Published online Sep 26, 2025. doi: 10.4330/wjc.v17.i9.110756
Revised: June 26, 2025
Accepted: August 12, 2025
Published online: September 26, 2025
Processing time: 95 Days and 20.4 Hours
Postoperative atrial fibrillation (POAF) is a complication after cardiac surgeries associated with increased morbidity and hospital stay. Surgical cardiac dener
To evaluate the impact of surgical cardiac denervation on the incidence of POAF and related clinical outcomes.
This meta-analysis adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A literature search was conducted across PubMed, Cochrane, ScienceDirect, and EMBASE up to April 2025 using a preformed search strategy using Medical Subject Headings terms and free-text keywords. Risk of bias assessment was done via Risk of Bias 2.0 and Risk Of Bias In Non-randomized Studies - of Interventions tools. Study analysis was performed using Review Manager version 5.4, with heterogeneity assessed via I2 values and appropriate fixed- or random-effects models applied.
Five studies (N = 1266) were included, with 627 patients undergoing cardiac denervation and 639 serving as controls. Denervation did not significantly reduce overall POAF [odds ratio = 0.71; 95% confidence interval (CI): 0.32-1.58; P = 0.40; I2 = 83%], but was associated with a significant reduction in persistent atrial fibrillation (odds ratio = 0.19; 95%CI: 0.10-0.36; P < 0.00001; I2 = 0%). Among secondary outcomes, only postoperative serum magnesium levels significantly reduced the denervation group (mean difference: -0.07 mmol/L; 95%CI: -0.08 to
Surgical cardiac denervation does not significantly reduce overall POAF but does lower the incidence of persistent atrial fibrillation. It is also shown to decrease serum magnesium levels. Other outcomes, such as stroke, reope
Core Tip: This systematic review and meta-analysis assessed the effectiveness of surgical cardiac denervation in preventing postoperative atrial fibrillation after cardiac surgery. While denervation did not significantly lower the overall incidence of postoperative atrial fibrillation, it was strongly associated with a marked reduction in persistent atrial fibrillation. These findings highlight the potential role of autonomic modulation in targeting sustained arrhythmias, even though its impact on other clinical outcomes such as stroke, mortality, and hospital stay was minimal.
- Citation: Bakht D, Amir M, Saleem F, Asif A, Mubashir MM, Farooq AS, Malik MZ, Hassan A, Bakht K, Arham M, Bokhari SFH, Awais MN, Buhadur Ali MK, Dad A, Akram MR. Systematic review and meta-analysis: Is surgical cardiac denervation effective against postoperative atrial fibrillation? World J Cardiol 2025; 17(9): 110756
- URL: https://www.wjgnet.com/1949-8462/full/v17/i9/110756.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i9.110756
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice characterized by uncoordinated atrial activation with subsequent deterioration of heart function[1]. Global trends have reported an increase of 33% in AF prevalence during the last 20 years with the prevalence almost doubling in the last decade[2,3]. Postoperative atrial fibrillation (POAF) is the most common cause of secondary AF and the most frequent complication following cardiac surgery, occurring in 15% to 45% patients in the early post-operative period[4,5]. There is currently no universally accepted definition of POAF, as its characterization differs across studies and among authors. Broadly, POAF has been defined in the literature as AF requiring intervention, episodes lasting more than 30 seconds in the postoperative period, any new-onset AF following surgery regardless of duration, or AF persisting for more than ten minutes after the operation[4]. While the exact pathophysiology behind the development of POAF is unknown, multiple etiologies have been proposed to drive the process including autonomic neuromodulation, atrial structural alterations and postoperative inflammation[4-6]. POAF has largely been attributed to variety of adverse cardiovascular events such as stroke, heart failure and mortality[7,8].
Despite various pharmacological strategies to mitigate the risk of POAF, it still poses a compelling challenge to address in patients undergoing cardiac surgery especially coronary artery bypass grafting (CABG). This unmet clinical need has fueled efforts in targeted interventions that modulate POAF’s underlying mechanism of sympathetic hyperactivity and impaired vagal tone[4,6,9]. Prophylactic surgical cardiac denervation as an adjunct to CABG, achieved through mechanical nerve transection offers a targeted strategy to attenuate sympathetic overactivity by selectively interrupting cardiac autonomic nervous system[9-11]. The potential role of epicardial adipose tissue in pathogenesis of AF has been well established in the literature[12,13], and therefore is the primary site of focus in these treatment modalities. Recent partial cardiac denervation-POAF trial has demonstrated that cardiac denervation significantly reduced POAF and initiated a direction in the exploration of cardiac denervation as a promising preventive strategy[10]. Although several other clinical studies have explored prophylactic nerve transection during CABG for POAF prevention, results have been inconsistent, with differences in surgical technique, anatomical targets, and patient selection contributing to variability[11,14,15]. This meta-analysis seeks to comprehensively assess the effectiveness and safety of prophylactic surgical cardiac denervation performed during CABG. The key emphasis is on its influence on the incidence of POAF, perioperative outcomes, and associated procedural risks. By synthesizing available evidence, the study aims to clarify the role of nerve transection - based autonomic modulation within modern cardiac surgical practice.
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to maintain methodological rigor and ensure transparent reporting[16]. The study protocol was prospectively registered with International Prospective Register of Systematic Reviews prior to commence
A thorough and methodical literature search was performed using four key electronic databases - PubMed, Cochrane Library, ScienceDirect, and EMBASE - covering records from their inception until April 2025. The search approach incorporated both controlled vocabulary (such as Medical Subject Headings terms) and free-text keywords associated with “surgical cardiac denervation”, “atrial fibrillation”, and “coronary artery bypass grafting”, along with their synonyms and related terms. Boolean operators (“AND” and “OR”) were applied to balance search sensitivity and precision. Additionally, manual screening of reference lists from existing meta-analyses and systematic reviews was conducted to ensure no eligible studies were overlooked in the database searches. The detailed search strategy has been provided in the Supplementary material.
Studies were included in this meta-analysis if they met the following criteria: (1) Randomized controlled trials (RCTs), original research, or cohort studies; (2) Enrolled patients who underwent any major cardiac surgery; (3) Evaluated surgical cardiac denervation compared to no denervation; and (4) Reported post-operative AF or at least one other predefined outcome of interest. Exclusion criteria were as follows: (1) Studies involving patients who did not undergo major cardiac surgery; (2) Research focused on ventricular tachycardia, pharmacological denervation, ablation techniques, or the Maze procedure; (3) Case reports, reviews, abstracts, or unpublished studies; (4) Insufficient or incomplete outcome data; and (5) Non-English publications.
Three independent reviewers performed data extraction using a standardized Google sheets form, collecting key study characteristics including first author and publication year, sample size, type of study, country of study, procedure details, patient demographics (age and number of male patients), comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, left main disease, and two-vessel or triple-vessel disease), medication history (beta-blocker use), surgical procedure details (cardiopulmonary bypass use, cardiopulmonary bypass time, cross-clamp time, number of grafts, and denervation time), and postoperative outcomes with specific focus on AF incidence. Any discrepancies in the extracted data were resolved through discussion among reviewers, with a fourth reviewer consulted when consensus could not be reached.
The outcomes assessed in the included studies were categorized as primary and secondary endpoints. The primary outcomes were POAF and persistent AF. The secondary outcomes consisted of all additional clinically significant endpoints documented across the studies.
The methodological quality of RCTs was evaluated using the Cochrane Risk of Bias tool, which examines six key domains: (1) Randomization process; (2) Deviation from intended interventions; (3) Missing outcome data; (4) Outcome measurement; (5) Selection of reported results; and (6) Overall bias risk. Each domain was classified as ‘low risk’, ‘some concerns’, or ‘high risk’ based on predefined criteria[17]. For non-randomized studies, the Risk Of Bias In Non-randomized Studies - of Interventions tool was applied to assess potential bias in intervention effect estimates, including evaluations of benefit, harm, or safety outcomes[18].
All meta-analyses were performed using Review Manager version 5.4. Dichotomous outcomes were analyzed using odds ratios (OR), and continuous outcomes were assessed through mean differences (MD), both reported with 95% confidence intervals (CI). The degree of statistical heterogeneity was assessed via the I2 statistic. Low heterogeneity (I2 < 50%) led to the selection of a fixed-effects model, while moderate or high heterogeneity (I2 ≥ 50%) mandated a random-effects model. Sensitivity analyses were conducted via leave-one-out methodology. For outcomes comprising ≥ 10 studies, publication bias was considered for assessment using Egger’s regression test. A P-value < 0.05 was termed insignificant.
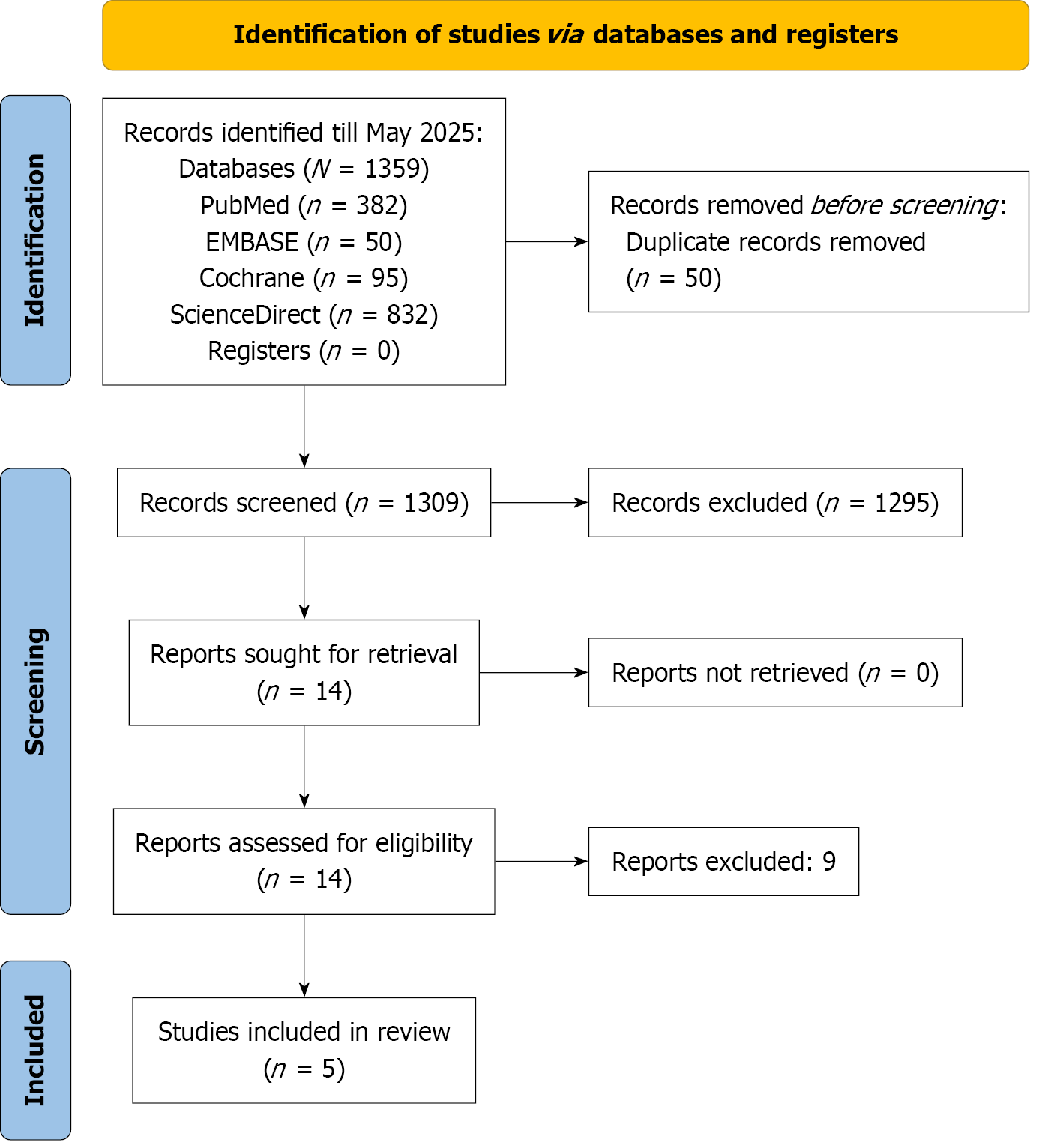
A comprehensive literature search yielded 1359 records across four electronic databases: PubMed (n = 382), Cochrane Library (n = 95), ScienceDirect (n = 832), and EMBASE (n = 50). After removing 50 duplicate entries, 1309 unique records remained for title and abstract screening. Of these, 14 articles were selected for full-text review, nine of which were subsequently excluded. Consequently, five studies met the inclusion criteria and were incorporated into the quantitative synthesis. The study selection process is illustrated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram (Figure 1).
A total of five studies were incorporated into this analysis, comprising three RCTs, one nonrandomized case - control study, and one prospective cohort study. Collectively, 627 patients underwent cardiac denervation, while 639 served as controls. Yang et al[10] evaluated partial cardiac denervation, whereas the remaining trials employed ventral cardiac denervation techniques. Individual group sizes ranged from 25 to 215 participants, with Yang et al[10] representing the largest cohort. The proportion of male subjects was comparable between arms (78.6% in the denervation group vs 78.7% in controls), reflecting adequate demographic matching. Baseline comorbidity profiles were similarly balanced: Diabetes mellitus was present in 219 patients (34.9%) in the denervation arm and 188 (29.4%) among controls, and hypertension occurred in 345 (55.0%) vs 369 (57.7%) participants, respectively. However, Breda et al[15] reported a higher hypertension prevalence in the intervention group (88% vs 68%). Denervation procedures were performed intraoperatively, although precise timing data were inconsistently reported. Reported denervation durations ranged from 5 minutes ± 2 minutes in Melo et al[19] to 7.64 minutes ± 2.33 minutes in Breda et al[15]. A detailed summary of baseline patient characteristics is presented in Table 1.
| Omran et al[11] | Melo et al[19] | Yang et al[10] | Alex et al[14] | Breda et al[15] | ||||||
| Denervation group | Control group | Denervation group | Control group | Denervation group | Control group | Denervation group | Control group | Denervation group | Control group | |
| Sample size | 110 | 110 | 207 | 219 | 215 | 215 | 70 | 70 | 25 | 25 |
| Year of publication | 2010 | 2004 | 2025 | 2005 | 2008 | |||||
| Type of study | RCT | Case control study | RCT | Prospective cohort study | RCT | |||||
| Country of study | Iran | Portugal, Türkiye, Netherlands | China | United Kingdom | Brazil | |||||
| Procedure | CABG with ventral cardiac denervation | CABG without ventral cardiac denervation | CABG with ventral cardiac denervation | CABG without ventral cardiac denervation | CABG with partial cardiac denervation | CABG without partial cardiac denervation | CABG with ventral cardiac denervation | CABG without ventral cardiac denervation | CABG with ventral cardiac denervation | CABG without ventral cardiac denervation |
| Denervation procedure | Removal of fat pads and surrounding nerve tissues around the great vessels at the heart’s base, starting at the ascending aorta and extending to the pulmonary artery and SVC using electrocautery | Excision of fat pads and nerve tissues around the SVC, aorta, and anterior pulmonary artery, starting from the right pericardial cavity and extending to the left border of the pulmonary artery | Cutting off the LOM and resecting the fat pad along the Waterston groove | Excision of the adventitia and periadventitial fat using scissors and forceps, starting around the superior vena cava, then the ascending aorta, and finally the pulmonary artery, without using cauterization | Removal of nerves and adipose tissue around the great vessels, starting at the SVC and ending at the pulmonary artery | |||||
| Baseline data | ||||||||||
| Age (years) | 63.47 ± 7.80 | 62.18 ± 7 | 60 ± 10 | 62 ± 8 | 61.9 ± 7.9 | 61.9 ± 7.6 | 64.8 ± 9.6 | 65.3 ± 9 | 57.6 ± 13.9 | 59.4 ± 7.57 |
| Male | 89 (80.9) | 88 (80) | 157 (76) | 164 (75) | 176 (81.9) | 175 (81.4) | 58 (83) | 60 (85.7) | 13 (52) | 16 (64) |
| Comorbidities | ||||||||||
| Diabetes mellitus | 34 (30.9) | 29 (26.4) | 54 (26) | 59 (27) | 108 (50.2) | 93 (43.3) | 11 (16) | 9 (13) | 12 (48) | 8 (32) |
| Hypertension | 50 (45.5) | 60 (54.5) | 75 (36) | 87 (40) | 152 (70.7) | 165 (76.7) | 46 (65) | 40 (57) | 22 (88) | 17 (68) |
| COPD | 7 (6.4) | 8 (7.3) | 6(3) | 4 (2) | 3 (1.4) | 1 (0.5) | 7 (10) | 8 (11) | NA | NA |
| Left- main disease | 9 (8.9) | 7 (6.6) | 29 (14) | 35 (16) | 36 (16.7) | 42 (19.5) | NA | NA | 3 (12) | 3 (12) |
| Triple vessel disease | NA | NA | 149 (72) | 145 (66) | 183 (85.1) | 188 (87.4) | 53 (76) | 53 (75) | 16 (64) | 13 (52) |
| Two vessel disease | NA | NA | 29 (14) | 39 (18) | NA | NA | 16 (23) | 15 (22) | 5 (20) | 5 (20) |
| Medication history | ||||||||||
| Beta-blockers | NA | NA | 155 (75) | 177 (81) | 188 (87.4) | 188 (87.4) | 48 (69) | 50 (72) | 23 (92) | 23 (92) |
| Operative data | ||||||||||
| CPB used (on-pump) | 110 (100) | 110 (100) | 170 (82) | 173 (79) | 86 (40) | 91 (42.3) | 70 (100) | 70 (100) | 24 (96) | 22 (88) |
| CPB time (minutes) | 66.62 ± 13.61 | 60.91 ± 16.44 | 95 ± 19 | 99 ± 19 | 101.67 ± 32.09 | 107.33 ± 38.06 | 57.1 ± 13.5 | 54.3 ± 12.2 | NA | NA |
| Cross-clamp time (minutes) | 38.74 ± 8.42 | 36.63 ± 17.35 | NA | NA | 72.17 ± 25.38 | 75.00 ± 28.36 | 34.3 ± 7.5 | 33.4 ± 7.5 | NA | NA |
| Number of grafts | 3.86 ± 0.77 | 3.27 ± 0.77 | 3.2 ± 0.4 | 2.9 ± 0.1 | 3.7 ± 0.8 | 3.8 ± 0.8 | 2.8 (NR) | 2.9 (NR) | 2.92 ± 0.81 | 2.48 ± 0.82 |
| Denervation (minutes) | NA | NA | 5 ± 2 | NA | NA | NA | 5 | NA | 7.64 + 2.33 | NA |
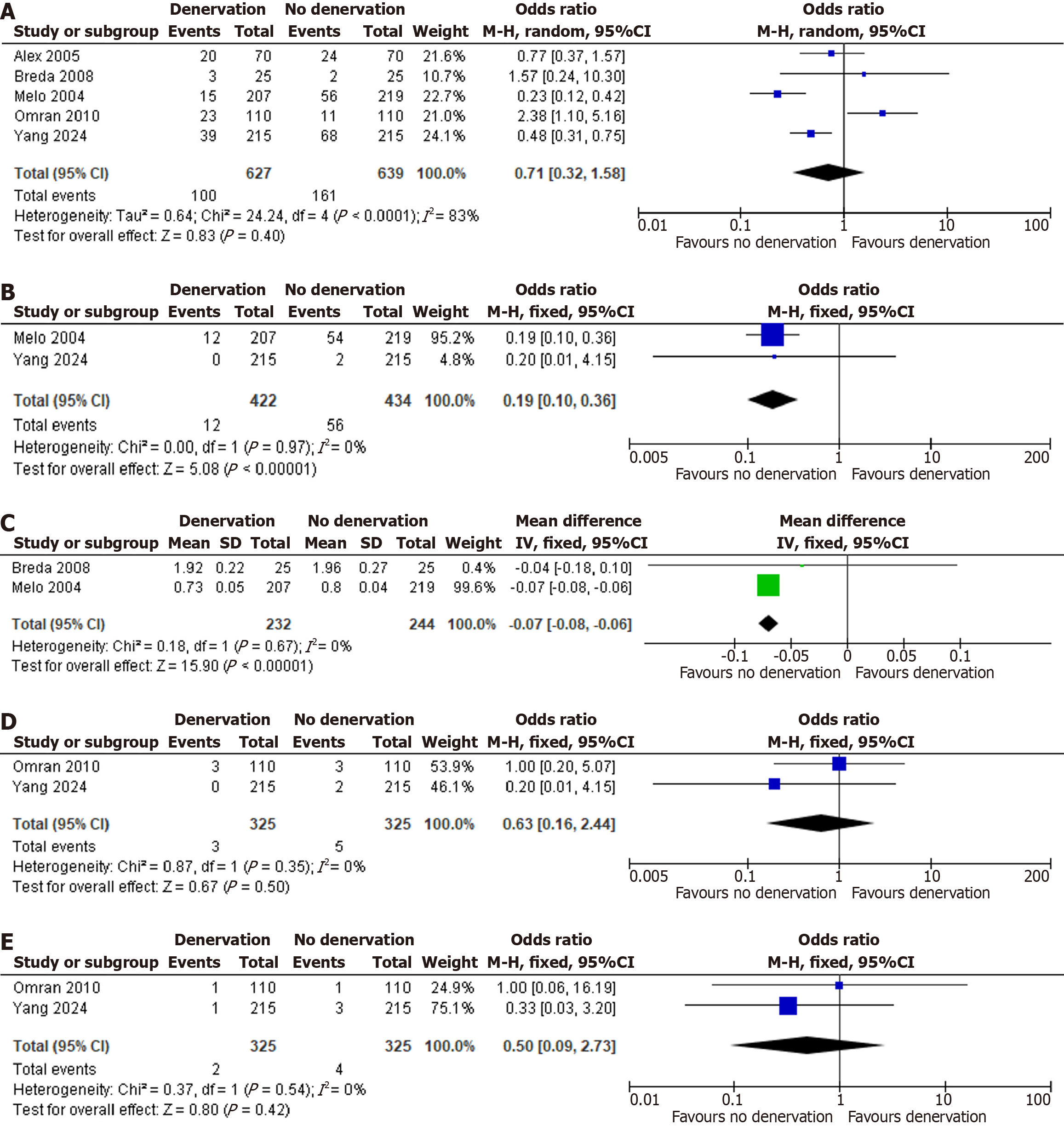
The primary endpoints evaluated were POAF and persistent AF following cardiac surgery. Meta-analysis demonstrated no significant reduction in overall POAF with denervation vs control (OR = 0.71; 95%CI: 0.32-1.58; P = 0.40; I2 = 83%), indicating that cardiac denervation did not confer protection against early postoperative arrhythmia. Conversely, denervation was associated with a marked decrease in postoperative persistent AF (OR = 0.19; 95%CI: 0.10-0.36; P < 0.00001; I2 = 0%). Figure 2A and B show the forest plots for POAF and persistent AF in denervation vs no denervation respectively.
Among the secondary endpoints, only postoperative serum magnesium concentration demonstrated a statistically significant benefit of cardiac denervation (MD: -0.07 mmol/L; 95%CI: -0.08 to -0.06; P < 0.00001; I2 = 0%). All other secondary outcomes yielded non-significant differences: Reoperation for bleeding (OR = 0.63; 95%CI: 0.16-2.44; P = 0.50; I2 = 0%); postoperative cerebrovascular events (transient ischemic attack or stroke) (OR = 0.50; 95%CI: 0.09-2.73; P = 0.42; I2 = 0%); length of hospital stay (MD: -0.14 days; 95%CI: -0.54 to 0.27; P = 0.50; I2 = 59%); 30-day mortality (OR = 0.58; 95%CI: 0.12-2.72; P = 0.49; I2 = 0%); and postoperative chest-tube drainage volume (MD: 2.22 mL; 95%CI: -23.60 to 28.03; P = 0.87; I2 = 0%). Thus, cardiac denervation conferred no demonstrable protective effect on secondary endpoints aside from preservation of postoperative magnesium levels. A detailed overview of both primary and secondary outcome measures is provided in Table 2. Figure 2C-E and Figure 3A-C show forest plots for bleeding, TIA/stroke, length of hospital stays, 30-day mortality, post op blood drainage and post op magnesium in denervation vs no denervation respectively.
| Outcomes | Number of studies | Effect model | MD/OR | I2 (%) | P value for heterogeneity | Effect size | P value |
| AF (number) | 5 | RE | OR | 83 | < 0.0001 | 0.71 (0.32-1.58) | 0.40 |
| Postoperative persistent AF | 2 | FE | OR | 0 | 0.97 | 0.19 (0.10-0.36) | < 0.00001 |
| Reoperation for bleeding | 2 | FE | OR | 0 | 0.35 | 0.63 (0.16-2.44) | 0.50 |
| TIA/stroke after cardiac surgery | 2 | FE | OR | 0 | 0.54 | 0.50 (0.09-2.73) | 0.42 |
| Length of hospital stay (days) | 5 | RE | MD | 59 | 0.04 | -0.14 (-0.54 to 0.27) | 0.50 |
| 30-day mortality | 2 | FE | OR | 0 | 0.69 | 0.58 (0.12-2.72) | 0.49 |
| Post-operative blood drainage (mL) | 3 | FE | MD | 0 | 0.72 | 2.22 (-23.60 to 28.03) | 0.87 |
| Post-operative magnesium (mmol/L) | 2 | FE | MD | 0 | 0.67 | -0.07 (-0.08 to -0.06) | < 0.00001 |
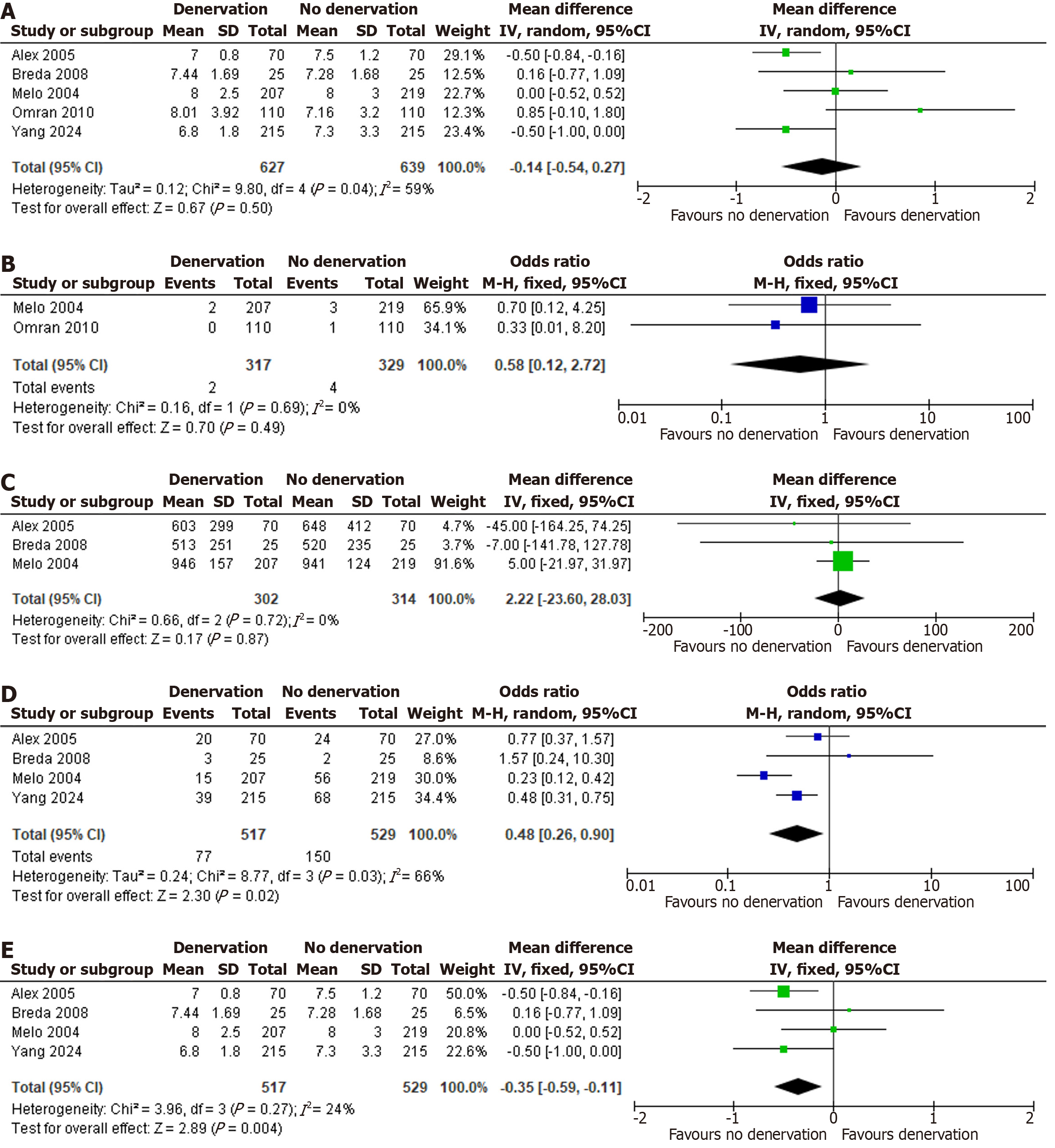
Leave-one-out analysis did not reveal any single study to be the sole cause of heterogeneity in the primary outcome of AF. However, the results became statistically significant after removing Omran et al[11] (OR = 0.48; 95%CI: 0.26-0.90, P = 0.02, I2 = 66%). For length of hospital stay, Omran et al[11] was found to be a major cause of heterogeneity and the results became statistically significant after excluding it in the sensitivity analysis (MD: -0.35; 95%CI: -0.59 to -0.11, P = 0.004, I2 = 24%). Figure 3D and E show forest plots for sensitivity analysis of POAF and length of hospital stay respectively.
General considerations do not recommend performing Egger’s regression test for assessment of publication bias for outcomes with fewer than 10 studies as test power is usually too low to differentiate chance from real asymmetry[20]. Therefore, we did not perform Egger’s regression test to assess the presence of publication bias.
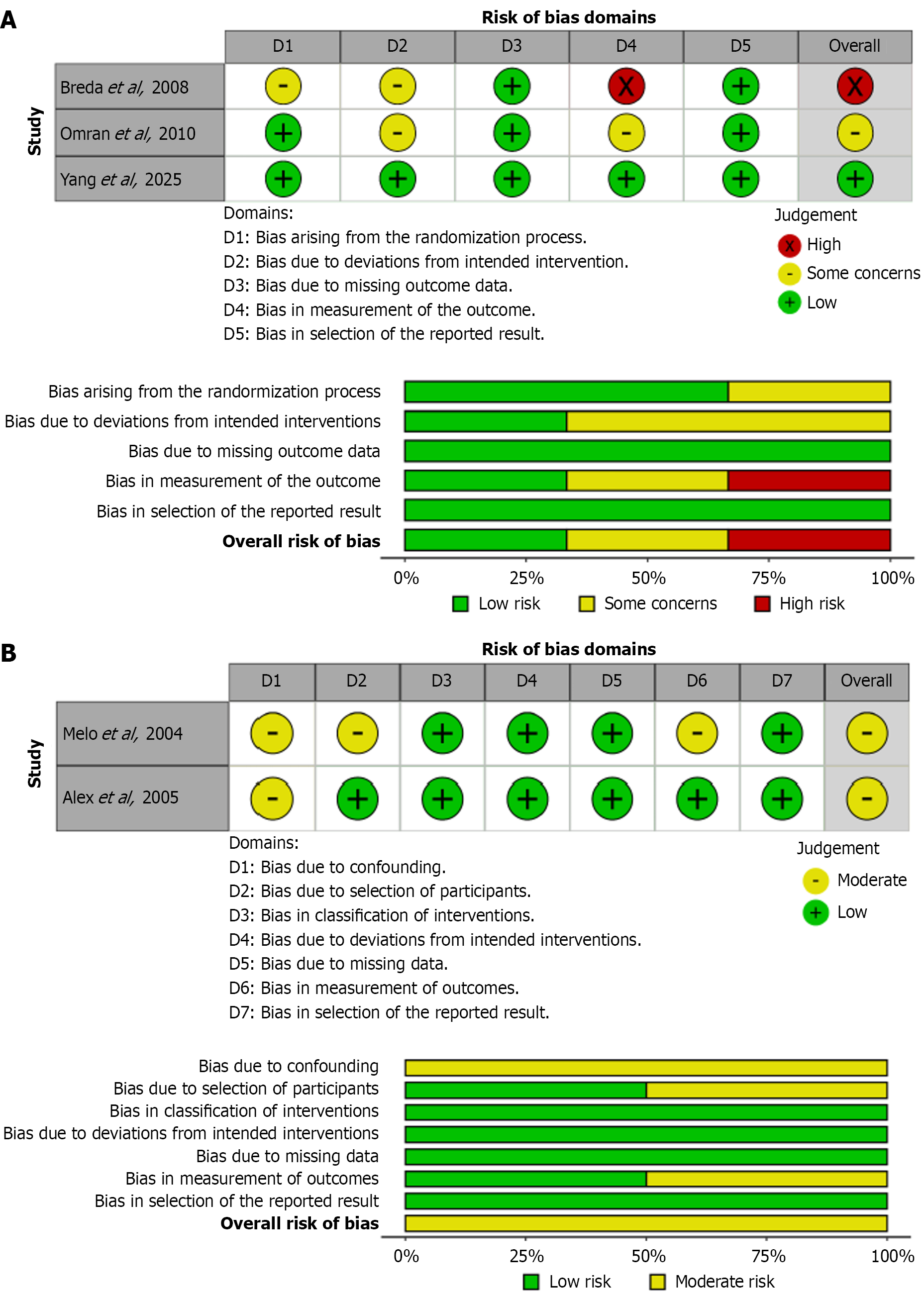
Risk of bias was assessed using Risk of Bias 2.0 for interventional studies and Risk Of Bias In Non-randomized Studies - of Interventions tool for non-randomized studies. Visualization was done using robvis tool. Out of three studies analyzed using Risk of Bias 2.0, there was high risk of bias in Breda et al[15], some concerns in Omran et al[11] and low risk in Yang et al[10]. Similarly for non-randomized studies, there was moderate risk of bias in both Melo et al[19] and Alex et al[14]. Figure 4 show assessment of risk of bias in randomized controlled trials and non-randomized studies of intervention respectively.
This meta-analysis, which included five studies comprising a total of 1266 participants, was conducted to evaluate the efficacy of surgical cardiac denervation in preventing POAF and improving related clinical outcomes in patients undergoing cardiac surgery. Regarding the primary outcomes, surgical cardiac denervation did not demonstrate a clear advantage in reducing the overall incidence of AF occurring shortly after surgery. However, the findings did reveal a compelling association between denervation and a reduction in persistent AF - suggesting that while short-term arrhythmic protection may be limited, surgical cardiac denervation may provide meaningful benefits in lowering the risk of sustained, longer-term AF following surgery. As for the secondary outcomes, most clinical and procedural endpoints - such as the need for reoperation due to bleeding, cerebrovascular complications (stroke or transient ischemic attack), duration of hospital stay, short-term mortality, and postoperative blood loss - showed no notable differences between the denervation and control groups. Interestingly, the only parameter that demonstrated a statistically significant benefit was postoperative serum magnesium concentration, which was better preserved in the denervation group. This may point toward a subtle physiological effect of autonomic modulation on electrolyte homeostasis, though its clinical relevance warrants further investigation.
Our meta-analysis, which exclusively included studies focusing on anterior fat pad (AFP) excision, did not demon
Several studies have compared surgical and catheter-based approaches for AF ablation, particularly in patients with refractory or persistent disease. Kearney et al[24] demonstrated that surgical ablation offers superior rates of sinus rhythm maintenance across multiple follow-up intervals compared to catheter ablation, especially in patients with paroxysmal AF. Boersma et al[25] echoed these findings in an RCT, showing that minimally invasive surgical ablation was more effective in achieving arrhythmia-free survival in patients with dilated left atria or prior failed ablations. However, they also reported a significantly higher incidence of procedural complications in the surgical group, such as bleeding, pneumothorax, and the need for pacemaker implantation[24,25]. Muston et al[26] provided long-term comparative data through a meta-analysis evaluating mid-to-long-term AF recurrence following catheter and surgical ablation. Despite including patients with potentially more advanced disease in the surgical cohort, they reported non-inferior freedom from AF recurrence in the surgical group over follow-ups extending to five years. Procedural times were comparable between the two methods. This contradicting evidence calls for further research comparing surgical cardiac denervation with catheter ablation. Surgical denervation performed concomitantly with other procedures like CABG or valve surgery poses minimal additional operative burden, and does not increase rate of complications making it a potentially attractive adjunctive therapy in selected high-risk populations. And this role of surgical cardiac denervation as an adjunctive therapy has been highlighted by a recently published trial by Yang et al[10]. Therefore, while current evidence does not yet support widespread routine use, surgical cardiac denervation may be considered in specific clinical scenarios where autonomic imbalance is suspected to play a significant role in arrhythmogenesis, particularly in patients with recurrent POAF despite conventional therapy.
This meta-analysis has several important limitations that must be acknowledged. First, there was variability in the denervation technique employed across studies: Four trials performed ventral cardiac denervation[11,14,15,19], while one utilized a partial approach[10]. This inconsistency may have introduced procedural heterogeneity that could have influenced the pooled outcomes. Second, the included studies differed in design - three were RCTs[10,11,15], one was a case-control study[19], and one was a prospective cohort study[14]. This heterogeneity in study design may affect internal validity and introduce inherent biases related to patient selection, intervention allocation, and follow-up assessment. Third, in certain instances, continuous data originally reported as medians with interquartile ranges were mathematically converted into means and standard deviations to allow for quantitative synthesis. This was done using meta-analysis accelerator tool[27]. Although commonly employed in meta-analyses, this conversion can introduce approximation errors and impact the reliability of statistical estimates. Furthermore, the relatively small number of included studies (n = 5) and modest sample sizes limited the ability to perform subgroup analyses or formally assess for publication bias. Variability in follow-up duration, outcome definitions, and reporting standards among trials may also contribute to residual heterogeneity. This meta-analysis offers a valuable starting point for future research aimed at evaluating surgical cardiac denervation as an adjunctive therapy rather than a standalone intervention. Across the included studies, denervation was performed alongside primary cardiac procedures such as CABG, which reflects how this technique is likely to be applied in real-world surgical settings. Although postoperative management following cardiac denervation was not the primary focus of this meta-analysis, limited data were available from some included studies. Yang et al[10] reported that all patients received beta-blockers preoperatively, which were continued in the postoperative period. Omran et al[11] provided safety-related outcomes such as transfusion requirements, delayed pericardial effusion, and critical arrhythmias necessitating clinical intervention. However, comprehensive postoperative management protocols were not uniformly described across studies and remain an area for further investigation. Future studies should continue to explore its effectiveness when used in this adjunctive capacity. Future trials should clearly define and follow uniform protocols for how and where denervation is performed during surgery. Future research should aim to include a larger number of RCTs to strengthen the evidence base for surgical cardiac denervation. Additionally, upcoming studies should focus on standardized reporting of postoperative management protocols and include outcomes that specifically address post-denervation care and its clinical implications. Second, larger RCTs with longer follow-up periods would provide stronger and more reliable evidence about whether this intervention truly reduces POAF. Third, research should aim to identify which patient groups are most likely to benefit - such as those with a history of AF, enlarged atria, or high autonomic tone - rather than applying the approach broadly. Additionally, future studies should explore whether surgical dener
Surgical cardiac denervation did not demonstrate statistically significant efficacy in reducing the risk of POAF, indicating that its benefit in routine clinical settings remains uncertain. Furthermore, the absence of significant differences in secondary outcomes such as duration of hospital stay and need for permanent pacemaker implantation suggests that the intervention does not compromise patient safety when compared to standard surgical approaches. However, this study was limited by variability in surgical techniques, heterogeneity in study designs, and reliance on estimated statistical conversions. These limitations highlight the need for future high-quality, large-scale RCTs that standardize surgical methods and report complete datasets to more accurately assess the potential role of surgical cardiac denervation as an adjunctive therapy.
| 1. | Nesheiwat Z, Goyal A, Jagtap M. Atrial Fibrillation. Treasure Island (FL): StatPearls, 2025. [PubMed] |
| 2. | Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021;16:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 861] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 3. | Linz D, Gawalko M, Betz K, Hendriks JM, Lip GYH, Vinter N, Guo Y, Johnsen S. Atrial fibrillation: epidemiology, screening and digital health. Lancet Reg Health Eur. 2024;37:100786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 216] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 4. | Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J. 2023;44:1020-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 159] [Reference Citation Analysis (0)] |
| 5. | Tzoumas A, Nagraj S, Tasoudis P, Arfaras-Melainis A, Palaiodimos L, Kokkinidis DG, Kampaktsis PN. Atrial Fibrillation Following Coronary Artery Bypass Graft: Where Do We Stand? Cardiovasc Revasc Med. 2022;40:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Lopes LA, Agrawal DK. Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication. J Surg Res (Houst). 2022;5:159-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 7. | Lubitz SA, Yin X, Rienstra M, Schnabel RB, Walkey AJ, Magnani JW, Rahman F, McManus DD, Tadros TM, Levy D, Vasan RS, Larson MG, Ellinor PT, Benjamin EJ. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 8. | Mostafa A, El-Haddad MA, Shenoy M, Tuliani T. Atrial fibrillation post cardiac bypass surgery. Avicenna J Med. 2012;2:65-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Zafeiropoulos S, Doundoulakis I, Farmakis IT, Miyara S, Giannis D, Giannakoulas G, Tsiachris D, Mitra R, Skipitaris NT, Mountantonakis SE, Stavrakis S, Zanos S. Autonomic Neuromodulation for Atrial Fibrillation Following Cardiac Surgery: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:682-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Yang Z, Tiemuerniyazi X, Xu F, Wang Y, Sun Y, Yan P, Tian L, Han C, Zhang Y, Pan S, Hu Z, Li X, Zhao W, Feng W. Partial Cardiac Denervation to Prevent Postoperative Atrial Fibrillation After Coronary Artery Bypass Grafting: The pCAD-POAF Randomized Clinical Trial. JAMA Cardiol. 2025;10:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Omran AS, Karimi A, Ahmadi H, Yazdanifard P, Sheikh Fahtollahi M, Tazik M. Prophylactic ventral cardiac denervation: does it reduce incidence of atrial fibrillation after coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2010;140:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Conte M, Petraglia L, Cabaro S, Valerio V, Poggio P, Pilato E, Attena E, Russo V, Ferro A, Formisano P, Leosco D, Parisi V. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front Cardiovasc Med. 2022;9:932262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 13. | Poggi AL, Gaborit B, Schindler TH, Liberale L, Montecucco F, Carbone F. Epicardial fat and atrial fibrillation: the perils of atrial failure. Europace. 2022;24:1201-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Alex J, Guvendik L. Evaluation of ventral cardiac denervation as a prophylaxis against atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2005;79:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Breda JR, Breda AS, Freitas AC, Meneghini A, Tavares CM, Abreu LC, Murad N, Pires AC. Effect of ventral cardiac denervation in the incidence of atrial fibrillation after coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2008;23:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51401] [Article Influence: 10280.2] [Reference Citation Analysis (2)] |
| 17. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18738] [Article Influence: 2676.9] [Reference Citation Analysis (0)] |
| 18. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12542] [Article Influence: 1254.2] [Reference Citation Analysis (2)] |
| 19. | Melo J, Voigt P, Sonmez B, Ferreira M, Abecasis M, Rebocho M, Timóteo A, Aguiar C, Tansal S, Arbatli H, Dion R. Ventral cardiac denervation reduces the incidence of atrial fibrillation after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;127:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3796] [Cited by in RCA: 5293] [Article Influence: 352.9] [Reference Citation Analysis (1)] |
| 21. | Liu S, Jing Y, Zhang J, Bian C, Zhang YU, Zhang X. Does Anterior Fat Pad Removal Reduce the Incidence of Atrial Fibrillation after CABG? A Meta-Analysis of Randomized Controlled Trials. Pacing Clin Electrophysiol. 2015;38:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Consoli LN, Cetinel E, Lajczak P, Koziakas IG, Majeed MW, Wijaya P, Salha I, Samanidis G. Surgical neuromodulation therapies to prevent postoperative atrial fibrillation: A meta-analysis, meta-regression, and trial sequential analysis of randomized controlled trials. Heart Rhythm. 2025;22:e301-e308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Cummings JE, Gill I, Akhrass R, Dery M, Biblo LA, Quan KJ. Preservation of the anterior fat pad paradoxically decreases the incidence of postoperative atrial fibrillation in humans. J Am Coll Cardiol. 2004;43:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Kearney K, Stephenson R, Phan K, Chan WY, Huang MY, Yan TD. A systematic review of surgical ablation versus catheter ablation for atrial fibrillation. Ann Cardiothorac Surg. 2014;3:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 25. | Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, Sandoval E, Calvo N, Brugada J, Kelder J, Wijffels M, Mont L. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Muston BT, Bilbrough J, Eranki A, Wilson-Smith C, Wilson-Smith AR. Mid-to-long-term recurrence of atrial fibrillation in surgical treatment vs. catheter ablation: a meta-analysis using aggregated survival data. Ann Cardiothorac Surg. 2024;13:18-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Abbas A, Hefnawy MT, Negida A. Meta-analysis accelerator: a comprehensive tool for statistical data conversion in systematic reviews with meta-analysis. BMC Med Res Methodol. 2024;24:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/