Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.111292
Revised: July 24, 2025
Accepted: October 17, 2025
Published online: November 26, 2025
Processing time: 147 Days and 15.8 Hours
Lysosomal acid lipase-deficiency (LAL-D) is a rare and systemic condition, secon
Core Tip: Lysosomal acid lipase deficiency is a rare disease caused by the mutation of the lysosomal acid lipase A gene and characterized by the lysosomal accumulation of cholesteryl esters and triglycerides in many tissues. Its late onset form, cholesteryl ester storage disease, is frequently misrecognized because of a mild and nonspecific presentation that resembles more common illnesses such as metabolically associated steatotic liver disease and dyslipidemia. This review summarizes the current knowledge on cholesteryl ester storage disease and aims to increase the awareness of the disease among clinicians.
- Citation: Fornengo P, Ferro A, Fagoonee S, Rinaudo E, Amione C, Durazzo M. Lysosomal acid lipase deficiency: The forgotten link between liver and cardiovascular disease. World J Cardiol 2025; 17(11): 111292
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/111292.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.111292
Lysosomal acid lipase deficiency (LAL-D) is a rare genetic disease characterized by the lysosomal accumulation of cholesteryl esters (CE) and triglycerides (TG) in many tissues. This condition is due to genetic mutations of the lipase A (LIPA) gene, which leads to the reduction of LAL activity and impairment of lipid metabolism[1]. Firstly described by Abramov et al[2] as an infant life-threatening disease [named Wolman disease (WD)] and typically occurring in the first weeks of life, it was successively discovered with milder manifestations and later-onset by Fredrickson et al[3], who described it as cholesteryl ester storage disease (CESD). The aim of this review is to summarize recent knowledge about LAL-D, focusing on phenotype and early identification of its most under-recognized form: CESD. In this contest, not only hepatic manifestations but also atherosclerotic implications will be discussed, showing LAL-D as a real link between liver and cardiovascular disease. The main available therapies for CESD will also be treated.
LAL-D is an autosomal recessive disease caused by mutations in the LIPA gene, located on chromosome 10q23.2. It consists of 10 exons (encoding a messenger RNA of approximately 26 Kb, for LAL protein synthesis) and 9 introns (as untranslated regions)[4]. Thus, both the type and site of the mutation are correlated with the residual enzymatic activity. To date, more than 100 LIPA mutations have been identified (including deletions, insertions, missense, nonsense, and frame-shift), which can lead to the alteration of LAL synthesis at various levels (trafficking, folding, aggregation, or stabilization)[5]. WD is usually caused by frame-shift, nonsense, or splice-site mutations, resulting in a preservation < 1% of LAL activity or its complete loss. Conversely, CESD is more often characterized by missense mutations, which allow it to retain between 1%-10% of LAL activity[6]. Nevertheless, the correlation between residual enzyme activity and phenotypic severity is still a debated topic. The most common mutation observed in CESD patients is c.894G>A (E8SJM). This splice-site mutation allows the production of a small percentage of functional LIPA transcripts and the expression of 3%-5% of LAL activity[7]. Although c.894G>A accounts for 50% of all LAL-D cases, many other disease-causing LIPA variants (such as c.891C>T, c.676-23T>C, c.966+3A>T) and predicted pathogenic mutations have been found[7]. Other novel non-classical genetic variants have been recently described in a few case reports[8,9]. The role of epigenetics in LAL-D has also been investigated, focusing on the possible correlation between DNA methylation and LIPA protein expression. Preliminary evidence is promising[10,11], but further specific investigations on LIPA epigenetic regulation will be necessary to determine the significance of these findings.
LAL-D is considered a rare pan-ethnic condition. However, the real incidence and prevalence in the various populations are difficult to establish due to the rarity of the disease, the presence of many underdiagnosed cases, and the lack of certain epidemiological data. Until 2015, epidemiological reports on LAL-D derived from a few observational studies performed on Caucasian populations, in which the number of affected patients was estimated using the prevalence of the c.894G>A mutation[12]. More recently, the use of the next-generation sequencing technique has allowed for obtaining more precise data: Considering all known LIPA variants, Carter et al[7] found a prevalence of LAL-D in the general population of 1 per 177452 (95% confidence interval: 149467-210683), which was significantly lower than previous estimates (about 1 per 40000). Differences were also observed between ethnic groups, with a higher prevalence of LAL-D in European and Latino ancestries and low prevalence in Asian and Ashkenazi Jewish ones[7].
In LAL-D, the extension of CE and TG deposition in tissues appears to be directly proportional to illness severity and inversely proportional to onset age. In fact, clinical manifestations are more severe in the early-onset form of LAL-D (WD), in which the heavy accumulation of lipids in visceral organs causes hepatosplenomegaly and adrenal calcification, presenting soon after birth (2-4 months)[6]. These symptoms are frequently accompanied by digestive disorders, such as steatorrhea/diarrhea, vomiting, and feeding difficulties, leading to malabsorption, malnutrition, and growth delay. Thus, the median life expectancy of an infant with WD rarely exceeds 1 year old[13]. On the contrary, CESD shows much later during childhood or adulthood, and its symptoms are often mild and nonspecific, so that the disease can remain misdiagnosed for a long time[14]. In a recent investigation by Balwani et al[15], the median time from symptom onset to diagnostic testing among adult patients was 9.8 years. The features that mainly characterize later-onset LAL-D include: Liver disease, dyslipidemia, accelerated atherosclerosis, and premature cardiovascular disease. Calcification of the adrenal glands is instead not frequent[16].
Alterations in hepatic function may be discovered after routine radiological or biochemical examinations, showing hepatosplenomegaly and/or hypertransaminasemia[14]. Hepatomegaly occurs in the majority of patients (63%-99%)[15,16]. Macroscopically, the liver appears yellow-orange in color, due to lipid accumulation, and thus indistinguishable from other causes of fatty liver disease [such as metabolic-associated steatotic liver disease (MASLD)]. However, the micro
At biochemical analysis, elevated serum transaminases [alanine aminotransferase (ALT), aspartate aminotransferase (AST), or both] are observed in the majority of patients. However, their values are often not markedly increased (ALT and AST ≥ 1.5 × upper limit of normal in 46% and 15% of CESD cases, respectively), making these parameters nonspecific and not pathognomonic[15]. Taken together, macroscopic, microscopic, and biochemical tests are challenging to recognize LAL-D, as about 77% of subjects report 3 or more liver manifestations, such as elevated ALT, raised AST, and hepatomegaly[15].
The impairment of lipid metabolism caused by LAL-D is evident in the lipid profile of affected patients. Dyslipidemia is present in the majority of them (70%-95%), with elevated levels of total cholesterol and LDL cholesterol (LDL-c), and reduced concentrations of HDL cholesterol (HDL-c)[15,16]. Instead, hypertriglyceridemia is observed in half of LAL-D cases, and it often consists of levels slightly higher than the normal range[17]. This lipid profile is quite similar to other types of common genetic dyslipidemia, such as familial hypercholesterolemia (IIA phenotype) and familial combined hypercholesterolemia (IIA phenotype)[18]. Thus, differential diagnosis is essential in order to identify the disease and reduce the associated cardiovascular risk.
The abnormality of lipid metabolism and the elevated concentrations of LDL-c among CESD patients promote a pro-inflammatory and pro-atherogenic environment, which can soon lead to premature atherosclerosis and cardiovascular diseases[16]. Recently, Guerreiro et al[19] have confirmed the role of inflammation in LAL-D, showing an increased production of free radicals in this kind of patients, which makes them more susceptible to oxidative stress. For all these reasons, LAL-D individuals must be considered at heightened risk for premature atherosclerotic cardiovascular disease, and other risk factors (such as hypertension, smoking, diabetes mellitus, etc.) should be appropriately managed[20]. Moreover, the last genome-wide association studies have identified LIPA as a risk gene for coronary artery disease (CAD)[21]. Although a weak association was observed with an altered lipid profile, the rise of CAD risk due to increased LIPA transcription could be explained by other mechanisms mediated by LAL activity, such as growing production of free fatty acids and increasing release of interstitial cholesterol, which all promote plaque inflammation[22].
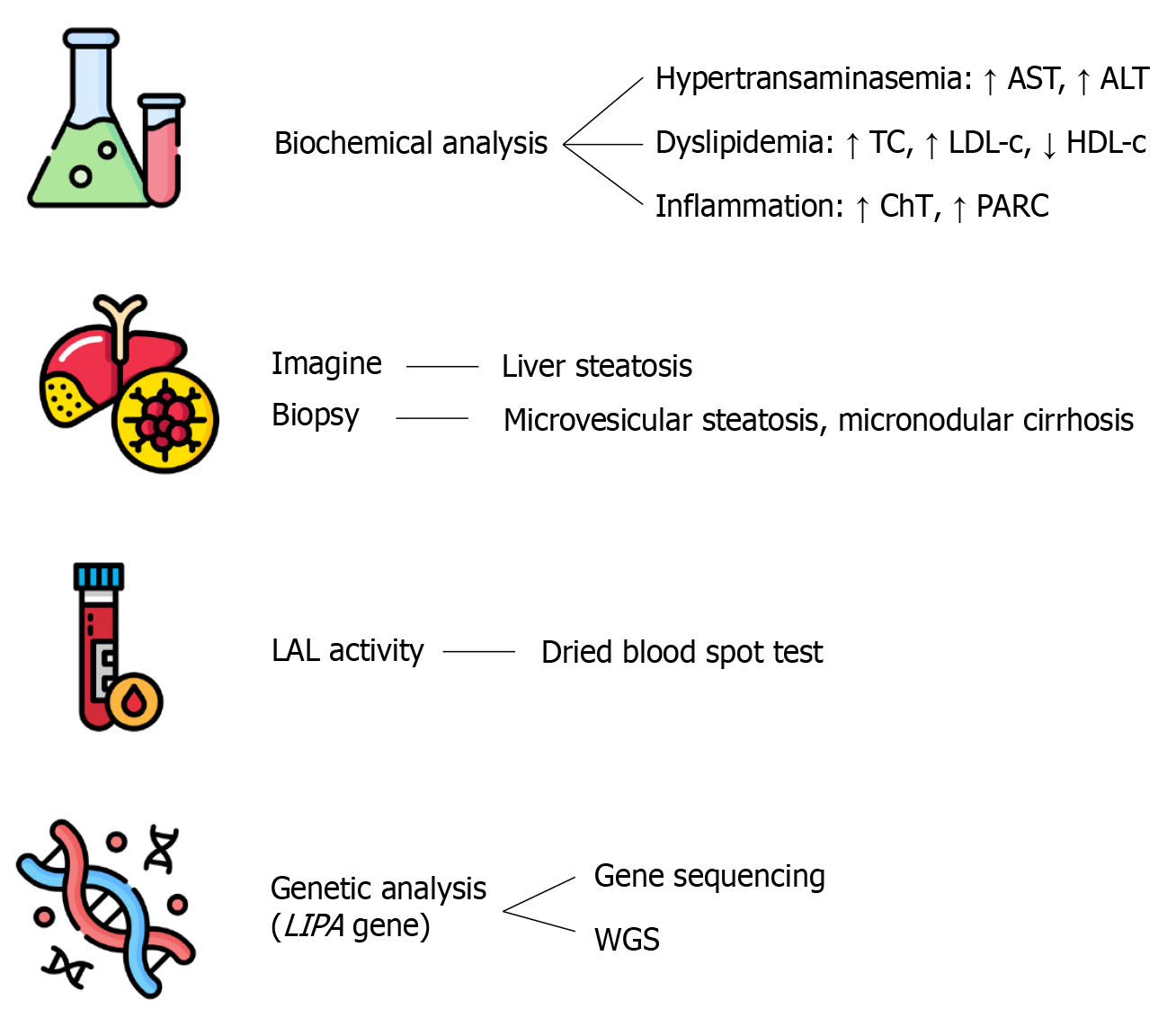
As LAL-D prevalence is low and clinical manifestations are often nonspecific, diagnosis can be very challenging. The diagnostic process is based on different assessments, which include: Biochemical analysis, imaging and histological tools, enzyme activity test, and genetics evaluation[14] (Figure 1).
The alteration of biochemical markers may occur precociously in LAL-D, but they are frequently detected during routine medical examinations. The most consistent abnormalities include elevated levels of liver enzymes (particularly ALT) and impairment in lipid profile (high LDL-c and low HDL-c concentrations). The former is suggestive of liver injury, while the latter represents a consequence of lipid metabolism alteration and can be classified as dyslipidemia[14]. According to the biochemical profile, three phenotypes can be distinguished, respectively characterized by hepatic impairment, dyslipidemia, or both (mixed phenotype)[14]. Although hepatic transaminases and cholesterol are available and routinely used dosages, they are nonspecific for LAL-D, and further studies are needed to identify disease-specific biomarkers. Recently, Cebolla et al[23] have investigated the possible role of parameters suggestive of secondary processes of LAL-D: Inflammation and oxidative stress. The assessment of inflammation state was performed through the measurement of plasma chitotriosidase activity and the concentration of pulmonary and activation-regulated chemokine (PARC, also called chemokine ligand 18 - CCL18), which were both higher among LAL-D patients[23]. Moreover, Cebolla et al[23] observed elevated levels of some oxysterols (e.g., 7-ketocholesterol and cholestane-3β, 5α, 6β-trio) in affected patients, which are known products of cholesterol oxidation and thus markers of oxidative stress. Taken together, plasma lipid-liver profile and inflammation-oxidative biomarkers are useful to diagnose LAL-D, but remain non-disease-specific.
As mentioned above, the liver is one of the main organs in which the lysosomal accumulation of CE and TG occurs. Thus, the quantification of hepatic fat is essential in the diagnostic pathway of LAL-D. This assessment can be performed with different image techniques, such as magnetic resonance and transient elastography[14]. Though the macroscopical appearance of LAL-D liver cannot be distinguished from other causes of fatty liver disease, the more invasive liver biopsy remains the gold standard for the evaluation of liver involvement[14].
Histological examination is a challenge to avoid misdiagnosis. In fact, histopathology of liver samples from patients with LAL-D shows the peculiar pattern of microvesicular steatosis and micronodular cirrhosis, which strongly suggests a lysosomal disease[6]. This is also confirmed by the use of lysosomal markers (such as cathepsin D, lysosomal-associated membrane protein 1 - LAMP1, LAMP2, and lysosomal integral membrane protein 2 - LIMP2) that can be stained through immunohistochemistry in paraffin-embedded liver specimens, helping to distinguish cytosolic from lysosomal lipid accumulation[24]. Moreover, polarized light can be used to see cholesterol crystals present in hepatocytes and Kupffer cells. The presence of these birefringent needle-shaped crystals is pathognomonic for LAL-D and can be examined with electron microscopy in frozen biopsy samples[24].
Whenever LAL-D is suspected, the residual assessment of LAL activity is essential to confirm the diagnosis. This analysis can be carried out on various biological samples, such as whole blood, peripheral leukocytes, fibroblasts, or liver tissue[25]. Due to easy execution and less invasiveness, the test is usually performed on a dried blood spot sample. LAL activity is evaluated using a fluorochrome-modified substrate (such as palmitic acid covalently modified with a 4-methylumbelliferone) and a specific LAL inhibitor (Lalistat-2). Residual LAL activity is assessed by quantifying overall blood lipase activity and then subtracting the activity measured in the presence of Lalistat-2, followed by fluorescence emission spectroscopy[26]. The LAL activity test is able to differentiate healthy individuals, carriers of LAL-D, and diseased patients. However, the test has a lower detection threshold of approximately 3% residual LAL activity; below this level, it is not possible to reliably differentiate between patients with WD and those with CESD, since both present with similarly low enzyme activity[26].
Although LAL residual activity represents a fundamental diagnostic step, genetic analysis is needed to confirm LAL-D. Sequencing of the LIPA gene allows for to detection and characterization of pathogenic variants, identifying patients with single-nucleotide mutations[27]. However, when changes are located in the intronic or regulatory regions of the LIPA gene, they cannot be detected with gene sequencing, and the use of further techniques such as transcriptome or whole genome sequencing is required[14]. In order to facilitate the identification of these patients, Halabi et al[28] have recently proposed the use of rapid whole genome sequencing for the LIPA gene.
Identifying LAL-D among other disorders that cause dyslipidemia and liver dysfunction is often difficult. In fact, LAL-D is often misdiagnosed, not only due to a lack of awareness of the disease but also because its presentation resembles more common conditions, such as familial hypercholesterolemia and MASLD[27]. Here, we report some “red flags” helpful to consider LAL-D as alternative diagnosis in patients with dyslipidemia and hepatic alterations[29]: (1) Absence of family history for metabolic syndrome; (2) Absence of other common components of metabolic syndrome (e.g., hypertension, diabetes mellitus, visceral obesity); (3) Presence of microvescicular steatosis at liver biopsy; (4) Early atherosclerotic disease; and (5) Poor response to traditional lipid-lowering interventions (e.g., statins, fibrates, lifestyle changes). Nevertheless, LAL-D remains a very rare disease. Even if a few cases have been identified in at-risk populations presenting with suggestive signs and symptoms of the disease[30], evidence from larger cohort studies did not support the use of routine testing or screening programs for the detection of LAL-D among hypercholesterolemic subjects[31,32].
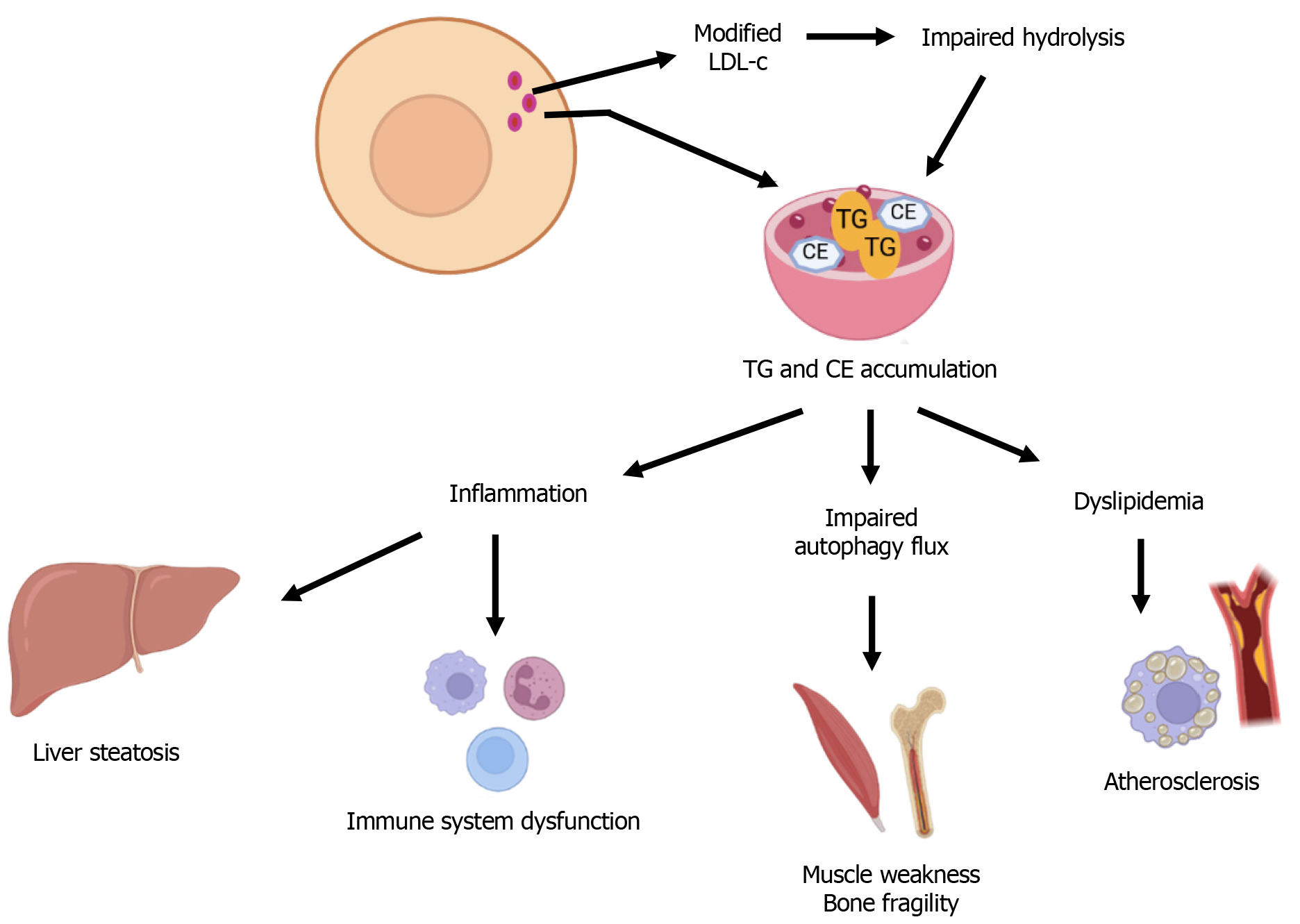
LAL-D leads to a systemic disturbance in intracellular lipid homeostasis, with far-reaching biological consequences. The primary biochemical defect involves impaired hydrolysis of CE and TG derived from modified LDL-c into free cholesterol and fatty acids within lysosomes. This impairment results in the progressive accumulation of these lipids in the liver, vasculature, immune cells, and bone spectroscopy[33] (Figure 2). The resulting metabolic derangements trigger a cascade of dysfunctions at the cellular and tissue levels, underpinning the clinical heterogeneity of LAL-D and its association with both hepatic and cardiovascular pathology, as discussed below.
As mentioned above, a hallmark of LAL-D is a profoundly abnormal lipid profile characterized by elevated LDL-c, low HDL-c, and increased serum TG - an atherogenic profile that promotes premature cardiovascular disease[34]. The impaired lysosomal hydrolysis of CE disrupts intracellular cholesterol trafficking, thereby activating sterol-regulatory element-binding protein (SREBP)-mediated upregulation of de novo cholesterol synthesis and LDL receptor expression. This paradoxically increases cellular cholesterol uptake and exacerbates lipid storage pathology[35,36]. Lipid-laden lysosomes accumulate in hepatocytes and macrophages, triggering microvesicular steatosis and activating transcription factors SREBP-1/2 and SREBP-1c, which drive de novo lipogenesis and LDL receptor expression while suppressing liver X receptors- adenosine triphosphate-binding cassette transporter A1 (ABCA1)-mediated HDL formation[29,22]. These dyslipidemic changes are tightly linked to hepato-steatosis and liver inflammation, as well as systemic atherogenesis, establishing a clear mechanistic bridge between hepatic dysfunction and cardiovascular disease in LAL-D.
In fact, LAL-D predisposes patients to early-onset and accelerated atherosclerosis driven by dyslipidemia as well as intrinsic cellular pathology and inflammation within the arterial wall. In particular, impaired hydrolysis of CE and TG in macrophages and smooth muscle cells leads to lysosomal CE accumulation, lysosomal dysfunction and enlargement, and foam cell formation, a key step in atherogenesis[37]. Macrophages and vascular smooth muscle cells transform into foam cells due to extensive intracellular lipid accumulation and subsequently secrete pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β, driving persistent vascular inflammation and plaque progression. Tumor necrosis factor-α causes an increase in cell adhesion molecules, favoring the recruitment of further vascular smooth muscle cells and immune cells[38]. While the phagocytosis of oxidized LDL induces macrophages to release interleukin-1β by activating the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 inflammasome, reinforcing chronic inflammatory signaling within the plaque[39]. In parallel, impaired lysosomal processing of cholesterol reduces free cholesterol availability for liver X receptor activation, leading to suppressed ABCA1 expression and diminished cholesterol efflux from foam cells, which further exacerbates atherosclerotic lesion development[40]. These intertwined mechanisms (lipid-stressed lysosomes, inflammatory cytokine release, and impaired cholesterol efflux) form a vicious cycle that fuels foam cell pathogenicity and atherosclerotic plaque progression in LAL-D[41].
Moreover, LAL-mediated lipid catabolism is important in adipose tissue biology and in cardiovascular risk. LIPA-/- mice exhibit significantly lower body weight compared to their wild-type counterparts, accompanied by a progressive reduction in both white and brown adipose tissue[36]. In contrast, obesity stimulates the formation of new lysosomes in adipose tissue macrophages (ATM), as shown by elevated expression of genes encoding lysosomal structural proteins (for instance, LAMP2), hydrolases (such as LIPA), and components of vacuolar proton pumps (like Atp6v1b2), without increasing markers of classical inflammatory activation. Consistently, chloroquine-treated ATM[36] and LIPA-deficient human induced pluripotent stem cell-derived macrophages[42] show significant lipid accumulation, but do not display a gene expression profile typical of inflammatory activation. Thus, the lipid accumulation resulting from LAL deficiency does not seem to be linked to an intrinsic pro-inflammatory response in macrophages[42]. In ATM-depleted adipose tissue, the inhibitory effect of chloroquine on lipolysis is lost, highlighting the importance of macrophage LAL in regulating lipid breakdown within adipose tissue. In atherosclerotic plaques, macrophages accumulate large amounts of CE and free cholesterol[43] in their lysosomes, causing impaired lysosomal lipid degradation. However, several studies have observed elevated LAL activity in atherosclerotic tissues as well as in lysosomes rich in lipids isolated from these tissues[44,45]. Though it remains uncertain whether this increase in LAL activity is due to a greater abundance of LAL-expressing cells within the lesion and/or an upregulation of LAL expression at the cellular level. Moreover, LAL messenger RNA levels or enzymatic activity measured in vitro do not necessarily reflect its true lipolytic function within atherosclerotic lesions, especially when lysosomal acidification is impaired. Indeed, resident aortic CD45þ+/CD64þ+/merTKþ+ macrophages of ApoE-deficient mice fed a western diet for 2 months displayed decreased lysotracker fluorescence, a dye that accumulates in late endosomes and lysosomes, indicating impaired lysosomal function[46]. Treating murine macrophages with lysosomal stressors such as chloroquine or atherogenic lipids, such as oxidized LDL and cholesterol crystals, results in significant lysosomal dysfunction, which is marked by altered lysosomal pH, reduced proteolytic activity, and compromised membrane integrity[46]. The impairment in lysosomal function is also linked to the nuclear translocation of the transcription factor EB as well as to an elevated expression of lysosome (LIPA, Atp6v0d2, LAMP1) and autophagy-related genes (BECN1 and p62/SQSTM1)[46]. Increasing transcription factor EB expression in macrophages stimulates the formation of new lysosomes and enhances autophagy, thus helping in counteracting the cell death and inflammation triggered by atherogenic lipid overload, without reducing the build-up of lipids in the cells despite increased LAL activity[47]. Thus, boosting the degradative capacity of macrophages is considered a promising therapeutic strategy for atherosclerosis[47], but the effect of increasing macrophage LIPA expression and LAL-mediated lipolysis on the development of atherosclerosis is not yet fully understood[48]. Multiple genome-wide association study[21,49,50] have identified LIPA as a novel locus for CAD. Despite the well-known role of LIPA in lipoprotein metabolism, the common LIPA CAD risk alleles, rs1412444 (T) and rs2246833 (T), clustered in introns 2/3 in high linkage disequilibrium, are not associated with altered plasma lipids[51], liver traits[51], and are not associated with LIPA expression (i.e., are not expression quantitative trait) locus in liver tissue[52]. These studies will shed light on the potential for benefit and risk in therapeutic targeting of LIPA in CAD, particularly in the context of the availability of LAL replacement therapy currently approved for use in patients with CESD. The significance of LAL in a broad spectrum of pathophy
The lysosomal accumulation of CE and TG in peripheral tissues triggers the release of chemokines and growth factors, which mediate the recruitment of T cells, monocyte-derived macrophages, and dendritic cells, thereby amplifying the local production of pro-inflammatory cytokines, impairing antigen presentation, and promoting chronic inflammation[53]. Lipid-laden macrophages, commonly observed in LAL-D tissues, adopt a pro-inflammatory phenotype due to chronic lysosomal stress and impaired cholesterol efflux, contributing to systemic immune dysregulation. Moreover, LAL-D disrupts the polarization of macrophages toward the anti-inflammatory M2 phenotype, skewing toward the M1-like, pro-inflammatory phenotype, and compromises their mitochondrial oxidative metabolism[54]. These alterations amplify hepatic fibrosis and accelerate atherosclerosis through local and systemic inflammatory signaling, reinforcing the role of LAL-D as an immune-metabolic disorder. There is evidence of an inverse correlation between LAL activity and the development of metabolic-associated fatty liver disease in humans. In preclinical models, in an effort to clarify the role of hepatic LAL activity and macrophages in metabolic dysfunction-associated steatohepatitis (MASH), it was shown that hepatocyte-specific depletion of LAL (hepLAL-/-) led to an augmentation of TC and CE levels in the liver[55]. However, Kupffer cells’ elimination worsens hepLAL-/- mice phenotype, and results in an increase in hepatic and plasma cholesterol levels with minimal effects on inflammation without significant changes in fibrotic lesions. This study points out the importance of immune cells in modulating metabolic-associated fatty liver disease progression to MASH, and that macrophage phenotype modulation rather than depletion could be a potential therapeutic strategy also in LAL-D[55].
In LIPA-/- mice, the absence of LAL led to a significant reduction in multipotent progenitor cells, accompanied by an increase in downstream myeloid precursors, including common myeloid progenitors and granulocyte/monocyte progenitors, compared to wild-type controls. These shifts in progenitor cell populations were reflected in the peripheral blood, where LIPA-/- mice exhibited a markedly decreased proportion of circulating monocytes and natural killer cells[56]. A similar pattern was observed in human patients with CESD. Thus, LAL may function as a critical immune-metabolic regulator, influencing the development and distribution of immune cell populations in both mice and humans.
The LAL enzyme plays a key role in the breakdown of CE within lipid droplets, a process triggered by cholesterol accumulation in macrophages, to produce free cholesterol primarily for ABCA1-mediated efflux from foam cells[57]. These lipid droplets are brought into lysosomes by autophagy. There is evidence implicating autophagic dysfunction in the pathophysiology of LAL-D[58]. Moriwaki et al[58] demonstrated that in LIPA-/- HeLa cells, autophagy is impaired under nutrient-rich conditions due to disrupted lysosomal acidity, triggered by increased sensitivity to ammonia derived from glutamine metabolism. This acidity defect is linked to enlarged lysosomes without a corresponding increase in vacuolar-type ATPase, the proton pump responsible for acidifying lysosomes. Although autophagy remains normal under starvation, the primary defect, lysosomal enlargement, persists, indicating a fundamental disruption in lysosomal homeostasis. These findings highlight LAL’s essential role in maintaining lysosomal integrity and autophagic function, offering insights into the pathology of WD and the potential impact of metabolic stressors like hyperlipidemia. The excessive lipid accumulation within lysosomes impairs autophagic flux by disrupting lysosome-autophagosome fusion, which exacerbates hepatocellular injury and may contribute to muscle pathology through defective mitophagy and energy depletion[59]. This defect perpetuates a vicious cycle of organelle stress and metabolic insufficiency, especially in tissues with high turnover or metabolic demand.
Skeletal muscle, though not classically emphasized in LAL-D, is increasingly recognized as a secondary site of pathology. Mitochondrial and lipid metabolic abnormalities in myocytes, likely driven by systemic lipo-toxicity and impaired autophagy, may contribute to muscle weakness and exercise intolerance reported in some patients[60]. Moreover, emerging data suggest that LAL-D may affect bone metabolism. Disruption of lipid signaling pathways, essential for osteoblast and osteoclast function, has been linked to decreased bone mineral density and altered bone remodeling, although the clinical relevance of these findings warrants further investigation[61]. Chronic inflammation further potentiates bone resorption, and impaired autophagy in osteocytes may disrupt their function, enhancing skeletal fragility.
Treatment of LAL-D in adults is focused on reducing the cardiovascular risk using lipid-lowering drugs, while liver and bone marrow transplantation are reserved for the most severe forms associated with organ failure[16,62].
Lipid-lowering drugs are one of the pivotal strategies of LAL-D traditional treatment. Among these, statins are widely used. They reduce total cholesterol and LDL-c and modestly increase HDL-c levels through the inhibition of cholesterol synthesis by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase, leading to an increase in serum lipoprotein uptake through the low-density lipoprotein receptor. Controversies on statin efficacy are related to the enhanced uptake of cholesterol ester by the liver, potentially leading to increased lysosomal content[1]. Most data derive from patients receiving simvastatin[63] or lovastatin, and demonstrate significant reduction of LDL-c levels[64-68] and Apolipoprotein B synthesis in adolescents affected by CESD[67].
Questions have been raised regarding the efficacy of statins in preventing cardiovascular diseases in patients with LAL-D[69], as these individuals continue to exhibit elevated levels of LDL-c[70] while the liver does not show improvement or even worsens[67,71]. Although reductions in liver size and improvements in liver profile have been observed during lovastatin therapy, cases of liver failure and poor long-term outcomes have also been reported in some instances[72]. Normalization of the lipid profile in children with LAL-D does not always halt the progression of liver disease or prevent hepatic failure.
As an alternative or in combination with statins, other lipid-lowering drugs can be used. Bile chelating resins and ezetimibe both act to decrease cholesterol absorption in the intestine. Ezetimibe specifically blocks cholesterol uptake at the brush border of the small intestine by inhibiting the Niemann Pick C1-like 1 transporter activity[73]. It enhances the plasma lipoprotein profile, particularly by further lowering LDL cholesterol when used alongside statin therapy, and this combination is generally well tolerated. However, in patients with LAL-D, combining statins with resins has not shown long-term improvements in cholesterol levels[74]. In a 10-year-old child with hepatomegaly and biopsy-confirmed liver fibrosis, ezetimibe, administered for 80 months, resulted in normalization of AST levels, decreased serum lipid levels, and a reduction of hepatomegaly. However, there was no report on post-treatment liver transplantation (LT) levels or liver biopsy[75]. In observational case series, ezetimibe was found effective, safe, and sustainable in a long-duration treatment (10 years), showing a significant reduction of ALT, cholesterol, and TG, with no progression of liver fibrosis at hepatic elastography[76]. Moreover, in order to improve lipid profile, a low-lipid diet may be recommended among CESD patients, although a strict regimen is not usually necessary[14].
The other pillar of LAL-D treatment is enzyme replacement therapy (ERT). The recombinant human LAL, sebelipase-α, has recently been approved in both Europe and the United States[77] as a long-term ERT for patients of all ages with LAL-D. This therapy is administered intravenously every 2 weeks at a dose of 1 mg/kg body weight, and has demon
In another long-term study performed on an adult population (LAL-CL04), sebelipase-α therapy for up to 5 years achieved sustained improvements in markers of liver and lipid dysfunction, decreased liver volume and fat content, and was well tolerated[81]. In 2022, Burton et al[82] tried to broaden inclusion criteria, analyzing a more heterogeneous population compared to previous ones (acid lipase replacement investigating safety and efficacy and LAL-CL04), with patients at different illness stages. In this cohort, they observed that the assessment of treatment response depended on different factors: The disease phenotype at baseline, the dosage of sebelipase-α, the dietary regimen employed, and concomitant diseases and medications[82].
Safety and efficiency of ERT were also confirmed in a recent systematic review. sebelipase-α was shown to be effective in decreasing serum biomarkers (AST, ALT, total cholesterol, TG, and LDL-c) among LAL-D patients, but it did not induce a significant reduction of liver volume[83]. Meanwhile, adverse pharmacological events were rare and mild/moderate in severity[83].
Although ERT treatment appears to attenuate clinical manifestations, it leads to improvement of liver fibrosis and cardiovascular outcomes in most but not all patients with CESD. Thus, suspected non-responders should be assessed for any underlying comorbidities that may be contributing to the severity and progression of disease before consideration of discontinuing sebelipase-α[82]. Moreover, ERT therapy shows various limitations, including invasiveness (usually 2 hours of intravenous administration are required weekly or bi-weekly) and costs[84].
Unlike treatments mentioned above, LT is generally reserved for patients with LAL-D who develop liver failure, due to the significant risks associated with the procedure, the need for lifelong immunosuppression, and the fact that extra-hepatic symptoms of the disease often persist after transplantation. It has been performed in a limited number of pediatric patients and one adult[16,62,85,86], being successful in four children and in the adult patient. However, a retrospective analysis by Bernstein et al[87] showed that 61% of transplanted patients developed multi-organ LAL-D progression even after LT. In fact, although LT is indispensable in patients suffering from end-stage liver failure or hepatocellular carcinoma, the deficiency il LAL enzyme activity persists, and disease recurrence may occur[87]. Moreover, a recent case report signaled the presence of an undiagnosed LAL-D patient among those enlisted for liver transplant in a Brazilian hospital, also suggesting the importance of a preoperative differential diagnosis[88].
Early treatment initiation for LAL-D is challenging. It may improve liver-lipid biomarkers and prevent potentially serious disease-related adverse outcomes. Further research is necessary to determine the impact of sebelipase-α on quality-of-life measures, how and the extent to which sebelipase-α improves liver histology, the role of concomitant dietary measures, and whether certain groups of patients with LAL-D benefit more from ERT than others. Further studies are also needed to assess the cardiovascular benefit[89].
Among innovative and alternative treatments, hematopoietic stem cell transplantation is the most investigated for WD. However, current data are conflicting and inconsistent: Some studies described hematopoietic stem cell transplantation as a successful treatment, while others reported disease progression and fatal transplant-related complications[29]. Gene therapy is instead a novel therapeutic approach currently under investigation for many lysosomal storage diseases, including CESD[90]. Recent preclinical studies have observed the efficacy of a gene treatment based on the in vivo administration of adeno-associated virus encoding human LIPA transgene in LIPA-/- mice[91,92]. This gene therapy led to significant improvement in disease symptoms (hepatosplenomegaly attenuation, serum liver-lipid biomarkers reduction) and to achievement of a stable long-term LAL expression sufficient to correct the disease phenotype[91,92]. Interestingly, treatment was more effective in adult mice than in neonates, suggesting that adeno-associated virus-mediated gene therapy should be a viable option, especially for CESD patients[91].
Because of the low prevalence and the nonspecific presentation, LAL-D is often misrecognized, especially in its late-onset form (CESD). However, the alteration of lipid metabolism caused by the disease puts LAL-D patients at high risk of developing not only liver dysfunction (MASLD, MASH, cirrhosis), but also premature atherosclerosis and cardiovascular disease. Thus, prompt identification and treatment are essential, and greater awareness of this condition among clinicians is required.
We thank Emanuela Tolosano for her help in figure editing.
| 1. | Reiner Ž, Guardamagna O, Nair D, Soran H, Hovingh K, Bertolini S, Jones S, Ćorić M, Calandra S, Hamilton J, Eagleton T, Ros E. Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | ABRAMOV A, SCHORR S, WOLMAN M. Generalized xanthomatosis with calcified adrenals. AMA J Dis Child. 1956;91:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Fredrickson DS, Sloan HR, Ferrans VJ, Demosky SJ Jr. Cholesteryl ester storage disease: a most unusual manifestation of deficiency of two lysosomal enzyme activities. Trans Assoc Am Physicians. 1972;85:109-119. [PubMed] |
| 4. | Aslanidis C, Ries S, Fehringer P, Büchler C, Klima H, Schmitz G. Genetic and biochemical evidence that CESD and Wolman disease are distinguished by residual lysosomal acid lipase activity. Genomics. 1996;33:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Rajamohan F, Reyes AR, Ruangsiriluk W, Hoth LR, Han S, Caspers N, Tu M, Ward J, Kurumbail RG. Expression and functional characterization of human lysosomal acid lipase gene (LIPA) mutation responsible for cholesteryl ester storage disease (CESD) phenotype. Protein Expr Purif. 2015;110:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Pericleous M, Kelly C, Wang T, Livingstone C, Ala A. Wolman's disease and cholesteryl ester storage disorder: the phenotypic spectrum of lysosomal acid lipase deficiency. Lancet Gastroenterol Hepatol. 2017;2:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Carter A, Brackley SM, Gao J, Mann JP. The global prevalence and genetic spectrum of lysosomal acid lipase deficiency: A rare condition that mimics NAFLD. J Hepatol. 2019;70:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Zhang JH, Lin AP, Zhang L, Ruan DD, Gao MZ, Chen Q, Yu HP, Liao LS, Lin XF, Fang ZT, Lin F, Lu SY, Luo JW, Zheng XL, Chen MS. Pedigree Analysis of Nonclassical Cholesteryl Ester Storage Disease with Dominant Inheritance in a LIPA I378T Heterozygous Carrier. Dig Dis Sci. 2024;69:2109-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Bychkov IO, Kamenets EA, Filatova AY, Skoblov MY, Mikhaylova SV, Strokova TV, Gundobina OS, Zakharova EY. The novel synonymous variant in LIPA gene affects splicing and causes lysosomal acid lipase deficiency. Mol Genet Metab. 2019;127:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Robinson SL, Mumford SL, Guan W, Zeng X, Kim K, Radoc JG, Trinh MH, Flannagan K, Schisterman EF, Yeung E. Maternal fatty acid concentrations and newborn DNA methylation. Am J Clin Nutr. 2020;111:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Płatek T, Polus A, Góralska J, Raźny U, Gruca A, Kieć-Wilk B, Zabielski P, Kapusta M, Słowińska-Solnica K, Solnica B, Malczewska-Malec M, Dembińska-Kieć A. DNA methylation microarrays identify epigenetically regulated lipid related genes in obese patients with hypercholesterolemia. Mol Med. 2020;26:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Scott SA, Liu B, Nazarenko I, Martis S, Kozlitina J, Yang Y, Ramirez C, Kasai Y, Hyatt T, Peter I, Desnick RJ. Frequency of the cholesteryl ester storage disease common LIPA E8SJM mutation (c.894G>A) in various racial and ethnic groups. Hepatology. 2013;58:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Jones SA, Valayannopoulos V, Schneider E, Eckert S, Banikazemi M, Bialer M, Cederbaum S, Chan A, Dhawan A, Di Rocco M, Domm J, Enns GM, Finegold D, Gargus JJ, Guardamagna O, Hendriksz C, Mahmoud IG, Raiman J, Selim LA, Whitley CB, Zaki O, Quinn AG. Rapid progression and mortality of lysosomal acid lipase deficiency presenting in infants. Genet Med. 2016;18:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | de Las Heras J, Almohalla C, Blasco-Alonso J, Bourbon M, Couce ML, de Castro López MJ, García Jiménez MC, Gil Ortega D, González-Diéguez L, Meavilla S, Moreno-Álvarez A, Pastor-Rosado J, Sánchez-Pintos P, Serrano-Gonzalo I, López E, Valdivielso P, Yahyaoui R, Quintero J. Practical Recommendations for the Diagnosis and Management of Lysosomal Acid Lipase Deficiency with a Focus on Wolman Disease. Nutrients. 2024;16:4309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Balwani M, Balistreri W, D'Antiga L, Evans J, Ros E, Abel F, Wilson DP. Lysosomal acid lipase deficiency manifestations in children and adults: Baseline data from an international registry. Liver Int. 2023;43:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Bernstein DL, Hülkova H, Bialer MG, Desnick RJ. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J Hepatol. 2013;58:1230-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Zhang B, Porto AF. Cholesteryl ester storage disease: protean presentations of lysosomal acid lipase deficiency. J Pediatr Gastroenterol Nutr. 2013;56:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Fouchier SW, Defesche JC. Lysosomal acid lipase A and the hypercholesterolaemic phenotype. Curr Opin Lipidol. 2013;24:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Guerreiro G, Deon M, Vargas CR. Evaluation of biochemical profile and oxidative damage to lipids and proteins in patients with lysosomal acid lipase deficiency. Biochem Cell Biol. 2023;101:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Maciejko JJ. Managing Cardiovascular Risk in Lysosomal Acid Lipase Deficiency. Am J Cardiovasc Drugs. 2017;17:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 562] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 22. | Besler KJ, Blanchard V, Francis GA. Lysosomal acid lipase deficiency: A rare inherited dyslipidemia but potential ubiquitous factor in the development of atherosclerosis and fatty liver disease. Front Genet. 2022;13:1013266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 23. | Cebolla JJ, Irún P, Mozas P, Giraldo P. Evaluation of two approaches to lysosomal acid lipase deficiency patient identification: An observational retrospective study. Atherosclerosis. 2019;285:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Hůlková H, Elleder M. Distinctive histopathological features that support a diagnosis of cholesterol ester storage disease in liver biopsy specimens. Histopathology. 2012;60:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Guy GJ, Butterworth J. Acid esterase activity in cultured skin fibroblasts and amniotic fluid cells using 4-methylumbelliferyl palmitate. Clin Chim Acta. 1978;84:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Hamilton J, Jones I, Srivastava R, Galloway P. A new method for the measurement of lysosomal acid lipase in dried blood spots using the inhibitor Lalistat 2. Clin Chim Acta. 2012;413:1207-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Kohli R, Ratziu V, Fiel MI, Waldmann E, Wilson DP, Balwani M. Initial assessment and ongoing monitoring of lysosomal acid lipase deficiency in children and adults: Consensus recommendations from an international collaborative working group. Mol Genet Metab. 2020;129:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Halabi N, Ramaswamy S, El Naofal M, Taylor A, Yaslam S, Jain R, Alfalasi R, Shenbagam S, Bitzan M, Yavuz L, Abulhoul H, Shankar S, Janjua D, Jadhav D, Al Maazmi MM, Abuhammour W, Alsheikh-Ali A, Al Awadhi M, Al Khayat A, Abou Tayoun AN. Rapid whole genome sequencing of critically ill pediatric patients from genetically underrepresented populations. Genome Med. 2022;14:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 29. | Strebinger G, Müller E, Feldman A, Aigner E. Lysosomal acid lipase deficiency - early diagnosis is the key. Hepat Med. 2019;11:79-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Tebani A, Sudrié-Arnaud B, Boudabous H, Brassier A, Anty R, Snanoudj S, Abergel A, Abi Warde MT, Bardou-Jacquet E, Belbouab R, Blanchet E, Borderon C, Bronowicki JP, Cariou B, Carette C, Dabbas M, Dranguet H, de Ledinghen V, Ferrières J, Guillaume M, Krempf M, Lacaille F, Larrey D, Leroy V, Musikas M, Nguyen-Khac E, Ouzan D, Perarnau JM, Pilon C, Ratzlu V, Thebaut A, Thevenot T, Tragin I, Triolo V, Vergès B, Vergnaud S, Bekri S. Large-scale screening of lipase acid deficiency in at risk population. Clin Chim Acta. 2021;519:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Vinje T, Wierød L, Leren TP, Strøm TB. Prevalence of cholesteryl ester storage disease among hypercholesterolemic subjects and functional characterization of mutations in the lysosomal acid lipase gene. Mol Genet Metab. 2018;123:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Ashfield-Watt P, Haralambos K, Edwards R, Townsend D, Gingell R, Wa Li K, Humphries SE, McDowell I. Estimation of the prevalence of cholesteryl ester storage disorder in a cohort of patients with clinical features of familial hypercholesterolaemia. Ann Clin Biochem. 2019;56:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Li F, Zhang H. Lysosomal Acid Lipase in Lipid Metabolism and Beyond. Arterioscler Thromb Vasc Biol. 2019;39:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 34. | Arnaboldi L, Ossoli A, Giorgio E, Pisciotta L, Lucchi T, Grigore L, Pavanello C, Granata A, Pasta A, Arosio B, Azzolino D, Baragetti A, Castelnuovo S, Corsini A, Catapano AL, Calabresi L, Gomaraschi M. LIPA gene mutations affect the composition of lipoproteins: Enrichment in ACAT-derived cholesteryl esters. Atherosclerosis. 2020;297:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Bianco V, Korbelius M, Vujic N, Akhmetshina A, Amor M, Kolb D, Pirchheim A, Bradic I, Kuentzel KB, Buerger M, Schauer S, Phan HTT, Bulfon D, Hoefler G, Zimmermann R, Kratky D. Impact of (intestinal) LAL deficiency on lipid metabolism and macrophage infiltration. Mol Metab. 2023;73:101737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Dubland JA, Francis GA. Lysosomal acid lipase: at the crossroads of normal and atherogenic cholesterol metabolism. Front Cell Dev Biol. 2015;3:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Javadifar A, Rastgoo S, Banach M, Jamialahmadi T, Johnston TP, Sahebkar A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding RNAs. Int J Mol Sci. 2021;22:2529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Jiang Y, Wang M, Huang K, Zhang Z, Shao N, Zhang Y, Wang W, Wang S. Oxidized low-density lipoprotein induces secretion of interleukin-1β by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation. Biochem Biophys Res Commun. 2012;425:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Dubland JA, Allahverdian S, Besler KJ, Ortega C, Wang Y, Pryma CS, Boukais K, Chan T, Seidman MA, Francis GA. Low LAL (Lysosomal Acid Lipase) Expression by Smooth Muscle Cells Relative to Macrophages as a Mechanism for Arterial Foam Cell Formation. Arterioscler Thromb Vasc Biol. 2021;41:e354-e368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Guardamagna O, Guaraldi F. Lysosomal Acid Lipase Deficiency: Could Dyslipidemia Drive the Diagnosis? Curr Pediatr Rev. 2017;13:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Zhang H, Shi J, Hachet MA, Xue C, Bauer RC, Jiang H, Li W, Tohyama J, Millar J, Billheimer J, Phillips MC, Razani B, Rader DJ, Reilly MP. CRISPR/Cas9-Mediated Gene Editing in Human iPSC-Derived Macrophage Reveals Lysosomal Acid Lipase Function in Human Macrophages-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:2156-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res. 2006;9:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Haley NJ, Fowler S, de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res. 1980;21:961-969. [PubMed] |
| 45. | Davis HR, Glagov S, Zarins CK. Role of acid lipase in cholesteryl ester accumulation during atherogenesis. Correlation of enzyme activity with acid lipase-containing macrophages in rabbit and human lesions. Atherosclerosis. 1985;55:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Emanuel R, Sergin I, Bhattacharya S, Turner J, Epelman S, Settembre C, Diwan A, Ballabio A, Razani B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014;34:1942-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 47. | Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, Javaheri A, Crowley JR, Ballabio A, Schilling JD, Epelman S, Weihl CC, Diwan A, Fan D, Zayed MA, Razani B. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat Commun. 2017;8:15750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 48. | Zhang H. Lysosomal acid lipase and lipid metabolism: new mechanisms, new questions, and new therapies. Curr Opin Lipidol. 2018;29:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Wild PS, Zeller T, Schillert A, Szymczak S, Sinning CR, Deiseroth A, Schnabel RB, Lubos E, Keller T, Eleftheriadis MS, Bickel C, Rupprecht HJ, Wilde S, Rossmann H, Diemert P, Cupples LA, Perret C, Erdmann J, Stark K, Kleber ME, Epstein SE, Voight BF, Kuulasmaa K, Li M, Schäfer AS, Klopp N, Braund PS, Sager HB, Demissie S, Proust C, König IR, Wichmann HE, Reinhard W, Hoffmann MM, Virtamo J, Burnett MS, Siscovick D, Wiklund PG, Qu L, El Mokthari NE, Thompson JR, Peters A, Smith AV, Yon E, Baumert J, Hengstenberg C, März W, Amouyel P, Devaney J, Schwartz SM, Saarela O, Mehta NN, Rubin D, Silander K, Hall AS, Ferrieres J, Harris TB, Melander O, Kee F, Hakonarson H, Schrezenmeir J, Gudnason V, Elosua R, Arveiler D, Evans A, Rader DJ, Illig T, Schreiber S, Bis JC, Altshuler D, Kavousi M, Witteman JC, Uitterlinden AG, Hofman A, Folsom AR, Barbalic M, Boerwinkle E, Kathiresan S, Reilly MP, O'Donnell CJ, Samani NJ, Schunkert H, Cambien F, Lackner KJ, Tiret L, Salomaa V, Munzel T, Ziegler A, Blankenberg S. A genome-wide association study identifies LIPA as a susceptibility gene for coronary artery disease. Circ Cardiovasc Genet. 2011;4:403-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | IBC 50K CAD Consortium. Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 2011;7:e1002260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Döring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2340] [Cited by in RCA: 2388] [Article Influence: 183.7] [Reference Citation Analysis (0)] |
| 52. | GTEx Consortium; Laboratory, Data Analysis &Coordinating Center (LDACC)—Analysis Working Group; Statistical Methods groups—Analysis Working Group; Enhancing GTEx (eGTEx) groups; NIH Common Fund; NIH/NCI; NIH/NHGRI; NIH/NIMH; NIH/NIDA; Biospecimen Collection Source Site—NDRI; Biospecimen Collection Source Site—RPCI; Biospecimen Core Resource—VARI; Brain Bank Repository—University of Miami Brain Endowment Bank; Leidos Biomedical—Project Management; ELSI Study; Genome Browser Data Integration &Visualization—EBI; Genome Browser Data Integration &Visualization—UCSC Genomics Institute, University of California Santa Cruz; Lead analysts:; Laboratory, Data Analysis &Coordinating Center (LDACC):; NIH program management:; Biospecimen collection:; Pathology:; eQTL manuscript working group:, Battle A, Brown CD, Engelhardt BE, Montgomery SB. Genetic effects on gene expression across human tissues. Nature. 2017;550:204-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3357] [Cited by in RCA: 2964] [Article Influence: 329.3] [Reference Citation Analysis (0)] |
| 53. | Pandey MK. Exploring Pro-Inflammatory Immunological Mediators: Unraveling the Mechanisms of Neuroinflammation in Lysosomal Storage Diseases. Biomedicines. 2023;11:1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 54. | Vassiliou E, Farias-Pereira R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. Int J Mol Sci. 2023;24:12032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 117] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 55. | Bradić I, Kuentzel KB, Pirchheim A, Rainer S, Schwarz B, Trauner M, Larsen MR, Vujić N, Kratky D. From LAL-D to MASLD: Insights into the role of LAL and Kupffer cells in liver inflammation and lipid metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 2025;1870:159575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Bellini R, Bonacina F, Kratky D, Gomaraschi M, Norata GD. Lysosomal acid lipase (LAL) deficiency alters immune cells distribution in mice and humans. Arteriosclerosis. 2022;355:6. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 627] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 58. | Moriwaki T, Terawaki S, Otomo T. Impaired lysosomal acidity maintenance in acid lipase-deficient cells leads to defective autophagy. J Biol Chem. 2024;300:105743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 59. | Raza S, Rajak S, Yen PM, Sinha RA. Autophagy and hepatic lipid metabolism: mechanistic insight and therapeutic potential for MASLD. NPJ Metab Health Dis. 2024;2:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 60. | Akhmetshina A, Bianco V, Bradić I, Korbelius M, Pirchheim A, Kuentzel KB, Eichmann TO, Hinteregger H, Kolb D, Habisch H, Liesinger L, Madl T, Sattler W, Radović B, Sedej S, Birner-Gruenberger R, Vujić N, Kratky D. Loss of lysosomal acid lipase results in mitochondrial dysfunction and fiber switch in skeletal muscles of mice. Mol Metab. 2024;79:101869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Helderman RC, Whitney DG, Duta-Mare M, Akhmetshina A, Vujic N, Jayapalan S, Nyman JS, Misra BB, Rosen CJ, Czech MP, Kratky D, Rendina-Ruedy E. Loss of function of lysosomal acid lipase (LAL) profoundly impacts osteoblastogenesis and increases fracture risk in humans. Bone. 2021;148:115946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Ambler GK, Hoare M, Brais R, Shaw A, Butler A, Flynn P, Deegan P, Griffiths WJ. Orthotopic liver transplantation in an adult with cholesterol ester storage disease. JIMD Rep. 2013;8:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Leone L, Ippoliti PF, Antonicelli R. Use of simvastatin plus cholestyramine in the treatment of lysosomal acid lipase deficiency. J Pediatr. 1991;119:1008-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Ginsberg HN, Le NA, Short MP, Ramakrishnan R, Desnick RJ. Suppression of apolipoprotein B production during treatment of cholesteryl ester storage disease with lovastatin. Implications for regulation of apolipoprotein B synthesis. J Clin Invest. 1987;80:1692-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 65. | Tarantino MD, McNamara DJ, Granstrom P, Ellefson RD, Unger EC, Udall JN Jr. Lovastatin therapy for cholesterol ester storage disease in two sisters. J Pediatr. 1991;118:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Glueck CJ, Lichtenstein P, Tracy T, Speirs J. Safety and efficacy of treatment of pediatric cholesteryl ester storage disease with lovastatin. Pediatr Res. 1992;32:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Levy R, Ostlund RE Jr, Schonfeld G, Wong P, Semenkovich CF. Cholesteryl ester storage disease: complex molecular effects of chronic lovastatin therapy. J Lipid Res. 1992;33:1005-1015. [PubMed] |
| 68. | Rassoul F, Richter V, Lohse P, Naumann A, Purschwitz K, Keller E. Long-term administration of the HMG-CoA reductase inhibitor lovastatin in two patients with cholesteryl ester storage disease. Int J Clin Pharmacol Ther. 2001;39:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Block RC, Razani B. Options to consider when treating lysosomal acid lipase deficiency. J Clin Lipidol. 2016;10:1280-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Quinn AG, Burton B, Deegan P, Di Rocco M, Enns G, Guardamagna O, Horslen S, Hovingh G, Lobritto S, Malinova V, Mclin V, Raiman J, Santra S, Sharma R, Sykut-cegielska J, Valayannopoulos V, Whitley CB, Eckert S, Schneider E. Sustained elevations in LDL cholesterol and serum transaminases from early childhood are common in lysosomal acid lipase deficiency. Mol Genet Metab. 2014;111:S89. [DOI] [Full Text] |
| 71. | Di Bisceglie AM, Ishak KG, Rabin L, Hoeg JM. Cholesteryl ester storage disease: hepatopathology and effects of therapy with lovastatin. Hepatology. 1990;11:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Grabowski G. Therapy for lysosomal acid lipase deficiency: replacing a missing link. Hepatology. 2013;58:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1297] [Cited by in RCA: 1311] [Article Influence: 59.6] [Reference Citation Analysis (1)] |
| 74. | McCoy E, Yokoyama S. Treatment of cholesteryl ester storage disease with combined cholestyramine and lovastatin. Ann N Y Acad Sci. 1991;623:453-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Tadiboyina VT, Liu DM, Miskie BA, Wang J, Hegele RA. Treatment of dyslipidemia with lovastatin and ezetimibe in an adolescent with cholesterol ester storage disease. Lipids Health Dis. 2005;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Di Rocco M, Pisciotta L, Madeo A, Bertamino M, Bertolini S. Long term substrate reduction therapy with ezetimibe alone or associated with statins in three adult patients with lysosomal acid lipase deficiency. Orphanet J Rare Dis. 2018;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Su K, Donaldson E, Sharma R. Novel treatment options for lysosomal acid lipase deficiency: critical appraisal of sebelipase alfa. Appl Clin Genet. 2016;9:157-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Demaret T, Lacaille F, Wicker C, Arnoux JB, Bouchereau J, Belloche C, Gitiaux C, Grevent D, Broissand C, Adjaoud D, Abi Warde MT, Plantaz D, Bekri S, de Lonlay P, Brassier A. Sebelipase alfa enzyme replacement therapy in Wolman disease: a nationwide cohort with up to ten years of follow-up. Orphanet J Rare Dis. 2021;16:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Burton BK, Balwani M, Feillet F, Barić I, Burrow TA, Camarena Grande C, Coker M, Consuelo-Sánchez A, Deegan P, Di Rocco M, Enns GM, Erbe R, Ezgu F, Ficicioglu C, Furuya KN, Kane J, Laukaitis C, Mengel E, Neilan EG, Nightingale S, Peters H, Scarpa M, Schwab KO, Smolka V, Valayannopoulos V, Wood M, Goodman Z, Yang Y, Eckert S, Rojas-Caro S, Quinn AG. A Phase 3 Trial of Sebelipase Alfa in Lysosomal Acid Lipase Deficiency. N Engl J Med. 2015;373:1010-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 80. | Burton BK, Feillet F, Furuya KN, Marulkar S, Balwani M. Sebelipase alfa in children and adults with lysosomal acid lipase deficiency: Final results of the ARISE study. J Hepatol. 2022;76:577-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Malinová V, Balwani M, Sharma R, Arnoux JB, Kane J, Whitley CB, Marulkar S, Abel F. Sebelipase alfa for lysosomal acid lipase deficiency: 5-year treatment experience from a phase 2 open-label extension study. Liver Int. 2020;40:2203-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Burton BK, Sanchez AC, Kostyleva M, Martins AM, Marulkar S, Abel F, Barić I. Long-Term Sebelipase Alfa Treatment in Children and Adults With Lysosomal Acid Lipase Deficiency. J Pediatr Gastroenterol Nutr. 2022;74:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Bashir A, Tiwari P, Duseja A. Enzyme replacement therapy in lysosomal acid lipase deficiency (LAL-D): a systematic literature review. Ther Adv Rare Dis. 2021;2:26330040211026928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Korbelius M, Kuentzel KB, Bradić I, Vujić N, Kratky D. Recent insights into lysosomal acid lipase deficiency. Trends Mol Med. 2023;29:425-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 85. | Ferry GD, Whisennand HH, Finegold MJ, Alpert E, Glombicki A. Liver transplantation for cholesteryl ester storage disease. J Pediatr Gastroenterol Nutr. 1991;12:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Leone L, Ippoliti PF, Antonicelli R, Balli F, Gridelli B. Treatment and liver transplantation for cholesterol ester storage disease. J Pediatr. 1995;127:509-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Bernstein DL, Lobritto S, Iuga A, Remotti H, Schiano T, Fiel MI, Balwani M. Lysosomal acid lipase deficiency allograft recurrence and liver failure- clinical outcomes of 18 liver transplantation patients. Mol Genet Metab. 2018;124:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Bastos KLM, Stephan BO, Linnenkamp BDW, Costa LA, Lima FR, Carvalho LML, Honjo RS, Tannuri U, Tannuri ACA, Kim CA. Evaluation of 73 Enlisted Patients for Liver Transplant with Unknown Etiology Reveals a Late-Diagnosed Case of Lysosomal Acid Lipase Deficiency. Int J Mol Sci. 2024;25:8648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 89. | Du H, Schiavi S, Wan N, Levine M, Witte DP, Grabowski GA. Reduction of atherosclerotic plaques by lysosomal acid lipase supplementation. Arterioscler Thromb Vasc Biol. 2004;24:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Massaro G, Geard AF, Liu W, Coombe-Tennant O, Waddington SN, Baruteau J, Gissen P, Rahim AA. Gene Therapy for Lysosomal Storage Disorders: Ongoing Studies and Clinical Development. Biomolecules. 2021;11:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 91. | Lam P, Ashbrook A, Zygmunt DA, Yan C, Du H, Martin PT. Therapeutic efficacy of rscAAVrh74.miniCMV.LIPA gene therapy in a mouse model of lysosomal acid lipase deficiency. Mol Ther Methods Clin Dev. 2022;26:413-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 92. | Laurent M, Harb R, Jenny C, Oustelandt J, Jimenez S, Cosette J, Landini F, Ferrante A, Corre G, Vujic N, Piccoli C, Brassier A, Van Wittenberghe L, Ronzitti G, Kratky D, Pacelli C, Amendola M. Rescue of lysosomal acid lipase deficiency in mice by rAAV8 liver gene transfer. Commun Med (Lond). 2025;5:110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/