Published online Sep 26, 2025. doi: 10.4330/wjc.v17.i9.109876
Revised: June 12, 2025
Accepted: August 28, 2025
Published online: September 26, 2025
Processing time: 116 Days and 10.5 Hours
Rheumatoid arthritis (RA) significantly increases the risk of cardiovascular di
Core Tip: In this review, we analyze both the positive and negative influences on the cardiovascular system of all drugs currently used in rheumatological practice, including the most up-to-date therapies. Particular attention is given to the safety analysis of long-established drugs, whose effectiveness in treating rheumatoid arthritis is well established, while their cardiovascular safety has been studied to a much lesser extent.
- Citation: Zotova LA, Enenkov NV. From joints to vessels: How rheumatoid arthritis therapy alters the fate of the heart. World J Cardiol 2025; 17(9): 109876
- URL: https://www.wjgnet.com/1949-8462/full/v17/i9/109876.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i9.109876
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by progressive joint destruction due to erosive arthritis and a generalized autoimmune process, in which immune complexes and cytokines damage blood vessels and internal organs. A distinctive feature of RA is systemic inflammation, which contributes to a more severe course of comorbidities, including cardiovascular disease (CVD).
The prognosis in RA is determined less by chronic erosive arthritis and more by comorbid conditions, especially atherosclerosis-related pathology. A meta-analysis of prospective studies showed that the risk of cardiovascular mortality in patients with RA is 48% higher than in the general population[1]. Atherosclerosis in RA is characterized by multiple coronary artery lesions, early recurrence of acute coronary syndrome, increased mortality after the first myocardial infarction (MI), and a high frequency of asymptomatic MI[2-4].
Even before the development of RA or in its early stages, markers of cardiovascular system involvement can be detected in 35%-50% of cases. These markers include endothelial dysfunction, reduced elasticity of both small and large blood vessels, and diastolic myocardial dysfunction, the intensity of which increases with disease duration[5-7].
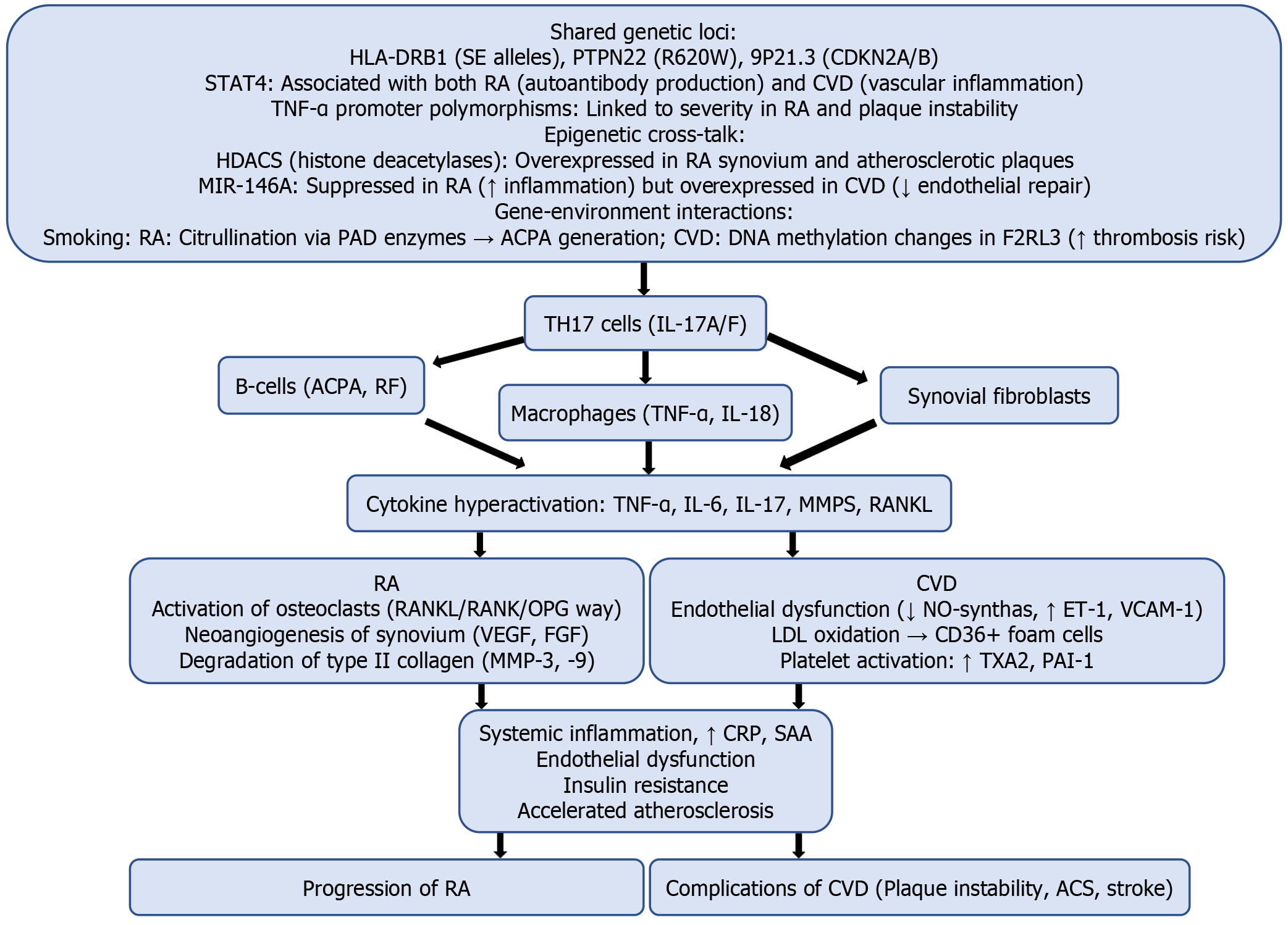
The accelerated development of atherosclerosis in patients with RA may be partly due to similar pathogenic mechanisms shared by both diseases (Table 1). Atherosclerosis can be considered a chronic inflammatory disease of blood vessels, characterized by lipid accumulation, leukocyte infiltration, and proliferation of vascular smooth muscle cells (VSMC)[8-10].
| Pathogenic mechanism | Rheumatoid arthritis | Atherosclerosis | Common features |
| Chronic inflammation | Synovial membrane activation, TNF-α, IL-1β, IL-6 release | Vascular wall inflammation, same cytokines | Systemic inflammation, |
| Autoimmune component | Autoantibodies (RF, ACPA) | Autoantibodies to oxidized LDL | Immune complexes, Th1 response |
| Macrophage activation | Synovial infiltration → pannus formation | Oxidized LDL uptake → foam cells | Macrophages as key effectors |
| Oxidative stress | ROS-mediated cartilage & synovium damage | LDL oxidation → plaque formation | ROS-driven tissue destruction |
| Endothelial dysfunction | Microangiopathy, synovial neovascularization | Impaired vascular barrier function | VCAM-1/ICAM-1 upregulation |
| Neoangiogenesis | Angiogenesis in synovium → arthritis progression | Plaque instability due to new vessels | Pathological vessel growth |
| Fibrosis/tissue remodeling | Joint deformity (excess collagen) | Fibrous cap formation on plaques | Fibroblast activation |
| Biomarkers | CRP, ACPA, RF | CRP, oxidized LDL, IL-6 | Shared markers (CRP, IL-6) |
An association has been established between elevated C-reactive protein (CRP) and erythrocyte sedimentation rate and the increased risk of MI and stroke in patients with RA[11,12]. Furthermore, the asymptomatic carrier state of rheumatoid factor or anti-cyclic citrullinated peptide antibodies is associated with increased CVD morbidity and mortality[13].
Cytokines link the pathogenesis of RA and CVD through shared mechanisms such as chronic inflammation, endothelial dysfunction, and oxidative stress (Table 2)[14-16]. Controlling the cytokine network is a key factor in reducing cardiovascular risk in patients with RA.
| Cytokine | Major sources | Effects in RA | Effects in CVD | Common pathogenic effects |
| TNF-α | Macrophages, Th1 cells, adipocytes | Activates synovial fibroblasts | Endothelial dysfunction | NF-κB activation |
| Stimulates osteoclasts (via RANKL) | Increased leukocyte adhesion | Induction of cellular apoptosis | ||
| Induces MMP-9 production | Atherosclerotic plaque destabilization | Stimulation of IL-6 production | ||
| IL-6 | Macrophages, Th1 cells, adipocytes | Stimulates B-cells (RF production) | Enhances fibrinogen synthesis | JAK/STAT pathway activation |
| Induces acute-phase proteins (CRP, SAA) | Promotes cardiomyocyte hypertrophy | Induction of insulin resistance | ||
| Causes anemia of chronic disease | Accelerates atherogenesis | |||
| IL-1β | Macrophages, neutrophils | Stimulates chondrocyte protease production | Increases platelet aggregation | NLRP3 inflammasome activation |
| Induces fever and pain | Upregulates adhesion molecules (VCAM-1) | Angiogenesis stimulation | ||
| Activates osteoclasts | Plaque destabilization | |||
| IL-17 | Th17 cells, γδT cells | Promotes synovial neoangiogenesis | Increases endothelial ET-1 production | MAPK pathway activation |
| Induces neutrophil infiltration | Promotes myocardial fibrosis | Stimulates IL-6 production | ||
| Synergizes with TNF-α | Enhances oxidative stress | |||
| IL-10 | Tregs, B-cells, macrophages | Suppresses TNF-α and IL-6 production | Stabilizes atherosclerotic plaques | NF-κB inhibition |
| Inhibits Th17 activation | Reduces leukocyte adhesion | SOCS3 stimulation | ||
| IFN-γ | Th1, natural killer cells | Activates synovial macrophages | Increases plaque vulnerability | STAT1 activation |
| Inhibits Th17 differentiation | Stimulates smooth muscle cell apoptosis | Enhances MHC II expression |
The increased risk of CVD typically manifests in the presence of genetic predisposition and largely depends on traditional risk factors, such as arterial hypertension, diabetes mellitus, and obesity. It is further exacerbated by dyslipidemia, smoking, a sedentary lifestyle, family history of CVD, and menopause (Figure 1)[16-18]. However, RA and atherosclerosis share only partial similarities in their pathogenic mechanisms, with different pathways contributing to varying extents. Therefore, it cannot be unequivocally stated that anti-inflammatory therapy always reduces the risk of cardiovascular events, as demonstrated in the CANTOS and CIRT studies (Table 3)[19,20].
| Criterion | CANTOS (2017) | CIRT (2019) |
| Study drug | Canakinumab (IL-1β inhibitor) | Methotrexate |
| Study objective | Evaluate IL-1β suppression on cardiovascular outcomes | Test if low-dose immunosuppression reduces CVD risk |
| Design | Double-blind, placebo-controlled, multicenter | Double-blind, placebo-controlled, multicenter |
| Patient population | 10061 patients with CAD and hs-CRP ≥ 2 mg/L | 4786 patients with CAD/metabolic syndrome + diabetes/obesity |
| Primary outcomes | 15% reduction in acute coronary syndrome (MI, unstable angina, cardiac death) (P = 0.007) | No significant effect: No difference in CVD outcomes vs placebo (P = 0.67) |
| 37%-41% reduction in hs-CRP | ||
| Effect on inflammation | Sustained reduction in IL-6 and hs-CRP | Minimal impact on CRP |
| Adverse effects | ↑ Fatal infections (0.18 vs 0.06 per 100 person-years) | ↑ Liver enzyme abnormalities |
| ↑ LDL levels | ↑ Leukopenia risk | |
| Conclusions | Hypothesis confirmed: IL-1β is a valid target for secondary CVD prevention | Hypothesis rejected: Methotrexate does not reduce CVD risk in this population |
RA is characterized by the so-called lipid paradox: Low levels of total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) in the presence of active inflammation. These levels tend to rise following the initiation of RA treatment, but this does not correlate with increased CVD risk. This paradox is likely due to the effects of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which increase LDL and SRB1 receptor activity on hepatocytes, leading to increased hepatic LDL uptake and cholesterol secretion into bile, thus reducing circulating LDL levels.
RA is also associated with an increased risk of heart failure (HF), predominantly with preserved ejection fraction[21]. Some data suggest that RA patients have twice the risk of developing HF compared to the general population[22]. This may result from accelerated atherosclerosis, impaired systolic and diastolic myocardial function, increased interstitial myocardial fibrosis, and reduced myofilament sensitivity to Ca2+ ions[23]. Rare complications of RA, such as myocarditis and amyloidosis, can also lead to HF.
Many disease-modifying antirheumatic drugs (DMARDs) are approved for RA treatment in clinical guidelines from different countries[24-26]. Continuous use of drugs from different classes of DMARDs is recommended: (1) Conventional synthetic DMARDs (csDMARDs): Methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF), hydroxychloroquine (HCQ); (2) Biologic DMARDs (bDMARDs); (3) TNF inhibitors: Adalimumab, Certolizumab, Etanercept, Golimumab, Infliximab; (4) Cytotoxic T-lymphocyte-associated protein 4 immunoglobulin that inhibits T-cell co-stimulation: Abatacept; (5) Recombinant human IL-1 type I receptor antagonist: Anakinra; (6) IL-6 receptor inhibitors: Sarilumab, Tocilizumab; (7) Anti-IL-6 monoclonal antibody: Olokizumab; (8) Chimeric monoclonal antibody against CD20: Rituximab; (9) Targeted synthetic DMARDs (tsDMARDs); and (10) Janus kinase (JAK) inhibitors: Baricitinib, Tofacitinib, Upadacitinib, Filgotinib.
First-line treatment for RA involves csDMARDs, with MTX being the preferred choice (if no contraindications exist). In cases of MTX intolerance, it may be substituted with LEF, SSZ, or HCQ, either as monotherapy or in combination. If csDMARDs prove insufficiently effective, bDMARDs or tsDMARDs may be added or substituted. TNF inhibitors are the first-line bDMARDs, especially in seropositive RA with high disease activity. Other bDMARDs are typically reserved for patients with an inadequate response or intolerance to TNF inhibitors, with agent selection based on safety profiles and individual risk factors. JAK inhibitors are usually employed following failure of bDMARD therapy. The mechanisms of action of DMARDs in the context of RA and CVD development are summarized in Table 4.
| Drug class/target | Mechanism of action | CVD benefit/risks | Specific drugs (examples) | Key characteristics |
| Conventional synthetic DMARDs | ||||
| Folate antagonists | Inhibits dihydrofolate reductase → ↓ purine synthesis → lymphocyte apoptosis | ↓Cardiovascular mortality | Methotrexate | Gold standard for RA |
| ↑ Extracellular adenosine | Weekly dosing (SC/PO) | |||
| ↓ TNF-α/IL-6 | Requires folate supplementation | |||
| Pyrimidine synthesis inhibitors | Blocks dihydroorotate dehydrogenase (DHODH) → ↓ lymphocyte proliferation | Neutral/↑ hypertension | Leflunomide | Teratogenic (requires washout) |
| Active metabolite (teriflunomide) | ||||
| NF-κB inhibitors | Scavenges ROS, inhibits NF-κB → ↓ TNF-α/IL-6 | ↓ Oxidative stress in endothelium | Sulfasalazine | Split into 5-ASA (gut) + sulfapyridine (systemic) |
| Safe in pregnancy | ||||
| Lysosomotropic agents | ↑ Lysosomal pH → ↓ TLR7/9 signaling and antigen presentation | Anti-thrombotic (↓ platelet aggregation) | Hydroxychloroquine | Slow onset (3-6 months) |
| Retinopathy risk at high cumulative doses | ||||
| Biologic DMARDs | ||||
| TNF-α | Neutralizes soluble/membrane-bound TNF ↓ IL-1/6/8, ↓ metalloproteinases | ↑ Endothelial function | Adalimumab, Infliximab | First-line biologics |
| Screen for TB/HBV | ||||
| IL-6 pathway | Blocks IL-6 receptor or ligand: ↓ JAK/STAT3, ↓ Th17 differentiation | ↓ LDL oxidation | Tocilizumab, Olokizumab | Rapid CRP reduction |
| May ↑ LDL | ||||
| T-cell Co-stimulation | CTLA4-Ig binds CD80/86 → ↓ T-cell activation | ↓ Atherosclerosis progression | Abatacept | Lower infection risk vs anti-TNF |
| B-cell Depletion | Anti-CD20 → B-cell lysis ↓ Autoantibodies, ↓ Antigen presentation | ↓ Atheroma progression | Rituximab | Preferred for seropositive RA |
| Prolonged hypogammaglobulinemia risk | ||||
| IL-1 | Recombinant IL-1 receptor antagonist | Reducing atherosclerotic progression and stabilizing plaques | Anakinra | Limited use in RA (more for autoinflammatory diseases) |
| Targeted synthetic DMARDs | ||||
| JAK inhibitors | Blocks JAK-STAT signaling: ↓ IFN-γ, IL-6, IL-15 signaling; ↓ GM-CSF, IL-12/23 pathways | ↑ LDL Potential ↑ thrombosis | Tofacitinib, Upadacitinib | Oral administration |
| Boxed warning for thrombosis | ||||
| Avoid in elderly smokers | ||||
Given the key role of chronic inflammation and autoimmune dysfunction in the development of atherosclerosis and related cardiovascular complications in RA, effective anti-inflammatory therapy plays a vital role in prevention. Adequate inflammation control, tailored to the pathogenic overlap between these diseases, theoretically reduces the risk of cardiovascular events. In this regard, studying the cardiovascular effects of DMARDs is of undeniable importance.
MTX is currently the most commonly used drug in the treatment of RA. Its high efficiency has earned it the status of the “gold standard” in the treatment of this condition. Over the past decades, MTX has become the first-line drug for RA and the most frequently used component of combination therapy.
As mentioned, the CIRT trial, a double-blind, placebo-controlled study, demonstrated that low doses of MTX did not reduce the frequency of cardiovascular events, nor the levels of inflammatory cytokines such as CRP, IL-1β, and IL-6, compared to the placebo group. However, the study included patients with established ischemic heart disease and diabetes, metabolic syndrome, or both, but not patients with RA[20].
Other studies have shown different results when assessing the impact of MTX on cardiovascular events in patients with rheumatic diseases. A multicenter, prospective cohort study by Johnson et al[27], involving long-term follow-up of 2044 United States veterans with RA receiving MTX therapy, showed a 24% reduction in the risk of cardiovascular events, along with a 57% decrease in hospitalizations due to HF progression. Similar results were reported by the Veterans Affairs RA registry[28], which included 1015 participants. The study found that men with RA had a twofold higher mortality risk compared to the general population, associated with inflammatory activity, the presence of rheumatoid nodules, low body weight, and glucocorticoid use. However, MTX therapy, by effectively controlling chronic rheumatoid inflammation, reduced mortality risk by 40%, primarily due to decreased cardiovascular mortality. A notable limitation of both studies is that most participants were male, which restricts the generalizability of the findings to the broader RA population, where women statistically predominate.
In the study by Wasko et al[29], which included 5626 RA patients (75% women), it was demonstrated that over a 25-year prospective follow-up, long-term MTX use (over 1 year) was associated with a 70% reduction in mortality risk. Male sex, older age, high HAQ scores, glucocorticoid use, presence of HF, and cancer were identified as risk factors for death.
In addition to its impact on prognosis, studies have also examined the effect of MTX on classical CVD risk factors. According to Wållberg-Jonsson et al[30], RA patients receiving MTX demonstrated a 17.6% reduction in carotid intima-media thickness compared to controls, provided effective disease activity suppression was achieved. This highlights the influence of MTX on early atherosclerosis markers through anti-inflammatory mechanisms.
To date, numerous studies have investigated the effects of MTX on blood lipid profiles in RA patients, but the findings remain contradictory. Some report a reduction in the atherogenic index and blood anti-atherogenic lipoproteins in MTX-treated RA patients, correlating with decreased acute-phase reactant levels[31,32]. In contrast, other studies have shown no significant changes in lipid parameters[33,34].
The exact mechanisms by which long-term MTX therapy reduces CVD risk remain unclear. However, it is evident that this effect occurs alongside improved control of RA activity and systemic inflammation. As a result, the intensity of vascular dysfunction, underlying the development and progression of atherothrombosis, is reduced. Moreover, not only long-term but even single-dose MTX administration can lead to a decrease in acute-phase proteins, inhibition of neutrophil chemotaxis, reduction of toxic oxygen metabolite production, and prevention of leukocyte adhesion to the vascular endothelium[35].
Indirect mechanisms by which MTX influences the development of atherosclerosis and its complications in RA have also been discussed. According to Cronstein and Reiss[36], the favorable cardiovascular action of MTX may be linked to its primary pharmacological effects, specifically, the enhanced production of adenosine. This effect is mediated through G-protein signaling, which activates adenylate cyclase and T-cell activation[37]. Adenylate cyclase is abundant in the basal ganglia, vascular walls, and platelets. A2A and A1 receptors for endogenous adenosine play an important role in regulating coronary blood flow, myocardial oxygen consumption, and brain metabolism. Activation of these receptors may contribute to coronary vasodilation and increased cardiac perfusion. Additionally, MTX protects the vascular endothelium by increasing the expression of anti-atherogenic proteins involved in reverse cholesterol transport and by scavenging superoxide, thereby reducing oxidative stress[38].
Despite its anti-inflammatory effects, MTX may have adverse cardiovascular effects by inducing hyperhomocysteinemia, endothelial dysfunction, accelerated atherosclerosis, increased thrombosis risk, and direct cardiotoxicity (Table 5). Nonetheless, most studies demonstrate a positive effect of MTX on cardiovascular event risk, early atherosclerotic changes, and traditional cardiovascular risk factors in RA.
| Effect | Pathogenesis |
| Cardiomyopathy | Folate depletion → impaired myocardial energy metabolism Oxidative stress and mitochondrial dysfunction |
| Accumulation of adenosine → vasodilation and reduced contractility | |
| Accelerated atherosclerosis | Hyperhomocysteinemia (due to folate antagonism) → endothelial dysfunction |
| Vascular toxicity | Endothelial injury due to oxidative stress |
| Reduced nitric oxide bioavailability | |
| Increased homocysteine → vascular smooth muscle proliferation | |
| Hypertension | Renal toxicity → sodium retention |
| Endothelial dysfunction → impaired vasoregulation | |
| Heart failure | Direct myocardial toxicity (similar to cardiomyopathy) |
| Fluid retention due to renal impairment | |
| Arrhythmias | Electrolyte imbalances (e.g., hypokalemia from nephrotoxicity) QT prolongation (rare, linked to high-dose MTX) |
LEF is traditionally considered a second-line drug for monotherapy in RA (e.g., in cases of MTX inefficacy or intolerance) or as an effective component of dual or triple combination therapy. Compared to MTX, the impact of LEF on the development and progression of CVD in patients with rheumatic conditions has been studied to a much lesser extent. Its active metabolite, teriflunomide, exerts anti-inflammatory, antiproliferative, and antioxidant properties, which may contribute to cardiovascular benefits. The following are the key mechanisms of action: (1) Anti-inflammatory effects: LEF inhibits pro-inflammatory cytokines (e.g., TNF-α, IL-6) and reduces endothelial dysfunction, a key factor in atherosclerosis development. Suppression of NF-κB signaling attenuates vascular inflammation and plaque instability; (2) Antiproliferative action on VSMCs: By inhibiting dihydroorotate dehydrogenase, LEF blocks pyrimidine synthesis, reducing VSMC proliferation and migration, thereby slowing atherosclerotic plaque progression; (3) Antioxidant properties: Teriflunomide decreases oxidative stress by scavenging reactive oxygen species and enhancing endogenous antioxidant defenses, improving endothelial function; and (4) Improvement of endothelial function: Enhanced nitric oxide bioavailability and reduced endothelin-1 secretion contribute to vasodilation and improved arterial compliance.
Despite its potential anti-inflammatory benefits, LEF may have adverse effects, with arterial hypertension being the most frequently reported (occurring in approximately 5% of patients). This is hypothesized to result from fluid retention and endothelial dysfunction due to impaired nitric oxide synthesis[39]. Given its potential cardiovascular protective effects, LEF might be considered for the treatment of RA patients with metabolic syndrome. However, the overall impact of the drug on cardiovascular mortality remains insufficiently studied and should be further investigated in larger clinical studies.
SSZ is a drug for combination therapy in RA. Consequently, there are no large-scale studies evaluating the effect of this drug as monotherapy on cardiovascular risk in patients with RA.
SSZ suppresses the activation of the NF-κB transcription factor. In both animal models and humans with CVD risk factors, NF-κB activation in the endothelium leads to impaired vasodilatory function[40]. A compound related to SSZ, salsalazine, improves flow-mediated dilation in people with obesity[41], and NF-κB is a central regulator of genes involved in inflammation and adhesion in cells participating in atherogenesis[42]. Suppression of NF-κB is an effective method for preventing the development of atherosclerosis[43]. However, the effect of NF-κB inhibition on established clinical atherosclerotic disease remains incompletely understood. For example, a randomized study by Tabit et al[44], which included patients with stable ischemic heart disease or a history of MI, evaluated the effect of SSZ vs placebo over 6 weeks. The results showed no effect of SSZ on flow-mediated dilation of the brachial artery (a marker of large-vessel endothelial function), the hyperemic response (a marker of small-vessel vasodilatory function), blood pressure (BP), lipid levels, glucose, or insulin levels. However, short-term SSZ treatment did reduce NF-κB activity. The authors suggested that endothelial dysfunction in chronic disease may be more resistant to improvement via NF-κB inhibition, which could explain the lack of a significant clinical effect with this drug.
Moreover, SSZ has been shown to positively influence the lipid profile by increasing HDL levels and reducing the atherogenic index[45]. However, long-term SSZ use may elevate homocysteine levels, as SSZ competitively inhibits folate absorption in the small intestine. Hyperhomocysteinemia, in turn, is associated with an increased risk of CVD and thrombosis[46]. In rare cases, SSZ can induce hypokalemia (due to renal potassium wasting), which may theoretically elevate the risk of arrhythmias. Overall, SSZ appears to have a neutral or possibly beneficial effect on the cardiovascular system due to its anti-inflammatory properties. However, in some cases, monitoring of electrolyte levels and folate status may be necessary.
HCQ is most widely used in the treatment of systemic lupus erythematosus, and in RA, it is traditionally considered primarily as a component of dual or triple combination therapy. The drug demonstrates potential anti-atherosclerotic properties, which are particularly relevant for patients with chronic inflammatory diseases. These effects are mediated through several key mechanisms. Multiple studies have demonstrated a direct effect of HCQ on lipid metabolism, including reductions in total cholesterol and LDL levels, achieved by inhibiting cholesterol synthesis in the liver via its action on the enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase similarly to statins, though less potently. As a result, HCQ use in RA patients has been associated with reduced total cholesterol and LDL levels[47,48]. HCQ also improves glucose utilization, resulting in decreased levels of triglycerides and very-LDL[49,50]. The drug helps suppress endothelial dysfunction by inhibiting pro-inflammatory cytokines (TNF-α, IL-6, and interferon-gamma), which contribute to endothelial damage and atherosclerotic plaque formation. HCQ also reduces CRP, a marker of systemic inflammation and atherosclerosis risk[51,52].
There is further evidence that HCQ inhibits nicotinamide adenine dinucleotide phosphate oxidase, an enzyme responsible for generating active oxygen species that accelerate atherosclerosis[53]. HCQ reduces collagen degradation in the fibrous cap of atherosclerotic plaques, and this lowers the risk of plaque rupture and thrombosis[54] by blocking the molecule of vascular cell adhesion protein-1 and intercellular adhesion molecule 1, which hinders the migration of inflammatory cells into the vessel wall[55].
A retrospective observational study by Cordova Sanchez et al[56], which included over 2 million patients with RA, showed that HCQ was associated with a slight reduction in the incidence of cardiovascular events and strokes. However, these outcomes were less favorable compared to patients taking MTX or bDMARDs. There is also evidence of potential adverse cardiovascular effects of HCQ, which exhibits dose-dependent cardiotoxicity, with adverse events typically manifesting after prolonged use.
Of particular concern is the potential of HCQ to prolong the QT interval due to blockade of hERG potassium channels, leading to impaired ventricular repolarization. This increases the risk of torsades de pointes and sudden cardiac death, especially in the setting of hypokalemia, concomitant use of other QT-prolonging drugs (e.g., macrolides, class III antiarrhythmics), or congenital long QT syndrome[57,58]. HCQ may also suppress sinoatrial node function and impair atrioventricular (AV) conduction, resulting in sinus bradycardia or first- and second-degree AV block (rarely third-degree). These effects are particularly dangerous in patients with pre-existing conduction abnormalities.
Another serious complication is toxic cardiomyopathy, which is associated with the cumulative dose and duration of therapy, especially in patients treated with HCQ for more than 10 years[59]. Cardiomyopathy usually presents as concentric hypertrophy with restrictive features, with or without conduction abnormalities. While HCQ may offer modest cardiovascular benefits due to its anti-inflammatory, anti-thrombotic, and metabolic effects, its use should be carefully justified and monitored.
TNF-α is one of the key mediators of atherogenesis. It induces endothelial dysfunction, upregulates cell adhesion molecules that facilitate leukocyte migration into the vascular wall, contributes to the destabilization of atherosclerotic plaques, suppresses anticoagulant properties while promoting procoagulant activity of the endothelium, impairs myocardial contractility, and stimulates CRP synthesis[60]. Experimental studies[61] have shown that mice lacking the TNF-α gene do not develop intimal hyperplasia after arterial injury. Clinical studies[62-64] have further demonstrated that elevated TNF-α concentrations correlate with the development of atherosclerosis and its complications. TNF-α also affects lipid metabolism, glucose metabolism, and energy homeostasis. TNF-α inhibitors, widely used biologic drugs for RA, are associated with a reduced overall risk of CVD in RA patients[65]. Anti-TNF-α therapy suppresses the production of pro-inflammatory and pro-atherogenic mediators such as CRP, IL-6, and TNF-α itself, as well as adhesion molecules. It also causes depletion of CD4+CD28- T cells, which play an important role in destabilizing atherosclerotic plaques, and increases the number of endothelial progenitor cells, whose deficiency is linked to higher cardiovascular risk[66]. TNF-α accelerates plaque formation by activating macrophages and promoting LDL oxidation. Blocking TNF-α may help stabilize plaques and reduce the risk of acute coronary events.
Barnabe et al[65] demonstrated that long-term anti-TNF-α therapy reduced cardiovascular event frequency in RA patients. However, a study by Mann et al[67] found no significant effect of etanercept on atherosclerosis progression, suggesting possible differences between agents. A systematic review and meta-analysis by Roubille et al[68], which included 34 studies, confirmed that anti-TNF-α therapy was associated with a decreased risk of overall CVD, including MI, stroke, and major adverse cardiovascular events (MACE). More recent studies reported a 2.1% improvement in endothelium-dependent vasodilation in RA patients receiving anti-TNF-α therapy. Randomized controlled trials have also shown that anti-TNF-α therapy (infliximab, adalimumab, etanercept) reduces markers of subclinical atherosclerosis, such as carotid intima-media thickness and pulse wave velocity[69].
Data on the impact of anti-TNF-α therapy on BP are contradictory. Some studies show a moderate BP reduction due to improved endothelial function and reduced systemic inflammation[70]. However, a study by Faria et al[71] found no significant increase in hypertension risk during treatment, although some patients experienced transient BP elevations requiring adjustment of antihypertensive therapy.
TNF-α also has a negative inotropic effect on the myocardium and contributes to left ventricular remodeling and the progression of HF[72]. Clinical trials, including the anti-TNF Therapy Against Congestive Heart (ATTACH) trial, showed that high-dose infliximab in patients with severe HF worsened outcomes: In the high-dose group (10 mg/kg), hospitalization and mortality rates increased (14% vs 3% in placebo). The trial was terminated early due to the risk of decompensation[73]. Consequently, anti-TNF-α therapy is not recommended for patients with reduced ejection fraction. In addition, TNF-α promotes tissue factor expression and inhibits fibrinolysis, thereby increasing thrombosis risk. Anti-TNF-α therapy may reduce prothrombotic activity, as evidenced by decreased levels of D-dimer and fibrinogen in patients with chronic inflammation[74].
Overall, anti-TNF-α therapy exerts a complex influence on the cardiovascular system, primarily through suppression of systemic inflammation and improvement of endothelial function. However, its use in patients with HF requires caution, and further research is needed to clarify its long-term safety profile.
Among IL-6 inhibitors used in rheumatology, the following monoclonal antibodies are of particular interest: Tocilizumab, sarilumab, olokizumab, and levilimab. Most studies have focused on the safety and efficacy of tocilizumab. The literature presents contradictory data regarding its effects on lipid profiles. For example, the randomized placebo-controlled MEASURE trial demonstrated that LDL levels increased by approximately 15%-20% in patients receiving tocilizumab. However, lipoprotein(a) levels decreased by more than 30%, and HDL levels, as well as HDL composition, shifted toward patterns associated with anti-inflammatory effects[75].
The ADACTA trial compared the influence of tocilizumab and adalimumab on lipid profiles in RA patients[76]. During the first 8 weeks of treatment, patients who received tocilizumab monotherapy had higher LDL but lower HDL and lipoprotein(a) levels compared to those treated with adalimumab. Nevertheless, later findings did not show any increased CVD risk associated with tocilizumab-induced hyperlipidemia[77].
A study by Toussirot et al[78] found that tocilizumab treatment in RA patients was associated with a significant in
Several studies have found no clinically significant differences in treatment-related side effects among sarilumab, olokizumab, and levilimab[79,80]. The MONARCH trial demonstrated a smaller increase in LDL levels in patients treated with sarilumab compared to those receiving tocilizumab[81].
Olokizumab is unique among anti-IL-6 monoclonal antibodies in its mechanism of action. The randomized controlled trials CREDO 1 and CREDO 2 evaluated the efficacy and safety of olokizumab in RA. These studies demonstrated reductions in inflammatory markers, including CRP and fibrinogen, which may theoretically reduce cardiovascular risk[82]. A moderate increase in both LDL and HDL was observed, but without a significant rise in cardiovascular event rates[83].
Despite the absence of a clear increase in cardiovascular risk, some studies have reported a paradoxical rise in LDL without worsening prognosis. This may be related to changes in the functional properties of lipoproteins. Therefore, caution is advised in patients with high cardiovascular risk (e.g., those with diabetes). Long-term data remain limited, highlighting the need for further research.
Anakinra is a drug that provides dual inhibition of IL-1α and IL-1β. Several studies have examined its impact on cardiovascular risk, not only in RA patients but also in those with acute MI. For example, a study by Abbate et al[84] demonstrated that IL-1 blockade with anakinra was safe and improved left ventricular remodeling in patients with ST-elevation MI.
A meta-analysis by Zheng et al[85] showed that IL-1 blockers were associated with a reduced risk of adverse cardiovascular events, although they did not significantly reduce all-cause mortality, including mortality from acute MI. The likely mechanism by which IL-1 inhibition lowers CVD risk involves suppression of the inflammatory response and cytokine production, particularly CRP, as confirmed by numerous studies.
The multicenter randomized TRACK trial demonstrated that anakinra therapy significantly improved glycemic control and reduced RA activity, which may counteract the synergy between traditional CVD risk factors and inflammation that accelerates atherosclerosis[86]. A 1% reduction in HbA1c is associated with approximately a 15% decrease in CVD risk[87]. This conclusion is further supported by the CANTOS trial, where IL-1β antagonism reduced CVD risk in post-MI patients with elevated CRP levels, confirming the role of inflammation in CVD development[88]. However, the long-term use of anakinra has also been linked to the development of dyslipidemia[89]. Additional studies are needed to further clarify these risks and benefits.
Among this class of drugs, rituximab is used in rheumatology and was approved in 2006 for the treatment of patients with RA who had an inadequate response to at least one TNF-α inhibitor. In a study by Provan et al[90], the 12-month effect of rituximab on traditional cardiovascular risk factors and early signs of cardiovascular involvement (e.g., arterial stiffness) was assessed. The results showed no significant changes in CVD risk markers during the first 3 months of therapy. However, after 1 year, there was a trend toward reduced arterial stiffness, with an average decrease of 0.46 m/s, approximately corresponding to a 10-year reduction in vascular aging.
In contrast, an earlier study by Mathieu et al[91] demonstrated no reduction in arterial stiffness after 6 and 12 months of rituximab therapy. Moreover, a significant increase in total cholesterol and LDL levels was observed, while HDL and triglyceride levels remained unchanged. Kerekes et al[92] and Gonzalez-Juanatey et al[69] reported significant improvements in endothelial function, as measured by flow-mediated dilation, indicating reduced CVD risk in patients treated with rituximab. Additionally, in the study by Kerekes et al[92], a 3%-11% reduction in total cholesterol and a 14%-35% increase in HDL levels were observed. Similar anti-atherogenic effects of rituximab were demonstrated in a study by Raterman[93].
Rituximab rarely causes direct cardiotoxic effects, but infusion reactions may lead to hemodynamic disturbances. Reported complications following drug administration include the following: (1) Arrhythmias (rare)-including cases of atrial fibrillation and ventricular tachycardia; (2) Hypotension due to cytokine release syndrome; and (3) Cute coronary syndrome (very rare), triggered by histamine and other mediator release.
Thus, in patients with autoimmune diseases, rituximab may reduce systemic inflammation, potentially lowering cardiovascular risk. However, in patients with pre-existing cardiac conditions, close monitoring during infusion is essential.
Within this class, abatacept is the only drug currently used in rheumatology. The literature presents conflicting data regarding its influence on cardiovascular risk across different patient age groups. A study by Kang et al[94] showed that patients treated with abatacept had a lower risk of MI compared to those treated with TNF-α inhibitors. However, for other secondary endpoints (such as stroke and venous thromboembolism), the risk was similar between the two groups. The authors also conducted a stratified analysis of high-risk patients (age > 65 years and/or a history of diabetes mellitus). In this subgroup, the risk of combined cardiovascular outcomes was 26% lower with abatacept compared to TNF-α inhibitors. Conversely, among patients without diabetes, the combined cardiovascular risk was similar in both treatment groups[94]. These findings may be explained by the additional metabolic effects of abatacept. Evidence suggests that abatacept improves insulin sensitivity in target cells and may exert anti-atherogenic effects[95].
The risk of venous thromboembolism is significantly higher in patients receiving abatacept compared to those treated with TNF-α inhibitors, both in patients with and without diabetes[94]. In a retrospective cohort study by Shih et al[95], cardiovascular risk was assessed in RA patients with interstitial lung disease who were treated with abatacept, compared with those receiving TNF-α inhibitors. The study found a higher risk of all-cause mortality among patients without cardiovascular risk factors who were treated with abatacept, compared with TNF-α inhibitors. However, among patients with cardiovascular risk factors, this difference was not statistically significant. Additionally, in patients aged 18-64 years, abatacept use was associated with a higher likelihood of requiring mechanical ventilation. The authors suggested that abatacept may provide relatively greater benefits in patients over 65 years of age, consistent with the findings of Kang et al[94].
A study by Mathieu et al[96] evaluated the 6-month effect of abatacept on traditional cardiovascular risk factors and early signs of cardiovascular involvement (e.g., arterial stiffness). After 6 months of treatment, with concurrent reductions in RA disease activity, there was a modest increase in arterial stiffness parameters, a slight elevation in total cholesterol, LDL, and HDL, and a decrease in the atherogenic index. The authors suggested that the lack of improvement in arterial stiffness could be due to insufficient reduction of systemic inflammation. At the same time, abatacept showed favorable effects on lipid profiles, warranting longer-term studies for clearer conclusions.
Unlike TNF-α inhibitors, which may exacerbate HF, abatacept has not been associated with an increased risk of decompensation. Therefore, abatacept may be a preferable option for patients with high cardiovascular risk or those experiencing adverse lipid profile changes with other therapies.
Among tsDMARDs, the following are currently included in various international clinical guidelines for the treatment of RA: Tofacitinib, baricitinib, upadacitinib, and filgotinib. All of them belong to the class of JAK inhibitors. This group of drugs demonstrates efficiency comparable to that of other DMARDs in the treatment of RA[97].
One practical advantage of JAK inhibitors is their oral formulation, which improves adherence. This allows for long-term outpatient treatment (no need for hospital visits or parenteral administration), eliminates the requirement for refrigerated storage, and offers a more convenient option for patients who dislike injections. A specific drawback of tofacitinib is its twice-daily dosing, though an extended-release formulation requiring once-daily administration is now available. However, the cardiovascular safety profile of this drug class remains inconclusive, prompting close scrutiny by regulatory authorities (FDA, EMA).
In 2022, to minimize the risk of serious adverse events associated with JAK inhibitors, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency recommended restrictions on their use for certain indications. These included chronic inflammatory diseases such as RA, psoriatic arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, ulcerative colitis, atopic dermatitis, and alopecia areata. PRAC identified high-risk groups in which JAK inhibitors should be used only if no suitable alternatives exist. These groups include patients aged ≥65 years, those with increased CVD risk (e.g., prior MI or stroke), current or long-term smokers, and patients with high cancer risk. Caution was also recommended when prescribing JAK inhibitors to patients with thromboembolic risk factors.
The basis for this decision was the open-label clinical trial A3921133, which compared the safety of tofacitinib with TNF-α inhibitors in 4362 RA patients aged ≥ 50 years with at least one cardiovascular risk factor[98]. In this study, the incidence of pulmonary embolism per 100 patient-years was 0.54 with tofacitinib 5 mg twice daily, 0.27 with tofacitinib 10 mg twice daily, and 0.09 with TNF-α inhibitors. The incidence of deep vein thrombosis was 0.38, 0.30, and 0.18, respectively. The highest increase in pulmonary embolism risk was observed in patients with pre-existing risk factors for venous thromboembolism. Notably, an observational cohort study using the Truven and Medicare databases found that the risk of venous thromboembolism was comparable between RA patients treated with tofacitinib and those treated with TNF-α inhibitors[99]. Other studies assessing the safety and efficacy of JAK inhibitors have reported more reassuring results. For example, the incidence of MACE in patients receiving tofacitinib was 0.4 per 100 patient-years, with no significant differences between dose regimens and no increase with longer treatment duration[100]. The main cardiovascular events observed were atrial fibrillation, MI, and coronary artery disease. Similarly, clinical trials of baricitinib and upadacitinib have shown a low incidence of cardiovascular complications[101,102]. Moreover, a meta-analysis of randomized clinical trials found no increased risk of cardiovascular outcomes with JAK inhibitor therapy[103].
In the RA-BUILD trial, which studied dose-dependent effects of baricitinib, an elevated risk of venous thromboembolism was observed in patients receiving 4 mg/day. However, comprehensive safety analyses showed that overall risk was not higher than in the general population, regardless of whether baricitinib was taken at 2 mg or 4 mg/day. Risk was more closely associated with age, comorbid conditions (obesity, chronic lung disease, varicose veins), a history of venous thromboembolism, and concomitant use of selective cyclooxygenases-2 inhibitors[104,105].
An analysis of data from a phase III clinical trial of upadacitinib in the SELECT-RA program, in which 54% of patients were considered high risk due to age and smoking status, showed that despite an increased risk of cardiovascular events, venous thromboembolism, and malignant tumors, the incidence rates were comparable across all therapy groups[106].
The cardiovascular safety of filgotinib is currently regarded as satisfactory. The incidence of cardiovascular events and venous thromboembolism in patients treated with this JAK inhibitor did not differ significantly from those observed with adalimumab, MTX, or placebo[107].
The safety profile of various JAK inhibitors in RA was analyzed by Alves et al[108] in a systematic review that included 42 randomized clinical trials. The review found no significant differences among JAK inhibitors with respect to cardiovascular outcomes or venous thromboembolism risk. Moreover, no differences were identified between JAK inhibitors and bDMARDs, including abatacept, adalimumab, and MTX.
The mechanisms potentially linking JAK inhibitor therapy to increased cardiovascular risk remain unclear and require further investigation. Therefore, the current approach to prescribing these drugs involves a careful risk–benefit assessment for each patient.
RA is associated with an increased risk of CVD, driven by chronic inflammation, traditional risk factors, and the potential cardiotoxicity of some antirheumatic drugs. Modern treatment strategies should not only focus on controlling inflammation but also on minimizing cardiovascular risk. The use of DMARDs has demonstrated beneficial effects on the cardiovascular system through the reduction of systemic inflammation. However, some drugs, such as JAK inhibitors, require close monitoring due to a possible increase in the risk of thrombosis and other cardiovascular events. To improve prognosis in RA patients, it is essential to conduct regular screening for cardiovascular risk factors (hypertension, dyslipidemia, smoking), tailor therapy to each patient’s cardiovascular profile, and adopt a multidisciplinary approach involving both rheumatologists and cardiologists. A summary of cardiovascular safety profiles for DMARDs, preventive measures, and preferred patient categories is provided in Table 6. Future research should focus on clarifying the mechanisms of cardioprotection in RA, optimizing treatment strategies, and developing personalized approaches for patients at high cardiovascular risk.
| Drug class | Drug | Prevention of adverse cardiovascular effects methods | Preferred patient category | Safety comparison |
| csDMARDs | Methotrexate | Homocysteine control (target level < 10 μmol/L); Folic acid supplementation (5-10 mg/day)-reduces the risk of hyperhomocysteinemia by 50%-70%. Regular monitoring: Blood pressure (BP), ECG, echocardiography (EchoCG) (with long-term use). Lipid profile, homocysteine levels (every 6-12 months) | Patients without severe cardiovascular disease | Safer than bDMARDs and tsDMARDs but requires monitoring |
| Sulfasalazine | Caution in patients with conduction disorders; Use validated risk scores: SCORE2/SCORE2-OP for estimating 10-year CVD risk, QRISK3; Baseline & periodic evaluation: Lipid profile (LDL-C, HDL-C, triglycerides), hs-CRP, homocysteine (if high CVD risk). BP monitoring; ECG/Echocardiography | Patients with mild RA | Safer than biologics but less effective | |
| Leflunomide | BP control, salt restriction | Patients without a history of HTN | Similar to MTX in safety but more likely to cause HTN | |
| Hydroxychloroquine | ECG monitoring (QT interval). Before starting therapy: Measure baseline QT (corrected using Fridericia’s formula- QTc). Assess risks if QTc > 450 ms in men or > 470 ms in women (consider alternative medications). Repeat ECG 3-5 days after initiation and after each dose increase. Correction of electrolyte imbalances: Hypokalemia (K+ < 3.5 mmol/L) and hypomagnesemia (Mg2+ < 0.7 mmol/L) increase arrhythmia risk | Patients with very mild RA or SLE | Safest in this group but requires QT interval monitoring | |
| bDMARDs (TNF inhibitors) | Adalimumab | Avoid in patients with HF class III-IV | Patients without severe CVD | Higher infection risk but lower CV risk than JAK inhibitors |
| Certolizumab | BP monitoring, cardiac function assessment | Pregnant women (low placental transfer) | Similar to other TNF inhibitors | |
| Etanercept | Caution in HF | Patients with moderate CV risk | Considered safer than infliximab | |
| Golimumab | Monitor BP and HF symptoms | Patients intolerant to other TNF inhibitors | Comparable to other TNF inhibitors | |
| Infliximab | Avoid in HF class II–IV | Patients with severe RA but no CVD | Highest HF risk among TNF inhibitors | |
| Other bDMARDs | Abatacept | BP control, ECG if risk factors present | Patients at high infection risk | Safer than TNF inhibitors regarding HF |
| Anakinra | Not required | Patients with concomitant atherosclerosis | One of the safest biologics | |
| Sarilumab/Tocilizumab | Lipid monitoring, statins if needed | Patients without severe dyslipidemia | Higher CV risk than TNF inhibitors but lower than JAK inhibitors | |
| Olokizumab | Lipid profile monitoring | Patients resistant to other IL-6 inhibitors | Presumed similar to tocilizumab | |
| Rituximab | Premedication, slow infusion | Patients with lymphoproliferative disorders | Neutral CV effects but risk of infusion-related hypotension | |
| tsDMARDs (JAK inhibitors) | Baricitinib | Avoid in patients with thrombosis history, BP control, anticoagulants for AF | Younger patients without thrombosis risk factors | Least safe regarding CV risk (FDA, EMA warning-thrombosis, MI, stroke) |
| Tofacitinib | Lipid monitoring, BP control, avoid in smokers/obese patients | Patients unresponsive to bDMARDs | High CV risk, especially in smokers | |
| Upadacitinib | Thrombosis risk assessment before prescribing | Patients intolerant to other JAK inhibitors | Similar to other JAK inhibitors | |
| Filgotinib | General precautions as for other JAK inhibitors | Limited use, caution required | Presumed similar to tofacitinib |
| 1. | Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 1073] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 2. | Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 662] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Krougly LB, Fomicheva OA, Karpov YA, Popkova TV, Novikova DS, Nasonov EL. [Cardiovascular Complications of Rheumatoid Arthritis: Prevalence and Pathogenesis]. Kardiologiia. 2016;56:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Udachkina HV, Novikova DS, Popkova TV, Kirillova IG, Markelova EI, Luchikhina EL, Lukina GV, Sinitsyn VE, Karateev DE, Nasonov EL. Calcification of coronary arteries in early rheumatoid arthritis prior to anti-rheumatic therapy. Rheumatol Int. 2018;38:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kerola AM, Kauppi MJ, Kerola T, Nieminen TV. How early in the course of rheumatoid arthritis does the excess cardiovascular risk appear? Ann Rheum Dis. 2012;71:1606-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Kirillova IG, Novikova DS, Popkova TV, Gorbunova YN, Markelova EI, Korsakova YO, Volkov AV, Alexandrova EN, Novikov AA, Fomicheva OA, Luchikhina EL, Karateev DE, Nasonov EL. [Left and right ventricular diastolic dysfunction in patients with early rheumatoid arthritis before prescribing disease-modifying antirheumatic therapy]. Ter Arkh. 2015;87:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Gerasimova EV, Popkova TV, Novikova DS, Aleksandrova EN, Novikov AA, Nasonov EL. [Ten-year risk of cardiovascular complications in patients with rheumatoid arthritis]. Ter Arkh. 2011;83:14-19. [PubMed] |
| 8. | Fomicheva OA, Popkova TV, Krougly LB, Gerasimova EV, Novikova DS, Pogorelova OA, Tripoten MI, Balakhonova TV, Karpov YA, Nasonov EL. Factors of Progression and Occurrence of Atherosclerosis in Rheumatoid Arthritis. Kardiologiia. 2021;61:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kotlyarov SN, Kotlyarova AA. Role of lipid metabolism and systemic inflammation in the development of atherosclerosis in animal models. I P Pavlov Russ Med Biol Her. 2021;29:134-146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 740] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 11. | Venetsanopoulou AI, Pelechas E, Voulgari PV, Drosos AA. The lipid paradox in rheumatoid arthritis: the dark horse of the augmented cardiovascular risk. Rheumatol Int. 2020;40:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, Millan IY, Crowson CS, Curtis JR. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Sanghavi N, Ingrassia JP, Korem S, Ash J, Pan S, Wasserman A. Cardiovascular Manifestations in Rheumatoid Arthritis. Cardiol Rev. 2024;32:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3395] [Cited by in RCA: 4039] [Article Influence: 269.3] [Reference Citation Analysis (0)] |
| 15. | Sokolove J, Brennan MJ, Sharpe O, Lahey LJ, Kao AH, Krishnan E, Edmundowicz D, Lepus CM, Wasko MC, Robinson WH. Brief report: citrullination within the atherosclerotic plaque: a potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013;65:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | López-Mejías R, Genre F, Corrales A, González-Juanatey C, Ubilla B, Llorca J, Miranda-Filloy JA, Pina T, Blanco R, Castañeda S, Martín J, González-Gay MA. Investigation of a PON1 gene polymorphism (rs662 polymorphism) as predictor of subclinical atherosclerosis in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73:1749-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kotlyarov SN. Place of lipid theory in history of study of atherosclerosis. I P Pavlov Russ Med Biol Her. 2024;32:681-689. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Rebrov AP, Nikitina NM. [Risk factors of cardiovascular diseases in patients with rheumatoid arthritis]. Klin Med (Mosk). 2008;86:56-59. [PubMed] |
| 19. | Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The CANTOS Trial: One Important Step for Clinical Cardiology but a Giant Leap for Vascular Biology. Arterioscler Thromb Vasc Biol. 2017;37:e174-e177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 1024] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 21. | Ahlers MJ, Lowery BD, Farber-Eger E, Wang TJ, Bradham W, Ormseth MJ, Chung CP, Stein CM, Gupta DK. Heart Failure Risk Associated With Rheumatoid Arthritis-Related Chronic Inflammation. J Am Heart Assoc. 2020;9:e014661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Voskuyl AE. The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford). 2006;45 Suppl 4:iv4-iv7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Mantel Ä, Holmqvist M, Andersson DC, Lund LH, Askling J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J Am Coll Cardiol. 2017;69:1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, Caporali R, Edwards CJ, Hyrich KL, Pope JE, de Souza S, Stamm TA, Takeuchi T, Verschueren P, Winthrop KL, Balsa A, Bathon JM, Buch MH, Burmester GR, Buttgereit F, Cardiel MH, Chatzidionysiou K, Codreanu C, Cutolo M, den Broeder AA, El Aoufy K, Finckh A, Fonseca JE, Gottenberg JE, Haavardsholm EA, Iagnocco A, Lauper K, Li Z, McInnes IB, Mysler EF, Nash P, Poor G, Ristic GG, Rivellese F, Rubbert-Roth A, Schulze-Koops H, Stoilov N, Strangfeld A, van der Helm-van Mil A, van Duuren E, Vliet Vlieland TPM, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1030] [Article Influence: 343.3] [Reference Citation Analysis (0)] |
| 25. | Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, Kremer J, Nakamura MC, Russell LA, Singh JA, Smith BJ, Sparks JA, Venkatachalam S, Weinblatt ME, Al-Gibbawi M, Baker JF, Barbour KE, Barton JL, Cappelli L, Chamseddine F, George M, Johnson SR, Kahale L, Karam BS, Khamis AM, Navarro-Millán I, Mirza R, Schwab P, Singh N, Turgunbaev M, Turner AS, Yaacoub S, Akl EA. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73:924-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 26. | Harigai M, Kaneko Y, Tanaka E, Hirata S, Kameda H, Kaneko K, Kishimoto M, Kohno M, Kojima M, Kojima T, Morinobu A, Nakajima A, Sugihara T, Fusama M, Yajima N, Yanai R, Kawahito Y. 2024 Update of the Japan College of Rheumatology Clinical Practice Guidelines for the Management of Rheumatoid Arthritis: Secondary publication. Mod Rheumatol. 2025;35:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Johnson TM, Sayles HR, Baker JF, George MD, Roul P, Zheng C, Sauer B, Liao KP, Anderson DR, Mikuls TR, England BR. Investigating changes in disease activity as a mediator of cardiovascular risk reduction with methotrexate use in rheumatoid arthritis. Ann Rheum Dis. 2021;80:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, Johnson D, Richards JS, Kerr GS, Cannon GW, Reimold A. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford). 2011;50:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Wasko MC, Dasgupta A, Hubert H, Fries JF, Ward MM. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Wållberg-Jonsson S, Ohman M, Rantapää-Dahlqvist S. Which factors are related to the presence of atherosclerosis in rheumatoid arthritis? Scand J Rheumatol. 2004;33:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Georgiadis AN, Papavasiliou EC, Lourida ES, Alamanos Y, Kostara C, Tselepis AD, Drosos AA. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment--a prospective, controlled study. Arthritis Res Ther. 2006;8:R82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Toms TE, Panoulas VF, John H, Douglas KM, Kitas GD. Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60- more than just an anti-inflammatory effect? A cross sectional study. Arthritis Res Ther. 2009;11:R110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Park YB, Choi HK, Kim MY, Lee WK, Song J, Kim DK, Lee SK. Effects of antirheumatic therapy on serum lipid levels in patients with rheumatoid arthritis: a prospective study. Am J Med. 2002;113:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Dessein PH, Joffe BI, Stanwix AE. Effects of disease modifying agents and dietary intervention on insulin resistance and dyslipidemia in inflammatory arthritis: a pilot study. Arthritis Res. 2002;4:R12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Segal R, Yaron M, Tartakovsky B. Methotrexate: mechanism of action in rheumatoid arthritis. Semin Arthritis Rheum. 1990;20:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Cronstein B, Reiss A. Immunologic reactants in the pathogenesis of atherosclerosis in rheumatic diseases. Arthritis Res Ther. 2003;5 (Suppl 3):44. [DOI] [Full Text] |
| 37. | Morita Y, Fukazawa T, Hirashima M, Kaga K, Kusaoi M, Morita T, Touyama S, Morita K, Takasaki Y, Hashimoto H. The effect of methotrexate (MTX) on expression of signalling lymphocytic activation molecule (SLAM) in patients with rheumatoid arthritis (RA) and its role in the regulation of cytokine production. Scand J Rheumatol. 2006;35:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Giollo A, Bissell LA, Buch MH. Cardiovascular outcomes of patients with rheumatoid arthritis prescribed disease modifying anti-rheumatic drugs: a review. Expert Opin Drug Saf. 2018;17:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Osiri M, Shea B, Robinson V, Suarez-Almazor M, Strand V, Tugwell P, Wells G. Leflunomide for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2003;2002:CD002047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 42. | Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614-7620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 589] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 43. | Pateras I, Giaginis C, Tsigris C, Patsouris E, Theocharis S. NF-κB signaling at the crossroads of inflammation and atherogenesis: searching for new therapeutic links. Expert Opin Ther Targets. 2014;18:1089-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, Kim BH, Duess MA, Kluge MA, Levit A, Keaney JF Jr, Vita JA, Hamburg NM. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med. 2012;17:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Atzeni F, Turiel M, Caporali R, Cavagna L, Tomasoni L, Sitia S, Sarzi-Puttini P. The effect of pharmacological therapy on the cardiovascular system of patients with systemic rheumatic diseases. Autoimmun Rev. 2010;9:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Haagsma CJ, Blom HJ, van Riel PL, van't Hof MA, Giesendorf BA, van Oppenraaij-Emmerzaal D, van de Putte LB. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann Rheum Dis. 1999;58:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Restrepo JF, Del Rincon I, Molina E, Battafarano DF, Escalante A. Use of Hydroxychloroquine Is Associated With Improved Lipid Profile in Rheumatoid Arthritis Patients. J Clin Rheumatol. 2017;23:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Wahlin B, Braune A, Jönsson E, Wållberg-Jonsson S, Bengtsson C. Beneficial effects of hydroxychloroquine on blood lipids and glycated haemoglobin: A randomised interventional study in patients with rheumatoid arthritis and systemic lupus erythematosus. PLoS One. 2024;19:e0312546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 49. | Solomon DH, Garg R, Lu B, Todd DJ, Mercer E, Norton T, Massarotti E. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res (Hoboken). 2014;66:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care. 2016;39 Suppl 2:S244-S252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 51. | Shukla AM, Bose C, Karaduta OK, Apostolov EO, Kaushal GP, Fahmi T, Segal MS, Shah SV. Impact of Hydroxychloroquine on Atherosclerosis and Vascular Stiffness in the Presence of Chronic Kidney Disease. PLoS One. 2015;10:e0139226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Yang DH, Leong PY, Sia SK, Wang YH, Wei JC. Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. J Clin Med. 2019;8:796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Müller-Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis. 2017;76:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 54. | Frostegård J. Systemic lupus erythematosus and cardiovascular disease. J Intern Med. 2023;293:48-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 55. | Wong SK. Repurposing New Use for Old Drug Chloroquine against Metabolic Syndrome: A Review on Animal and Human Evidence. Int J Med Sci. 2021;18:2673-2688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Cordova Sanchez A, Khokhar F, Olonoff DA, Carhart RL. Hydroxychloroquine and Cardiovascular Events in Patients with Rheumatoid Arthritis. Cardiovasc Drugs Ther. 2024;38:297-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Michaud V, Dow P, Al Rihani SB, Deodhar M, Arwood M, Cicali B, Turgeon J. Risk Assessment of Drug-Induced Long QT Syndrome for Some COVID-19 Repurposed Drugs. Clin Transl Sci. 2021;14:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Tselios K, Deeb M, Gladman DD, Harvey P, Urowitz MB. Antimalarial-induced cardiomyopathy: a systematic review of the literature. Lupus. 2018;27:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Kaplan MJ. Cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Zimmerman MA, Selzman CH, Reznikov LL, Miller SA, Raeburn CD, Emmick J, Meng X, Harken AH. Lack of TNF-alpha attenuates intimal hyperplasia after mouse carotid artery injury. Am J Physiol Regul Integr Comp Physiol. 2002;283:R505-R512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Blann AD, McCollum CN. Increased levels of soluble tumor necrosis factor receptors in atherosclerosis: no clear relationship with levels of tumor necrosis factor. Inflammation. 1998;22:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Elkind MS, Cheng J, Boden-Albala B, Rundek T, Thomas J, Chen H, Rabbani LE, Sacco RL. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke. 2002;33:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 646] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 65. | Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 66. | Hürlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Béchir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Lüscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 439] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 67. | Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 954] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 68. | Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 692] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 69. | Gonzalez-Juanatey C, Llorca J, Vazquez-Rodriguez TR, Diaz-Varela N, Garcia-Quiroga H, Gonzalez-Gay MA. Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor alpha blocker therapy. Arthritis Rheum. 2008;59:1821-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Vlachopoulos C, Gravos A, Georgiopoulos G, Terentes-Printzios D, Ioakeimidis N, Vassilopoulos D, Stamatelopoulos K, Tousoulis D. The effect of TNF-a antagonists on aortic stiffness and wave reflections: a meta-analysis. Clin Rheumatol. 2018;37:515-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 71. | Faria AP, Ritter AMV, Santa-Catharina A, Souza DP, Naseri EP, Bertolo MB, Pioli MR, Carvalho CC, Modolo R, Moreno H. Effects of Anti-TNF alpha Therapy on Blood Pressure in Resistant Hypertensive Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Arq Bras Cardiol. 2021;116:443-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 746] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 73. | Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1219] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 74. | Manfredi AA, Baldini M, Camera M, Baldissera E, Brambilla M, Peretti G, Maseri A, Rovere-Querini P, Tremoli E, Sabbadini MG, Maugeri N. Anti-TNFα agents curb platelet activation in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 76. | Gabay C, McInnes IB, Kavanaugh A, Tuckwell K, Klearman M, Pulley J, Sattar N. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1806-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | Alsulaim T, Alhassan N, Khalil H, Almutlaq A. Tocilizumab Effect on Lipid Profile in Correlation to Cardiovascular Events: A Retrospective Cohort Study. Int J Rheumatol. 2021;2021:5535486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Toussirot E, Marotte H, Mulleman D, Cormier G, Coury F, Gaudin P, Dernis E, Bonnet C, Damade R, Grauer JL, Abdesselam TA, Guillibert-Karras C, Lioté F, Hilliquin P, Sacchi A, Wendling D, Le Goff B, Puyraveau M, Dumoulin G. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: a 12-month multicentre study. Arthritis Res Ther. 2020;22:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Emery P, Rondon J, Parrino J, Lin Y, Pena-Rossi C, van Hoogstraten H, Graham NMH, Liu N, Paccaly A, Wu R, Spindler A. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58:849-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Feist E, Fleischmann RM, Fatenejad S, Bukhanova D, Grishin S, Kuzkina S, Luggen M, Nasonov E, Samsonov M, Smolen JS. Olokizumab plus methotrexate: safety and efficacy over 106 weeks of treatment. Ann Rheum Dis. 2024;83:1454-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Burmester GR, Lin Y, Patel R, van Adelsberg J, Mangan EK, Graham NM, van Hoogstraten H, Bauer D, Ignacio Vargas J, Lee EB. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76:840-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 82. | Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022;387:715-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 83. | Genovese MC, Durez P, Fleischmann R, Tanaka Y, Furst D, Yamanaka H, Korneva E, Vasyutin I, Takeuchi T. Long-term safety and efficacy of olokizumab in patients with rheumatoid arthritis and inadequate response to tumor necrosis factor inhibitor therapy in phase II studies. Eur J Rheumatol. 2021;8:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 85. | Zheng ZH, Zeng X, Nie XY, Cheng YJ, Liu J, Lin XX, Yao H, Ji CC, Chen XM, Jun F, Wu SH. Interleukin-1 blockade treatment decreasing cardiovascular risk. Clin Cardiol. 2019;42:942-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, Cantatore FP, Carlino G, D'Abrosca V, Frassi M, Frediani B, Iacono D, Liakouli V, Maggio R, Mulè R, Pantano I, Prevete I, Sinigaglia L, Valenti M, Viapiana O, Cipriani P, Giacomelli R. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. 2019;16:e1002901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 87. | Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 88. | Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4997] [Cited by in RCA: 7156] [Article Influence: 795.1] [Reference Citation Analysis (0)] |
| 89. | Interleukin 1 Genetics Consortium. Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2015;3:243-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Provan SA, Berg IJ, Hammer HB, Mathiessen A, Kvien TK, Semb AG. The Impact of Newer Biological Disease Modifying Anti-Rheumatic Drugs on Cardiovascular Risk Factors: A 12-Month Longitudinal Study in Rheumatoid Arthritis Patients Treated with Rituximab, Abatacept and Tociliziumab. PLoS One. 2015;10:e0130709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Mathieu S, Pereira B, Dubost JJ, Lusson JR, Soubrier M. No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology (Oxford). 2012;51:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Kerekes G, Soltész P, Dér H, Veres K, Szabó Z, Végvári A, Szegedi G, Shoenfeld Y, Szekanecz Z. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. 2009;28:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis. 2013;72:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Kang EH, Jin Y, Brill G, Lewey J, Patorno E, Desai RJ, Kim SC. Comparative Cardiovascular Risk of Abatacept and Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis With and Without Diabetes Mellitus: A Multidatabase Cohort Study. J Am Heart Assoc. 2018;7:e007393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 95. | Shih PC, Lai CC, Zou QH, Wang SI, Huang XY, Wei JCC. Abatacept versus tumor necrosis factor inhibitors on mortality and medical utilizations in the treatment of rheumatoid arthritis associated interstitial lung disease: a large-scale real-world retrospective cohort study. Clin Exp Med. 2024;24:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 96. | Mathieu S, Couderc M, Glace B, Pereira B, Tournadre A, Dubost JJ, Soubrier M. Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics. 2013;7:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Emery P, Pope JE, Kruger K, Lippe R, DeMasi R, Lula S, Kola B. Efficacy of Monotherapy with Biologics and JAK Inhibitors for the Treatment of Rheumatoid Arthritis: A Systematic Review. Adv Ther. 2018;35:1535-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 98. | Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, Germino R, Menon S, Sun Y, Wang C, Shapiro AB, Kanik KS, Connell CA; ORAL Surveillance Investigators. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 1178] [Article Influence: 294.5] [Reference Citation Analysis (0)] |
| 99. | Desai RJ, Pawar A, Weinblatt ME, Kim SC. Comparative Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Receiving Tofacitinib Versus Those Receiving Tumor Necrosis Factor Inhibitors: An Observational Cohort Study. Arthritis Rheumatol. 2019;71:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 100. | Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, Winthrop KL, Charles-Schoeman C, Wang L, Chen C, Kwok K, Biswas P, Shapiro A, Madsen A, Wollenhaupt J. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020;6:e001395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 101. | Smolen JS, Genovese MC, Takeuchi T, Hyslop DL, Macias WL, Rooney T, Chen L, Dickson CL, Riddle Camp J, Cardillo TE, Ishii T, Winthrop KL. Safety Profile of Baricitinib in Patients with Active Rheumatoid Arthritis with over 2 Years Median Time in Treatment. J Rheumatol. 2019;46:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 102. | Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, Zhang Y, Khan N, Hendrickson B, Enejosa JV, Burmester GR. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2021;80:304-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 103. | Lee YH, Song GG. Impact of Janus kinase inhibitors on the risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2020;79:e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Szekanecz Z, Buch MH, Charles-Schoeman C, Galloway J, Karpouzas GA, Kristensen LE, Ytterberg SR, Hamar A, Fleischmann R. Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician. Nat Rev Rheumatol. 2024;20:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 105. | Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, Issa M, Salinas CA, Saifan C, Zhang X, Cardoso A, González-Gay MA, Takeuchi T. Cardiovascular Safety During Treatment With Baricitinib in Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71:1042-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 106. | Fleischmann R, Curtis JR, Charles-Schoeman C, Mysler E, Yamaoka K, Richez C, Palac H, Dilley D, Liu J, Strengholt S, Burmester G. Safety profile of upadacitinib in patients at risk of cardiovascular disease: integrated post hoc analysis of the SELECT phase III rheumatoid arthritis clinical programme. Ann Rheum Dis. 2023;82:1130-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 107. | Winthrop KL, Tanaka Y, Takeuchi T, Kivitz A, Matzkies F, Genovese MC, Jiang D, Chen K, Bartok B, Jahreis A, Besuyen R, Burmester GR, Gottenberg JE. Integrated safety analysis of filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 1.6 years. Ann Rheum Dis. 2022;81:184-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 108. | Alves C, Penedones A, Mendes D, Marques FB. Risk of Cardiovascular and Venous Thromboembolic Events Associated With Janus Kinase Inhibitors in Rheumatoid Arthritis: A Systematic Review and Network Meta-analysis. J Clin Rheumatol. 2022;28:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/