Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.110900
Revised: August 7, 2025
Accepted: September 23, 2025
Published online: October 26, 2025
Processing time: 128 Days and 18.1 Hours
Subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation re
To compare perioperative outcomes of GA and US-ICNB in S-ICD implantation.
This retrospective single-center study included 64 patients who received S-ICD implantation between February 2021 and December 2024. They were divided into GA and US-ICNB groups based on anesthesia type. Demographic data, peri
This study included 64 patients (20 in the GA group and 44 in the US-ICNB group). Baseline left ventricular ejection fraction was significantly lower in the US-ICNB group (39.20% ± 12.00% vs 56.20% ± 11.50% in GA, P < 0.001), while American Society of Anesthesiologists scores and comorbidities were comparable. US-ICNB showed superior pain control, with significantly lower numeric rating scale scores at 6-48 hours (P < 0.001) and fewer patients requiring analgesics (P = 0.02). The US-ICNB group had shorter operation times (P < 0.001), total hospital stays (P < 0.001), and later first analgesia times (P < 0.001). No anesthesia-related complications occurred in either group.
Both anesthetic methods were safe in the short term. However, US-ICNB was superior in reducing operation and hospital stay times and alleviating peri-operative pain. It has high safety in S-ICD implantation and deserves further clinical promotion, though large-scale, multi-center, randomized controlled trials are needed to confirm these findings.
Core Tip: This study compares general anesthesia and ultrasound-guided intercostal nerve block in perioperative subcutaneous implantable cardioverter-defibrillator placement, evaluating their effects on pain management, recovery, and complications.
- Citation: Wen CJ, Cheng JF, Jiang SB, Wang M, Yin XX, Liu R, Shen W, Zhong Y. Comparative analysis of general anesthesia and ultrasound-guided intercostal nerve block in subcutaneous implantable cardioverter-defibrillator perioperative care. World J Cardiol 2025; 17(10): 110900
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/110900.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.110900
Sudden cardiac death (SCD) remains a critical global public health challenge, accounting for millions of deaths annually and underscoring the urgent need for effective prophylactic interventions[1,2]. Implantable cardioverter-defibrillators (ICDs) have emerged as a cornerstone in SCD prevention, with transvenous ICDs (TV-ICDs) traditionally serving as the standard of care[3]. However, TV-ICD implantation is associated with significant risks, including lead-related complications (e.g., infection, fracture, venous obstruction) and procedure-related morbidity, which are particularly pronounced in high-risk populations such as those with prior infections, vascular anomalies, or advanced heart failure[4,5].
Subcutaneous ICDs (S-ICDs), a novel alternative that avoids transvenous lead placement, have gained traction for their ability to reduce lead-dependent complications while maintaining efficacy in terminating life-threatening arrhythmias[6,7]. By positioning the pulse generator and lead subcutaneously, S-ICD eliminates vascular and intracardiac interactions, offering a safer profile for patients with contraindications to transvenous approaches[8,9]. However, S-ICD implantation requires adequate anesthesia to manage the discomfort associated with submuscular pocket creation, lead tunneling, and defibrillation threshold (DFT) testing. General anesthesia (GA), though commonly used, carries risks of hemodynamic instability, airway complications, and prolonged recovery, which are particularly relevant in vulnerable populations with reduced left ventricular ejection fraction (LVEF) or comorbidities[10-12].
Regional anesthesia techniques, such as ultrasound-guided intercostal nerve block (US-ICNB), have emerged as promising alternatives to GA for chest wall procedures, offering targeted analgesia with minimal systemic effects[13,14]. US-ICNB provides sensory blockade of the intercostal nerves, reducing postoperative pain and opioid requirements while preserving hemodynamic stability[15]. Despite its theoretical advantages, the application of US-ICNB in S-ICD implantation remains underexplored, with limited data on its safety, efficacy, and comparative outcomes against GA.
Against this backdrop, this study aims to compare the perioperative safety and clinical outcomes of GA vs US-ICNB in patients undergoing S-ICD implantation. By analyzing a single-center cohort, we sought to evaluate whether US-ICNB could serve as a viable alternative to GA, particularly in high-risk patients, by reducing complications, optimizing pain management, and improving procedural efficiency. Given the paucity of comparative data, this analysis addresses a critical gap in the literature, informing anesthetic selection for S-ICD implantation and potentially expanding access to this life-saving therapy.
This retrospective study analyzed 64 patients who underwent S-ICD (model A209, Boston Scientific, United States) implantation in the Department of Cardiology, Second Affiliated Hospital of Zhejiang University School of Medicine, between February, 2021, and December, 2024. Patients before February 2022 underwent GA, and patients after that underwent US-ICNB.
Eligibility criteria included: (1) Class I or IIa indications for ICD implantation in accordance with the 2022 European Society of Cardiology Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death[16]; and (2) Successful preimplantation electrocardiogram (ECG) screening for S-ICD suitability. Exclusion criteria were: (1) Indications for bradycardia pacing or cardiac resynchronization therapy; (2) Predicted survival < 1 year; and (3) Patient refusal of S-ICD. Patients were stratified into GA and US-ICNB groups based on intraoperative anesthesia type. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine, adhering to the 2013 revised Declaration of Helsinki.
GA: GA was induced with intravenous propofol (1-2 mg/kg) and fentanyl (1-2 μg/kg), followed by tracheal intubation for airway management. Anesthesia was maintained with sevoflurane (1.5%-2.5%) and remifentanil (0.1-0.2 μg/kg/minute). Hemodynamic parameters (heart rate, mean arterial pressure, oxygen saturation) were continuously monitored. Muscle relaxation was achieved with rocuronium (0.6 mg/kg) and adjusted as needed. Postoperative analgesia included intravenous tramadol (1-2 mg/kg) or patient-controlled analgesia with fentanyl, titrated to a numeric rating scale (NRS) pain score ≤ 3.
US-ICNB: US-ICNB was performed by anesthesiologists with ≥ 5 years of regional anesthesia experience using a high-frequency linear ultrasound probe (10-12 MHz). Patients were positioned in the lateral decubitus position with the affected side uppermost. The intercostal nerves at the 5th-7th intercostal spaces were visualized in the mid-axillary line. A 22-gauge block needle was advanced in-plane toward the inferior border of the rib, and after negative aspiration, 3-5 mL of 0.375% ropivacaine was injected per space, with a total volume of 15-20 mL. Lidocaine 1% (5-10 mL) was infiltrated at the incision sites. Sedation was maintained with intravenous midazolam (0.03-0.05 mg/kg) and dexmedetomidine (0.5-1.0 μg/kg/hour) to achieve a Ramsay sedation score of 2-3. Postoperative analgesia consisted of oral paracetamol (1 g q6h) and oxycodone (5-10 mg q12h) as needed, with NRS monitoring.
Preimplantation ECG screening: Preoperative screening utilized the manual screening tool and automatic screening tool of the Boston Scientific programmer. Three ECG vectors were assessed in supine (mandatory) and standing/sitting positions. Automatic screening tool automatically analyzed QRS-T wave morphology, amplitude, and consistency across positions. A successful screen required at least one vector passing in ≥ 2 positions with stable QRS morphology, confirmed by visual inspection.
S-ICD implantation technique: Surface marking: Under fluoroscopy, the generator pocket was marked in the left axillary line (5th-6th intercostal space), with the lead trajectory marked from the xiphoid process to the upper sternum. The goal was to maximize the cardiac area between the generator and lead.
Surgical steps: Before incision, 1% lidocaine was locally infiltrated into the skin and subcutaneous tissue at the planned incision sites (left axilla, xiphoid, and upper sternum) by the electrophysiologist. A 6-8 cm incision was made in the left axilla, with blunt dissection to the fascial layer. The intermuscular pocket was created between the latissimus dorsi and serratus anterior muscles. A 2-3 cm xiphoid incision and a 2 cm upper sternal incision were made. The defibrillation lead was tunneled subcutaneously using a curved tunneling tool, fixed to the fascia, and connected to the generator. DFT testing was performed with 50 Hz/200 mA alternating current to induce ventricular fibrillation. The S-ICD was programmed to deliver a 65 J shock after a 2-second blanking period, with successful conversion defined as sinus rhythm restoration within 30 seconds.
Postoperative programming and follow-up: Post-implantation settings included a defibrillation zone ≥ 230 bpm and a conditional zone ≥ 200 bpm (or 10-20 bpm below the patient’s clinical ventricular tachycardia frequency). Post-shock pacing was activated (VVI mode, 50 bpm, 30-second duration). Patients were followed up at 3 months and every 6-12 months thereafter with ICD interrogation, blood biochemistry, and clinical assessments. Events were classified as appropriate (correct VT/VF detection) or inappropriate (oversensing, noise, or false detection) based on intracardiac electrograms.
Demographic data and clinical characteristics of patients were retrospectively collected and analyzed. NRS pain scores and analgesic usage were recorded for patients undergoing S-ICD implantation. The impacts of the two anesthesia methods on postoperative complications, surgical success rate, and operation time (defined as the period from skin incision by the surgeon to completion of incision suturing after S-ICD implantation) were analyzed. Follow-up management data were obtained to understand postoperative event records and defibrillation occurrences.
Data were analyzed using SPSS 26.0 (IBM). Normally distributed continuous variables are presented as mean ± SD, while non-normally distributed data are reported as median (IQR). Categorical variables are expressed as counts and percentages. Between-group comparisons used independent t-tests for normal data, Mann-Whitney U tests for non-normal data, and χ2 tests for categorical variables. A two-sided P < 0.05 was considered statistically significant.
A total of 64 patients who underwent S-ICD implantation were included in this study. They were divided into the GA group and the US-ICNB group. The baseline data of the patients are shown in Table 1. Regarding the indications for S-ICD implantation, class I indications accounted for 60.00% in the GA group and class IIa indications accounted for 40.00%; class I indications accounted for 56.82% in the US-ICNB group and class IIa indications accounted for 43.18%. There was no significant difference between the two groups (P = 0.752).
| Items | General anesthesia group | Ultrasound-guided intercostal nerve block group | χ2 | P value |
| Number of cases | 20 | 44 | - | - |
| Male [n (%)] | 15 (75.00) | 35 (79.55) | 0.150 | 0.698 |
| Age (years, mean ± SD) | 50.25 ± 11.80 | 56.80 ± 15.30 | -1.850 | 0.065 |
| BMI (kg/m2, mean ± SD) | 27.20 ± 4.20 | 26.30 ± 4.00 | 0.850 | 0.395 |
| LVEF (%, mean ± SD) | 56.20 ± 11.50 | 39.20 ± 12.00 | 5.120 | < 0.001 |
| ASA score [point, n (%)] | -1.550 | 0.121 | ||
| 2 | 3 (15.00) | 15 (34.09) | ||
| 3 | 17 (85.00) | 29 (65.91) | ||
| Type 2 diabetes mellitus [n (%)] | 3 (15.00) | 10 (22.73) | 0.600 | 0.438 |
| Hypertension [n (%)] | 11 (55.00) | 22 (50.00) | 0.200 | 0.655 |
| Aspartate aminotransferase [U/L, M (P25, P75)] | 19.20 (14.50, 24.00) | 24.50 (19.00, 35.50) | -2.100 | 0.036 |
| Alanine aminotransferase [U/L, M (P25, P75)] | 19.50 (12.00, 28.00) | 23.50 (15.00, 36.00) | -1.300 | 0.193 |
| Creatinine [μmol/L, M (P25, P75)] | 73.00 (61.00, 93.00) | 79.00 (63.00, 94.00) | -0.550 | 0.582 |
| Indication | ||||
| Class I | 12 (60.00) | 25 (56.82) | 0.100 | 0.752 |
| Class IIa | 8 (40.00) | 19 (43.18) |
The 64 patients undergoing S-ICD implantation were divided into GA (n = 20) and US-ICNB (n = 44) groups. Pre-operative NRS was 0 for all. Post-operative pain was mild (0-2). From 6-48 hours post-operative, NRS differed significantly (P < 0.05), with GA higher (Table 2).
| Items | General anesthesia group | Ultrasound-guided intercostal nerve block group | t value | P value |
| Number of cases | 20 | 44 | ||
| NRS (points) | ||||
| Immediately after operation | 1.82 ± 0.31 | 1.77 ± 0.33 | 0.682 | 0.501 |
| 1 hour after operation | 1.72 ± 0.36 | 1.62 ± 0.39 | 1.085 | 0.281 |
| 6 hours after operation | 1.62 ± 0.41 | 1.17 ± 0.56 | 3.883 | < 0.001 |
| 12 hours after operation | 1.02 ± 0.56 | 0.52 ± 0.46 | 3.387 | 0.001 |
| 24 hours after operation | 0.72 ± 0.61 | 0.22 ± 0.36 | 4.285 | < 0.001 |
| 48 hours after operation | 0.42 ± 0.51 | 0.12 ± 0.26 | 3.187 | 0.002 |
| 72 hours after operation | 0.16 ± 0.41 | 0 ± 0 | 2.183 | 0.033 |
| Total hospital stays (day) | 12.03 ± 4.03 | 7.53 ± 3.03 | 4.885 | < 0.001 |
| Average post operative hospital stays (day) | 4.03 ± 2.03 | 2.33 ± 1.23 | 4.185 | < 0.001 |
| Operation time (minutes) | 65.54 ± 15.25 | 45.09 ± 10.59 | 5.885 | < 0.01 |
| Time to first analgesia after operation (hour) | 8.03 ± 1.83 | 22.03 ± 15.03 | -3.585 | 0.001 |
| Usage rate of analgesics (%) | 43.75 | 7.89 | 9.59 | 0.02 |
Ultrasound-guided group had shorter total and post-operative average hospital stays (P < 0.05). GA had a longer operation time (P < 0.01). First analgesia time was later in the ultrasound-guided group (P = 0.043). Analgesic use rate was 43.75% in GA (7 on tramadol injection) and 7.89% in the other group (3 on tramadol tablets), a significant difference (P = 0.02; Table 2).
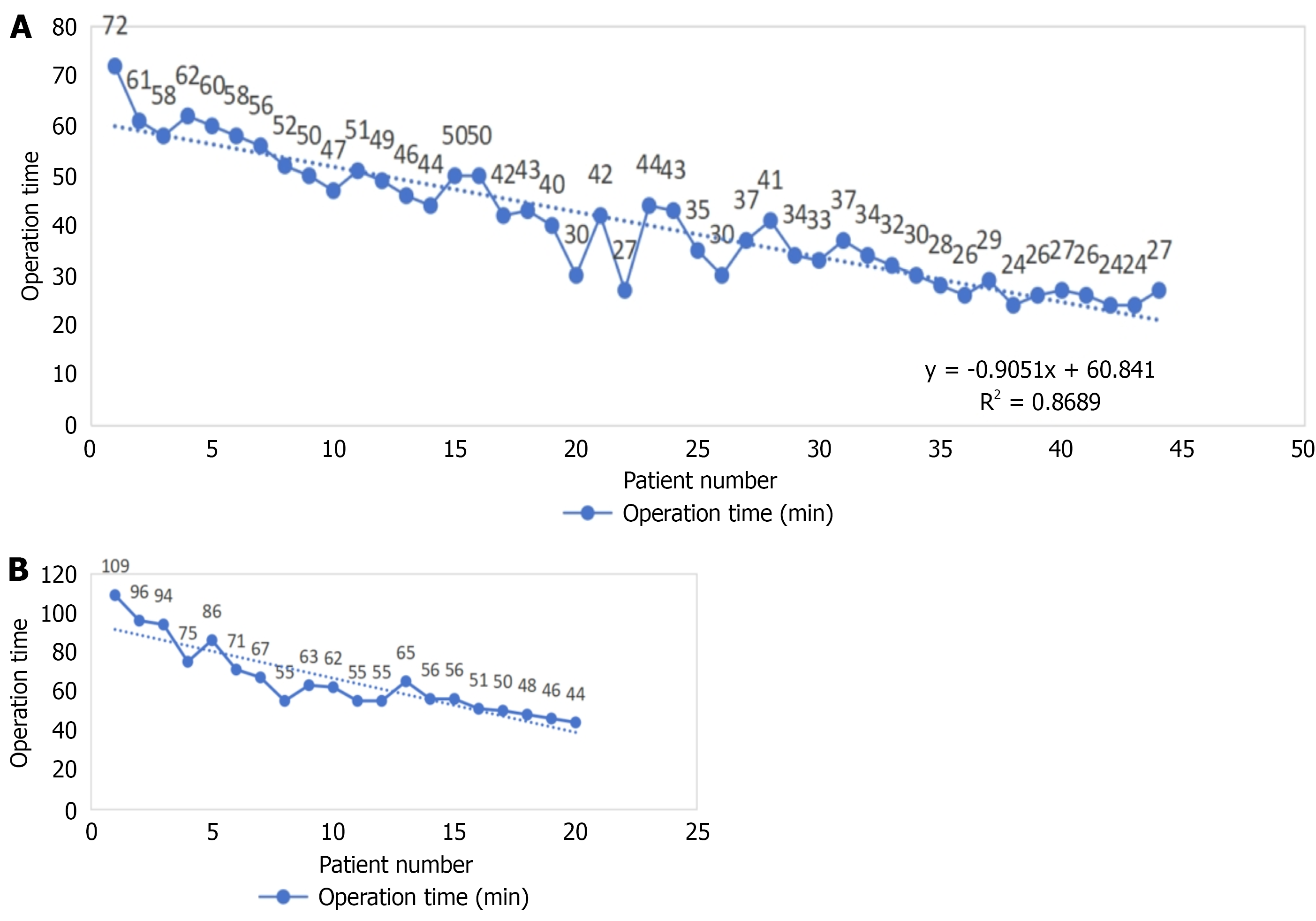
Regarding operation time, both groups demonstrated a gradual reduction in procedure duration as the number of surgeries increased (Figure 1), suggesting a short learning curve for S-ICD implantation. In the US-ICNB group, the decline was more pronounced and consistent, with a regression equation of y = -0.9051X + 60.841 and R2 = 0.8689, reflecting improved efficiency with surgical experience. The GA group also showed a downward trend but with greater initial variability and longer starting times, potentially due to differences in anesthetic management affecting procedural flow.
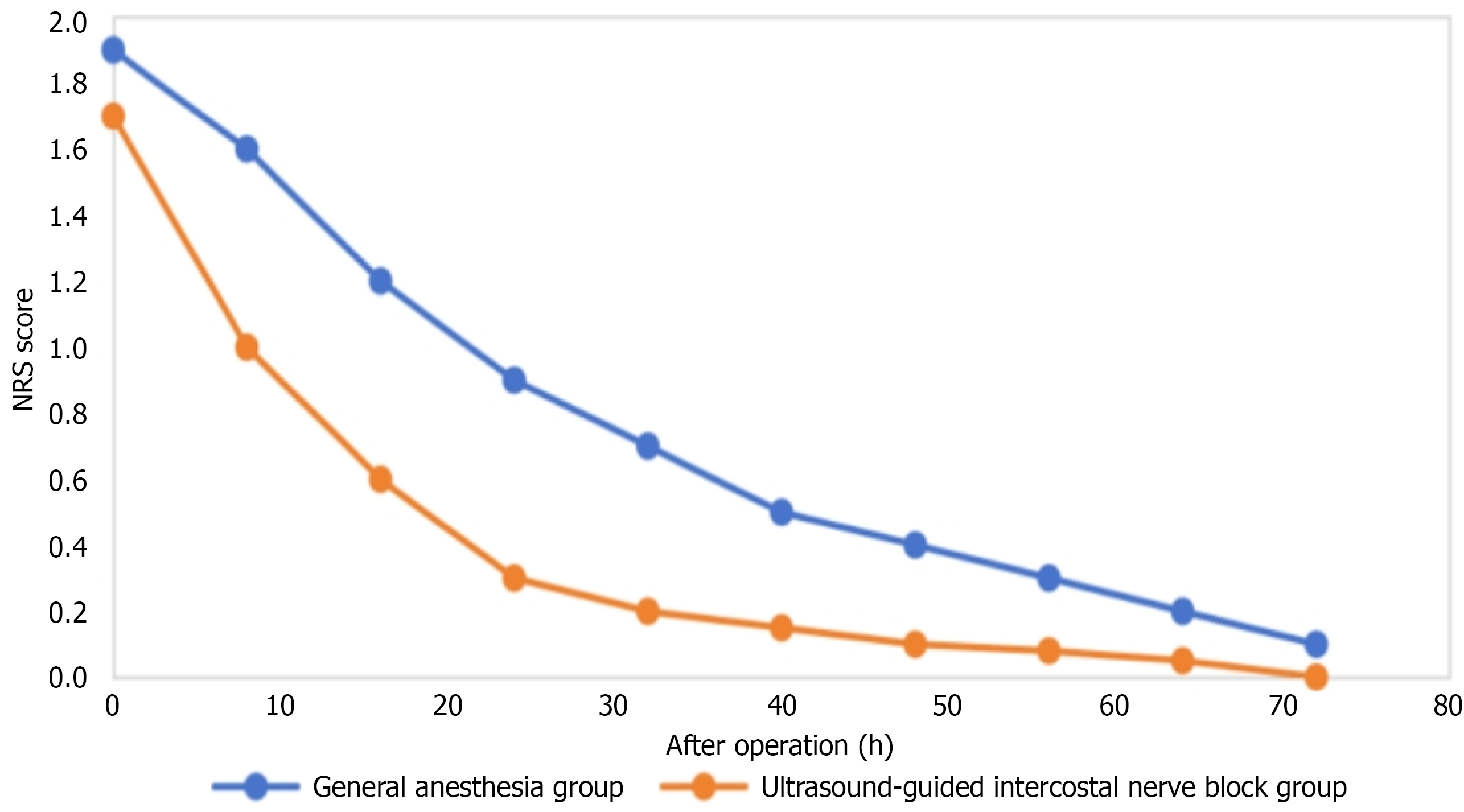
The trend of postoperative NRS score changes (Figure 2) showed that in the early postoperative period (0-10 hours), the scores of both groups decreased rapidly, and then the decline slowed down. The GA group had relatively higher scores at each time point, indicating that US-ICNB had a better postoperative analgesic effect.
All 64 patients were followed up until the end of the observation period.
In the GA group (20 patients), 8 patients (8/20, 40.0%) received a total of 25 defibrillation treatments. Among them, 6 patients (6/20, 30.0%) received 18 appropriate defibrillation treatments, mainly for ventricular tachycardia or fibrillation events that met the preset detection criteria of the S-ICD. Two patients (2/20, 10.0%) had 7 inappropriate defibrillation events. The reasons for inappropriate defibrillation were 1 case of T-wave oversensing, 1 case of R-wave double counting, and in the other cases, suspected baseline instability (possibly due to air interference).
In the US-ICNB group (44 patients), 19 patients (19/44, 43.2%) received 50 defibrillation treatments. The 16 patients (16/44, 36.4%) underwent 33 appropriate defibrillation treatments for life-threatening arrhythmias. The 3 patients (3/44, 6.8%) had 17 inappropriate defibrillation episodes. The causes included 3 cases of T-wave oversensing, 1 case of R-wave double counting, 1 case of baseline instability (suspected air interference), and 2 cases of electromagnetic interference.
Compared to traditional TV-ICDs, S-ICDs avoid direct contact with the heart and venous system, effectively reducing major complications associated with transvenous lead implantation or long-term indwelling, such as lead dislodgement, lead fracture, infective endocarditis, and pneumothorax[17,18]. Studies like IDE and EFFORTLESS have shown that S-ICDs have comparable defibrillation efficiency to TV-ICDs while significantly reducing transvenous implantation - related complications[19]. The UNTOUCHED study presented at the 2020 Heart Rhythm Society Annual Scientific Sessions further supported the value of S-ICDs in primary prevention of SCD in patients with reduced ejection fraction[20]. These findings provide strong evidence-based support for the effectiveness and safety of S-ICDs in preventing SCD. In 2015, S-ICDs were first included in guidelines as an alternative to TV-ICDs, and the 2017 ACC/AHA/HRS guidelines upgraded S-ICDs to a class I recommendation for patients with ICD implantation indications, insufficient venous access, or high infection risk who do not require bradycardia pacing, ventricular tachycardia termination pacing, or cardiac resynchronization therapy[21].
S-ICDs, with their subcutaneous implantation approach, have become the preferred option for patients at high risk of infection or with vascular problems. Although GA has been recommended as the primary choice for S-ICD implantation due to the complex surgical procedures involved, it is limited by resource requirements and high costs, and is associated with increased complications and mortality. US-ICNB, first introduced in 2013, has become an important method for peri- and post-operative analgesia in thoracic and chest wall surgeries because of its simplicity, effective pain control, and low complication rate[14]. We hypothesized that this anesthetic approach could be optimal for S-ICD implantation.
In our study, the American Society of Anesthesiologists scores were similar between the two groups, indicating comparable physical status and surgical risks. However, patients in the ultrasound-guided intercostal nerve block group had a lower LVEF, suggesting relatively poorer cardiac function. Notably, neither group experienced nerve or vascular injury, inflammation, infection, hematoma, nor anesthesia failure. The operation time decreased with increasing surgical experience in both groups, suggesting a short learning curve for S-ICD implantation. The NRS pain scores decreased over time in both groups, with significant differences from 6-48 hours post-operation. More patients in the GA group required post-operative analgesia with injectable agents, likely due to the short-acting nature of general anesthetics, while fewer patients in the US-ICNB group needed analgesia, mainly with oral medications that have a slower onset and weaker efficacy. This may be attributed to ropivacaine used in US-ICNB, which has a long-lasting effect (6-19 hours) and gradually weakens, helping patients tolerate pain and reducing the need for additional analgesics[22]. Additionally, the US-ICNB group had shorter operation and post-operative hospital stay times, indicating its superiority in S-ICD clinical application.
However, US-ICNB, as a form of local anesthesia, has potential risks. Ropivacaine, despite its high safety profile, has potential toxicity. Although the procedure is guided by ultrasound with clear visualization of structures like the pleural line, there is still a risk of pneumothorax. Also, while the needle is directed towards the nerve plane rather than the nerve directly, individual variability may lead to neurovascular injury.
Our study has limitations, including a small, non-random sample size, a single-center design which may introduce selection bias, and differences in postoperative analgesic protocols. To confirm our findings, large-scale, multi-center, randomized controlled trials with standardized analgesic regimens are needed.
In conclusion, based on our center's data, both anesthetic methods for S-ICD implantation were safe in the short term. However, US-ICNB had advantages in reducing operation and hospital stay times and alleviating peri-operative pain. It shows high safety in S-ICD implantation and is worthy of further clinical promotion.
| 1. | Marijon E, Narayanan K, Smith K, Barra S, Basso C, Blom MT, Crotti L, D'Avila A, Deo R, Dumas F, Dzudie A, Farrugia A, Greeley K, Hindricks G, Hua W, Ingles J, Iwami T, Junttila J, Koster RW, Le Polain De Waroux JB, Olasveengen TM, Ong MEH, Papadakis M, Sasson C, Shin SD, Tse HF, Tseng Z, Van Der Werf C, Folke F, Albert CM, Winkel BG. The Lancet Commission to reduce the global burden of sudden cardiac death: a call for multidisciplinary action. Lancet. 2023;402:883-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 2. | Grubic N, Puskas J, Phelan D, Fournier A, Martin LJ, Johri AM. Shock to the Heart: Psychosocial Implications and Applications of Sudden Cardiac Death in the Young. Curr Cardiol Rep. 2020;22:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA. 2006;295:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Sousonis V, Jacon P, Kerkouri F, Garcia R, Marquié C, Amara W, Anselme F, Badenco N, Behar N, Belhameche M, Bouzeman A, Fareh S, Guy-Moyat B, Hermida A, Hourdain J, Jesel L, Khattar P, Khoueiry Z, Laurent G, Manenti V, Mechulan A, Menet A, Milhem A, Mondoly P, Ollitrault P, Perrot D, Peyrol M, Pierre B, Sadoul N, Scarlatti D, Taieb J, Vanesson C, Winum P, Probst V, Marijon E, Defaye P, Boveda S; HONEST study investigators. S-ICD Implantation Following TV-ICD: Insights Into Patients With Infections and Abandoned Leads-the HONEST Cohort. JACC Clin Electrophysiol. 2025;S2405-500X(25)00277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Waldmann V, Marquié C, Bessière F, Perrot D, Anselme F, Badenco N, Barra S, Bertaux G, Blangy H, Bordachar P, Boveda S, Chauvin M, Clémenty N, Clerici G, Combes N, Defaye P, Deharo JC, Durand P, Duthoit G, Eschalier R, Fauchier L, Garcia R, Geoffroy O, Gitenay E, Gourraud JB, Guenancia C, Iserin L, Jacon P, Jesel-Morel L, Kerkouri F, Klug D, Koutbi L, Labombarda F, Ladouceur M, Laurent G, Leclercq C, Maille B, Maltret A, Massoulié G, Mondoly P, Ninni S, Ollitrault P, Pasquié JL, Pierre B, Pujadas P, Champ-Rigot L, Sacher F, Sadoul N, Schatz A, Winum P, Milliez PU, Probst V, Marijon E. Subcutaneous Implantable Cardioverter-Defibrillators in Patients With Congenital Heart Disease. J Am Coll Cardiol. 2023;82:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Lenarczyk R, Boveda S, Haugaa KH, Potpara TS, Syska P, Jedrzejczyk-Patej E, Chauvin M, Sadoul N, Dagres N. Peri-procedural routines, implantation techniques, and procedure-related complications in patients undergoing implantation of subcutaneous or transvenous automatic cardioverter-defibrillators: results of the European Snapshot Survey on S-ICD Implantation (ESSS-SICDI). Europace. 2018;20:1218-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Adduci C, Palano F, Silvetti G, Cosentino P, Francia P. Prevention of Sudden Cardiac Death: Focus on the Subcutaneous Implantable Cardioverter-Defibrillator. High Blood Press Cardiovasc Prev. 2020;27:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Van Der Stuijt W, Quast ABE, Baalman SWE, Wilde AAM, Knops RE. 5969 The experiences of women with a subcutaneous implantable cardioverter defibrillator. Eur Heart J. 2019;40:ehz746.0110. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Nishii N, Tachibana M, Morimoto Y, Kawada S, Miyoshi A, Sugiyama H, Nakagawa K, Watanabe A, Nakamura K, Morita H, Ito H. Initial experience with the subcutaneous implantable cardioverter-defibrillator in a single Japanese center. J Arrhythm. 2017;33:338-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Russo V, Viani S, Migliore F, Nigro G, Biffi M, Tola G, Bisignani G, Dello Russo A, Sartori P, Rordorf R, Ottaviano L, Perego GB, Checchi L, Segreti L, Bertaglia E, Lovecchio M, Valsecchi S, Bongiorni MG. Lead Abandonment and Subcutaneous Implantable Cardioverter-Defibrillator (S-ICD) Implantation in a Cohort of Patients With ICD Lead Malfunction. Front Cardiovasc Med. 2021;8:692943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Gasperetti A, Schiavone M, Biffi M, Casella M, Compagnucci P, Mitacchione G, Angeletti A, Vogler J, Proietti R, Ziacchi M, Dello Russo A, Natale A, Tilz RR, Forleo GB. Intraprocedural PRAETORIAN score for early assessment of S-ICD implantation: A proof-of-concept study. J Cardiovasc Electrophysiol. 2021;32:3035-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Frommeyer G, Reinke F, Rath B, Wolfes J, Willy K, Wegner FK, Köbe J, Eckardt L. Long-Term Follow-Up of the S-ICD: A 10-Years Follow-Up Study of a Large Single Center Cohort. Pacing Clin Electrophysiol. 2025;48:443-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Elders J, AlHashimi H, Gomes M, Panhuizen I, van Kuijk S, Vernooy K. Subcutaneous ICD implantation under ultrasound-guided serratus anterior plane block: Single-center experience in the Netherlands. Int J Cardiol Heart Vasc. 2022;38:100949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Marrone F, Paventi S, Tomei M, Bosco M. 153 Ultrasound-guided serratus anterior plane block (US-SAP block) and ultrasound-guided parasternal block (US-PsB) for s-ICD implantation in severe dilated post-ischaemic cardiomyopathy: a case study. Reg Anesth Pain Med. 2021;70:A80-A81. [DOI] [Full Text] |
| 15. | Roriz D, Brandão J, Graça R, Caramelo S, Correia C, Abrunhosa R. S-ICD implantation under the serratus plane block and transversus thoracis muscle plane block. A clinical case. Rev Esp Anestesiol Reanim (Engl Ed). 2022;69:102-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Könemann H, Dagres N, Merino JL, Sticherling C, Zeppenfeld K, Tfelt-Hansen J, Eckardt L. Spotlight on the 2022 ESC guideline management of ventricular arrhythmias and prevention of sudden cardiac death: 10 novel key aspects. Europace. 2023;25:euad091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 17. | Gold MR, Aasbo JD, Weiss R, Burke MC, Gleva MJ, Knight BP, Miller MA, Schuger CD, Carter N, Leigh J, Brisben AJ, El-Chami MF. Infection in patients with subcutaneous implantable cardioverter-defibrillator: Results of the S-ICD Post Approval Study. Heart Rhythm. 2022;19:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 18. | Gold MR, El-Chami MF, Burke MC, Upadhyay GA, Niebauer MJ, Prutkin JM, Herre JM, Kutalek S, Dinerman JL, Knight BP, Leigh J, Lucas L, Carter N, Brisben AJ, Aasbo JD, Weiss R; S-ICD System Post Approval Study Investigators. Postapproval Study of a Subcutaneous Implantable Cardioverter-Defibrillator System. J Am Coll Cardiol. 2023;82:383-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Burke MC, Gold MR, Knight BP, Barr CS, Theuns DAMJ, Boersma LVA, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD. Safety and Efficacy of the Totally Subcutaneous Implantable Defibrillator: 2-Year Results From a Pooled Analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 397] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 20. | Bozkurt B, Mullens W, Leclercq C, Russo AM, Savarese G, Böhm M, Hill L, Kinugawa K, Sato N, Abraham WT, Bayes-Genis A, Mebazaa A, Rosano GMC, Zieroth S, Linde C, Butler J. Cardiac rhythm devices in heart failure with reduced ejection fraction - role, timing, and optimal use in contemporary practice. European Journal of Heart Failure expert consensus document. Eur J Heart Fail. 2025;27:1242-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Bögeholz N, Willy K, Niehues P, Rath B, Dechering DG, Frommeyer G, Kochhäuser S, Löher A, Köbe J, Reinke F, Eckardt L. Spotlight on S-ICD™ therapy: 10 years of clinical experience and innovation. Europace. 2019;21:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Shen L, Lu J, He W. Effects of varying concentrations of ropivacaine on pain relief in ultrasound-guided brachial plexus block surgery in the intercostal space. Trop J Pharm Res. 2024;23:977-983. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/