studies included if they reported perioperative or clinical outcomes in obese patients undergoing AVR via PUS or FMS.
Data were extracted independently by two reviewers (Gupta A and Chikhradze T) using a standardized, pre-designed Google Sheet to ensure consistency. Prior to extraction, reviewers underwent training to align on variable definitions and data entry protocols. Extracted data included study characteristics, patient demographics, surgical approaches, and clinical outcomes (e.g., operative time, intraoperative blood loss, ICU and hospital stay duration, complication rates, mortality). Discrepancies were resolved through consensus discussions, with a third reviewer consulted if agreement could not be reached. Authors were contacted for clarification when needed. For outcomes reported as medians with interquartile ranges, validated methods were used to convert these to means and standard deviations. A random sample of 20% of extracted data was cross-checked by an independent reviewer to verify accuracy and enhance reproducibility.
The Newcastle-Ottawa Scale (NOS) was used to evaluate study quality[17]. Two independent authors (Sakrani RA and Khan Z) assessed each study across three domains: Selection of study groups (up to 4 points, evaluating representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, and demonstration that outcome was not present at start); comparability of groups (up to 2 points, based on control for key confounders such as age, sex, and comorbidities); and outcome assessment (up to 3 points, evaluating adequacy of follow-up, completeness of follow-up, and independent blind assessment or record linkage). Total scores ranged from 0 (worst) to 9 (best), with classifica
This meta-analysis followed PRISMA guidelines, analyzing dichotomous and continuous outcomes from cohort studies. Dichotomous outcomes were reported as odds ratios (OR) with 95% confidence intervals (CI), and continuous outcomes as mean differences (MDs) with 95%CI, based on measurement scale consistency. Heterogeneity was assessed using the I² statistic. The choice of model was based on heterogeneity levels: A fixed-effects model was applied for low heterogeneity (I² ≤ 50%), assuming studies estimate the same underlying effect; a random-effects model was used for moderate to high heterogeneity (I² > 50%) to account for between-study variability, providing more conservative estimates in the presence of differences in study populations or methods. Analyses were performed using RevMan version 5.4, with significance defined as P < 0.05. For each outcome, a forest plot was constructed to visually analyze the data and funnel plots were generated to check the publication bias. Sensitivity analyses, including leave-one-out approaches, were conducted to assess the robustness of findings by evaluating the impact of individual studies on overall heterogeneity, pooled esti
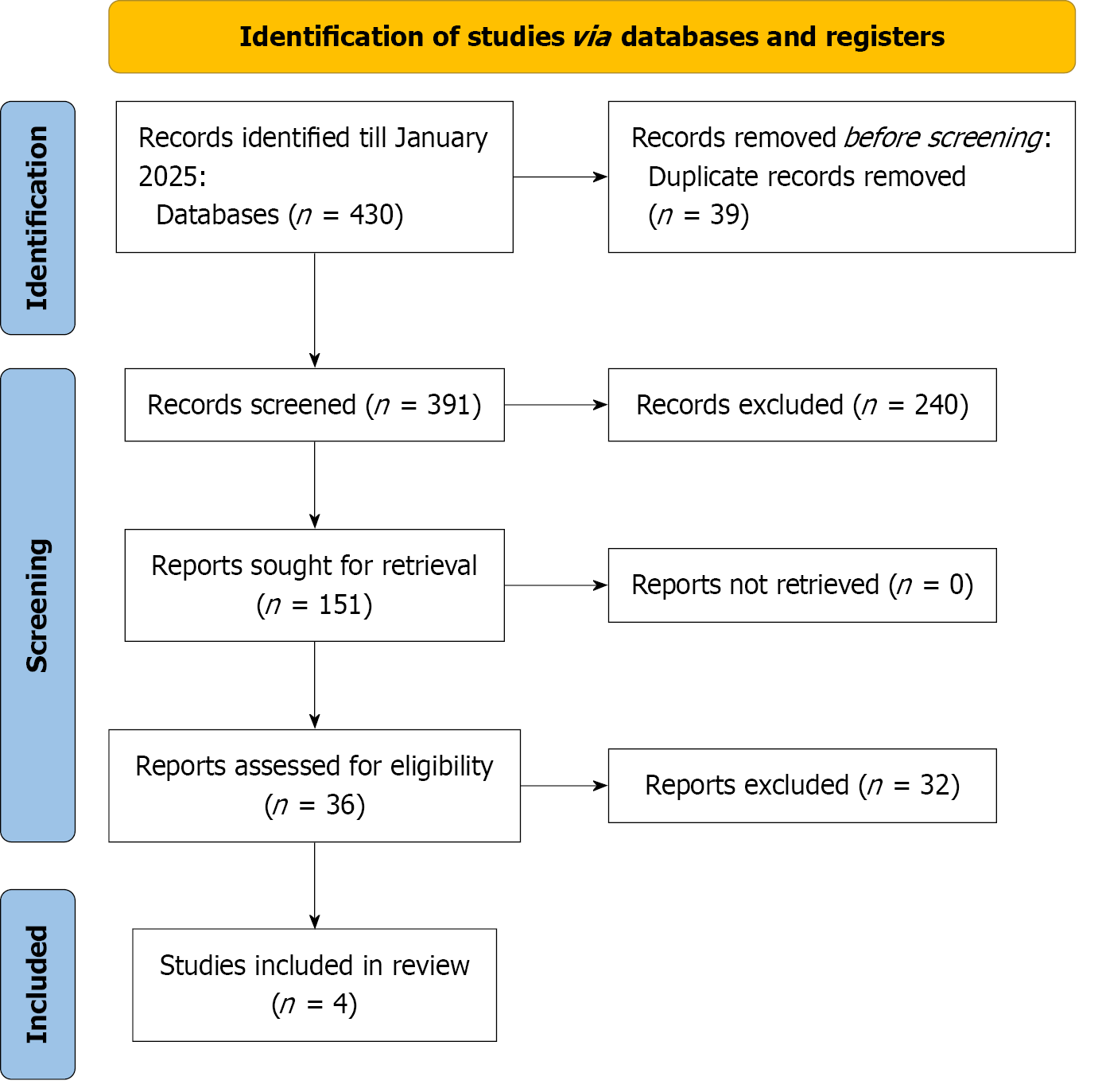
The PRISMA flow chart (Figure 1) outlines the literature screening, study selection, and exclusion criteria. The initial search yielded 430 articles, with 36 full-text articles assessed for eligibility. Four observational studies[12,18-20] met the criteria and were included in both qualitative and quantitative meta-analyses.
The four studies conducted between 2014 and 2023, compared PUS and FMS in obese adults undergoing isolated AVR. Mean ages ranged from 54.0-69.8 years for PUS and 55.9-70.0 years for FMS. Male patients comprised 46.0%-78.6% of PUS and 54.8%-79.5% of FMS groups. Mean BMI ranged from 30.56-38.3 kg/m² for PUS and 30.11-35.5 kg/m² for FMS. Diabetes mellitus prevalence was 6.0%-28.6% for PUS and 8.0%-38.5% for FMS. Hypertension affected 52.0%-90.0% of PUS and 62.0%-95.6% of FMS patients. Smoking, reported in three studies, ranged from 28.6%-29.4% for PUS and 26.4%-35.2% for FMS. Chronic obstructive pulmonary disease prevalence was 4.0%-9.5% for PUS and 5.0%-8.8% for FMS. Dyslipidemia, reported in two studies, affected 46.2%-90.0% of PUS and 48.4%-89.8% of FMS patients. Previous stroke, reported in two studies, occurred in 2.2%-6.3% of PUS and 4.4%-14.3% of FMS patients. Mean left ventricular ejection fraction ranged from 55.16%-62.5% for PUS and 58.33%-61.7% for FMS. Hemoglobin levels were 12.69-13.71 g/dL for PUS and 12.22-13.5 g/dL for FMS. Creatinine levels ranged from 1.12-1.68 mg/dL for PUS and 1.22-1.85 mg/dL for FMS. Hematocrit, reported in two studies, was 40.50%-41.57% for PUS and 38.32%-41.20% for FMS. Baseline characteristics were comparable, with minor variations in sex, diabetes, and stroke prevalence, supporting valid meta-analysis compa
| Ref. | Year | Age (mean ± SD) | Male (%) | BMI (kg/m2) | Diabetes mellitus (%) | Hypertension (%) | Smoking (%) | COPD (%) | Peripheral vascular disease (%) | LVEF | Hemoglobin (mean ± SD) | Creatinine (mean ± SD) | Hematocrit (mean ± SD) | Dyslipidemia (%) | Previous stroke (%) | ||||||||||||||
| PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | PUS | FS | ||
| Cammertoni et al[18] | 2024 | 68.6 ± 14.5 | 69.2 ± 13.1 | 60.4 | 59.3 | 32.9 ± 1.3 | 32.6 ± 1.2 | 19.8 | 24.2 | 83.5 | 82.4 | 28.6 | 26.4 | 4.4 | 7.7 | 5.5 | 7.7 | 61.3 ± 6.2 | 60.1 ± 5.5 | 13.2 ± 1.4 | 13.5 ± 1.3 | NA | NA | NA | NA | 46.2 | 48.4 | 2.2 | 4.4 |
| Luo et al[12] | 2022 | 54.0 ± 11.5 | 55.9 ± 10.8 | 78.6 | 79.5 | 30.56 ± 3.09 | 30.11 ± 3.04 | 17.1 | 18.2 | 90 | 89.8 | N/A | N/A | 8.6 | 8 | N/A | N/A | 62.5 ± 10.2 | 61.7 ± 8.9 | 12.69 ± 2.01 | 12.66 ± 2.88 | 120.5 ± 88.5 | 119.6 ± 98.6 | 41.57 ± 3.88 | 40.94 ± 3.87 | 90 | 89.8 | NA | NA |
| Welp et al[19] | 2018 | 69.8 ± 10.4 | 70.0 ± 10.5 | 56 | 54.8 | 32.56 ± 3.09 | 33.11 ± 3.04 | 28.6 | 38.5 | 84.1 | 95.6 | 29.4 | 35.2 | 9.5 | 8.8 | 7.1 | 7.7 | 55.16 ± 14.90 | 58.33 ± 10.82 | 13.71 ± 1.48 | 13.39 ± 1.85 | 1.12 ± 0.77 | 1.22 ± 0.81 | 40.50 ± 4.81 | 41.20 ± 3.82 | 59.5 | 67 | 6.3 | 14.3 |
| Xie et al[20] | 2022 | 62.9 ± 10.7 | 57.8 ± 12.3 | 46 | 58 | 38.3 ± 5.3 | 35.5 ± 4.2 | 6 | 8 | 52 | 62 | 29 | 34 | 4 | 5 | NA | NA | 57 ± 11.31 | 58.33 ± 11.29 | 13.68 ± 1.68 | 12.22 ± 1.35 | NA | NA | 41.32 ± 3.68 | 38.32 ± 4.68 | NA | NA | NA | NA |
Study quality was assessed using the NOS. Three studies were rated as good quality (scores 8/9), and one was rated moderate (score 7/9). All studies met the threshold for good quality (≥ 7), with consistent patient selection, comparability, and outcome ascertainment. Discrepancies between assessors were resolved by consensus (Supplementary Table 1).
Outcomes are presented below under separate subheadings for clarity, with effect sizes (ORs or MDs) and 95%CI re
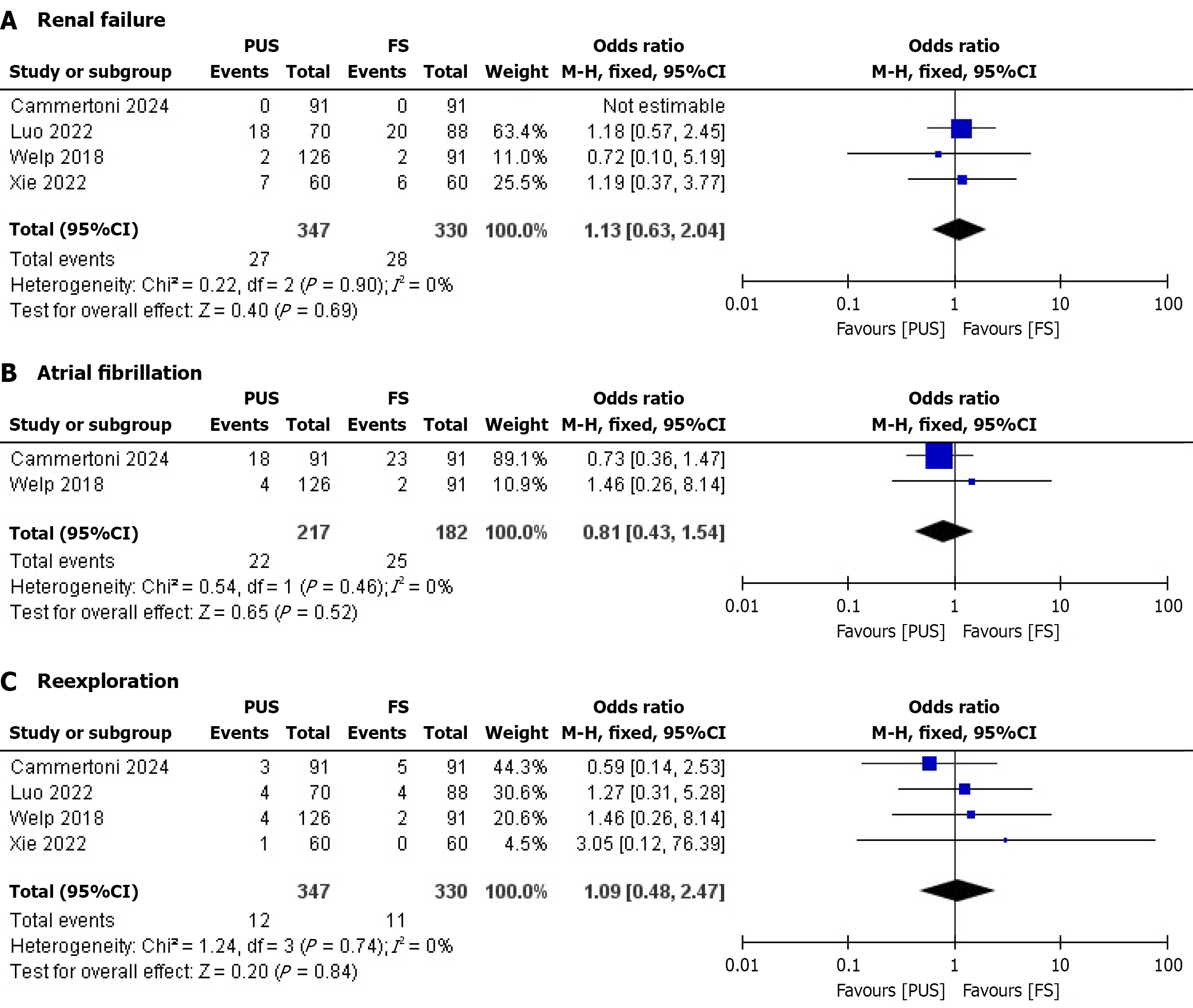
Renal failure: Four studies reported renal failure outcomes. No significant difference was observed between the PUS and full sternotomy (FS) groups. The pooled OR was 1.13 (95%CI: 0.63-2.94; P = 0.69), with no heterogeneity (I² = 0%) (Figure 2A). The lack of difference suggests comparable renal safety between PUS and FS, though the wide CI indicates limited precision, reducing clinical confidence in this finding.
Atrial fibrillation: Two studies assessed atrial fibrillation. No significant difference was found between the PUS and FS groups. The pooled OR was 0.81 (95%CI: 0.43-1.54; P = 0.84), with no heterogeneity (I² = 0%) (Figure 2B). This finding indicates similar arrhythmic risk, but the small number of studies and wide CI limit its clinical applicability.
Reexploration: Four studies reported reexploration rates. No significant difference was observed between the PUS and FS groups. The pooled OR was 1.09 (95%CI: 0.48-2.47; P = 0.84), with no heterogeneity (I² = 0%) (Figure 2C). The comparable reexploration rates suggest equivalent surgical precision, though the wide CI warrants cautious interpretation.
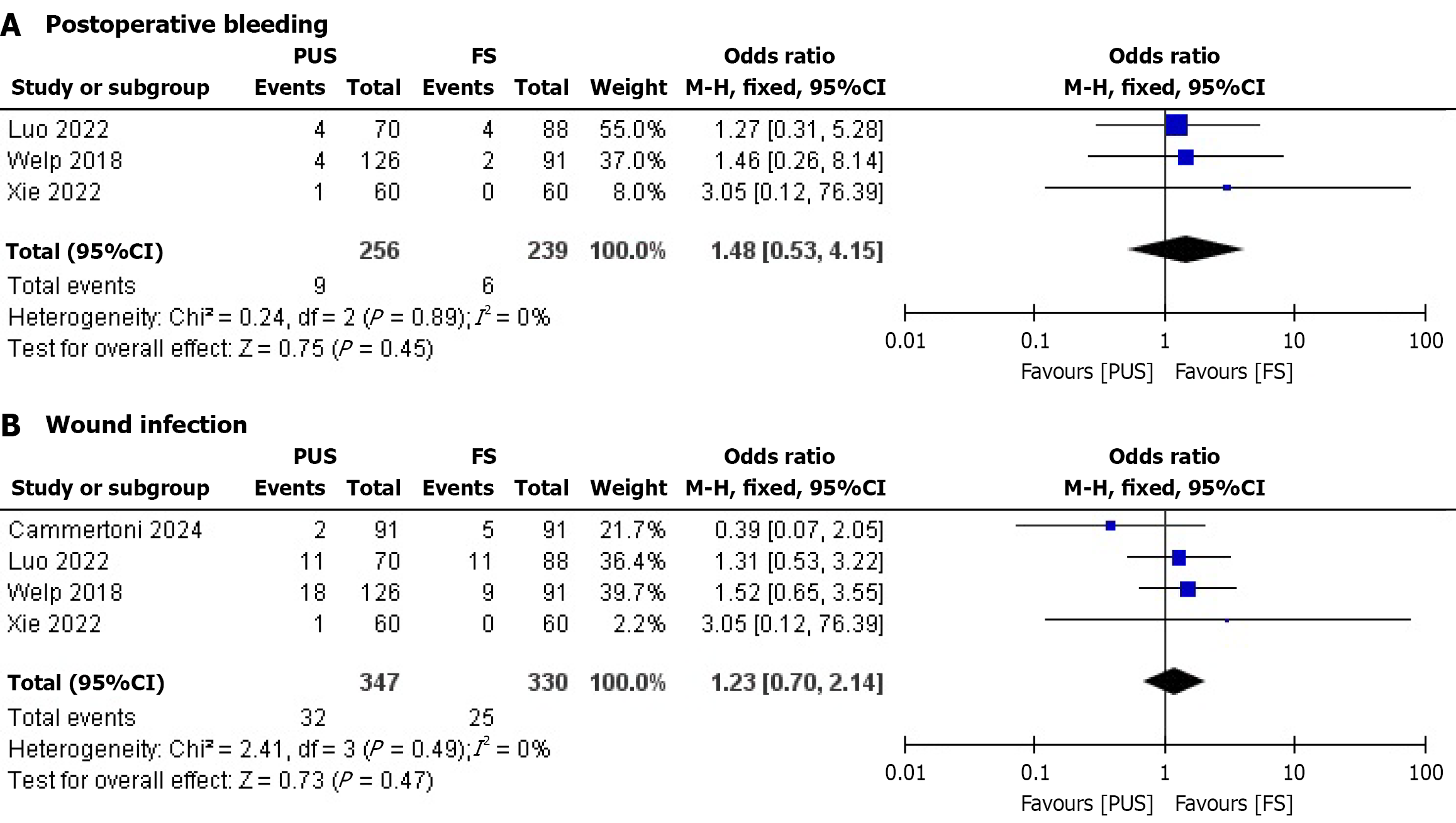
Postoperative bleeding: Three studies evaluated postoperative bleeding. No significant difference was found between the PUS and FS groups. The pooled OR was 1.48 (95%CI: 0.53-4.15; P = 0.45), with no heterogeneity (I² = 0%) (Figure 3A). This suggests similar bleeding risks, but the wide CI indicates uncertainty in clinical decision-making.
Wound infection: Four studies reported wound infection outcomes. No significant difference was observed between the PUS and FS groups. The pooled OR was 1.23 (95%CI: 0.70-2.14; P = 0.47), with no heterogeneity (I² = 0%) (Figure 3B). The lack of difference is notable given the expected benefit of PUS in reducing infections in obese patients, though the CI suggests further research is needed.
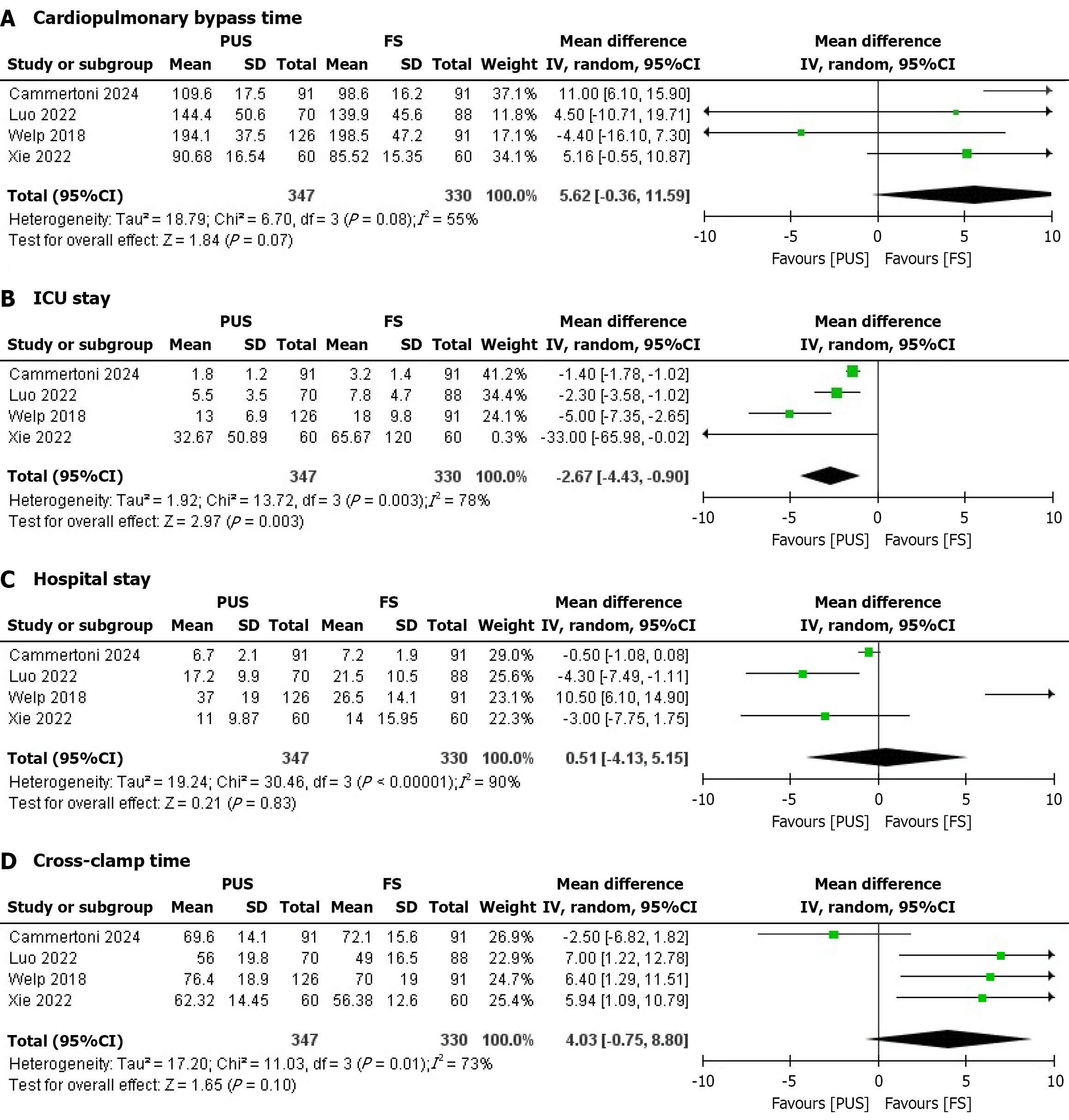
Cardiopulmonary bypass time: Four studies reported cardiopulmonary bypass time. No significant difference was found between the PUS and FS groups. The pooled MD was 5.62 minutes (95%CI: -0.36 to 11.59; P = 0.07), with moderate heterogeneity (I² = 55%) that dropped significantly (I² = 22%) upon removal of Welp et al[19] (Figure 4A). Heterogeneity may stem from variations in surgical expertise or patient comorbidities (e.g., diabetes severity). Subgroup analyses by study design or patient BMI were considered but not feasible due to limited study numbers; meta-regression was not performed due to insufficient covariates. The small MD suggests minimal clinical impact on operative efficiency.
ICU stay: Six studies assessed ICU stay duration. The PUS was associated with a significantly shorter ICU stay compared to the FS group. The pooled MD was -2.67 days (95%CI: -4.43 to -0.90; P = 0.003), with considerable heterogeneity (I² = 78%) that reduced significantly (I² = 62%) upon removal of Welp et al[19] (Figure 4B). Heterogeneity may arise from differences in ICU protocols or patient characteristics (e.g., age, obesity severity). Subgroup analyses by study quality or hospital setting were planned but not conducted due to limited data; meta-regression was infeasible. The 2.67-day reduc
Hospital stay: Four studies reported hospital stay duration. No significant difference was observed between the PUS and FS groups. The pooled MD was 0.51 days (95%CI: -4.13 to 5.15; P = 0.83), with considerable heterogeneity (I² = 90%) that reduced significantly (I² = 68%) upon removal of Welp et al[19] (Figure 4C). Heterogeneity likely reflects variations in discharge criteria or postoperative care across centers. Subgroup analyses or meta-regression were not performed due to insufficient study numbers and covariate data. The lack of difference suggests limited clinical impact on overall hospital resource use.
Four studies evaluated cross-clamp time. No significant difference was found between the PUS and FS groups. The pooled MD was 4.03 minutes (95%CI: -0.75 to 8.80; P = 0.10), with considerable heterogeneity (I² = 73%) that reduced significantly (I² = 0%) upon removal of Cammertoni et al[18] (Figure 4D). Heterogeneity may be due to differences in surgical techniques or patient complexity. Subgroup analyses and meta-regression were considered but not feasible due to limited data. The small MD indicates negligible clinical impact on operative duration.
Publication bias was assessed using funnel plots for all outcomes. No evidence of publication bias was observed for renal failure, atrial fibrillation, reexploration, post-operative bleeding, and wound infection. Cardiopulmonary bypass time, ICU stay, hospital stay, and cross-clamp time showed evidence of publication bias, indicated by asymmetrical plots with gaps in the lower left quadrant, suggesting potential underrepresentation of smaller studies with non-significant or negative results (Supplementary Figures 1 and 2).
The GRADE approach evaluated evidence certainty for clinical outcomes (Supplementary Table 2). Based on four observational studies (two for atrial fibrillation, three for postoperative bleeding), certainty started at low. Low risk of bias was noted (NOS scores 7-9/9). Renal failure, atrial fibrillation, reexploration, postoperative bleeding, and wound infection had no heterogeneity (I² = 0%), no publication bias, but serious imprecision (wide CIs, e.g., OR 1.13, 95%CI: 0.63-2.94 for renal failure), yielding low certainty. Cardiopulmonary bypass time (MD 5.62 minutes, I² = 55%), hospital stay (MD 0.51 days, I² = 90%), and cross-clamp time (MD 4.03 minutes, I² = 73%) showed serious inconsistency, imprecision, and publication bias, resulting in very low certainty. ICU stay (MD -2.67 days, I² = 78%) had serious inconsistency and publication bias but no imprecision, maintaining low certainty. No outcomes were downgraded for indirectness.
This meta-analysis of four observational studies compared PUS and FMS in obese adults undergoing isolated AVR. PUS showed comparable safety to FMS for renal failure, atrial fibrillation, reexploration, postoperative bleeding, and wound infection (all I² = 0%), as well as hospital stay (I² = 90%) and cross-clamp time (I² = 73%), with no significant differences. However, PUS significantly reduced ICU stay (MD -2.67 days, P = 0.003, I² = 78%) but showed a trend toward longer cardiopulmonary bypass time (MD 5.62 minutes, P = 0.07, I² = 55%).
The equivalence in most outcomes suggests PUS is as safe as FMS for obese AVR patients. Studies like Eqbal et al[21] and Khalid et al[22] report similar complication rates for minimally invasive and conventional sternotomy in AVR, even in non-obese populations. Santana et al[23] found no differences in major complications for obese patients undergoing minimally invasive AVR, supporting these findings. In the context of existing literature, these results align with broader evidence indicating that minimally invasive techniques like PUS offer benefits in reducing surgical trauma without compromising safety, particularly in high-risk groups such as obese patients where traditional FMS may exacerbate wound healing issues due to excess adipose tissue. Low heterogeneity for renal failure, atrial fibrillation, reexploration, postoperative bleeding, and wound infection indicates consistent results, likely due to standardized surgical protocols and comparable patient characteristics (e.g., hypertension prevalence: 52.0%-95.6%). Comorbidities (e.g., diabetes: 6.0%-38.5%) and postoperative care may outweigh surgical approach effects. For hospital stay, high heterogeneity (I² = 90%) may reflect non-clinical factors like bed availability, as noted in prior research[24]. Sensitivity analysis revealed that removing Welp et al[19] reduced heterogeneity to I² = 68%, suggesting that differences in hospital discharge protocols or patient management in that study may have contributed to variability. This heterogeneity complicates direct comparisons of hospital stay, indicating that local practices may influence this outcome more than the surgical approach itself.
The shorter ICU stay with PUS is a key advantage, particularly for obese patients prone to prolonged recovery. Wilbring et al[25] and Olds et al[26] attribute reduced ICU stays with minimally invasive approaches to less surgical trauma and faster mobilization. These align with our results, suggesting PUS may optimize ICU resource use. Consi
The trend toward longer cardiopulmonary bypass time with PUS reflects technical challenges in obese patients. Ilcheva et al[28] note that minimally invasive approaches often extend bypass times due to limited exposure and complex anatomy. In obese patients, adipose tissue can displace the heart and aortic valve, requiring incision adjustments, as reported by D'Oria et al[29]. Moderate heterogeneity (I² = 55%) may stem from differences in surgeon experience or institutional protocols. Sensitivity analysis indicated that removing Welp et al[19] lowered heterogeneity to I² = 22%, suggesting that specific surgical techniques or equipment used in that study may have influenced bypass times. This heterogeneity implies that the extent of bypass time differences may depend on center-specific factors, and the modest increase observed may not universally apply. Despite concerns that prolonged bypass times increase risks like acute kidney injury or atrial fibrillation[30], our findings show no such complications, indicating the modest increase is clini
Cross-clamp time also showed considerable heterogeneity (I² = 73%), which sensitivity analysis traced to Cammertoni et al[18], as its removal reduced heterogeneity to I² = 0%. This suggests that study-specific factors, such as variations in surgical team expertise, aortic valve pathology, or intraoperative techniques, may have driven variability. This heterogeneity indicates that cross-clamp time differences between PUS and FMS may not be consistent across all settings, and results should be interpreted with caution in contexts with differing surgical practices.
The benefits of PUS include reduced ICU stay, potentially leading to faster recovery, lower healthcare costs, and decreased exposure to ICU-related risks like infections in obese patients. Limitations of PUS encompass technical challenges that may prolong operative times, requiring experienced surgeons to mitigate risks, and potential variability in outcomes due to institutional differences.
The comparable safety of PUS and FMS supports their use in obese AVR patients. The shorter ICU stay with PUS suggests benefits in recovery and resource utilization, making it preferable where technical expertise exists. The reduced ICU stay (MD -2.67 days, P = 0.003) accelerates recovery for obese patients, who are at higher risk of prolonged hospitalization due to comorbidities. This can lower ICU-related complications and enhance mobility, while also optimizing hospital resources by freeing up critical care beds. Skilled surgical teams are essential to manage the modest increase in bypass time (MD 5.62 minutes, P = 0.07). High heterogeneity in ICU stay, hospital stay, bypass time, and cross-clamp time underscores the influence of center-specific factors like ICU protocols, discharge criteria, and surgical expertise. Standardized perioperative protocols (e.g., incision techniques, perfusion strategies, ICU discharge criteria) could reduce variability and strengthen the reliability of PUS benefits. Future research should prioritize larger, multicenter studies controlling for confounders (e.g., obesity severity, surgeon experience) and explore long-term outcomes like quality of life, valve durability, and cost-effectiveness.
This meta-analysis has limitations. Only four observational studies were included, limiting statistical power and generalizability. Observational designs are prone to selection bias and confounding, despite NOS ratings of good to moderate quality. Considerable heterogeneity for ICU stay (I² = 78%), hospital stay (I² = 90%), bypass time (I² = 55%), and cross-clamp time (I² = 73%) likely stems from differences in surgical expertise, patient characteristics (e.g., BMI: 30.56-38.3 kg/m²), and perioperative protocols, as confirmed by sensitivity analyses identifying Welp et al[19] and Cammertoni et al[18] as key contributors. This heterogeneity limits the precision of pooled estimates for these outcomes and suggests that findings may not be uniformly applicable across all settings. Long-term outcomes (e.g., quality of life, valve durability) were not reported, restricting analysis to short-term results. Findings are specific to obese patients undergoing isolated AVR and may not apply to non-obese populations or combined procedures.
To address current limitations, future research should include larger sample sizes to enhance statistical power and reduce heterogeneity. RCTs are recommended to minimize selection bias and confounding inherent in observational designs. Additionally, studies with long-term follow-up (e.g., 5-10 years) are essential to evaluate outcomes such as valve durability, quality of life, and late complications. Multicenter collaborations could improve generalizability by incorporating diverse patient populations and surgical practices. Exploring subgroup analyses by obesity severity (e.g., class I vs class III) and surgeon experience, as well as cost-effectiveness evaluations, would further inform clinical decision-making.
PUS is a safe and effective alternative to FMS for obese patients undergoing isolated AVR, significantly reducing ICU stay (MD -2.67 days, P = 0.003) while maintaining comparable complication rates, despite a slight increase in cardiopulmonary bypass time. These key findings highlight the clinical significance of PUS in shortening critical care duration, which can mitigate risks associated with prolonged ICU exposure in obese patients, such as ventilator-associated pneumonia and deconditioning. This shorter ICU stay enhances patient recovery and optimizes hospital resource utilization, making PUS a valuable option in centers with surgical expertise. Standardized protocols are needed to address heterogeneity in outcomes and ensure consistent benefits, with future research focusing on long-term outcomes to support broader adoption in clinical practice.
| 1. | Yang C, Xu H, Jia R, Jin Z, Zhang C, Yuan J. Global Burden and Improvement Gap of Non-Rheumatic Calcific Aortic Valve Disease: 1990-2019 Findings from Global Burden of Disease Study 2019. J Clin Med. 2022;11:6733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 2. | Wu DA, Lang P, Varghese D, Al-Attar N, Shaikhrezai K, Zamvar V, Nair S. Short-term outcomes after surgical aortic valve replacement in elderly patients - results of a comparative cohort study. J Cardiothorac Surg. 2024;19:474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Taghizadeh-Waghefi A, Petrov A, Arzt S, Alexiou K, Matschke K, Kappert U, Wilbring M. Minimally Invasive Aortic Valve Replacement for High-Risk Populations: Transaxillary Access Enhances Survival in Patients with Obesity. J Clin Med. 2024;13:6529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Seo J, Kharawala A, Borkowski P, Singh N, Akunor H, Nagraj S, Avgerinos DV, Kokkinidis DG. Obesity and Transcatheter Aortic Valve Replacement. J Cardiovasc Dev Dis. 2024;11:169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Blüher M. An overview of obesity-related complications: The epidemiological evidence linking body weight and other markers of obesity to adverse health outcomes. Diabetes Obes Metab. 2025;27 Suppl 2:3-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 6. | Shi N, Liu K, Fan Y, Yang L, Zhang S, Li X, Wu H, Li M, Mao H, Xu X, Ma SP, Xiao P, Jiang S. The Association Between Obesity and Risk of Acute Kidney Injury After Cardiac Surgery. Front Endocrinol (Lausanne). 2020;11:534294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Liu X, Xie L, Zhu W, Zhou Y. Association of body mass index and all-cause mortality in patients after cardiac surgery: A dose-response meta-analysis. Nutrition. 2020;72:110696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Chien S, Clark C, Maheshwari S, Koutsogiannidis CP, Zamvar V, Giordano V, Lim K, Pessotto R. Benefits of rapid deployment aortic valve replacement with a mini upper sternotomy. J Cardiothorac Surg. 2020;15:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Kirmani BH, Jones SG, Muir A, Malaisrie SC, Chung DA, Williams RJ, Akowuah E. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst Rev. 2023;12:CD011793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Abdelaal SA, Abdelrahim NA, Mamdouh M, Ahmed N, Ahmed TR, Hefnawy MT, Alaqori LK, Abozaid M. Comparative effects of minimally invasive approaches vs. conventional for obese patients undergoing aortic valve replacement: a systematic review and network meta-analysis. BMC Cardiovasc Disord. 2023;23:392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Bakir I, Casselman FP, Wellens F, Jeanmart H, De Geest R, Degrieck I, Van Praet F, Vermeulen Y, Vanermen H. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg. 2006;81:1599-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Luo ZR, Chen YX, Chen LW. Surgical outcomes associated with partial upper sternotomy in obese aortic disease patients. J Cardiothorac Surg. 2022;17:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Boukhris M, Forcillo J, Potvin J, Noiseux N, Stevens LM, Badreddine M, Gobeil JF, Masson JB. Does "Obesity Paradox" Apply for Patients Undergoing Transcatheter Aortic Valve Replacement? Cardiovasc Revasc Med. 2022;38:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hlavicka J, Janda D, Budera P, Tousek P, Maly M, Fojt R, Linkova H, Holubec T, Kacer P. Partial upper sternotomy for aortic valve replacement provides similar mid-term outcomes as the full sternotomy. J Thorac Dis. 2022;14:857-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51705] [Article Influence: 10341.0] [Reference Citation Analysis (2)] |
| 16. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26290] [Article Influence: 1752.7] [Reference Citation Analysis (4)] |
| 17. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1844] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 18. | Cammertoni F, Bruno P, Pavone N, Nesta M, Chiariello GA, Grandinetti M, D'Avino S, Sanesi V, D'Errico D, Massetti M. Outcomes of Minimally Invasive Aortic Valve Replacement in Obese Patients: A Propensity-Matched Study. Braz J Cardiovasc Surg. 2024;39:e20230159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Welp HA, Herlemann I, Martens S, Deschka H. Outcomes of aortic valve replacement via partial upper sternotomy versus conventional aortic valve replacement in obese patients. Interact Cardiovasc Thorac Surg. 2018;27:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Xie XB, Dai XF, Qiu ZH, Jiang DB, Wu QS, Dong Y, Chen LW. Do obese patients benefit from isolated aortic valve replacement through a partial upper sternotomy? J Cardiothorac Surg. 2022;17:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Eqbal AJ, Gupta S, Basha A, Qiu Y, Wu N, Rega F, Chu FV, Belley-Cote EP, Whitlock RP. Minimally invasive mitral valve surgery versus conventional sternotomy mitral valve surgery: A systematic review and meta-analysis of 119 studies. J Card Surg. 2022;37:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Khalid S, Hassan M, Ali A, Anwar F, Siddiqui MS, Shrestha S. Minimally invasive approaches versus conventional sternotomy for aortic valve replacement in patients with aortic valve disease: a systematic review and meta-analysis of 17 269 patients. Ann Med Surg (Lond). 2024;86:4005-4014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Santana O, Reyna J, Grana R, Buendia M, Lamas GA, Lamelas J. Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg. 2011;91:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Eskandari M, Alizadeh Bahmani AH, Mardani-Fard HA, Karimzadeh I, Omidifar N, Peymani P. Evaluation of factors that influenced the length of hospital stay using data mining techniques. BMC Med Inform Decis Mak. 2022;22:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Wilbring M. Advancing Minimally Invasive Cardiac Surgery-Let's Take a Look into the Future. J Clin Med. 2025;14:904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Olds A, Saadat S, Azzolini A, Dombrovskiy V, Odroniec K, Lemaire A, Ghaly A, Lee LY. Improved operative and recovery times with mini-thoracotomy aortic valve replacement. J Cardiothorac Surg. 2019;14:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Snell A, Lobaina D, Densley S, Moothedan E, Baker J, Al Abdul Razzak L, Garcia A, Skibba S, Dunn A, Follin T, Mejia M, Kitsantas P, Sacca L. Disparities in Postoperative Pain Management: A Scoping Review of Prescription Practices and Social Determinants of Health. Pharmacy (Basel). 2025;13:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Ilcheva L, Risteski P, Tudorache I, Häussler A, Papadopoulos N, Odavic D, Rodriguez Cetina Biefer H, Dzemali O. Beyond Conventional Operations: Embracing the Era of Contemporary Minimally Invasive Cardiac Surgery. J Clin Med. 2023;12:7210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | D'Oria R, Genchi VA, Caccioppoli C, Calderoni I, Marrano N, Biondi G, Borrelli A, Di Gioia L, Giorgino F, Laviola L. Impact of Dysfunctional Adipose Tissue Depots on the Cardiovascular System. Int J Mol Sci. 2022;23:14296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 30. | Feng Z, Cao X, Zhao C, Niu J, Yan Y, Shi T, Hao J, Zheng X. Serum CIRP increases the risk of acute kidney injury after cardiac surgery. Front Med (Lausanne). 2023;10:1258622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/