Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.110793
Revised: July 16, 2025
Accepted: September 17, 2025
Published online: October 26, 2025
Processing time: 131 Days and 13.5 Hours
Postoperative complications such as atrial fibrillation and pericardial effusion are frequent after coronary artery bypass grafting (CABG), contributing to increased morbidity and prolonged hospital stays. Posterior pericardiotomy (PP), a surgical technique involving incision of the posterior pericardium to allow drainage, has been suggested as a preventive measure. However, its overall efficacy and safety profile, including potential risks like pleural effusion, require comprehensive evaluation amid varying study qualities. We hypothesized that PP reduces key post-CABG complications compared to standard care.
To determine the efficacy of PP in reducing postoperative complications following CABG.
This systematic review and meta-analysis included randomized controlled trials (RCTs) from PubMed, Cochrane, ClinicalTrials.gov, and Ovid, comparing PP vs no PP in adult CABG patients. Studies were conducted in tertiary care hospital settings. Twenty RCTs with 5331 participants were selected based on predefined inclusion criteria. The intervention involved intraoperative PP. Primary outcome was postoperative atrial fibrillation (POAF); secondary outcomes included effusions, tamponade, hospital/intensive care unit stay, and bleeding revisions. Risk ratios (RRs), mean differences, and 95% confidence intervals (CIs) were calculated using random-effects models; heterogeneity assessed via I2 statistic.
Twenty RCTs analyzed 5331 patients (2665 with PP vs 2666 without). PP significantly lowered POAF (10% vs 21%; RR = 0.48, 95%CI: 0.36-0.65, P < 0.00001; I2 = 70%), cardiac tamponade (0.5% vs 3%; RR = 0.16, 95%CI: 0.08-0.34, P < 0.00001; I2 = 0%), early pericardial effusion (2% vs 6%; RR = 0.31, 95%CI: 0.14-0.68, P = 0.004; I2 = 96%), and late pericardial effusion (1% vs 9%; RR = 0.11, 95%CI: 0.05-0.21, P < 0.00001; I2 = 0%). Hospital stay decreased (mean difference = -1.23 days, 95%CI: -1.87 to -0.59, P = 0.0002; I2 = 85%). Pleural effusion risk increased (25% vs 17%; RR = 1.46, 95%CI: 1.21-1.76, P < 0.0001; I2 = 0%). No significant effects on mortality (RR = 0.92, 95%CI: 0.48-1.76, P = 0.80; I2 = 0%), intensive care unit stay, or bleeding revisions.
PP effectively reduces POAF, pericardial effusions, tamponade, and hospital stay in CABG patients, though it increases pleural effusion risk and shows heterogeneity in some outcomes.
Core Tip: Posterior pericardiotomy is considered a safe and effective intervention for coronary artery bypass grafting to reduce complications. Our meta-analysis reported a significant reduction in postoperative atrial fibrillation, cardiac tamponade, early and late pericardial effusion, and the length of hospital stay, while improving postoperative outcomes. However, due to the limitations of our study and heterogeneity recorded in some of our outcomes calls for a systematic approach for postoperative protocols and surgical interventions.
- Citation: Khawar M, Shah SA, Khan A, Waseem A, Saeed H, Fatima A, Saifullah M, Mehdi AM, Qadeer A, Hadeed Khawar MM. Posterior pericardiotomy: An effective strategy for reducing post-coronary artery bypass grafting complications, with considerations for pleural effusion risk. World J Cardiol 2025; 17(10): 110793
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/110793.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.110793
Coronary artery bypass grafting (CABG) is a critical intervention for managing severe coronary artery disease, signifi
Traditional management of post-CABG complications involves pharmacological interventions, such as antiarrhythmic drugs and anticoagulants, and mechanical approaches like pacemaker placement[9,10]. These strategies often address symptoms rather than underlying causes and may introduce risks such as bradycardia, hypotension, or bleeding[9,10]. Posterior pericardiotomy (PP), a surgical technique involving a longitudinal incision in the posterior pericardium to facilitate drainage of pericardial fluid into the pleural space, has emerged as a promising preventive approach[3,8,11,12]. Evidence suggests that PP reduces the incidence of POAF and pericardial effusion by preventing fluid accumulation and mitigating inflammatory responses[3,8,11,12]. Despite these benefits, the long-term outcomes and potential risks of PP, such as pleural effusion or infection, remain under debate due to limited data[6,12].
This meta-analysis synthesizes evidence from randomized controlled trials (RCTs) to evaluate the efficacy and safety of PP in reducing postoperative complications in CABG patients compared to standard care. By providing comprehensive evidence, this study aims to inform clinical decision-making and contribute to evidence-based guidelines for optimizing patient outcomes.
This study is a systematic review and meta-analysis conducted to analyze the outcomes of pericardiotomy after CABG. The review is in accordance with the PRISMA guidelines (Prospero ID: CRD42025645108)[13].
A systematic literature search was conducted across PubMed, Cochrane Library, ClinicalTrials.gov, and Ovid to identify studies published between January 1980 and January 2025, focusing on cardiac surgery, pericardiotomy, pericardiectomy, atrial fibrillation (AF), and pericardial effusion. The search strategy integrated Medical Subject Headings (MeSH) and free-text terms to ensure comprehensive retrieval of relevant studies while minimizing irrelevant results. MeSH terms included “Cardiac Surgical Procedures” [MeSH], “Thoracic Surgery” [MeSH], “Coronary Artery Bypass” [MeSH], “Pericardiectomy” [MeSH], “Atrial Fibrillation” [MeSH], “Arrhythmias, Cardiac” [MeSH], and “Pericardial Effusion” [MeSH]. Free-text terms comprised “cardiac surgery”, “cardiothoracic surgery”, “CABG”, “pericardiotomy”, “peri
This meta-analysis included RCTs published in English that investigated adult patients (≥ 18 years) undergoing isolated CABG. The intervention of interest was prophylactic posterior left pericardiotomy, compared to a control group that did not undergo the procedure. The primary outcome assessed was the incidence of POAF.
Studies were excluded if they involved pediatric or adolescent patients, were observational in nature (including cohort studies, case reports, case series, reviews, and abstracts), or were conducted on animals. Additionally, non-English publications were not considered.
Studies were selected through a systematic screening process based on predefined inclusion and exclusion criteria. Two independent reviewers assessed the titles and abstracts of all retrieved studies to identify those meeting the eligibility criteria. Full-text articles of potentially relevant studies were then reviewed to confirm their inclusions. Any discrepancies between the reviewers were resolved through discussion and consensus among the research team. This rigorous selection process ensured the inclusion of only high-quality, relevant studies in the meta-analysis.
Data extraction was performed independently by two authors to ensure accuracy and consistency. A standardized template was used to collect relevant information from each included study. Key details extracted included study characteristics such as the authors, publication year, country, study design, and participant numbers, demographics and comorbidities. Additionally, outcome data related to postoperative cardiovascular complications such as AF, early and late pericardial effusion, pericardial tamponade, hospital stay time, mortality were recorded. In cases of disagreement between the reviewers, consensus discussions were held to resolve discrepancies. This process helped maintain the reliability and thoroughness of the data collection.
The risk of bias assessment for the included RCTs was conducted using the Revised Cochrane Risk of Bias tool (RoB 2.0), which evaluates potential biases across five key domains: (1) Bias arising from the randomization process; (2) Bias due to deviations from intended interventions; (3) Bias due to missing outcome data; (4) Bias in measurement of the outcome; and (5) Bias in selection of the reported result. Each study was independently assessed by two reviewers, with discre
Data were synthesized using random-effects meta-analysis to account for the expected heterogeneity across studies. The following statistical measures were calculated: Risk ratio (RR) for dichotomous outcomes (e.g., AF, mortality, etc). Mean difference (MD) for continuous outcomes (e.g., length of hospital stays, etc). Both RRs and MDs were accompanied by 95% confidence intervals (CI). Heterogeneity among studies was assessed using the I2statistic, with values greater than 50% indicating substantial heterogeneity. To assess potential publication bias, we performed the Egger's Regression test for studies that include at least 10 studies. This test evaluates the asymmetry of the funnel plot for each key outcome and was used to determine if there was any evidence of publication bias. All statistical analyses were conducted using RevMan 5.3 (Cochrane Collaboration). The Egger’s Regression test for publication bias was performed using R version 4.4.1.
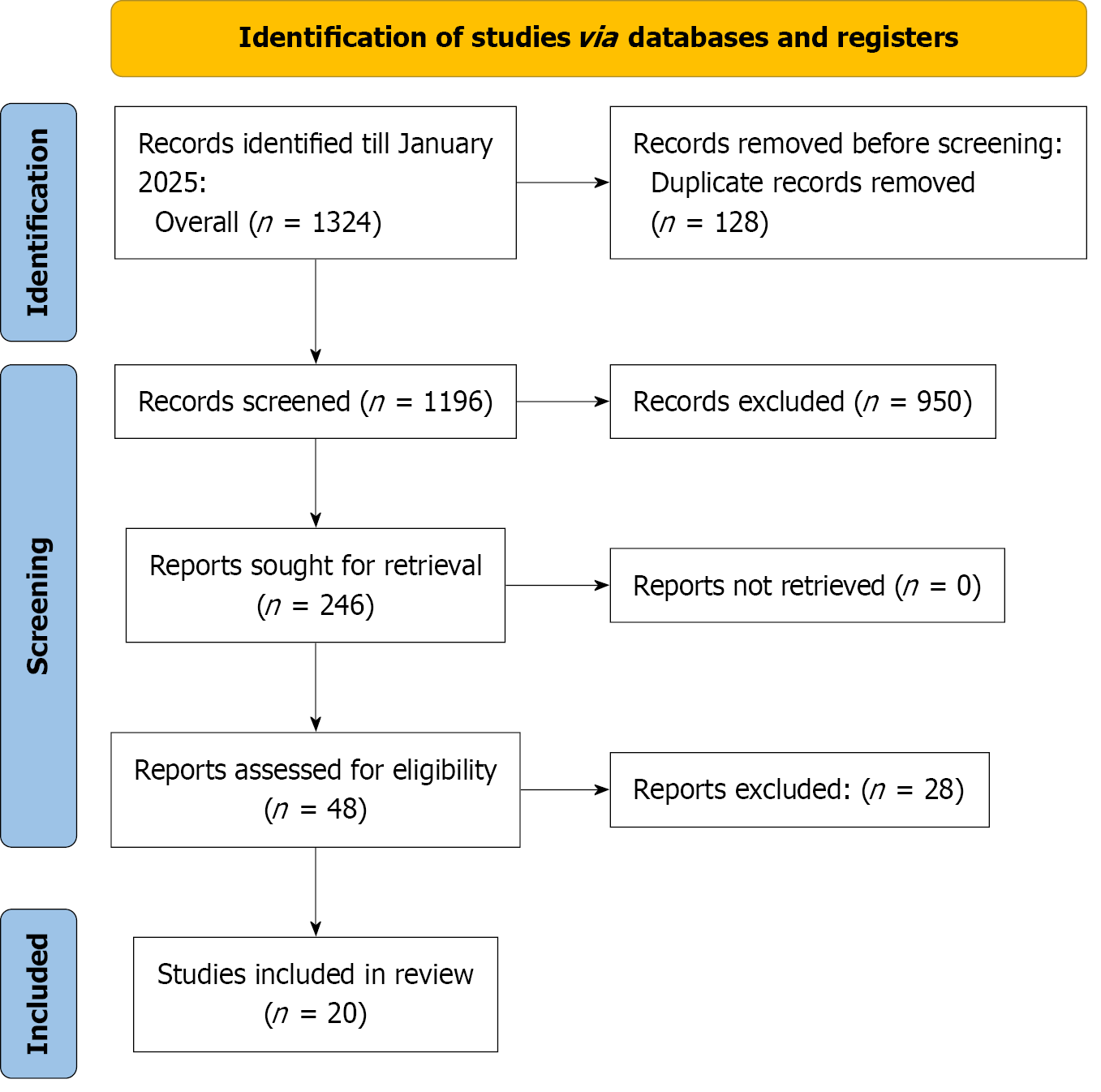
The systematic search of PubMed, Cochrane, ClinicalTrials.gov, and Ovid, conducted up to July 2025, yielded 1324 records. After removing 128 duplicates, 1106 records underwent title and abstract screening, of which 960 were excluded due to irrelevance, non-human studies, or inappropriate study designs. The full-text review was performed for 46 articles, with 26 excluding due to lack of comparative data, insufficient outcome reporting, single-arm studies, and duplicate or overlapping data. Ultimately, 20 studies[15-34] were included in the meta-analysis (Figure 1).
The meta-analysis included 20 RCTs conducted across nine countries, with the majority from Egypt (5 studies) and Turkey (6 studies), along with contributions from Iran, Pakistan, the United Kingdom, Thailand, Australia, China, and Greece. The mean age of participants ranged from 54 years to 67 years, with comparable distributions between inter
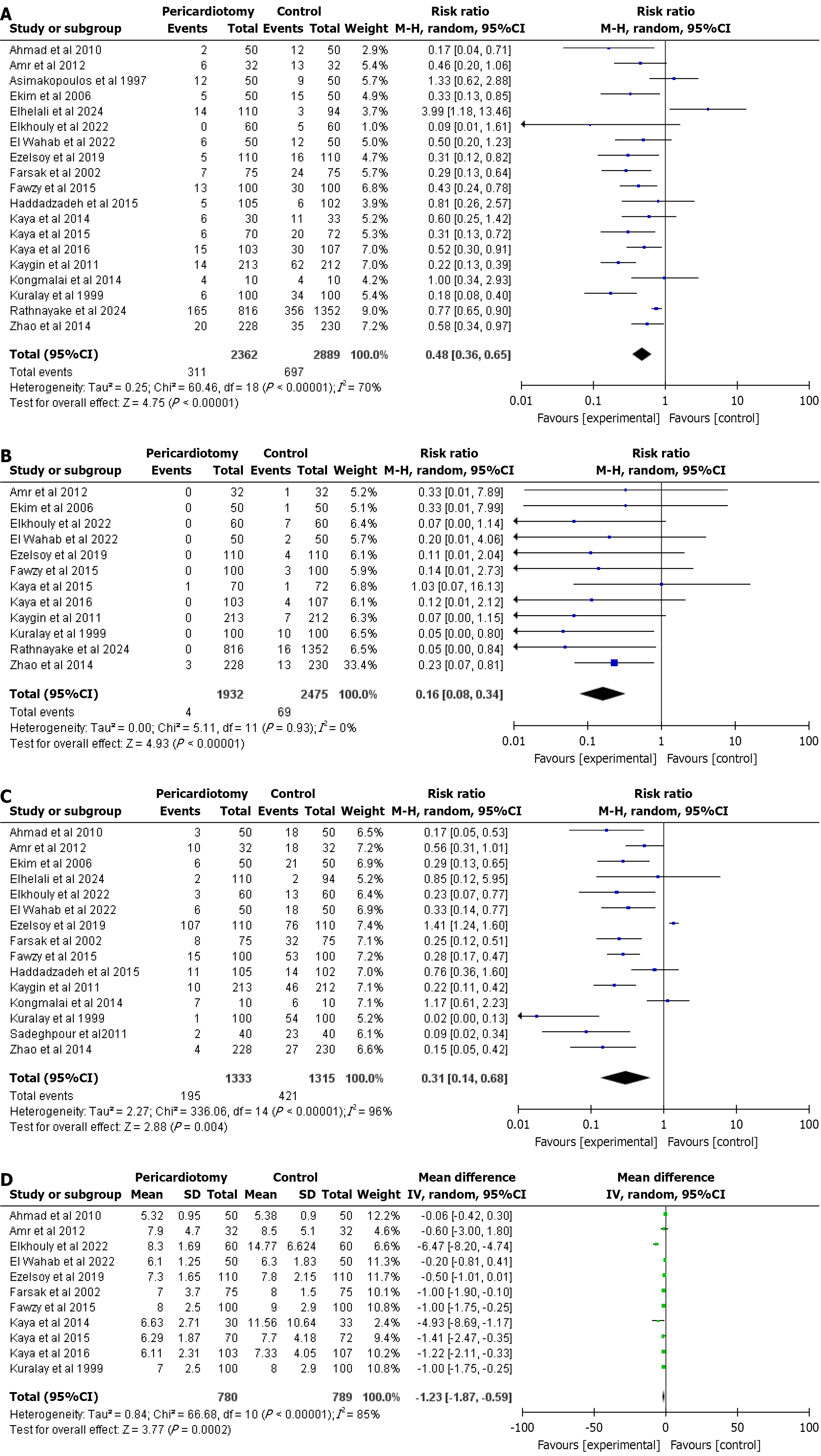
AF: AF was reported by 19 studies. The pooled RR of AF for pericardiotomy is 0.48 (95%CI: 0.36-0.65, P < 0.00001), with heterogeneity of I2 = 70% that dropped significantly upon removal of studies like Rathnayake et al[32] and Kongmalai et al[30] (I2 = 42%) (Figure 2A).
Cardiac tamponade: A total of 12 studies reported cardiac tamponade. PP was associated with significantly lesser rate of cardiac tamponade with the estimated pooled RR for cardiac tamponade is 0.16 (95%CI: 0.08-0.34, P < 0.00001), and heterogeneity I2 = 0% (Figure 2B).
Early pericardial effusion: 15 studies reported this outcome. The estimated pooled RR for early pericardial effusion is 0.31 (95%CI: 0.14-0.68, P = 0.004) with a heterogeneity I2 value of 96% that dropped significantly upon removal of studies like Rathnayake et al[32] and Kongmalai et al[30] (I2 = 54%) (Figure 2C).
Hospital length of stay: Hospital length of stay was reported by a total of 11 studies. The combined MD for hospital length of stay was estimated to be -1.23 (95%CI: -1.87 to 0.59, P = 0.0002), and a heterogeneity I2 = 85% that dropped significantly upon removal of studies like Rathnayake et al[32] and Sadeghpour et al[33] (I2 = 48%) (Figure 2D).
Mortality: A total of 7 studies reported about the outcome. No significant difference was observed between the groups. The estimated pooled RR for Mortality is 0.92 (95%CI: 0.48-1.76, P = 0.80) and no heterogeneity I2 = 0% (Supplementary Figure 1).
Late pericardial effusion: Late pericardial effusion was reported by 9 studies. The estimated pooled RR for late pericardial effusion resulting from the studies is 0.11 (95%CI: 0.05-0.21, P < 0.00001) with a heterogeneity I2 of 0% (Supplementary Figure 2).
Pleural effusion: 12 studies reported this outcome. The combined RR for pleural effusion is estimated to be 1.46 (95%CI: 1.21-1.76, P < 0.0001) with no heterogeneity (I2 = 0%) (Supplementary Figure 3).
Revision surgery for bleeding: The outcome was reported by a total of 15 studies. No significant difference was observed. The estimated pooled RR for revision surgery for bleeding is 0.77 (95%CI: 0.53-1.12, P = 0.17) and heterogeneity I2 = 2% (Supplementary Figure 4).
Quality assessment: Among the 20 RCTs assessed using RoB 2.0, 15 studies (75%) were classified as having a high risk of bias, 3 studies (15%) had some concerns, and only 2 studies (10%) were deemed to have a low risk of bias. This indicates that the majority of included studies had methodological limitations that could influence the overall findings of the meta-analysis (Supplementary Figures 5 and 6).
Postoperative complications following CABG, such as AF, cardiac tamponade, pericardial effusion, pleural effusion, and the need for revision surgery, contribute significantly to morbidity and prolonged hospital stays[14]. Our meta-analysis of 20 RCTs involving 5331 CABG patients demonstrates that PP significantly reduces the incidence of postoperative AF (RR = 0.48, 95%CI: 0.36-0.65, P < 0.00001), early pericardial effusion (RR = 0.31, 95%CI: 0.13-0.51, P < 0.0001), late pericardial effusion (RR = 0.11, 95%CI: 0.04-0.15, P < 0.00001), and cardiac tamponade, despite significant heterogeneity (I2 = 71%-83%). These findings align with prior studies[35-38] and support PP’s efficacy in mitigating complications by facilitating pericardial fluid drainage into the pleural space, reducing mechanical irritation and electrical instability that trigger AF[16]. However, PP was associated with a higher incidence of pleural effusion (RR = 1.46, 95%CI: 1.27-2.04, P < 0.0001, I2 = 0%) and no significant reduction in revision surgery for bleeding or intensive care unit stay.
Postoperative AF, affecting 5%-40% of CABG patients, peaks on postoperative day two and is driven by multifactorial etiologies, including pericardial inflammation, oxidative stress, and autonomic dysregulation[14,36]. PP, involving a 4-5 cm longitudinal incision posterior to the phrenic nerve, addresses these by draining posterior pericardial effusions that are difficult to manage with substernal chest drains due to their proximity to the heart and bypass grafts[37]. This drainage reduces localized pressure on the left atrium and ventricle, which can precipitate AF or tamponade[39,40]. Our results confirm PP’s antiarrhythmic effect, consistent with Mulay et al’s study[11] and subsequent meta-analyses[1].
The observed heterogeneity (I2 = 71%-85%) likely stems from variations in surgical techniques, patient characteristics, and perioperative management. Moreover, the high risk of bias in 15 of the 20 included studies, primarily due to lack of blinding and deviations from intended interventions, may have influenced our findings, particularly for subjective outcomes like AF detection[41-44]. Lack of blinding in these trials could introduce detection bias, as clinicians aware of the intervention status might have been more vigilant in monitoring for AF in the control group, potentially overestimating its incidence. This is particularly relevant for AF, which often requires electrocardiographic confirmation and clinical judgment, both susceptible to subjective interpretation. For instance, unblinded clinicians might prioritize more frequent electrocardiogram monitoring or interpret borderline arrhythmic events more stringently in the control arm, inflating the apparent benefit of PP. Similarly, the assessment of pericardial effusion via echocardiography could be subject to observer bias in unblinded settings, potentially exaggerating the reported reduction in effusion rates. While objective outcomes, such as cardiac tamponade, are less prone to detection bias due to their clear clinical presentation, the overall reliability of our pooled estimates may be compromised by these biases. Sensitivity analyses excluding high-risk-of-bias studies could help quantify this impact, but only two studies provided low-risk, unbiased data, limiting such analyses[20,41].
Despite these limitations, PP significantly reduced both early and late pericardial effusions, likely due to effective drainage and reduced pericardial constraint[45-47]. The increased pleural effusion incidence is an expected consequence of PP, as pericardial fluid drains into the pleural cavity, but this rarely leads to respiratory complications[11,36]. Hospital stay was reduced (MD = -1.23 days, 95%CI: -1.87 to -0.59, P = 0.0002), though high heterogeneity (I2 = 85%) suggests variability in institutional protocols. No significant reduction in ICU stay was observed (MD = -0.34 days, 95%CI: -0.83 to 0.14, P = 0.17), possibly due to factors beyond PP, such as postoperative care practices.
Despite these promising results, several limitations temper the strength of our conclusions. The high heterogeneity (I2 = 71%-85%) across outcomes likely stems from variations in surgical techniques, patient characteristics, and perioperative medication use. For example, differences in beta-blocker or amiodarone use could confound AF outcomes, yet most studies did not adequately report or adjust for these factors, limiting our ability to assess their impact. Similarly, va
A critical limitation is the high risk of bias in 15 of the 20 included studies[3-7,9,10,12-14,36-40], primarily due to lack of blinding, deviations from intended interventions, and inconsistent randomization criteria. Only two studies were deemed low risk for bias[20,41]. Lack of blinding introduces detection bias, particularly for subjective outcomes like AF, which relies on electrocardiographic confirmation and clinical judgment. Unblinded clinicians may have monitored control groups more vigilantly, potentially overestimating AF incidence and inflating PP’s apparent benefit. Similarly, echocardiographic assessment of pericardial effusions is susceptible to observer bias in unblinded settings. Objective outcomes, such as cardiac tamponade, are less affected due to their clear clinical presentation, but the overall reliability of pooled estimates is compromised. Additionally, the absence of patient-level data prevented subgroup analyses to explore how factors like age, comorbidities, or surgical approach modify PP’s effects, further limiting our ability to account for confounding variables.
Missing outcome data in several trials introduces attrition bias, potentially underestimating event rates for AF and effusions. The geographic concentration of studies, with eight conducted in Turkey[6,8-9,11-14,36] and five in Egypt[4,7,10,38,39], raises concerns about generalizability. For instance, differences in patient demographics, such as prevalence of diabetes or hypertension, or variations in surgical practices between these regions and global populations, were not reported in most studies, precluding a robust assessment of regional bias. This geographic limitation suggests caution in extrapolating our findings to diverse populations.
Given these limitations, particularly the high risk of bias and lack of patient-level data, strong recommendations for PP’s routine adoption in CABG are premature. While PP appears effective in reducing AF and pericardial effusions, the increased risk of pleural effusion and the lack of impact on ICU stay or revision surgery highlight the need for a balanced risk-benefit assessment. Future research should prioritize blinded, multicenter trials with standardized protocols and diverse populations to confirm PP’s efficacy and address confounding factors. Such studies could also explore non-invasive predictors of AF, such as left atrial volume index or P-wave dispersion, to refine patient selection for PP.
Our meta-analysis suggests that PP shows promise in reducing POAF, cardiac tamponade, early and late pericardial effusion, and hospital stay duration in CABG patients. However, the high risk of bias in 15 of the 20 included RCTs, along with significant heterogeneity in some outcomes, necessitates cautious interpretation of these findings. Limitations such as lack of blinding, inconsistent randomization, and missing data undermine the strength of the evidence, precluding definitive recommendations for PP’s routine use. A future study, which includes well-designed RCTs, is essential to further investigate patient selection criteria and streamlined surgical strategies, ensuring the optimal clinical advantages of PP in CABG patients. Higher-quality, low-bias RCTs with standardized protocols and diverse populations are needed to confirm PP’s efficacy and safety, providing a robust basis for clinical recommendations.
| 1. | Hakala T, Hedman A. Predicting the risk of atrial fibrillation after coronary artery bypass surgery. Scand Cardiovasc J. 2003;37:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Brookes JDL, Williams M, Mathew M, Yan T, Bannon P. Pleural effusion post coronary artery bypass surgery: associations and complications. J Thorac Dis. 2021;13:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Xiong T, Pu L, Ma YF, Zhu YL, Li H, Cui X, Li YX. Posterior pericardiotomy to prevent new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis of 10 randomized controlled trials. J Cardiothorac Surg. 2021;16:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 904] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 5. | Haghjoo M. Pharmacological and nonpharmacological prevention of atrial fibrillation after coronary artery bypass surgery. J Tehran Heart Cent. 2012;7:2-9. [PubMed] |
| 6. | LaPar DJ, Speir AM, Crosby IK, Fonner E Jr, Brown M, Rich JB, Quader M, Kern JA, Kron IL, Ailawadi G; Investigators for the Virginia Cardiac Surgery Quality Initiative. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98:527-33; discussion 533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J. 2023;44:1020-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 159] [Reference Citation Analysis (0)] |
| 8. | Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017;52:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 9. | Amin S, Mahmoud GM, Omar I. The Preventive Role of Posterior Pericardial Window in the Complications Following Coronary Artery Bypass Grafting. J Clin Diagn Res. 2020;20:264-272. |
| 10. | Dan GA, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita F, van Gelder I, Gorenek B, Kaski JC, Kjeldsen K, Lip GYH, Merkely B, Okumura K, Piccini JP, Potpara T, Poulsen BK, Saba M, Savelieva I, Tamargo JL, Wolpert C; ESC Scientific Document Group. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace. 2018;20:731-732an. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Mulay A, Kirk AJ, Angelini GD, Wisheart JD, Hutter JA. Posterior pericardiotomy reduces the incidence of supra-ventricular arrhythmias following coronary artery bypass surgery. Eur J Cardiothorac Surg. 1995;9:150-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Soletti GJ, Perezgrovas-Olaria R, Harik L, Rahouma M, Dimagli A, Alzghari T, Demetres M, Bratton BA, Yaghmour M, Satija D, Lau C, Girardi LN, Salemo TA, Gaudino M. Effect of posterior pericardiotomy in cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2022;9:1090102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51401] [Article Influence: 10280.2] [Reference Citation Analysis (2)] |
| 14. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18738] [Article Influence: 2676.9] [Reference Citation Analysis (0)] |
| 15. | Ahmad M, Iqbal A, Paracha V, Rashid A, Rashid A. Role of posterior pericardiotomy in prevention of pericardial effusion and atrial fibrillation after coronary artery bypass grafting surgery. Pak Armed Forces Med J. 2011;61:84-87. |
| 16. | Amr MA, Elkassas MH. The effect of posterior pericardiotomy on postoperative pericardial effusion in coronary artery bypass surgery. Suez Canal University Med J. 15:49-53. |
| 17. | Asimakopoulos G, Della Santa R, Taggart DP. Effects of posterior pericardiotomy on the incidence of atrial fibrillation and chest drainage after coronary revascularization: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;113:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ekim H, Kutay V, Hazar A, Akbayrak H, Başel H, Tuncer M. Effects of posterior pericardiotomy on the incidence of pericardial effusion and atrial fibrillation after coronary revascularization. Med Sci Monit. 2006;12:CR431-CR434. [PubMed] |
| 19. | Abd El-wahab M, El-shihy E, Sayed A, Mahfouz AO. The Role of Posterior Pericardiotomy on The Incidence of Atrial Fibrillation and Pericardial Effusion after Coronary Revascularization. Egypt J Hosp Med. 2022;88:2619-2624. [DOI] [Full Text] |
| 20. | Elhelali M, Elhelw E, Sabri RA, Bastawisy A. Impact of Posterior Pericardiotomy on Prevention of Post Operative Atrial Fibrillation in Patients Undergoing Coronary Bypass Surgery. Cardiol Angiol Int J. 2024;13:139-147. [DOI] [Full Text] |
| 21. | Elkhouly M, Fouad A. Influence of Posterior Pericardiotomy on Early and Late Pericardial Effusions Post CABG. Egypt J Hosp Med. 2022;89:4735-4739. [DOI] [Full Text] |
| 22. | Ezelsoy M, Oral K, Saracoglu KT, Saracoglu A, Akpınar B. Posterior pericardial window technique to prevent postoperative pericardial effusion in cardiac surgery. Kocaeli Med J. 2019;8:78-83. |
| 23. | Farsak B, Günaydin S, Tokmakoğlu H, Kandemir O, Yorgancioğlu C, Zorlutuna Y. Posterior pericardiotomy reduces the incidence of supra-ventricular arrhythmias and pericardial effusion after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2002;22: 278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Fawzy H, Elatafy E, Elkassas M, Elsarawy E, Morsy A, Fawzy A. Can posterior pericardiotomy reduce the incidence of postoperative atrial fibrillation after coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. 2015;21:488-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Haddadzadeh M, Motavaselian M, Rahimianfar AA, Forouzannia SK, Emami M, Barzegar K. The effect of posterior pericardiotomy on pericardial effusion and atrial fibrillation after off-pump coronary artery bypass graft. Acta Med Iran. 2015;53:57-61. [PubMed] |
| 26. | Kaya M, İyigün T, Yazıcı P, Melek Y, Göde S, Güler S, Karaçalılar M, Satılmışoğlu MH, Erek E. The effects of posterior pericardiotomy on pericardial effusion, tamponade, and atrial fibrillation after coronary artery surgery. Kardiochir Torakochirurgia Pol. 2014;11:113-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Kaya M, Utkusavaş A, Erkanlı K, Güler S, Kyaruzi M, Birant A, Karaçalılar M, Akkuş M, Bakır İ. The Preventive Effects of Posterior Pericardiotomy with Intrapericardial Tube on the Development of Pericardial Effusion, Atrial Fibrillation, and Acute Kidney Injury after Coronary Artery Surgery: A Prospective, Randomized, Controlled Trial. Thorac Cardiovasc Surg. 2016;64:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Kaya M, Satılmışoğlu MH, Buğra AK, Kyaruzi M, Kafa Ü, Utkusavaş A, Bakır İ. Impact of the total pericardial closure using bilateral trap door incision and pericardial cavity intervention on outcomes following coronary artery bypass grafting: a randomized, controlled, parallel-group prospective study. Interact Cardiovasc Thorac Surg. 2015;21:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Kaygin MA, Dag O, Güneş M, Senocak M, Limandal HK, Aslan U, Erkut B. Posterior pericardiotomy reduces the incidence of atrial fibrillation, pericardial effusion, and length of stay in hospital after coronary artery bypasses surgery. Tohoku J Exp Med. 2011;225:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Kongmalai P, Karunasumetta C, Kuptarnond C, Prathanee S, Taksinachanekij S, Intanoo W, Wongbuddha C, Senthong V. The posterior pericardiotomy. Does it reduce the incidence of postoperative atrial fibrillation after coronary artery bypass grafting? J Med Assoc Thai. 2014;97 Suppl 10:S97-S104. [PubMed] |
| 31. | Kuralay E, Ozal E, Demirkili U, Tatar H. Effect of posterior pericardiotomy on postoperative supraventricular arrhythmias and late pericardial effusion (posterior pericardiotomy). J Thorac Cardiovasc Surg. 1999;118:492-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Rathnayake A, Goh SS, Fenton C, Hardikar A. Posterior pericardiotomy and the prevention of post-operative atrial fibrillation and cardiac tamponade in isolated coronary artery bypass grafting - A retrospective analysis. J Cardiothorac Surg. 2024;19:263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Sadeghpour A, Baharestani B, Ghasemzade GB, Baghaei R, Givhtaje N. Influences of posterior pericardiotomy in early and late postoperative effusion of pericardium. Iranian J Cardiac Surg. 2011;3:42-43. |
| 34. | Zhao J, Cheng Z, Quan X, Zhao Z. Does posterior pericardial window technique prevent pericardial tamponade after cardiac surgery? J Int Med Res. 2014;42:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Montrief T, Koyfman A, Long B. Coronary artery bypass graft surgery complications: A review for emergency clinicians. Am J Emerg Med. 2018;36:2289-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Abdelaziz A, Hafez AH, Elaraby A, Roshdy MR, Abdelaziz M, Eltobgy MA, Elsayed H, El-Samahy M, Elbehbeh NA, Philip KG, Abdelaty AM, Rizk MA, Al-Tawil M, AboElfarh HE, Ramadan A, Ghaith HS, Wahsh EA, Abdelazeem B, Fayed B. Posterior pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: a systematic review and meta-analysis of 25 randomised controlled trials. EuroIntervention. 2023;19:e305-e317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Gaudino M, Di Franco A, Rong LQ, Cao D, Pivato CA, Soletti GJ, Chadow D, Cancelli G, Perezgrovas Olaria R, Gillinov M, DiMaio JM, Girardi LN. Pericardial Effusion Provoking Atrial Fibrillation After Cardiac Surgery: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:2529-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 38. | San TMM, Han KPP, Ismail M, Thu LM, Thet MS. Pericardiotomy and atrial fibrillation after isolated coronary artery bypass grafting: A systematic review and meta-analysis of 16 randomised controlled trials. Cardiovasc Revasc Med. 2024;66:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Achmad C, Tiksnadi BB, Akbar MR, Karwiky G, Sihite TA, Pramudya A, Iqbal M, Febrianora M. Left Volume Atrial Index and P-wave Dispersion as Predictors of Postoperative Atrial Fibrillation After Coronary Artery Bypass Graft: A Retrospective Cohort Study. Curr Probl Cardiol. 2023;48:101031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Hu XL, Chen Y, Zhou ZD, Ying J, Hu YH, Xu GH. Posterior pericardiotomy for the prevention of atrial fibrillation after coronary artery bypass grafting: A meta-analysis of randomized controlled trials. Int J Cardiol. 2016;215:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:CD003611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Karic A, Mujaric E, Krajnovic A, Busevac E, Selimovic T, Milaimi A, Sljivo A. Posterior Pericardiotomy and Its Impact on Clinical Outcomes in Off-Pump Coronary Artery Bypass Grafting Complications. Mater Sociomed. 2025;37:159-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Makarem A, Paneitz D, Winship T, Singh R, Bloom JP, Hosseini M, Jassar AS, Kreso A, Langer N, Melnitchouk S, Michel E, Rabi SA, Akeju O, D'Alessandro D, Sundt TM, Osho AA. Pericardiotomy and Amiodarone for Prophylaxis Against Postoperative Atrial Fibrillation in Cardiac Surgery (PAPPA). Ann Thorac Surg. 2025;120:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Crystal E, Connolly SJ, Sleik K, Ginger TJ, Yusuf S. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation. 2002;106:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 274] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Akhtar M, Meecham L, Birkett R, Pherwani AD, Fairhead JF. Conservative Treatment of an Infected Aortic Graft with Antibiotic Irrigation. Int J Angiol. 2016;25:e118-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Cohen AJ, Lockman J, Lorberboym M, Bder O, Cohen N, Medalion B, Schachner A. Assessment of sternal vascularity with single photon emission computed tomography after harvesting of the internal thoracic artery. J Thorac Cardiovasc Surg. 1999;118:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Gray KD, Molena D. Commentary: To cut is a chance to cure? Lessons to be learned from the PulMiCC trial. J Thorac Cardiovasc Surg. 2022;163:493-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/