Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.112018
Revised: July 28, 2025
Accepted: October 17, 2025
Published online: November 26, 2025
Processing time: 128 Days and 22.3 Hours
Given the clinical challenges posed by drug-eluting stents, drug-coated balloons offer a promising alternative by delivering antiproliferative medications directly to the vessel wall.
To compare the efficacy of paclitaxel-coated balloon (PCB) angioplasty vs sirolimus-coated balloon (SCB) angioplasty in the treatment of coronary artery disease (CAD), focusing on both in-stent restenosis (ISR) and de-novo lesions (DNL).
A comprehensive literature search on PubMed, EMBASE, and Cochrane Central from inception to 5th February 2025. Only randomized controlled trials and observational studies comparing outcomes of PCB vs SCB angioplasty in patients with ISR or DNL were included.
A total of nine studies with 1981 patients (949 in PCB arm and 1032 in SCB arm) were included for further quantitative analysis. The results indicated that both PCB and SCB angioplasty are effective in treating CAD, with PCB showing a greater minimal lumen diameter for DNL [mean difference: -0.11 (95% confidence interval: -0.22 to -0.01, P = 0.03)]. However, the risk of target lesion revascularization and diameter stenosis was identical for both PCB and SCB during the 9-12-month follow-up period.
This meta-analysis highlights that PCB angioplasty may offer superior angiographic outcomes compared to SCB angioplasty, specifically in achieving greater minimal lumen diameter in patients with DNL. These findings suggest that while PCB has certain advantages in terms of tissue retention and immediate efficacy, both PCB and SCB are viable options for treating ISR or DNL in CAD patients. Further large-scale studies are required to conclusively determine the long-term benefits and potential risks associated with each type of drug-coated balloons angioplasty.
Core Tip: This meta-analysis presents the most up-to-date comparison of paclitaxel- vs sirolimus-coated balloon angioplasty for coronary artery disease, encompassing 1981 patients across diverse lesion types. Our findings reveal comparable clinical and angiographic outcomes for both devices but identify a potential advantage for paclitaxel-coated balloons in de novo lesions. These results underscore the need for personalized lesion-specific strategies and highlight the importance of further large-scale trials to refine the role of drug-coated balloons in percutaneous coronary intervention.
- Citation: Rath S, Khan A, Khan H, Cheema AAA, Ud Din Z, Ullah W, Ahmed R. Assessing paclitaxel-coated vs sirolimus-coated balloon angioplasty for coronary artery diseases: A systematic review and meta-analysis. World J Cardiol 2025; 17(11): 112018
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/112018.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.112018
Coronary artery disease (CAD) is the most common cause of mortality and loss of disability adjusted life years, which is particularly prevalent in low and middle-income countries[1], and requires effective revascularization strategies to restore myocardial perfusion.
With the advent of drug-eluting stents (DES), the treatment of patients with CAD has undergone an unprecedented change, with a significant rise in the proportion of patients undergoing percutaneous revascularization rather than going for surgery. In the United States, almost 90% of patients undergoing percutaneous procedures now have DES, a huge rise in utilization since their introduction[2].
The currently approved DES have significantly reduced the complications by employing a polymer-impregnated coating that elutes either sirolimus or paclitaxel to suppress smooth muscle growth. The pivotal “treatment of de novo coronary disease using a single paclitaxel-eluting stent”[3]and “the SIRolImUS-coated stent in treatment of patients with de novo coronary artery lesions”[4] trials that contrasted DES with standard bare-metal stents, target vessel revascularization (TVR) rates in patients with stable CAD ranged from 3.1% to 4.1%, which is much less than what was previously observed with standard bare-metal stents. The use of DES in clinical practice rapidly grew when they were approved in April 2003. Even though DES have reduced in-stent restenosis (ISR), that have greatly improved outcomes during percutaneous coronary intervention (PCI)[5-7]. However, compared to drug-coated balloons (DCB), DES greatly increased target artery revascularization. Additionally, they have a low incidence of stent thrombosis and late atherosclerosis, which are significant drawbacks of DES implantation[5,8].
DCB is an alternative therapeutic technique that allows quick and efficient transfer of antiproliferative medications into the vessel wall after brief balloon inflation[9]. According to treatment rating, DES were most likely (61.4%) to be the most effective treatment for vascularization of the target lesion, while DCB were most likely (70.3%) to be the most effective treatment for late lumen loss (LLL)[6]. DCB is superior to DES by getting rid of the need to implant a permanent metal stent in the vessel while still providing antiproliferative effects[7,9].
Sirolimus and its derivatives are safer and more effective than paclitaxel-eluting stents; they are the most widely used antiproliferative drugs in current-generation DES[9,10]. For DCB applications, however, paclitaxel offers clear potential benefits because sirolimus and its derivatives are less lipophilic, have lower transfer to tissues and retention rates during brief contact durations, and rapidly decrease their concentration in tissue[9]. In a meta-analysis of patients receiving DCB-only PCI, the minimal lumen diameter (MLD) was greater for paclitaxel-coated balloon (PCB) than sirolimus-coated balloon (SCB) on follow-up angiography, but the risk of target lesion failure was identical during the 9-12 months of follow-up following PCB and SCB treatment[11]. Many of these studies have been limited by insufficient power to assess clinical outcomes, and the findings on angiographic outcomes remain inconsistent. To address this gap, we conducted a meta-analysis to compare the efficacy of PCB- and SCB-only PCI in the treatment of CAD.
This meta-analysis is conducted according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions and PRISMA guidelines[12,13]. Our protocol is registered with PROSPERO (CRD420251022011).
A comprehensive literature search was carried out on PubMed, Embase, and the Cochrane Central Register of Controlled Trials from inception till 5th February 2025. Reference lists of included studies and similar systematic reviews were also screened to identify further relevant studies. Keywords included angioplasty, balloon dilatation, paclitaxel, sirolimus, including others. A detailed search strategy is provided in Supplementary Table 1.
Randomized control trials (RCTs) and observational studies that aimed to compare outcomes after PCI with PCB angioplasty vs SCB angioplasty as an intervention in patients with ISR were included. Study designs other than these two were excluded. Case reports and studies conducted on animals were also excluded.
We used Rayyan to find and remove duplicates of each study that came up in our literature search. Two authors independently screened abstracts and titles to exclude any irrelevant articles (Rath S, Khan A). Included articles were further assessed by a full-text screening, and a third author finalized the included studies (Cheema AAA). Relevant data from the selected studies were then extracted in an Excel sheet comprised of a baseline and outcome sheet.
The main outcomes of our study were ISR, cardiac death, TVR, and myocardial infarction (MI). Other outcomes were rehospitalization due to heart failure, in-segment LLL on follow-up angiography, stenosis diameter on follow-up angiography, and minimal lesion diameter on follow-up angiography.
The revised Cochrane Risk of Bias tool (RoB 2.0) was used to evaluate the risk of bias in the included randomized trials[14]. The quality of the observational studies was assessed using the ROBINS-I tool[15]. The risk of bias for each included study was assessed by two investigators with high, low, or some concerns of bias being reported (Khan A, Ullah W). Any discord was settled by the main investigator (Rath S).
Statistical analysis was performed using RevMan 5.4 software. For dichotomous outcomes, risk ratios (RR) with 95% confidence intervals (CI) were calculated. For continuous outcomes, mean differences with 95%CI were computed. A random-effects model was utilized to account for variability among studies.
Heterogeneity was assessed using the I2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. Subgroup analyses were conducted based on the type of lesion (ISR and de-novo lesion). Sensitivity analyses were performed by leave-one-out analysis. Statistical significance was defined at P < 0.05.
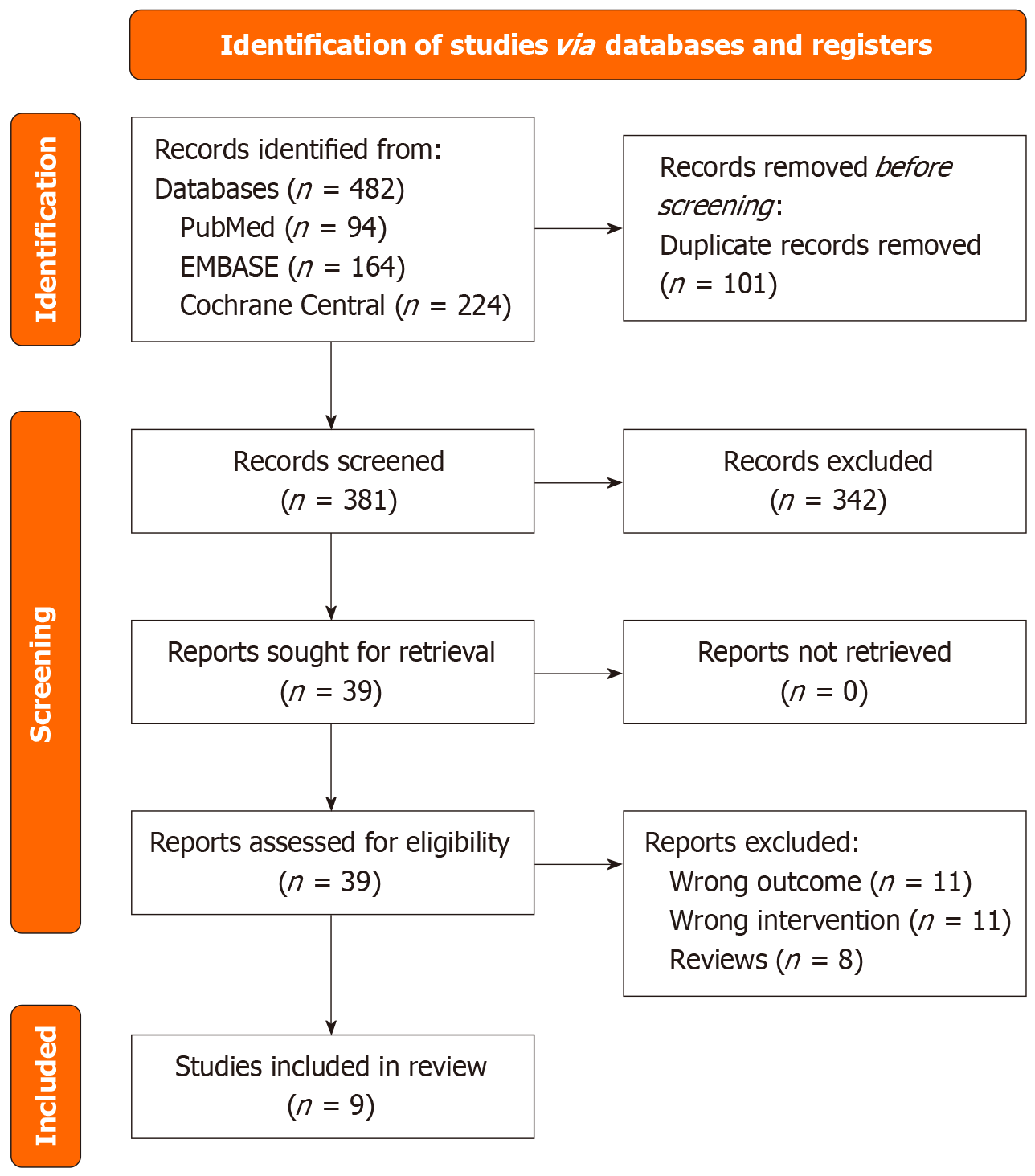
A search of PubMed, EMBASE, and Cochrane databases initially identified 482 results. Following the removal of duplicates, 381 studies were screened. Of these, 342 studies were excluded based on title and abstract screening, leaving 39 studies for full-text review. These 9 studies, involving a total of 1981 patients, were included in the meta-analysis[16-24]. The PRISMA flow chart summarizes the search and trial selection (Figure 1). The baseline characteristics for patients in each study are presented in Table 1. Follow-up time ranged from 6 months to 12 months. The study time span included dates from 2019 to 2025, covering various geographical locations like Malaysia, Europe, and China.
| Ref. | Sample size: Paclitaxel | Sample size: Sirolimus | Follow-up duration (months) | Age (years) | Male | Hypertension | Diabetes | Dyslipidemia | Prior MI | ACS presentation | |||||||
| Paclitaxel, mean | Sirolimus, mean | Paclitaxel (%) | Sirolimus (%) | Paclitaxel (%) | Sirolimus (%) | Paclitaxel (%) | Sirolimus (%) | Paclitaxel (%) | Sirolimus (%) | Paclitaxel (%) | Sirolimus (%) | Paclitaxel (%) | Sirolimus (%) | ||||
| Liu et al[23], 2025 | 128 | 130 | 12 | 63.7 | 63.8 | 75.8 | 73.1 | 70.3 | 66.9 | 41.4 | 33.8 | 14.1 | 17.7 | 6.3 | 4.6 | 89 | 92.3 |
| Scheller et al[24], 2024 | 35 | 35 | 12 | 67 | 66 | 77 | 86 | 89 | 83 | 43 | 31 | 71 | 77 | 34 | 37 | NR | NR |
| Ali et al[16], 2019 | 25 | 25 | 12 | 58.6 | 61.6 | 76 | 88 | 92 | 96 | 76 | 72 | 84 | 92 | 36 | 32 | NR | NR |
| Cortese et al[17], 2021 | 290 | 290 | 12 | 67 | 66 | 75 | 77 | 69 | 74 | 35 | 45 | 61 | 67 | 43 | 48 | 52 | 53 |
| Scheller et al[18], 2022 | 51 | 50 | 12 | 63 | 67 | 76 | 86 | 94 | 98 | 59 | 54 | 84 | 88 | 55 | 56 | 90 | 94 |
| Ahmad et al[22], 2022 | 35 | 35 | 12 | 59 | 60 | 86 | 74 | 66 | 69 | 49 | 54 | 57 | 51 | 46 | 34 | NR | NR |
| Ninomiya et al[19], 2023 | 60 | 61 | 6 | 66 | 69 | 83 | 90 | 73 | 84 | 25 | 25 | 82 | 82 | 33 | 28 | NR | NR |
| Briguori et al[20], 2023 | 186 | 186 | 12 | 68 | 67 | 77 | 75 | 92 | 90 | 55 | 56 | 90 | 86 | 25 | 21 | 25 | 25 |
| Cuculi et al[21], 2023 | 139 | 220 | 12 | 66 | 67 | NR | NR | NR | NR | 25 | 33 | NR | NR | NR | NR | 41 | 40 |
All studies were adjudged as either low risk of bias or some concerns, primarily due to biases in randomization (Supplementary Figures 1-4).
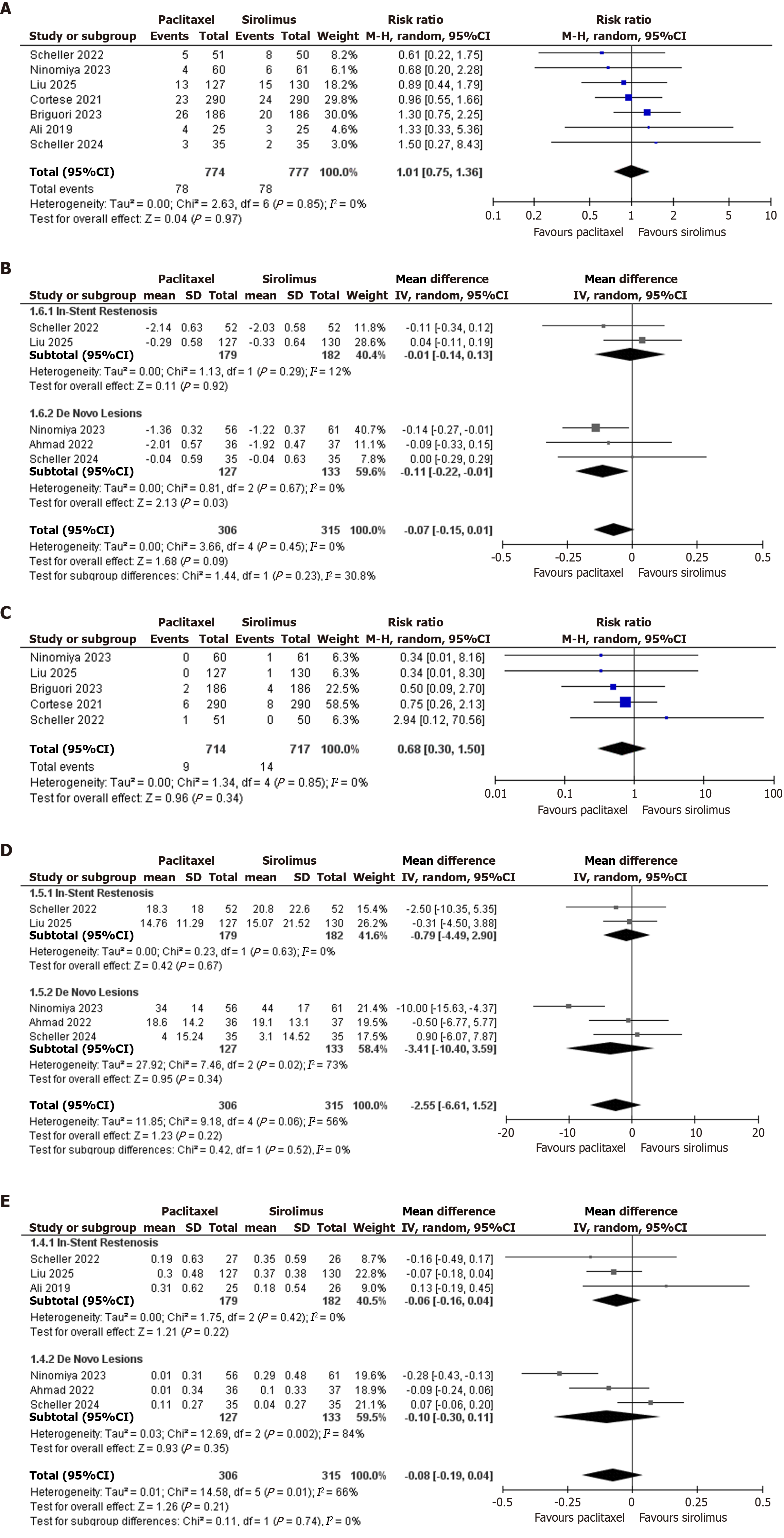
TVR: Seven out of ten included studies reported TVR. A total of 1551 patients were included in the analysis, out of which 774 were in the paclitaxel arm and 777 were in the sirolimus arm. Pooled RR was 1.01 (95%CI: 0.75-1.36, P = 0.97, I2: 0%), indicating no significant difference between paclitaxel-coated and sirolimus-coated stents (Figure 2A).
Minimal lesion diameter: Five out of nine included studies reported mean lesion diameter. A total of 621 patients were included in the analysis, out of which 306 were in the paclitaxel arm and 315 were in the sirolimus arm. Pooled mean difference was -0.07 (95%CI: -0.15 to 0.01, P = 0.09, I2: 0%), indicating no significant difference between paclitaxel-coated and sirolimus-coated stents.
In subgroup analysis, ISR was reported by 2 studies and included a total of 361 patients. Pooled mean difference was -0.01 (95%CI: -0.14 to 0.13, P = 0.92, I2: 12%). De Novo lesions were reported by 3 studies and included a total of 260 patients. Pooled mean difference was -0.11 (95%CI: -0.22 to -0.01, P = 0.03, I2: 0%), indicating paclitaxel as a superior option for minimal lesion diameter in de novo lesions (Figure 2B).
MI: Five out of ten included studies reported MI. A total of 1431 patients were included in the analysis, out of which 714 were in the paclitaxel arm and 717 were in the sirolimus arm. Pooled RR was 0.68 (95%CI: 0.30-1.50, P = 0.34, I2: 0%), indicating no significant difference between paclitaxel-coated and sirolimus-coated stents (Figure 2C).
Diameter stenosis on follow-up angiography: Five out of ten included studies reported diameter stenosis on follow-up angiography. A total of 621 patients were included in the analysis, out of which 306 were in the paclitaxel arm and 315 were in the sirolimus arm. Pooled mean difference was -2.55 (95%CI: -6.61 to 1.52, P = 0.22, I2: 56%), indicating no significant difference between paclitaxel-coated and sirolimus-coated stents.
In subgroup analysis, ISR was reported by two studies and included a total of 361 patients, and the pooled mean difference was -0.79 (95%CI: -4.49 to 2.90, P = 0.63, I2: 0%). De novo lesions were reported by three studies and included a total of 260 patients. Pooled mean difference was -3.41 (95%CI: -10.40 to 3.59, P = 0.34, I2: 73%) (Figure 2D).
In-segment LLL on follow-up angiography: Six out of ten included studies reported in-segment LLL on follow-up angiography. A total of 621 patients were included in the analysis, out of which 306 were in the paclitaxel arm and 315 were in the sirolimus arm. Pooled mean difference was -0.08 (95%CI: -0.19 to 0.04, P = 0.21, I2: 66%), indicating no significant difference between paclitaxel-coated and sirolimus-coated stents.
In subgroup analysis, in stent restenosis was reported by three studies and included a total of 361 patients, and the pooled mean difference was -0.06 (95%CI: -0.19 to 0.04), P = 0.42, I2: 0%). De novo lesions were reported by three studies and included a total of 260 patients. Pooled mean difference was -0.10 (95%CI: -0.30 to 0.11, P = 0.35, I2: 84%) (Figure 2E).
In this meta-analysis of 1981 patients undergoing DCB-only PCI, we compared the clinical and angiographic outcomes of PCBs vs SCBs. Our findings showed no significant difference in key outcomes such as TVR, MI, MLD, LLL, or diameter stenosis across the two balloon types, both in the overall population and in subgroup analyses of ISR and de novo lesions.
DCB-only PCI, which avoids permanent stent placement, is a growing strategy aimed at reducing restenosis risk. Both PCBs and SCBs deliver antiproliferative agents directly to the vessel wall to suppress neointimal hyperplasia[25,26,17]. While PCBs deliver paclitaxel - a cytotoxic agent that arrests cell division - SCBs use sirolimus to inhibit mammalian target of rapamycin-mediated pathways involved in smooth muscle proliferation and migration[27,28]. The clinical efficacy and safety of both approaches have been explored in ISR and de novo lesions, with PCBs traditionally favored due to earlier clinical adoption, though SCBs are now emerging as potential alternatives with a safer pharmacological profile.
Early trials such as PACCOCATH and SABRE provided foundational evidence for the feasibility and efficacy of PCBs and SCBs, respectively[24,29]. Our updated meta-analysis builds on prior evidence, including a recent meta-analysis by Shin et al[11], which reported no significant differences in TVR or MI and showed marginal angiographic benefits favoring PCBs. While Shin et al[11] found a greater MLD with PCBs (weighted mean difference: 0.10 mm; 95%CI: 0.02-0.17), our pooled analysis (n = 621) found no significant difference (P = 0.09), reinforcing the notion that both devices are largely comparable.
Similarly, previous estimates for LLL and diameter stenosis were not statistically significant[11], a trend that persisted in our updated analysis. Our results demonstrated no difference between PCBs and SCBs in diameter stenosis (P = 0.22) or LLL (P = 0.21), further supporting comparable angiographic performance.
Notably, subgroup analysis revealed a consistent pattern where PCBs may have an edge in treating de novo lesions. For MLD, PCBs showed a significant advantage over SCBs in de novo lesions (P = 0.03), whereas no meaningful difference was observed in ISR. This is aligned with Shin et al’s findings[11] (weighted mean difference: 0.13 mm; 95%CI: 0.02-0.24) and suggests that the benefit of paclitaxel might be lesion-specific. While there was no overall heterogeneity (I2 = 0%), mild heterogeneity in the ISR subgroup (I2 = 12%) points to potential variability in patient selection or procedural techniques. In contrast, the consistency in de novo lesions reinforces the reliability of the trend favoring PCBs in this subgroup.
Among the five studies reporting MI incidence, none showed a statistically significant difference, and our pooled analysis confirmed comparable outcomes between balloon types, supported by the absence of heterogeneity. The same was true for diameter stenosis and LLL, both of which showed no significant differences across the overall and subgroup analyses.
While individual studies such as TRANSFORM I indicated potential benefits of PCBs for de novo lesions, others reported no difference. These trends, though not statistically robust, hint at a selective advantage for PCBs in certain lesion types, particularly de novo disease. The consistency of these findings, despite limitations in sample size and follow-up duration, adds to the emerging body of evidence suggesting lesion-specific efficacy patterns.
Both PCBs and SCBs represent effective stent-free treatment modalities. A recent observational study also supported their use in treating DES-related ISR, reporting similar outcomes[30]. Overall, our analysis affirms that PCBs and SCBs offer comparable clinical and angiographic outcomes in most settings, though paclitaxel may provide modest benefits in de novo lesions. Further large-scale randomized controlled trials with extended follow-up are warranted to confirm these trends and refine lesion-specific therapeutic strategies.
Our meta-analysis comprised a total of nine investigations. RCTs were given top priority to minimize the risk of unaccounted confounding while also including observational studies in the meta-analysis to ensure comprehensiveness. However, our study has several limitations. First, like any other meta-analysis, this study is not immune to confounding, especially within the observational study cohort, where variations in study design and inherent biases may influence the findings. Although the clinical outcomes appeared consistent between RCTs and observational studies, the possibility of an underlying difference cannot be entirely dismissed. Second, while the previous meta-analysis reported moderate heterogeneity[31], likewise, our meta-analysis also had moderate heterogeneity, which could partly be explained by treatment effect, differences in patient characteristics such as comorbidities, disease severity, age distribution, and gender. Additionally, variations in the intervention protocols, including differences in dosing, timing, and treatment duration as well as discrepancies in comparator groups such as variations in control treatments or additional therapies administered alongside the primary intervention, could contribute to the observed heterogeneity[32]. Previous meta-analysis has analyzed angiographic outcomes considering both types of SCBs and lesion type. However, one of the limitations of our study is that our analysis was restricted to angiographic outcomes based solely on the type of lesions. Moreover, our meta-analysis did not report the cardiac death outcome. This should be acknowledged as a limitation in the discussion, as it may affect the overall clinical applicability of the findings[11]. While our meta-analysis reports valuable insights into PCB-only vs SCB-only PCI, several limitations must be acknowledged, including heterogeneity, limited follow-up, employing particular types of PCBs and SCBs, and small sample sizes[33]. The absence of intravascular ultrasound and Optical coherence tomography in the included trials highlights the need for advanced imaging in future studies[34,35]. Beyond comparing PCB and SCB, optimizing DCB technology remains an area for further exploration. Large-scale, well-powered trials with standardized protocols and extended follow-up are essential to refine treatment strategies and improve patient outcomes.
In this meta-analysis, we compared the efficacy of PCBs and SCBs in PCIs for CAD. Our findings indicate that there are no significant differences between paclitaxel-coated and SCBs in terms of TVR, minimal lesion diameter, MI, diameter stenosis on follow-up angiography, and in-segment LLL. While paclitaxel demonstrated a selective benefit in de novo lesions, both balloon types performed similarly across other lesion types. Further large-scale, well-powered randomized clinical trials with standardized protocols and extended follow-up are essential to refine treatment strategies and improve patient outcomes. Long-term studies are needed to evaluate the safety and effectiveness of PCBs and SCBs beyond 12 months of follow-up.
| 1. | Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res. 2014;114:1959-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Thanigaraj S, Wollmuth JR, Zajarias A, Chemmalakuzhy J, Lasala JM. From randomized trials to routine clinical practice: an evidence-based approach for the use of drug-eluting stents. Coron Artery Dis. 2006;17:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Ellis SG, Stone GW, Cox DA, Hermiller J, O'Shaughnessy C, Mann T, Turco M, Caputo R, Bergin PJ, Bowman TS, Baim DS; TAXUS IV Investigators. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc Interv. 2009;2:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1293] [Cited by in RCA: 1215] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 5. | Tarigan T, Sukmadja D, Prasiddha KC, Triatmaja R. Comparative efficacy of drug-eluting balloon and drug-eluting stent for treatment of in-stent restenosis: a systematic review and meta-analysis. J Pak Med Assoc. 2024;74:S51-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ. 2015;351:h6364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Neumann FJ, Sousa-Uva M. 'Ten commandments' for the 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur Heart J. 2019;40:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 277] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 8. | Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2103] [Cited by in RCA: 2151] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 9. | Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J, Scheller B, Kleber FX; International DCB Consensus Group. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 368] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 10. | Sato Y, Kuntz SH, Surve D, Jinnouchi H, Sakamoto A, Cornelissen A, Virmani R, Kolodgie F, Finn AV. What are the Pathological Concerns and Limitations of Current Drug-coated Balloon Technology? Heart Int. 2019;13:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Shin D, Singh M, Shlofmitz E, Scheller B, Latib A, Kandzari DE, Zaman A, Mylotte D, Dakroub A, Malik S, Sakai K, Jeremias A, Moses JW, Shlofmitz RA, Stone GW, Ali ZA. Paclitaxel-coated versus sirolimus-coated balloon angioplasty for coronary artery disease: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2024;104:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J Public Health (Oxf). 2022;44:e588-e592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 287] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51476] [Article Influence: 10295.2] [Reference Citation Analysis (2)] |
| 14. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18757] [Article Influence: 2679.6] [Reference Citation Analysis (0)] |
| 15. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12554] [Article Influence: 1255.4] [Reference Citation Analysis (2)] |
| 16. | Ali RM, Abdul Kader MASK, Wan Ahmad WA, Ong TK, Liew HB, Omar AF, Mahmood Zuhdi AS, Nuruddin AA, Schnorr B, Scheller B. Treatment of Coronary Drug-Eluting Stent Restenosis by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc Interv. 2019;12:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Cortese B, Caiazzo G, Di Palma G, De Rosa S. Comparison Between Sirolimus- and Paclitaxel-Coated Balloon for Revascularization of Coronary Arteries: The SIRPAC (SIRolimus-PAClitaxel) Study. Cardiovasc Revasc Med. 2021;28:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Scheller B, Mangner N, Abdul Kader MASK, Wan Ahmad WA, Jeger R, Wöhrle J, Ong TK, Liew HB, Gori T, Mahfoud F, Nuruddin AA, Woitek F, Abidin IZ, Schwenke C, Schnorr B, Mohd Ali R. Combined Analysis of Two Parallel Randomized Trials of Sirolimus-Coated and Paclitaxel-Coated Balloons in Coronary In-Stent Restenosis Lesions. Circ Cardiovasc Interv. 2022;15:e012305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 19. | Ninomiya K, Serruys PW, Colombo A, Reimers B, Basavarajaiah S, Sharif F, Testa L, Di Mario C, Nerla R, Ding D, Huang J, Kotoku N, Kageyama S, Kageyama M, Sevestre E, Fezzi S, Dijkstra J, O'Leary N, Morel MA, Garg S, Cortese B, Onuma Y. A Prospective Randomized Trial Comparing Sirolimus-Coated Balloon With Paclitaxel-Coated Balloon in De Novo Small Vessels. JACC Cardiovasc Interv. 2023;16:2884-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 20. | Briguori C, Visconti G, Golino M, Focaccio A, Scarpelli M, Nuzzo S, Biondi-Zoccai G. Paclitexel versus sirolimus-coated balloon in the treatment of coronary instent restenosis. Panminerva Med. 2023;65:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Cuculi F, Madanchi M, Cioffi GM, Majcen I, Gnan E, Gjergjizi V, Zhi Y, Seiler T, Attinger-Toller A, Bossard M. Treatment of coronary artery disease with sirolimus- versus paclitaxel-coated balloons. Eur Heart J. 2023;44:ehad655.2147. [DOI] [Full Text] |
| 22. | Ahmad WAW, Nuruddin AA, Abdul Kader MASK, Ong TK, Liew HB, Ali RM, Mahmood Zuhdi AS, Ismail MD, Yusof AKM, Schwenke C, Kutschera M, Scheller B. Treatment of Coronary De Novo Lesions by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc Interv. 2022;15:770-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Liu H, Li Y, Fu G, An J, Chen S, Zhong Z, Liu B, Qiu C, Ma L, Cong H, Li H, Tong Q, He B, Jin Z, Zhang J, Yuan H, Qiu M, Zhang R, Han Y. Sirolimus- vs Paclitaxel-Coated Balloon for the Treatment of Coronary In-Stent Restenosis: The SIBLINT-ISR Randomized Trial. JACC Cardiovasc Interv. 2025;18:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Scheller B, Mangner N, Jeger RV, Afan S, Mahfoud F, Woitek FJ, Fahrni G, Schwenke C, Schnorr B, Kleber F. A randomised trial of sirolimus- versus paclitaxel-coated balloons for de novo coronary lesions. EuroIntervention. 2024;20:e1322-e1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Gurgoglione FL, De Gregorio M, Benatti G, Donelli D, Vignali L, Solinas E, Tadonio I, Denegri A, Covani M, Dallaglio G, Cortese B, Niccoli G. Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives. Future Pharmacol. 2024;4:775-787. [DOI] [Full Text] |
| 26. | Loh JP, Waksman R. Paclitaxel drug-coated balloons: a review of current status and emerging applications in native coronary artery de novo lesions. JACC Cardiovasc Interv. 2012;5:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, Limbruno U. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart. 2010;96:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Habib A, Finn AV. Antiproliferative Drugs for Restenosis Prevention. Interv Cardiol Clin. 2016;5:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Verheye S, Vrolix M, Kumsars I, Erglis A, Sondore D, Agostoni P, Cornelis K, Janssens L, Maeng M, Slagboom T, Amoroso G, Jensen LO, Granada JF, Stella P. The SABRE Trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): Angiographic Results and 1-Year Clinical Outcomes. JACC Cardiovasc Interv. 2017;10:2029-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Ray S, Bandyopadhyay S, Bhattacharjee P, Mukherjee P, Karmakar S, Bose P, Choudhury B, Paul D, Karak A. Drug-coated balloon in patients with in-stent restenosis: A prospective observational study. Indian Heart J. 2025;77:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med. 2002;21:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Norrie J. The importance of long-term follow-up in clinical trials. Lancet Glob Health. 2023;11:e995-e996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Inclusion of Nonrandomized Studies of Interventions in Systematic Reviews of Intervention Effectiveness: An Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022 Sep- . [PubMed] |
| 34. | Zhang YB, Liu HD, Xing JH, Chen BW, Zhao YY, Gu HP, Tao HL. Safety and Efficacy of Drug-Coated Balloons in Patients with Acute Coronary Syndromes and Vulnerable Plaque. Clin Appl Thromb Hemost. 2022;28:10760296221130063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Ali ZA, Landmesser U, Maehara A, Matsumura M, Shlofmitz RA, Guagliumi G, Price MJ, Hill JM, Akasaka T, Prati F, Bezerra HG, Wijns W, Leistner D, Canova P, Alfonso F, Fabbiocchi F, Dogan O, McGreevy RJ, McNutt RW, Nie H, Buccola J, West NEJ, Stone GW; ILUMIEN IV Investigators. Optical Coherence Tomography-Guided versus Angiography-Guided PCI. N Engl J Med. 2023;389:1466-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 181] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/