Published online Dec 26, 2025. doi: 10.4330/wjc.v17.i12.110450
Revised: July 25, 2025
Accepted: November 7, 2025
Published online: December 26, 2025
Processing time: 201 Days and 4.3 Hours
Observational studies reported characteristics and outcomes of patients with secondary mitral valve regurgitation (MR) who underwent transcatheter edge-to-edge repair of the mitral valve. No study investigated the temporal trend of patient characteristics and outcomes in comparison with the published ran
To investigate the temporal trend in baseline characteristics and outcomes of patients with secondary MR who underwent transcatheter edge-to-edge repair of the mitral valve in the real world compared with those from the published landmark trials.
A comprehensive systematic literature search was conducted using MEDLINE, EMBASE, and CENTRAL databases, and the identified observational studies were divided into two five-year recruitment periods. The first period included 36 studies that enrolled patients between 2008 and 2012, and the second period included 25 studies that recruited patients between 2013 and 2017-2018. Pooled variables of each five-year recruitment period were compared with those of the landmark trials. A random-effects model was used for statistical comparisons. RStudio and RevMan software were used for the analysis.
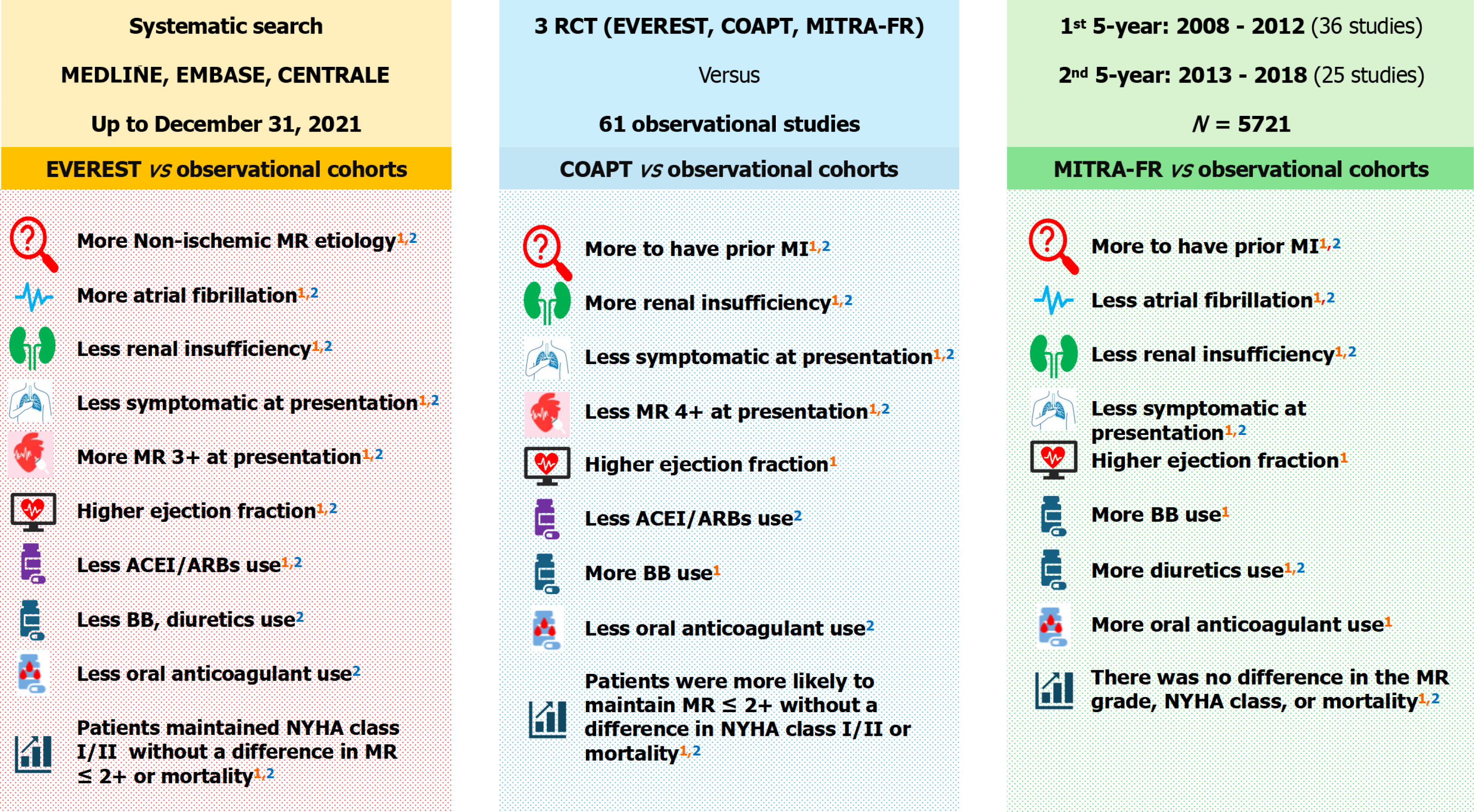
Overall, there were no major variations in the findings between the first and the second five-year recruitment periods. EVEREST program vs observational studies: Patients in the EVEREST program were more likely to have non-ischemic MR etiology [odds ratio (OR) = 3.59, 95% confidence interval (CI): 2.92-4.42] and atrial fibrillation (OR = 1.71, 95%CI: 1.42-2.06). They were less likely to receive angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (OR = 0.72, 95%CI: 0.58-0.90) and implantable cardiac device (OR = 0.41, 95%CI: 0.33-0.49) as well as less likely to be symptomatic at hospital presentation without a difference in MR grade ≤ 2+ or mortality at 12-month follow-up. COAPT trial vs observational studies: COAPT patients were more likely to have prior myocardial infarction (OR = 1.62, 95%CI: 1.27-2.06) and renal insufficiency (OR = 2.66, 95%CI: 2.05-3.45). They were more likely to receive beta-blockers (OR = 2.54, 95%CI: 1.68-3.85) and an implanted cardiac device (OR = 2.20, 95%CI: 1.71-2.84). There was no difference in procedure success or mortality. MITRA-FR trial vs observational studies: MITRA-FR patients were less likely to have atrial fibrillation (OR = 0.49, 95%CI: 0.34-0.69) and renal insufficiency (OR = 0.18, 95%CI: 0.11-0.28) but more likely to have a history of myocardial infarction (OR = 1.48, 95%CI: 1.06-2.05) and to receive diuretics (OR = 19.81, 95%CI: 2.75-142.48) and implantable cardiac devices (OR = 1.69, 95%CI: 1.21-2.37). At hospital presentation, they were less likely to be symptomatic (OR = 0.25, 95%CI: 0.18-0.35) without a difference in MR grades 3+ and 4+. There was no difference in terms of MR grade or mortality at 12-month follow-up.
Patients in the landmark studies may have favourable or unfavourable characteristics when compared to those in the observational studies, but this did not translate into different outcomes over time.
Core Tip: Patients with secondary mitral valve regurgitation from the real world who underwent transcatheter edge-to-edge repair of the mitral valve may show different characteristics and outcomes from those recruited in the landmark trials. Patients from the landmark trials may have overall favorable characteristics, but this did not translate into better outcomes over time. There were no major variations over time in terms of characteristics and outcomes when comparing patients from real-world and those from the landmark trials.
- Citation: Kaddoura R, Dakhil Z, Al-Badriyeh D, Abushanab D, Rafie I, Al-Hijji M. Temporal trends in characteristics and outcomes of patients undergoing percutaneous mitral valve repair. World J Cardiol 2025; 17(12): 110450
- URL: https://www.wjgnet.com/1949-8462/full/v17/i12/110450.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i12.110450
Mitral valve regurgitation (MR) is considered the most common form of significant valvular disease in the United States. It affects 6.0% of the population older than 65 years old[1]. The overall prevalence of MR in the general population is approximately 2.0%[2]. The etiology of MR can be classified into primary (degenerative or organic) and secondary (functional)[2,3]. Primary MR is characterized by leaflet prolapse or flail leading to loss of leaflet tip or edge coaptation. Secondary MR, on the other hand, is the consequence of left ventricular or left atrial remodeling and dysfunction leading to coaptation loss due to leaflet tethering, preventing adequate leaflet tip or edge approximation[3]. Secondary MR is seen in approximately one-third of patients with chronic heart failure and contributes to the progression of the disease and a worsening prognosis[2,4]. In patients aged ≥ 50 years, yearly mortality rates for moderate to severe MR ranged from 3.0% to 6.0%[4]. Transcatheter edge-to-edge repair of the mitral valve (M-TEER) using MitraClip® has become an important management strategy for secondary MR to decrease MR severity by approximating the anterior and posterior leaflets of the valve[5]. Yet, there are controversial data from different MitraClip® trials that included patients with secondary MR. The Endovascular Valve Edge-to-Edge Repair Study II (EVEREST II) trial reported that at 12-month follow-up, M-TEER significantly reduced the primary composite endpoint in comparison with surgery. THE EVEREST II trial recruited only 27.0% of patients with secondary MR[6]. The EVEREST program enrolled patients with secondary MR from the EVEREST II trial, along with patients from the EVEREST II HR (EVEREST II high risk) registry, and the Multicenter Study of the MitraClip System registry. In the EVEREST program, M-TEER in patients with secondary MR was safe and effective in reducing MR severity, improving symptoms, and positively impacting ventricular remodeling[7]. In the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial, M-TEER significantly reduced the rates of primary (i.e., hospitalization for heart failure) and all 10 secondary endpoints in comparison with medical therapy at two-year follow-up[8]. However, these results were not supported by the Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR) trial, which did not find a difference in primary outcome (i.e., death or unplanned heart failure hospitalization) between M-TEER and medical therapy at 12-month follow-up[9]. The explanation for the differences between the results of the COAPT and MITRA-FR trials was multifaceted that suggesting aspects including geographical region, methodology, primary efficacy endpoint, follow-up period, MR severity, ventricular remodeling, and medical therapy. More specifically, the eligibility criteria of both trials were different[5]. In the MITRA-FR trial, MR severity criteria [i.e., effective regurgitant orifice area (EROA) ≥ 0.20 cm2 and/or regurgitant volume ≥ 30 mL] were different from those of the COAPT trial (i.e., EROA ≥ 0.30 cm2 and/or regurgitant volume ≥ 45 mL). Consequently, the COPAT trial enrolled patients with more severe MR (mean EROA: 0.41 ± 0.15 cm2) in comparison with the MITRA-FR trial (mean EROA: 0.31 ± 0.01 cm2). However, patients in the COAPT trial had less severe left ventricular disease, evidenced by a smaller left ventricular end-diastolic volume (mean 194.4 ± 69.2 vs mean 258.8 ± 71.1 mL, respectively)[8,9]. The latter difference was likely due to the exclusion of patients with very severe left ventricular dilation in the COAPT trial, if compared with the MITRA-FR trial, which did not have such limits. It has been reported that severe left ventricular dilation and dysfunction were associated with unfavourable outcomes such as reverse remodeling, recurrent MR, and less favourable outcomes after surgical procedure for ischemic MR; hence, potentially better outcomes were reported in the patients enrolled in the COAPT trial[5]. In our previous study, we compared patient characteristics and clinical outcomes in observational studies that were published in 2020 and 2021, i.e., after the publication of the COAPT and MITRA-FR trials, and recruited patients with secondary MR who underwent the MitraClip® procedure (Figure 1). Patients from real-world experience showed more favorable outcomes than those from the MITRA-FR trial and probably less favourable than those from the COAPT trial[5]. Although observational studies reported characteristics and outcomes of MR patients who underwent M-TEER, no study investigated the temporal trend of patient characteristics and outcomes in comparison with the published randomized trials[7-9]. Thus, this systematic review investigated this temporal trend in patients with secondary MR who underwent M-TEER with MitraClip® and explored whether patient characteristics and outcomes from observational studies have differed over the years prior to the publication of the COAPT and MITRA-FR trials.
This systematic review was conducted according to the Cochrane Handbook for Systematic Reviews, Preferred Reporting Items for Systematic Reviews and Meta-analyses statement, and Meta-analysis of Observational Studies in Epidemiology checklist[10-12]. The protocol was registered in the International Prospective Register of Systematic Reviews (No. PROSPERO 2022 CRD42022343248).
A systematic literature search was conducted by two independent authors using MEDLINE, Embase, and CENTRAL databases to identify the observational studies that were published up to December 31, 2021, to reduce the influence of the published landmark trials on the real-world practice, since their enrolment periods should be mostly before or shortly after the publication of landmark trials. The studies should recruit adult heart failure patients who underwent MitraClip® intervention for secondary or functional MR from the approval of the MitraClip® device (i.e., 2008) to the publication of COAPT and MITRA-FR trials (i.e., September 2018). Broad search terms were used, such as “MitraClip”, “transcatheter mitral valve repair”, “percutaneous mitral valve repair”, “MitraClip” AND “mitral valve”, and “edge-to-edge” AND “mitral valve”. Search limits included “Trial”, “Clinical Trial”, “Article”, and “Human”. Manual search by screening the reference lists of the included studies was performed to identify additional publications. The literature search strategy is detailed in Supplementary Table 1. Transcatheter mitral valve intervention using the MitraClip® device was the intervention group, irrespective of the presence of a comparison group. Exclusion criteria included studies that recruited 10 patients or fewer, were published in a non-English language, or used devices other than MitraClip®. Moreover, primary MR, MR of mixed aetiology, and specific patient population (e.g., fibroelastic deficiency, acute myocardial infarction, or cardiogenic shock) were also excluded. When there were studies that recruited patients from the same center or registry with potential overlapping or similar recruitment periods, the study with a larger sample size was considered in the analysis. In addition, if studies from the same centers were included, they may have reported different outcomes to be considered.
The literature search records were screened at the title and abstract levels. After eliminating the duplicate and irrelevant publications, potential abstracts were retrieved in full text. Study selection was conducted by two authors, and any discrepancy was resolved by discussion or involvement of a third author. The data that were extracted from the selected studies included study and patient characteristics, medical or device therapy, pre- and post-procedure echocardiographic measurements, and clinical outcomes.
For the temporal trend, aggregate data of baseline characteristics, procedural and clinical outcomes, or variables were aggregated on a year-by-year basis to present the variations over time. In addition, the studies were divided into two five-year recruitment periods before the publication of the COAPT and MITRA-FR trials (i.e., September 2018). The first five-year recruitment period included the studies that recruited patients between 2008 and 2012, and the second five-year recruitment period included the studies that enrolled patients between 2013 and 2017-2018. The pooled data of each five-year recruitment period were compared with those from the three main studies (EVEREST program, COAPT, and MITRA-FR)[7-9]. The variables from observational studies were compared with those from the randomised studies using a random-effects model. Odds ratio (OR) and mean difference (MD) with 95% confidence interval (CI) were used to compare categorical and continuous data, respectively. Sensitivity analysis was also performed for the additional studies with a long enrollment period, which overlap with the two five-year recruitment periods. Heterogeneity was examined by Q statistics and inconsistency factor (I2), with statistical significance for the Q statistic being a P-value of less than 0.1 and for the I2 value of more than 50%. A significance level of P-value of less than 0.05 was used. RStudio® and RevMan® software were used for non-comparative pooling of variables and comparative meta-analysis, respectively.
The literature search identified 130 observational studies that were retrieved in full text after removing duplicates and studies that did not meet the eligibility criteria. Fifty-four studies were eliminated due to potential duplicates of patients from similar centers or registries (Supplementary Table 2). Fifteen studies were only included in the sensitivity analysis due to having a long recruitment period, which overlaps with the five-year recruitment periods (Supplementary Table 3). Finally, 61 studies were included and divided into two five-year recruitment periods (Supplementary Figure 1). Supplementary Table 3 cites the 61 included studies and the 15 studies included in the sensitivity analyses. The first five-year recruitment period included 36 studies with recruitment periods between 2008 and 2012, and the second five-year recruitment period included 25 studies with recruitment periods between 2013 and 2017-2018. Most studies were published between 2008 and 2012 (approximately 10 studies per year).
The 61 studies recruited 5721 patients from 2008 to 2018 in different countries, mostly in Europe. All the studies reported the characteristics and outcomes of patients with secondary MR who underwent MitraClip® implantation, irrespective of the presence of a comparison group. General study characteristics are summarized in Supplementary Tables 4 and 5.
In the first and second five-year recruitment periods, the pooled mean age was 71.99 ± 0.16 and 73.10 ± 0.24 years, with more than half of the patients being males (67.5% and 66.5%), and had atrial fibrillation (51.7% and 58.1%). Most patients were on beta-blockers (84.3% and 87.2%) and diuretics (90.4% and 92.4%), with approximately half of them (49.4% and 48.8%) having an implantable cardiac device. At hospital presentation, most patients were symptomatic [89.8% and 90.6%; i.e., New York Heart Association (NYHA) class III/IV]with MR grade 3+ (40.5% and 57.3%) and grade 4+ (60.8% and 57%), and left ventricular ejection fraction (LVEF) of 25.81 ± 0.22% and 35.15 ± 0.17. With outcomes at 12-month follow-up, MR grade ≤ 2+ was reported in 85.1% and 87.4%, whereas all-cause mortality was reported in 20.0% and 18.1% of patients, respectively. The pooled variables of included studies in both five-year recruitment periods are shown in Tables 1, 2 and 3 and Supplementary Figures 2-24.
| Variable | Pooled real-life variables | EVEREST program, population size | EVEREST vs pooled variable, MD or OR (95%CI) | COAPT trial, population size | COAPT vs pooled variable, MD or OR (95%CI) | MITRA-FR trial, population size | MITRA-FR vs pooled variable, MD or OR (95%CI) |
| First five-year period | |||||||
| Age (year) | 71.99 ± 0.16; Q = 3.32 | 73.3 ± 10.5 (n = 616) | 1.40 (0.57-2.23) | 71.7 ± 11.8 (n = 302) | -0.20 (-1.53 to 1.13) | 70.1 ± 10.1 (n = 152) | -1.80 (-3.41 to |
| Male | 67.5% (62.4-72.5); I2 = 97 | 364/616 (59.1) | 0.97 (0.82-1.15) | 201/302 (66.6) | 1.33 (1.04-1.71) | 120/152 (78.9) | 2.51 (1.69-3.73) |
| Diabetes mellitus | 33.9% (30.2-37.6); I2 = 79 | 239/616 (38.8) | 1.20 (1.01-1.43) | 106/302 (35.1) | 1.03 (0.80-1.31) | 50/152 (32.9) | 0.93 (0.66-1.31) |
| Non-ischemic etiology | 35.3 (30.8-40.0); I2 = 57 | 407/615 (66.2) | 3.59 (2.92-4.42) | 118/302 (39.1) | 1.18 (0.91-1.53) | 57/152 (37.5) | 1.10 (0.77-1.56) |
| Prior myocardial infarction | 41.9% (35.9-48.0); I2 = 84 | 334/602 (55.5) | 1.89 (1.58-2.26) | 156/302 (51.7) | 1.62 (1.27-2.06) | 75/152 (49.3) | 1.48 (1.06-2.05) |
| Prior percutaneous coronary intervention | 42.3% (35.9-48.9); I2=95 | 292/615 (47.5) | 1.20 (1.01-1.43) | 130/302 (43.0) | 1.00 (0.79-1.27) | 71/152 (46.7) | 1.16 (0.84-1.61) |
| Atrial fibrillation | 51.7% (46.2-57.1); I2 = 92 | 359/552 (65.0) | 1.71 (1.42-2.06) | 173/302 (57.3) | 1.24 (0.98-1.57) | 49/142 (34.5) | 0.49 (0.34-0.69) |
| Renal insufficiency | 46.1% (35.4-56.9); I2 = 98 | 163/616 (26.5) | 0.38 (0.31-0.46) | 214/299 (71.6) | 2.66 (2.05-3.45) | 22/152 (14.5) | 0.18 (0.11-0.28) |
| ACEI/ARB | 69.8% (64.4-74.9); I2 = 57 | 390/616 (63.3) | 0.72 (0.58-0.90) | 204/302 (71.9) | 0.87 (0.66-1.16) | 111/152 (82.2) | 1.13 (0.77-1.67) |
| Beta-blocker | 84.3% (79.5-88.6); I2 = 77 | 491/616 (79.7) | 0.98 (0.78-1.24) | 275/302 (91.1) | 2.54 (1.68-3.85) | 134/152 (88.2) | 1.86 (1.12-3.09) |
| Diuretics | 90.4% (87.7-92.8); I2 = 51 | 543/616 (88.1) | 0.98 (0.98-1.31) | 270/302 (89.4) | 1.11 (0.74-1.65) | 151/152 (99.3) | 19.81 (2.75-142.48) |
| Oral anticoagulation agents | 50.0% (23.5-77.3); I2 = 95 | 253/616 (41.1) | 0.87 (0.66-1.15) | 140/302 (46.4) | 1.08 (0.78-1.48) | 93/152 (61.2) | 1.97 (1.32-2.92) |
| ICD device | 30.1% (21.7-39.2); I2 = 91 | 170/600 (28.3) | 0.92 (0.75-1.12) | 91/302 (30.1) | 1.00 (0.77-1.30) | 48/151 (31.8) | 1.08 (0.76-1.55) |
| Any cardiac device | 49.4% (38.6-60.1); I2 = 96 | 170/600 (28.3) | 0.41 (0.33-0.49) | 206/302 (68.1) | 2.20 (1.71-2.84) | 94/151 (62.3) | 1.69 (1.21-2.37) |
| Second five-year period | |||||||
| Age (year) | 73.10 ± 0.24; Q = 3.66 | 73.3 ± 10.5 (n = 616) | 0.20 (-0.63 to 1.03) | 71.7 ± 11.8 (n = 302) | -1.40 (-2.73 to -0.07) | 70.1 ± 10.1 (n = 152) | -3.00 (-4.61 to |
| Male | 66.5% (63.4-69.6); I2 = 56 | 364/616 (59.1) | 0.69 (0.58-0.83) | 201/302 (66.6) | 0.95 (0.74-1.23) | 120/152 (78.9) | 1.80 (1.21-2.68) |
| Diabetes mellitus | 30.8% (27.2-34.4); I2 = 56 | 239/616 (38.8) | 1.32 (1.10-1.58) | 106/302 (35.1) | 1.12 (0.87-1.44) | 50/152 (32.9) | 1.02 (1.02-1.44) |
| Non-ischemic etiology | 48.3% (38.1-58.6); I2 = 70 | 407/615 (66.2) | 2.18 (1.66-2.86) | 118/302 (39.1) | 0.71 (0.52-0.98) | 57/152 (37.5) | 0.67 (0.45-0.99) |
| Prior myocardial infarction | 24.9% (18.3-32.0); I2 = 79 | 334/602 (55.5) | 3.03 (2.51-3.66) | 156/302 (51.7) | 2.60 (2.03-3.33) | 75/152 (49.3) | 2.37 (1.70-3.31) |
| Prior percutaneous coronary intervention | 38.6% (32.7-44.7); I2 = 62 | 292/615 (47.5) | 1.53 (1.24-1.89) | 130/302 (43.0) | 1.28 (0.98-1.67) | 71/152 (46.7) | 1.49 (1.05-2.10) |
| Atrial fibrillation | 58.1% (51.2-64.8); I2 = 91 | 359/552 (65.0) | 1.40 (1.16-1.69) | 173/302 (57.3) | 1.01 (0.79-1.28) | 49/142 (34.5) | 0.40 (0.28-0.57) |
| Renal insufficiency | 47.8% (41.2-54.4); I2 = 67 | 163/616 (26.5) | 0.35 (0.28-0.42) | 214/299 (71.6) | 2.41 (1.85-3.15) | 22/152 (14.5) | 0.16 (0.10-0.26) |
| ACEI/ARB | 78.1% (72.4-83.3); I2 = 71 | 390/616 (63.3) | 0.46 (0.37-0.58) | 204/302 (71.9) | 0.56 (0.42-0.74) | 111/152 (82.2) | 0.72 (0.49-1.07) |
| Beta-blocker | 87.2% (84.5-89.6); I2 = 49 | 491/616 (79.7) | 0.67 (0.53-0.85) | 275/302 (91.1) | 1.74 (1.15-2.64) | 134/152 (88.2) | 1.27 (0.77-2.12) |
| Diuretics | 92.4% (89.8-94.8); I2 = 61 | 543/616 (88.1) | 0.61 (0.45-0.82) | 270/302 (89.4) | 0.69 (0.46-1.03) | 151/152 (99.3) | 12.38 (1.72-89.08) |
| Oral anticoagulation agents | 56.3% (26.7-83.7); I2 = 92 | 253/616 (41.1) | 0.48 (0.35-0.66) | 140/302 (46.4) | 0.60 (0.42-0.85) | 93/152 (61.2) | 1.09 (0.71-1.67) |
| ICD device | 33.9% (24.1-44.4); I2 = 87 | 170/600 (28.3) | 0.89 (0.71-1.12) | 91/302 (30.1) | 0.97 (0.73-1.29) | 48/151 (31.8) | 1.05 (0.72-1.52) |
| Any cardiac device | 48.8% (34.8-62.9); I2 = 96 | 170/600 (28.3) | 0.46 (0.38-0.56) | 206/302 (68.1) | 2.49 (1.93-3.21) | 94/151 (62.3) | 1.91 (1.36-2.68) |
| Variable | Pooled real-life variables | EVEREST program, population size | EVEREST vs pooled variable, MD or OR (95%CI) | COAPT trial, population size | COAPT vs pooled variable, MD or OR (95%CI) | MITRA-FR trial, population size | MITRA-FR vs pooled variable, MD or OR (95%CI) |
| First five-year period | |||||||
| NYHA II | 10.5 (7.2-14.2); I2 = 84 | 107/615 (17.4) | 1.60 (1.26-2.02) | 129/302 (42.7) | 5.66 (4.40-7.28) | 56/152 (36.8) | 4.43 (3.13-6.26) |
| NYHA III | 24.9 (18.3-32.0); I2 = 79 | 387/615 (62.9) | 4.13 (3.41-5.00) | 154/302 (50.9) | 2.53 (1.98-3.24) | 82/152 (53.9) | 2.85 (2.04-3.98) |
| NYHA IV | 23.3 (18.9-27.9); I2 = 84 | 108/615 (17.6) | 0.77 (0.62-0.96) | 18/302 (5.9) | 0.23 (0.14-0.37) | 14/152 (9.2) | 0.37 (0.21-0.64) |
| NYHA III/IV | 89.8 (86.2-92.9); I2 = 87 | 495/615 (80.5) | 0.60 (0.48-0.75) | 172/302 (57.0) | 0.19 (0.15-0.25) | 96/152 (63.1) | 0.25 (0.18-0.35) |
| MR grade 3+ | 40.5% (31.3-50.0); I2 = 97 | 355/616 (58.1) | 2.21 (1.86-2.63) | 148/302 (49.0) | 0.95 (0.74-1.22) | 46/123 (37.4) | 0.97 (0.67-1.41) |
| MR grade 4+ | 60.8% (52.4-68.9); I2 = 96 | 139/616 (22.7) | 0.17 (0.14-0.20) | 154/302 (51.0) | 0.59 (0.47-0.75) | 76/123 (61.8) | 0.92 (0.64-1.34) |
| LVEDV (ml) | 177.34 ± 1.22; Q = 7.73 | 162.2 ± 52.8 (558) | -15.10 (-19.48 to -10.72) | 194.4 ± 69.2 (n = 302) | 17.10 (9.30-24.90) | 258.8 ± 71.11 (n = 152) | 80.70 (69.40-92.00) |
| LVEF (%) | 25.81 ± 0.22; Q = 50.71 | 43.2 ± 11.7 (558) | 17.40 (16.43-18.37) | 31.3 ± 9.1 (n = 302) | 5.50 (4.47-6.53) | 33.3 ± 6.5 (n = 152) | 7.50 (6.47-8.53) |
| Second five-year period | |||||||
| NYHA II | 11.2 (5.4-18.6); I2 = 93 | 107/615 (17.4) | 1.11 (0.87-1.41) | 129/302 (42.7) | 3.93 (3.03-5.09) | 56/152 (36.8) | 3.07 (2.16-4.37) |
| NYHA III | 68.9 (60.4-76.8); I2 = 90 | 387/615 (62.9) | 0.88 (0.72-1.08) | 154/302 (50.9) | 0.54 (0.42-0.70) | 82/152 (53.9) | 0.61 (0.43-0.85) |
| NYHA IV | 21.8 (14.6-29.9); I2 = 86 | 108/615 (17.6) | 0.69 (0.54-0.89) | 18/302 (5.9) | 0.21 (0.13-0.34) | 14/152 (9.2) | 0.33 (0.19-0.58) |
| NYHA III/IV | 90.6 (84.1-95.7); I2 = 94 | 495/615 (80.5) | 0.69 (0.55-0.87) | 172/302 (57.0) | 0.22 (0.17-0.29) | 96/152 (63.1) | 0.29 (0.20-0.41) |
| MR grade 3+ | 57.3 (37.6-75.8); I2 = 98 | 355/616 (58.1) | 2.27 (1.90-2.73) | 148/302 (49.0) | 1.61 (1.26-2.04) | 46/123 (37.4) | 1.00 (0.69-1.45) |
| MR grade 4+ | 57 (36.3-76.5); I2 = 98 | 139/616 (22.7) | 0.15 (0.13-0.19) | 154/302 (51.0) | 0.55 (0.43-0.70) | 76/123 (61.8) | 0.86 (0.59-1.25) |
| LVEDV (ml) | 155.38 ± 0.31; Q = 8.26 | 162.2 ± 52.8 (558) | 6.80 (2.42-11.18) | 194.4 ± 69.2 (n = 302) | 39.00 (31.20-46.80) | 258.8 ± 71.11 (n = 152) | 102.60 (91.30-113.90) |
| LVEF | 35.15 ± 0.17; Q = 15.97 | 43.2 ± 11.7 (558) | 8.00 (7.03-8.97) | 31.3 ± 9.1 (n = 302) | -3.90 (-4.93, -2.87) | 33.3 ± 6.5 (n = 152) | -1.90 (-2.93 to |
| Variable | Pooled real-life variables | EVEREST program, population size | EVEREST vs pooled variable, MD or OR (95%CI) | COAPT trial, population size | COAPT vs pooled variable, MD or OR (95%CI) | MITRA-FR trial, population size | MITRA-FR vs pooled variable, MD or OR (95%CI) |
| First five-year period | |||||||
| Post procedure | |||||||
| One device implanted | 50.6 (41.2-60.0); I2 = 92 | - | - | 106/293 (36.2) | 0.78 (0.61-1.00) | 63/138 (45.7) | 0.83 (0.59-1.18) |
| Two devices implanted | 42.4 (35.2-49.8); I2 = 88 | - | - | 157/293 (53.6) | 1.56 (1.22-1.99) | 62/138 (44.9) | 1.10 (0.78-1.56) |
| Three or more devices implanted | 4.2 (2.9-5.7); I2 = 14 | - | - | 24/293 (8.2) | 1.96 (1.19-3.21) | 13/138 (9.4) | 2.28 (1.21-4.29) |
| MR grade 1+ | 56.1 (48.5-63.5); I2 = 90 | 307/589 (52.1) | 0.82 (0.69-0.98) | 214/260 (82.3) | 3.51 (2.53-4.86) | 54/123 (43.9) | 0.59 (0.41-0.85) |
| MR grade 2+ | 39.5 (33.0-46.2); I2 = 87 | 215/589 (36.5) | 1.02 (0.84-1.22) | 33/260 (12.7) | 0.26 (0.18-0.37) | 23/123 (18.7) | 0.41 (0.26-0.64) |
| MR grade ≤ 2+ | 93.2 (89.6-96.2); I2 = 90 | 523/589 (88.8) | 0.58 (0.44-0.78) | 247/260 (95.0) | 1.40 (0.79-2.48) | 115/123 (93.5) | 1.06 (0.71-2.19) |
| MR grade ≥ 3+ | 8.2 (5.4-11.6); I2 = 87 | 66/589 (11.2) | 1.43 (1.07-1.91) | 13/260 (5.0) | 0.60 (0.34-1.06) | 8/123 (6.25) | 0.79 (0.38-1.64) |
| At follow-up | |||||||
| NYHA I/II at 12 months | 74.4 (68.6-79.9); I2 = 77 | 511/616 (83.0) | 1.74 (1.38-2.19) | 171/237 (72.2) | 0.931 (0.69-1.25) | 76/112 (67.8) | 0.76 (0.50-1.13) |
| MR grade ≤ 2+ at 12 months | 85.1 (79.7-89.8); I2 = 88 | 349/413 (84.5) | 1.20 (0.90-1.60) | 199/210 (94.8) | 3.98 (2.15-7.37) | 79/97 (81.4) | 0.97 (0.57-1.63) |
| All-cause mortality within 12 months | 20 (15.7-24.6); I2 = 74 | 138/616 (22.4) | 1.15 (0.92-1.44) | 57/302 (19.1) | 0.92 (0.68-1.26) | 37/152 (24.3) | 1.28 (0.87-1.89) |
| Second five-year period | |||||||
| Post procedure | |||||||
| One device implanted | 46.6 (40.6-52.5); I2 = 86 | - | - | 106/293 (36.2) | 0.74 (0.57-0.95) | 63/138 (45.7) | 1.09 (0.77-1.55) |
| Two devices implanted | 46.9 (43.2-50.7); I2 = 64 | - | - | 157/293 (53.6) | 1.22 (0.96-1.56) | 62/138 (44.9) | 0.86 (0.61-1.22) |
| Three or more devices implanted | 6.3 (3.2-10.2); I2 = 81 | - | - | 24/293 (8.2) | 1.04 (0.66-1.62) | 13/138 (9.4) | 1.21 (0.67-2.19) |
| MR grade 1+ | 57.7 (49.4-65.7); I2 = 82 | 307/589 (52.1) | 0.65 (0.54-0.78) | 214/260 (82.3) | 2.76 (1.98-3.84) | 54/123 (43.9) | 0.46 (0.32-0.67) |
| MR grade 2+ | 24.9 (18.3-32.0); I2 = 79 | 215/589 (36.5) | 1.40 (1.15-1.70) | 33/260 (12.7) | 0.35 (0.24-0.52) | 23/123 (18.7) | 0.56 (0.35-0.89) |
| MR grade ≤ 2+ | 89.7 (82.6-95.3); I2 = 90 | 523/589 (88.8) | 0.60 (0.44-0.81) | 247/260 (95.0) | 1.43 (0.80-2.56) | 115/123 (93.5) | 1.08 (0.52-2.26) |
| MR grade ≥ 3+ | 11.4 (2.9-23.6); I2 = 90 | 66/589 (11.2) | 1.38 (0.97-1.96) | 13/260 (5.0) | 0.57 (0.31-1.05) | 8/123 (6.25) | 0.76 (0.36-1.62) |
| At follow-up | |||||||
| NYHA I/II at 12 months | 75.1 (56.6-90.0); I2 = 93 | 511/616 (83.0) | 1.54 (1.16-2.03) | 171/237 (72.2) | 0.82 (0.58-1.15) | 76/112 (67.8) | 0.76 (0.43-1.03) |
| MR grade ≤ 2+ at 12 months | 87.4 (81.1-92.6); I2 = 83 | 349/413 (84.5) | 0.73 (0.52-1.02) | 199/210 (94.8) | 2.41 (1.27-4.58) | 79/97 (81.4) | 0.58 (0.34-1.02) |
| All-cause mortality within 12 months | 18.1 (11.5-25.8); I2 = 86 | 138/616 (22.4) | 1.14 (0.90-1.46) | 57/302 (19.1) | 0.92 (0.67-1.28) | 37/152 (24.3) | 1.28 (0.85-1.91) |
In the first five-year recruitment period, patients in the EVEREST program were more likely to have non-ischemic MR etiology (OR = 3.59, 95%CI: 2.92-4.42) and atrial fibrillation (OR = 1.71, 95%CI: 1.42-2.06). However, they were less likely to have renal insufficiency (OR = 0.38, 95%CI: 0.31-0.46), receive angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEI/ARB) (OR = 0.72, 95%CI: 0.58-0.90), and have an implantable cardiac device (OR = 0.41, 95%CI: 0.33-0.49). In the second five-year recruitment period, there was no change from the former comparisons, but also patients in the EVEREST program were less likely to receive beta-blockers (OR = 0.67, 95%CI: 0.53-0.85), diuretics (OR = 0.61, 95%CI: 0.45-0.82), and oral anticoagulation (OR = 0.48, 95%CI: 0.35-0.66) (Table 1). In both five-year recruitment periods, patients in the EVEREST program were less likely to be symptomatic at hospital presentation (i.e., NYHA III/IV) and have MR grade 4+ with higher LVEF (Table 2).
In the first five-year recruitment period, COAPT patients were more likely to have prior myocardial infarction (OR = 1.62, 95%CI: 1.27-2.06) and renal insufficiency (OR = 2.66, 95%CI: 2.05-3.45). They were more likely to receive beta-blockers (OR = 2.54, 95%CI: 1.68-3.85) and implanted cardiac device (OR = 2.20, 95%CI: 1.71-2.84) but less likely to receive ACEI/ARB (OR = 0.87, 95%CI: 0.66-1.16) (Table 1). In the second five-year recruitment period, there was no change from the former comparisons. In both periods, COAPT patients were less likely to present to the hospital with severe heart failure symptoms (i.e., NYHA class III/IV) and severe MR (i.e., grade 4+) (Table 2).
In both periods, MITRA-FR patients were less likely to have atrial fibrillation (OR = 0.49, 95%CI: 0.34-0.69) and renal insufficiency (OR = 0.18, 95%CI: 0.11-0.28). They were more likely to have a history of myocardial infarction (OR = 1.48, 95%CI: 1.06-2.05), receive diuretics (OR = 19.81, 95%CI: 2.75-142.48), and have an implantable cardiac device (OR = 1.69, 95%CI: 1.21-2.37) (Table 1). At hospital presentation, they were less likely to be symptomatic (i.e., NYHA class III/IV; OR = 0.25, 95%CI: 0.18-0.35) without a difference in MR grades 3+ and 4+ (Table 2).
EVEREST program vs observational studies: In the first five-year recruitment period, although the patients in the EVEREST program were less likely to have MR grade ≤ 2+ (OR = 0.58, 95%CI: 0.44-0.78) post procedure, they maintained NYHA class I/II (OR = 1.74, 95%CI: 1.38-2.19) without a difference in MR grade ≤ 2+ or mortality at 12-month follow-up. In the subsequent period, there was no change from the former comparisons (Table 3).
COAPT trial vs observational studies: In both periods, although there was no difference in the procedure success (i.e., MR grade ≤ 2+), COAPT patients were more likely to maintain MR grade ≤ 2+ without a difference in NYHA class I/II or mortality at 12-month follow-up (Table 3).
MITRA-FR trial vs observational studies: In the first period, MITRA-FR patients were less likely to have MR grade 1+ (OR = 0.59, 95%CI: 0.41-0.85) and 2+ (OR = 0.41, 95%CI: 0.26-0.64) post procedure without a difference in the MR grade, NYHA class, or mortality within 12-month follow-up. In the second period, there was no major change from the previous comparisons of the first period (Table 3).
The temporal trend for selected variables according to the start of the recruitment year showed variations over the years. The proportion of males notably varied over time with an overall downtrend. The non-ischemic etiology of MR and NYHA class III/IV presentation at baseline showed subtle variations over time with a subtle overall downtrend. Whereas, medications at baseline (i.e., ACEI/ARB and beta-blockers) showed a subtle overall uptrend. The proportions of MR grades 3+ and 4+ notably varied over time. Procedure success at follow-up showed subtle variations over time with a subtle overall uptrend, unlike mortality, which showed notable variations over time. Supplementary Figures 25-44 demonstrate the pooled variables per start of recruitment year, and Supplementary Figures 45 and 46 show a graphical presentation of each selected variable over the years.
Sensitivity analysis was performed for the additional 15 studies with a long enrollment period, which overlaps with the two five-year recruitment periods. The comparisons between patients in the EVEREST program and those in the observational studies were consistent with the comparisons in both five-year recruitment periods in terms of baseline characteristics (non-ischemic etiology, atrial fibrillation, and renal insufficiency), hospital presentation (NYHA class III/IV and MR grade 4+), and outcomes (procedure success, NYHA class, and mortality) but not in terms of medications at baseline (ACEI/ARB, beta-blockers, diuretics, and anticoagulation therapy). The comparisons between the COAPT patients and those in the observational studies were consistent with the comparisons in both five-year recruitment periods in terms of baseline characteristics (prior myocardial infarction and renal insufficiency), receiving implanted cardiac devices and beta-blockers, but not ACEI/ARB, and hospital presentation (NYHHA class III/IV and MR grade 4+). However, there were discrepancies in terms of less favorable outcomes (procedural success, NYHA class I/II, and mortality). The comparisons between the MITRA-FR patients and those in the observational studies were consistent with the comparisons in both five-year recruitment periods in terms of baseline characteristics (prior myocardial infarction and renal insufficiency), receiving diuretics and implanted cardiac devices and beta-blockers, and severity of MR (grades 3+ and 4+). There were discrepancies in terms of less favorable outcomes at follow-up in terms of procedural success, NYHA class I/II, and mortality (Supplementary Tables 6-8).
This systematic review investigated this temporal trend in characteristics and outcomes of patients with secondary MR who underwent M-TEER with MitraClip® and compared them with those from the published landmark randomized trials. In comparison with patients in the observational studies, patients in the EVEREST program were less likely to receive ACEI/ARB and implantable cardiac devices and less likely to have MR grade ≤ 2+ post procedure, but without a difference in MR grade ≤ 2+ or mortality at follow-up. COAPT patients were more likely to receive beta-blockers and implanted cardiac devices and less likely to present to the hospital with severe heart failure symptoms and severe MR without a difference in mortality at follow-up. Mitra-FR patients were more likely to receive diuretics and implantable cardiac devices, but they were less likely to be symptomatic at hospital presentation and without a difference in severe MR grades or mortality at follow-up. In general, there were no major variations in the findings between the first and the second five-year recruitment periods. Overall, patients in the randomized trials, namely the COAPT and MITRA-FR, might have favourable characteristics, but this was not reflected as an improvement in outcomes over time. To the best of our knowledge, this is the first meta-analysis that assessed the temporal trend in patient characteristics and outcomes in pooled observational cohorts of patients with secondary MR who underwent M-TEER.
The evolution of several aspects, such as patient selection, procedural optimization, and technological refinement in the M-TEER procedure, is evident in the differences observed between early trials such as EVEREST II and more recent trials such as MITRA-FR and COAPT, especially when compared with observational cohorts. These differences, particularly in baseline characteristics and outcomes, underscore the dynamic nature of the M-TEER procedure for treating secondary MR[5]. Initially, the EVEREST II trial population had predominantly primary MR and non-ischemic in nature, along with less symptomatic heart failure and more preserved left ventricular function[6]. Whereas, the population of the COAPT and MITRA-FR trials had secondary MR with more advanced heart failure in terms of symptoms, functional status, and left ventricular function[8,9]. This shift in etiology and severity reflects a broader indication expansion for M-TEER over time. Our analysis revealed that the pooled observational cohort was sicker in terms of NYHA classification (i.e., fewer NYHA class I and more NYHA class III/IV), MR grade (i.e., more MR grade 4+), and degree of systolic dysfunction (i.e., lower LVEF) upon hospital presentation. Patients in the observational studies were less likely to receive beta-blockers and implantable cardiac devices but more likely to receive ACEI/ARB than the COAPT and MITR-FR trials, specifically. Angiotensin receptor-neprilysin inhibitors and sodium-glucose cotransporter-2 inhibitors in heart failure were not widely used in the included studies. Overall, the more favourable characteristics of patients in the landmark randomized trials did not translate into better durable outcomes, especially mortality, over time.
Although the comparisons between the included observational studies and the randomized trials (i.e., COAPT and MITRA-FR) were comparable, MITRA-FR patients showed a trend towards less favourable outcomes in terms of trends towards higher mortality rates and lower procedural success (i.e., MR grade ≤ 2+) at 12-month follow-up which is may be consistent with the lower proportions of patients having MR grades 1+ and 2+ post procedure. Interestingly, our sensitivity analysis showed higher mortality in the COAPT and MITRA-FR patients. This may confirm the outcome trend in the MITRA-FR patients (OR = 1.28, 95%CI: 0.85-1.91) but probably contradict that of COAPT, who had higher procedural success (OR = 2.17, 95%CI: 1.23-3.82) which was maintained at 12-month follow-up (OR = 7.65, 95%CI: 4.15-14.11) according to our sensitivity analysis (Supplementary Table 8). However, our previous meta-analysis of 33 observational studies (n = 9200) that compared patient characteristics and outcomes in observational studies that were published after the publication of the COAPT and MITRA-FR trials and showed that patients from real-world experience showed more favorable outcomes than those from the MITRA-FR trial and probably less favourable than those from the COAPT trial, namely in terms of rates of death and heart failure hospitalization[5]. In our previous meta-analysis, we only included the studies that were published in 2020 and 2021, which may have affected the results in comparison to the present meta-analysis[5].
The divergent findings between the COAPT and MITRA-FR trials have raised many questions after their publications. The justifications were related to several varying aspects such as eligibility criteria, MR severity and characteristics, ventricular remodeling, primary endpoints, follow-up duration, and geographical regions[2,5]. However, the prospective single-arm COAPT Post-Approval Study (n = 5000) recruited patients participating in the Transcatheter Valve Therapy registry from 2019 to 2020 and divided them into two subgroups (i.e., COAPT- and MITRA-FR-like). Each subgroup was compared with its counterpart in the randomized trials. The study re-emphasized the efficacy and safety of M-TEER in patients with secondary MR who met the eligibility criteria of the COAPT or MITRA-FR trials[13]. Furthermore, a retrospective study (n = 22914), using the Nationwide Readmissions Database in the United States, assessed temporal trends in M-TEER short-outcomes between 2014 and 2018, which also occurred before the publication of the COAPT and MITRA-FR trials. The study demonstrated an increase of M-TEER utilization by 4.2-fold, accompanied by a significant reduction in hospital stay from four to two days and 30-day readmission for heart failure from 4.7% to 0.6%. The in-hospital mortality was significantly decreased from 3.9% to 2.0%[14].
Observational studies provide a more representative view of real-world clinical practice, which includes a broader spectrum of patients with comorbidities. Whereas randomized trials involve more uniform populations and strict elig
The temporal trends in patient characteristics and outcomes in observational studies in comparison with landmark trials in MR patients undergoing M-TEER revealed differences in patient selection and treatment evolution without a difference in mortality at follow-up. Patients in the landmark studies may have favourable or unfavourable characteristics when compared to those in the observational studies, but this did not translate into different outcomes over time.
| 1. | Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3465] [Article Influence: 173.3] [Reference Citation Analysis (0)] |
| 2. | Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20:620-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Huang AL, Dal-Bianco JP, Levine RA, Hung JW. Secondary Mitral Regurgitation: Cardiac Remodeling, Diagnosis, and Management. Struct Heart. 2023;7:100129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 4. | Dhaduk N, Chaus A, Williams D, Vainrib A, Ibrahim H. Secondary Mitral Regurgitation: Diagnosis and Management. US Cardiol. 2024;18:e05. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kaddoura R, Al-Badriyeh D, Abushanab D, Al-Hijji M. Percutaneous Mitral-Valve Intervention for Secondary Mitral Regurgitation: Data From Real-Life. Curr Probl Cardiol. 2023;48:101889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 6. | Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L; EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1728] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 7. | Ailawadi G, Lim DS, Mack MJ, Trento A, Kar S, Grayburn PA, Glower DD, Wang A, Foster E, Qasim A, Weissman NJ, Ellis J, Crosson L, Fan F, Kron IL, Pearson PJ, Feldman T; EVEREST II Investigators. One-Year Outcomes After MitraClip for Functional Mitral Regurgitation. Circulation. 2019;139:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 8. | Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ; COAPT Investigators. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 2281] [Article Influence: 285.1] [Reference Citation Analysis (0)] |
| 9. | Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N; MITRA-FR Investigators. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379:2297-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1387] [Article Influence: 173.4] [Reference Citation Analysis (0)] |
| 10. | Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. Version 6. The Cochrane Collaboration; 2019. Available from: https://training.cochrane.org/handbook/current. |
| 11. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 5674] [Article Influence: 1134.8] [Reference Citation Analysis (0)] |
| 12. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17246] [Article Influence: 663.3] [Reference Citation Analysis (0)] |
| 13. | Goel K, Lindenfeld J, Makkar R, Naik H, Atmakuri S, Mahoney P, Morse MA, Thourani VH, Yadav P, Batchelor W, Rogers J, Whisenant B, Rinaldi M, Hermiller J, Lindman BR, Barker CM. Transcatheter Edge-to-Edge Repair in 5,000 Patients With Secondary Mitral Regurgitation: COAPT Post-Approval Study. J Am Coll Cardiol. 2023;82:1281-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Abdelfattah OM, Saad AM, Hisung I, Abushouk AI, Gad MM, Okasha O, Isogai T, Ahuja KR, Shekhar S, Burns DJ, Krishnaswamy A, Kapadia SR. Temporal Trends of Transcatheter Edge-to-Edge Repair of the Mitral Valve Short-Term Outcomes in the United States: Nationwide Representative Study. Struct Heart. 2021;5:279-286. [DOI] [Full Text] |
| 15. | Wang MT, Bolland MJ, Grey A. Reporting of Limitations of Observational Research. JAMA Intern Med. 2015;175:1571-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Matsumoto T, Kubo S, Izumo M, Mizuno S, Shirai S; MitraClip Japan PMS Investigators. MitraClip Treatment of Moderate-to-Severe and Severe Mitral Regurgitation in High Surgical Risk Patients - Real-World 1-Year Outcomes From Japan. Circ J. 2022;86:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Pascual I, Arzamendi D, Carrasco-Chinchilla F, Fernández-Vázquez F, Freixa X, Nombela-Franco L, Avanzas P, Serrador Frutos AM, Pan M, Cid Álvarez AB, Hernández-Antolín RA, Andraka Ikazuriaga L, Cruz-González I, Díez Gil JL, Alcasena Juango MS, Berenguer Jofresa A, Alonso-Briales JH, Li CH, Benito González T, Regueiro A, Armijo G, León V, Amat-Santos IJ, Romero M, Trillo Nouche R, Fernández-Golfín C, Ruiz Gómez L, Campos-Arjona R, Millán X, Garrote Coloma C, Sanchis L, Jiménez-Quevedo P, Morís C, Hernández-García JM, Serra A, Pérez de Prado A, Estévez-Loureiro R. Transcatheter mitral repair according to the cause of mitral regurgitation: real-life data from the Spanish MitraClip registry. Rev Esp Cardiol (Engl Ed). 2020;73:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Benito-González T, Carrasco-Chinchilla F, Estévez-Loureiro R, Pascual I, Arzamendi D, Garrote-Coloma C, Nombela-Franco L, Pan M, Serrador A, Freixa X, Cid Alvarez AB, Hernández Antolín RA, Andraka L, Cruz-González I, López-Minguez JR, Díez Gil JL, Urbano-Carrillo C, Sanmiguel Cervera D, Sanchís J, Bosa F, Ruíz V, Molina E, Becerra-Muñoz VM, Gualis J, Avanzas P, Li CH, Baz JA, Jimenez-Quevedo P, Mesa D, Amat-Santos IJ, Regueiro A, Trillo R, Domínguez Franco AJ, Alonso-Briales JH, Fernández-Vázquez F. Clinical and echocardiographic outcomes of transcatheter mitral valve repair in atrial functional mitral regurgitation. Int J Cardiol. 2021;345:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Lüscher TF, Moat N, Price S, Dall'Ara G, Winter R, Corti R, Grasso C, Snow TM, Jeger R, Blankenberg S, Settergren M, Tiroch K, Balzer J, Petronio AS, Büttner HJ, Ettori F, Sievert H, Fiorino MG, Claeys M, Ussia GP, Baumgartner H, Scandura S, Alamgir F, Keshavarzi F, Colombo A, Maisano F, Ebelt H, Aruta P, Lubos E, Plicht B, Schueler R, Pighi M, Di Mario C; Transcatheter Valve Treatment Sentinel Registry Investigators of the EURObservational Research Programme of the European Society of Cardiology. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol. 2014;64:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, Butter C, Ussia GP, Sievert H, Richardt G, Widder JD, Moccetti T, Schillinger W. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 670] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 21. | Bedogni F, Popolo Rubbio A, Grasso C, Adamo M, Denti P, Giordano A, Tusa M, Bianchi G, De Marco F, Bartorelli AL, Montorfano M, Godino C, Citro R, De Felice F, Mongiardo A, Monteforte I, Villa E, Giannini C, Crimi G, Tarantini G, Testa L, Tamburino C. Italian Society of Interventional Cardiology (GIse) registry Of Transcatheter treatment of mitral valve regurgitaTiOn (GIOTTO): impact of valve disease aetiology and residual mitral regurgitation after MitraClip implantation. Eur J Heart Fail. 2021;23:1364-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/