Published online Aug 26, 2020. doi: 10.4330/wjc.v12.i8.427
Peer-review started: March 24, 2020
First decision: April 26, 2020
Revised: June 8, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 26, 2020
Processing time: 146 Days and 8.4 Hours
Treatment of congenitally corrected transposition of great arteries (cc-TGA) with anatomic repair strategy has been considered superior due to restoration of the morphologic left ventricle in the systemic circulation. However, data on long term outcomes are limited to single center reports and include small sample sizes.
To perform a systematic review and meta-analysis for observational studies reporting outcomes on anatomic repair for cc-TGA.
MEDLINE and Scopus databases were queried using predefined criteria for reports published till December 31, 2017. Studies reporting anatomic repair of minimum 5 cc-TGA patients with at least a 2 year follow up were included. Meta-analysis was performed using Comprehensive meta-analysis v3.0 software.
Eight hundred and ninety-five patients underwent anatomic repair with a pooled follow-up of 5457.2 patient-years (PY). Pooled estimate for operative mortality was 8.3% [95% confidence interval (CI): 6.0%-11.4%]. 0.2% (CI: 0.1%-0.4%) patients required mechanical circulatory support postoperatively and 1.7% (CI: 1.1%-2.4%) developed post-operative atrioventricular block requiring a pacemaker. Patients surviving initial surgery had a transplant free survival of 92.5% (CI: 89.5%-95.4%) per 100 PY and a low rate of need for pacemaker (0.3/100 PY; CI: 0.1-0.4). 84.7% patients (CI: 79.6%-89.9%) were found to be in New York Heart Association (NYHA) functional class I or II after 100 PY follow up. Total re-intervention rate was 5.3 per 100 PY (CI: 3.8-6.8).
Operative mortality with anatomic repair strategy for cc-TGA is high. Despite that, transplant free survival after anatomic repair for cc-TGA patients is highly favorable. Majority of patients maintain NYHA I/II functional class. However, monitoring for burden of re-interventions specific for operation type is very essential.
Core tip: This is a systematic review and meta-analysis looking at short- and long-term outcomes with the anatomic repair strategy (double switch or atrial switch Rastelli operation) for patients with congenitally corrected transposition of great arteries. Updated outcomes of operative mortality, long term survival free of transplantation and re-operation/re-intervention rates are provided. We find favorable long-term survival after anatomic repair despite the initial high operative mortality.
- Citation: Chatterjee A, Miller NJ, Cribbs MG, Mukherjee A, Law MA. Systematic review and meta-analysis of outcomes of anatomic repair in congenitally corrected transposition of great arteries. World J Cardiol 2020; 12(8): 427-436
- URL: https://www.wjgnet.com/1949-8462/full/v12/i8/427.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i8.427
Congenitally corrected transposition of the great arteries (cc-TGA) is an uncommon cardiac defect accounting for less than 0.5% of congenital heart disease[1,2]. Associated anatomic cardiac abnormalities include ventricular septal defects and pulmonary and subpulmonary obstruction, coarctation and Ebstein’s anomaly of the tricuspid valve (TV)[1]. Surgical anatomic correction, depending on underlying associated cardiac anomalies, can be obtained with either a double switch operation (Senning/Mustard atrial switch and arterial switch) or an atrial switch (Senning/Mustard) with a Rastelli operation (ventricular septal defect closure with a baffle to the aorta and a right ventricle to pulmonary artery conduit). An additional variation includes a hemi-Mustard with a bidirectional Glenn shunt. The anatomic repairs allow for the left ventricle to become the systemic ventricle. This reduces the long-term deleterious risk of systemic right ventricular (RV) failure, and the propensity for progressive systemic TV insufficiency[3,4].
There are multiple long-term complications that can occur after anatomic repair for cc-TGA. Patients with cc-TGA are prone to developing atrioventricular block (AVB) requiring pacemaker implantation regardless of operative intervention[5,6]. Furthermore, the baffles for systemic and pulmonary venous return have been shown to place patients at long-term risk for the development of atrial arrhythmia, though this risk might be mitigated by having a systemic left ventricle[6,7]. Postoperative baffle leaks and stenoses are an additional complication that can lead to a variety of symptomology including cyanosis, paradoxical embolism, or venous congestion. Finally, the long-term transplant free survival remains unknown without comparison to survival following a physiologic repair strategy[8].
Anatomic repair for cc-TGA is the preferred treatment strategy for many institutions; however, the short and long-term outcomes are mostly limited to single center studies with a limited number of patients. We therefore sought to better delineate the short-term surgical outcomes and long-term risks including need for pacing, baffle complications requiring reintervention, symptoms, and transplant free survival by pooling the data from multiple observational studies.
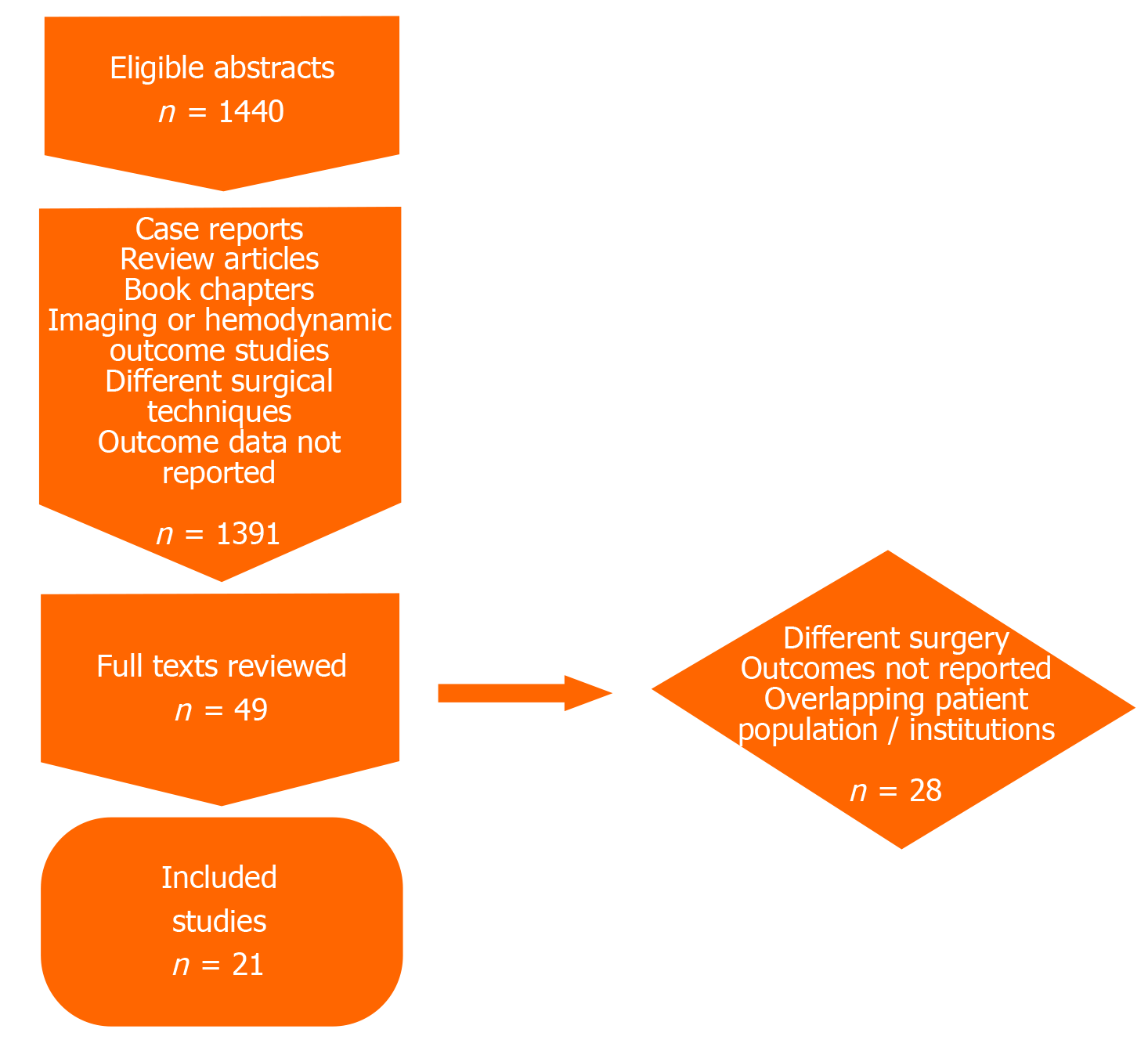
MEDLINE and Scopus databases were queried for manuscripts published till December 31, 2017 with the search items “transposition great arteries”; “TGA”; “double switch” and “anatomical repair”. All manuscripts reporting outcomes of anatomic repair in cc-TGA patients were considered in the initial review. Final inclusion criteria were a minimum sample size of 5 patients with at least 2 years of follow up. Many centers have published multiple reports of their experience at varying follow up durations. Thus, the most up to date manuscript from each group was selected. Two authors (Chatterjee A and Law MA) independently reviewed all studies considered to ensure no overlap amongst included studies. Figure 1 shows the relevant details of the study selection process.
Full texts for all potentially relevant studies were extracted and examined for alignment with inclusion criteria and verification of outcomes reported. All initially considered studies were discussed formally amongst experienced cardiologists (Chatterjee A, Cribbs MG, Law MA) and a final list was drawn up. Relevant data was then extracted from these manuscripts and reviewed for accuracy by two authors (Law MA, Chatterjee A) independently. Any disagreement was discussed in the group for resolution.
Primary outcome studied was operative mortality of the anatomic repair strategy. We also evaluated immediate operative complications including the need for extra corporeal membrane oxygenation (ECMO) or left ventricular assist devices (LVAD) and AVB requiring a pacemaker. Long term outcomes evaluated were transplant free survival, New York Heart Association (NYHA) class of patients, need for pacemaker, and rate of re-interventions. We also pooled data for development of left ventricular (LV) systolic dysfunction: LV dysfunction was defined as LV ejection fraction < 40% or when reported as moderate or severe.
Comprehensive meta-analysis (version 3; Biostat, Englewood, NJ) software was used to perform the meta-analysis. Short-term outcomes are reported as events (%) and long-term outcomes or reinterventions are reported as events per 100 patient years (PY). Heterogeneity in the data was assessed with the I2 test (I2 > 50 and Cochran’s Q statistic P value < 0.05 implying significant heterogeneity)[9]. Random effects modelling was used in keeping with the observational nature of the reports included and also heterogeneity. Publication bias was assessed using the standard funnel plot method using standard errors and any corrections assessed using the Duvall and Tweedie trim and fill method. Two-tailed P values were used with P < 0.05 implying statistical significance and confidence intervals (CI) were reported at the 95% level. PRISMA guidelines were followed in reporting the meta-analysis results[10].
Twenty-one reports of anatomic repair were included in the final analysis[4,11-30]. Table 1 lists the studies included and the type of anatomic repair used. A total of 895 patients with cc-TGA underwent anatomic repair: The pooled analysis yielded a total follow up of 5457.2 PY. The median/mean age at operation varied from 0.75–11.1 years. Four hundred and thirteen patients underwent the double switch operation (DS) while 482 patients underwent either an atrial switch-Rastelli operation or a hemi-Mustard Glenn-Rastelli operation (ASR). Sixteen studies reported patients with both types of operations; 4 studies reported experience with ASR operations only and 1 study reported only DS outcomes. Fifteen studies reported prevalence of moderate or more tricuspid regurgitation (TR): 22.7% (150/677) of the pooled sample had significant TR. Fifteen studies reported data on pre-existing AVB: This reveals that 15.6% (104/667) patients have a need for pacing even before any surgical repair.
| Ref. | Country | Total patients | Double (arterial/atrial) switch | Atrial switch-Rastelli or hemi-Mustard Glenn-Rastelli | Age at operation (yr) | Mean/median follow up (yr) |
| Ilbawi et al[11], 2002 | United States | 12 | 2 | 10 | 0.75 | 7.6 |
| Duncan et al[12], 2003 | United States | 46 | 26 | 20 | 2.3 | 2 |
| Hörer et al[13], 2008 | Germany | 6 | 0 | 6 | 3.5 | 7 |
| Gaies et al[14], 2009 | United States | 65 | 35 | 30 | 2.2 | 4.6 |
| Ly et al[15], 2009 | France | 20 | 20 | 0 | 2.2 | 5 |
| Sharma et al[16], 2009 | India | 68 | 31 | 37 | 5.2 | 4.9 |
| Lim et al[17], 2010 | South Korea | 44 | 10 | 34 | - | 5.4 |
| Malhotra et al[4], 2011 | United States | 48 | 23 | 25 | 3 | 4.9 |
| Murtuza et al[18], 2011 | United Kingdom | 113 | 68 | 45 | 3.2 | 6.9 |
| Hiramatsu et al[19], 2012 | Japan | 90 | 18 | 72 | 6.8 | 12.5 |
| Sojak et al[20], 2012 | Netherlands | 8 | 2 | 6 | 2.9 | 4.5 |
| Hoashi et al[21], 2013 | Japan | 47 | 0 | 47 | 5.5 | 11.6 |
| Bautista-Hernandez et al[22], 2014 | United States/Spain | 106 | 64 | 42 | 1.2 | 5.2 |
| Hsu et al[23], 2015 | Taiwan | 18 | 13 | 5 | 8.4 | 5.0 |
| Tocharoenchok et al[24], 2016 | Thailand | 22 | 0 | 22 | 10.9 | 5.3 |
| Brizard et al[25], 2017 | Australia | 32 | 27 | 5 | 1.9 | 5.4 |
| De León et al[26], 2017 | United States | 26 | 16 | 10 | 3 | 10 |
| Hraska et al[27], 2017 | United States/Germany | 63 | 38 | 25 | 1.6 | 5 |
| Ibrahimiye et al[28], 2017 | United States | 18 | 14 | 4 | 3 | 5 |
| Marathe et al[29], 2017 | Australia | 12 | 6 | 6 | 2.3 | 7.2 |
| Zhang et al[30], 2017 | China | 31 | 0 | 31 | 5.4 | 3.3 |
| 895 | 413 | 482 | Median age – 3 yr | Median f/u 5.2 yr |
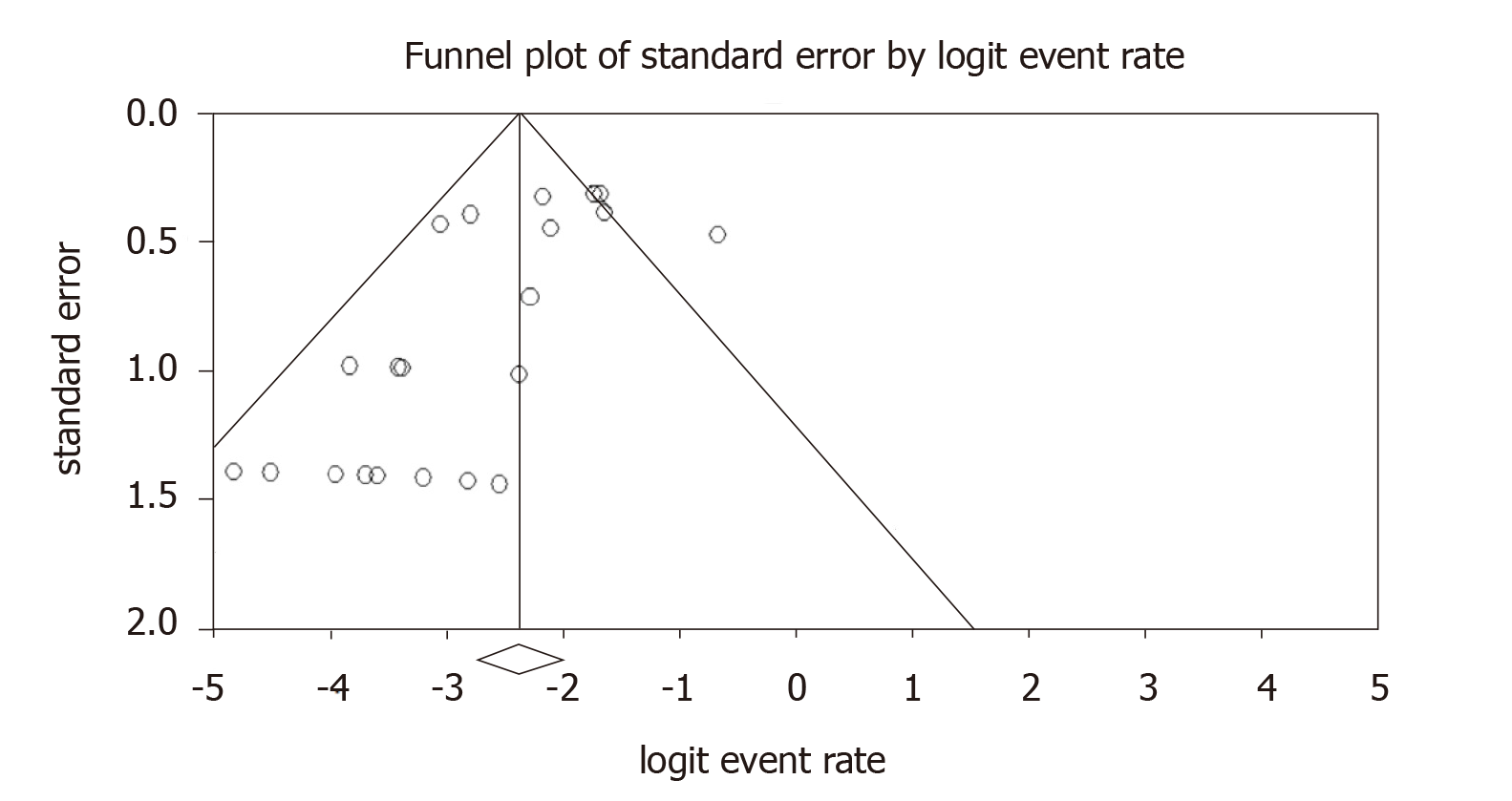
A total of 64 patients did not survive to discharge after initial operation giving a pooled estimate for operative mortality of 8.3% (95%CI: 6.0%-11.4%). Figure 2 shows the funnel plot for operative mortality for included studies which would suggest over-reporting of small studies with low operative mortality. Correcting for publication bias would raise the estimate of operative mortality to 10.9% (CI: 7.6%-15.5%). Need for mechanical circulatory support with ECMO / LVAD post operatively was 0.2% (CI: 0.1%-0.4%). Only 1.7% (CI: 1.1%-2.4%) patients developed AVB needing implantation of a permanent pacemaker.
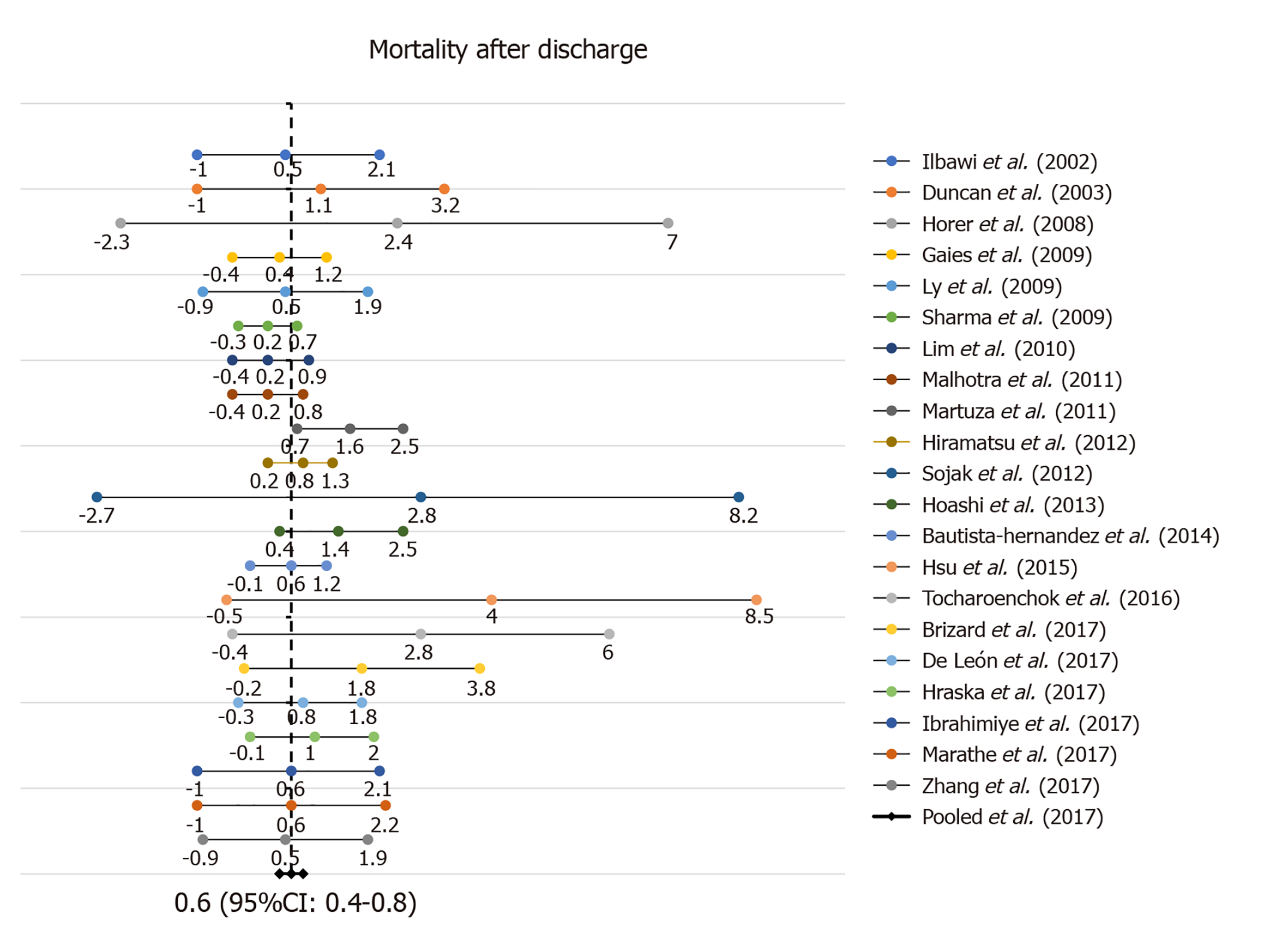
Long term outcomes are tabulated in Table 2. In patients surviving the initial operation, mortality (Figure 3) was estimated to be 0.6 per 100 PY (CI: 0.4-0.8). Fourty-eight patients died over the cumulative follow up of 5457.2 PY. In 20 studies that reported need for transplantation, 10 patients underwent heart transplantation giving an estimated overall survival free of death or needing transplant of 92.5% (CI: 89.5%-95.4%) per 100 PY. Thirteen studies reported data on functional class of surviving patients: 84.7% (CI: 79.6%-89.9%) patients were estimated to be in NYHA functional class I or II. Eighteen studies reported data on LV systolic function in long term follow up and LV dysfunction was estimated to happen in 1.7 patients/100 PY (CI: 1.0%-2.4%).
| Variable | No. of studies | Pooled estimate | 95%CI |
| Mortality after initial operation survival (n/100 PY) | 21 | 0.6 | 0.4-0.8 |
| Transplant free survival (%/100 PY) | 20 | 92.5 | 89.5-95.4 |
| NYHA I/II symptoms (%/100 PY) | 13 | 84.7 | 79.6-89.9 |
| Left ventricular systolic dysfunction (n/100 PY) | 19 | 1.7 | 1.0-2.4 |
| Left ventricular outflow tract obstruction (n/100 PY) | 16 | 0.2 | 0.1-0.3 |
| Permanent pacemaker | 19 | 0.3 | 0.1-0.4 |
| Total Reinterventions (n/100 PY) | 20 | 5.3 | 3.8-6.8 |
| Conduit interventions/operations | 20 | 1.5 | 0.9-2.1 |
| Baffle stenosis/leak | 19 | 1.1 | 0.8-1.5 |
| Tricuspid valve operations | 21 | 0.2 | 0.1-0.3 |
In long term follow up, further need for pacemaker was noted to be 0.3%/100 PY; (CI: 0.1%-0.4%).
Twenty studies reported data on total re-interventions and the pooled estimate was 5.3 per 100 PY (CI: 3.8-6.8) with incidence of baffle stenosis being estimated to be 1.1 (CI: 0.8%-1.5%)/100 PY. The most frequently needed reoperations/reinterventions were for conduit replacements or rehabilitation (1.5/100 PY; CI: 0.9-2.1).
This is a thorough attempt to use a meta-analysis study design in order to estimate the long-term outcomes with an anatomic repair strategy in this rare congenital heart disease subset. We report pooled outcomes from 895 patients with a sizeable follow up of > 5000 PY. Management patterns for patients with cc-TGA are dictated by variations in anatomic substrate and often by the experience of individual centers[3]. The long-term outcomes with a physiologic repair strategy keeping the right ventricle as the systemic ventricle, although considered safe unmistakably leads to progressive congestive heart failure. Graham et al[31] report that 67% patients with ccTGA and associated abnormalities develop congestive heart failure by age 45 and Hraska et al[32] estimate the 10-year survival after physiologic repair to be 68% only. With a low follow up mortality of 0.6/100 PY in a very large pooled sample, our results would certainly argue for anatomic repair being a default strategy for patients unless the substrate is not suitable. Most reports of anatomic repair would identify this unsuitable group as older patients whose morphological LV may not be able to sustain systemic pressures or not respond to training with a pulmonary artery band[33]. Other predictors of poor outcomes after anatomic repair are significant pre-existing TR, RV dysfunction and need for pacing[17,22].
This improved longer term survival outcome over physiological repair needs to be weighed over the apparent short-term safety of only correcting associated abnormalities in a physiologic repair strategy[34]. However, the surgical mortality in physiologic repair population is also not negligible, being reported at 3% in operations done after 1986 in a Mayo Clinic series (16% overall) and 6.7% in the Dutch series by Bogers et al[35,36]. The operative mortality in our pooled sample is slightly higher than previously reported estimates[37]. However, this is also a sample with a greater proportion of DS operations versus ASR operations. This is notable since multiple reports have considered the latter to have lower risk of operative mortality[38].
Staged single ventricle palliation with Fontan completion has been advocated as an alternative strategy in the management of cc-TGA[29,39]. The short-term results are comparable in regard to survival and symptomatology, but one must consider that long-term complications are not trivial with many patients experiencing morbid complications. In a study by Dennis et al[40], survival of patients was only 80% at the age of 40 years with many patients experiencing arrhythmia, embolic complications, and cardiac failure only after 16 years of age.
AVB in this population is consistently associated with poor outcomes[6]. However, our study results suggest that operative strategy may not make a huge incremental difference in the development of AVB. The post-operative need for pacing was only 1.7% with the long-term additional need for pacing in another 0.3% patients only.
Re-interventions after anatomic correction for cc-TGA are not rare as corroborated by our analysis. Especially in the ASR cohort, conduit replacements are very common and likely most patients will need at least one exchange to a larger conduit suitable for transcatheter replacement. However, after the first reoperation, multiple transcatheter replacements or rehabilitation procedures can be done avoiding the need for reoperation[41,42].
Baffle complications of residual leaks or stenosis are well described after the Mustard/Senning operations and thus account for the other common reoperations after anatomic repair for cc-TGA[43]. However, in the current era, most of the baffle related complications can be adequately managed with transcatheter techniques minimizing the need for re-operations[44,45]. Furthermore, residual LVOT obstruction is known after both types of anatomic repair[46]. Our analysis shows the incidence of this is low but not completely negligible.
However, the need for re-interventions should also be noted in the context of similar or higher need for reoperations and reinterventions in the physiologic repair strategy. In a sample of 111 children who underwent physiologic repair only, 41% patients required reoperations for conduit exchanges or TV repair/replacement at a mean follow up duration of 11.4 years[36]. Similarly, Bogers et al[35] have also reported a re-intervention rate of 32% at 20 years with a physiologic repair.
The meta-analysis study design lends itself to certain characteristic limitations. Systematic pooling of observational studies with differences in baseline patient characteristics, varied anatomic substrate, different sample sizes, surgical technique and different follow-up durations make the pooled sample more heterogenous. This is also not a patient level meta-analysis which can overcome some of these limitations. In congenital heart disease literature, reports often consist of small sample sizes and limited follow up, hence pre-disposing to lower than actual pooled estimates when included in a meta-analysis. Since all the studies included are observational, there is also the consideration of under-reporting of outcomes or exclusion of patients with poor outcomes. There is also no way to adequately account for refinement in surgical technique as experience with anatomic repair grew.
This large pooled analysis supports the overall favorable outcomes after anatomic repair for cc-TGA, especially beyond the early initial operative period. Majority of patients have good quality of life, falling in NYHA class I or II. Despite increase in the complexity of repair, there does not seem to be a large increase in the prevalence of heart block. Re-interventions are required but can be accomplished in many situations with transcatheter techniques in the modern era.
Anatomic repair for congenitally corrected transposition of great arteries (cc-TGA) is accomplished by either a double switch operation or one of many modifications of an atrial switch operation combined with a Rastelli operation. However, these operations are complex and a simpler physiologic repair strategy of correcting only associated defects like tricuspid valve regurgitation or ventricular septal defects is also adopted in many patients. Anatomic repair strategy has the benefit of restoring the left ventricle to the systemic circulation, thus decreasing the chances of development of congestive heart failure from a systemic right ventricle.
There are variations in practice regarding anatomic versus physiologic repair for cc-TGA. Long term data from a large set of patients regarding safety and outcomes of anatomic repair are lacking.
The objective of this study was to pool high quality observational studies reporting outcomes after anatomic repair in cc-TGA patients and perform a systematic review and meta-analysis to provide more comprehensive outcomes.
A search of MEDLINE and Scopus was conducted using pre-defined search criteria to identify manuscripts reporting outcomes after anatomic repair. Studies meeting inclusion criteria were reviewed and information regarding variables of interest were extracted. Meta-analysis was performed according to standard methods using Comprehensive meta-analysis software (version 3).
Eight hundred and ninety-five patients who were treated with an anatomic repair strategy were pooled from 21 studies with a total follow-up of 5457.2 patient-years (PY). Estimated operative mortality was 8.3%. Survivors had a transplant free survival of 92.5% (CI: 89.5%-95.4%) per 100 PY. 84.7% patients experienced a New York Heart Association functional class I or II after 100 PY follow up. There were 5.3 re-operations/re-interventions per 100 PY (CI: 3.8-6.8).
Our study reports a high operative mortality rate for anatomic repair strategy in cc-TGA patients. However, the long-term survival is excellent for survivors.
Our study suggests that the anatomic repair is worth pursuing in most patients with cc-TGA because of favorable long-term outcomes despite a high operative mortality risk. Re-intervention/reoperation risk remains – however with the advent of transcatheter therapies, most of these issues can be managed without a re-operation in the modern era.
| 1. | Wallis GA, Debich-Spicer D, Anderson RH. Congenitally corrected transposition. Orphanet J Rare Dis. 2011;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2512] [Cited by in RCA: 2317] [Article Influence: 154.5] [Reference Citation Analysis (1)] |
| 3. | Kutty S, Danford DA, Diller GP, Tutarel O. Contemporary management and outcomes in congenitally corrected transposition of the great arteries. Heart. 2018;104:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Malhotra SP, Reddy VM, Qiu M, Pirolli TJ, Barboza L, Reinhartz O, Hanley FL. The hemi-Mustard/bidirectional Glenn atrial switch procedure in the double-switch operation for congenitally corrected transposition of the great arteries: rationale and midterm results. J Thorac Cardiovasc Surg. 2011;141:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Anderson RH, Becker AE, Arnold R, Wilkinson JL. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 236] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Baruteau AE, Abrams DJ, Ho SY, Thambo JB, McLeod CJ, Shah MJ. Cardiac Conduction System in Congenitally Corrected Transposition of the Great Arteries and Its Clinical Relevance. J Am Heart Assoc. 2017;6:e007759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Roubertie F, Thambo JB, Bretonneau A, Iriart X, Laborde N, Baudet E, Roques X. Late outcome of 132 Senning procedures after 20 years of follow-up. Ann Thorac Surg. 2011;92:2206-13; discussion 2213-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Warnes CA. Transposition of the great arteries. Circulation. 2006;114:2699-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Higgings JPT, G. S.e. Cochrane Handbook for Systematic Reviews of Interventions. [cited 1 January 2020]. The Cochrane Collaboration 2011. Available from: www.handbook.cochrane.org. |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8997] [Article Influence: 529.2] [Reference Citation Analysis (0)] |
| 11. | Ilbawi MN, Ocampo CB, Allen BS, Barth MJ, Roberson DA, Chiemmongkoltip P, Arcilla RA. Intermediate results of the anatomic repair for congenitally corrected transposition. Ann Thorac Surg. 2002;73:594-599; discussion 599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Duncan BW, Mee RB, Mesia CI, Qureshi A, Rosenthal GL, Seshadri SG, Lane GK, Latson LA. Results of the double switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2003;24:11-19; discussion 19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Hörer J, Schreiber C, Krane S, Prodan Z, Cleuziou J, Vogt M, Holper K, Lange R. Outcome after surgical repair/palliation of congenitally corrected transposition of the great arteries. Thorac Cardiovasc Surg. 2008;56:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Gaies MG, Goldberg CS, Ohye RG, Devaney EJ, Hirsch JC, Bove EL. Early and intermediate outcome after anatomic repair of congenitally corrected transposition of the great arteries. Ann Thorac Surg. 2009;88:1952-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Ly M, Belli E, Leobon B, Kortas C, Grollmüss OE, Piot D, Planché C, Serraf A. Results of the double switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2009;35:879-83; discussion 883-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Sharma R, Talwar S, Marwah A, Shah S, Maheshwari S, Suresh P, Garg R, Bali BS, Juneja R, Saxena A, Kothari SS. Anatomic repair for congenitally corrected transposition of the great arteries. J Thorac Cardiovasc Surg. 2009;137:404-412.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Lim HG, Lee JR, Kim YJ, Park YH, Jun TG, Kim WH, Lee CH, Park HK, Yang JH, Park CS, Kwak JG. Outcomes of biventricular repair for congenitally corrected transposition of the great arteries. Ann Thorac Surg. 2010;89:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Murtuza B, Barron DJ, Stumper O, Stickley J, Eaton D, Jones TJ, Brawn WJ. Anatomic repair for congenitally corrected transposition of the great arteries: a single-institution 19-year experience. J Thorac Cardiovasc Surg. 2011;142:1348-1357.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Hiramatsu T, Matsumura G, Konuma T, Yamazaki K, Kurosawa H, Imai Y. Long-term prognosis of double-switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2012;42:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Sojak V, Kuipers I, Koolbergen D, Rijlaarsdam M, Hruda J, Blom N, Hazekamp M. Mid-term results of bidirectional cavopulmonary anastomosis and hemi-Mustard procedure in anatomical correction of congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2012;42:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Hoashi T, Kagisaki K, Miyazaki A, Kurosaki K, Shiraishi I, Yagihara T, Ichikawa H. Anatomic repair for corrected transposition with left ventricular outflow tract obstruction. Ann Thorac Surg. 2013;96:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Bautista-Hernandez V, Myers PO, Cecchin F, Marx GR, Del Nido PJ. Late left ventricular dysfunction after anatomic repair of congenitally corrected transposition of the great arteries. J Thorac Cardiovasc Surg. 2014;148:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Hsu KH, Chang CI, Huang SC, Chen YS, Chiu IS. 17-year experience in surgical management of congenitally corrected transposition of the great arteries: a single-centre's experience. Eur J Cardiothorac Surg. 2016;49:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Tocharoenchok T, Sriyoschati S, Tongcharoen P, Tantiwongkosri K, Subtaweesin T. Midterm results of anatomic repair in a subgroup of corrected transposition. Asian Cardiovasc Thorac Ann. 2016;24:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Brizard CP, Lee A, Zannino D, Davis AM, Fricke TA, d'Udekem Y, Konstantinov IE, Brink J, Cheung MMH. Long-term results of anatomic correction for congenitally corrected transposition of the great arteries: A 19-year experience. J Thorac Cardiovasc Surg. 2017;154:256-265.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | De León LE, Mery CM, Verm RA, Trujillo-Díaz D, Patro A, Guzmán-Pruneda FA, Adachi I, Heinle JS, Kane LC, McKenzie ED, Fraser CD. Mid-Term Outcomes in Patients with Congenitally Corrected Transposition of the Great Arteries: A Single Center Experience. J Am Coll Surg. 2017;224:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Hraska V, Vergnat M, Zartner P, Hart C, Suchowerskyj P, Bierbach B, Schindler E, Schneider M, Asfour B. Promising Outcome of Anatomic Correction of Corrected Transposition of the Great Arteries. Ann Thorac Surg. 2017;104:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Ibrahimiye AN, Mainwaring RD, Patrick WL, Downey L, Yarlagadda V, Hanley FL. Left Ventricular Retraining and Double Switch in Patients With Congenitally Corrected Transposition of the Great Arteries. World J Pediatr Congenit Heart Surg. 2017;8:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Marathe SP, Jones MI, Ayer J, Sun J, Orr Y, Verrall C, Nicholson IA, Chard RB, Sholler GF, Winlaw DS. Congenitally corrected transposition: complex anatomic repair or Fontan pathway? Asian Cardiovasc Thorac Ann. 2017;25:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Zhang S, Ma K, Li S, Hua Z, Zhang H, Yan J, Yang K, Pang K, Wang X, Qi L, Chen Q. The hemi-Mustard, bidirectional Glenn and Rastelli procedures for anatomical repair of congenitally corrected transposition of the great arteries/left ventricular outflow tract obstruction with positional heart anomalies†. Eur J Cardiothorac Surg. 2017;51:1058-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Graham TP, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 434] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 32. | Hraska V, Duncan BW, Mayer JE, Freed M, del Nido PJ, Jonas RA. Long-term outcome of surgically treated patients with corrected transposition of the great arteries. J Thorac Cardiovasc Surg. 2005;129:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Myers PO, del Nido PJ, Geva T, Bautista-Hernandez V, Chen P, Mayer JE, Emani SM. Impact of age and duration of banding on left ventricular preparation before anatomic repair for congenitally corrected transposition of the great arteries. Ann Thorac Surg. 2013;96:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Hirose K, Nishina T, Kanemitsu N, Mizuno A, Yasumizu D, Yada M, Onga Y, Yamanaka K. The long-term outcomes of physiologic repair for ccTGA (congenitally corrected transposition of the great arteries). Gen Thorac Cardiovasc Surg. 2015;63:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Bogers AJ, Head SJ, de Jong PL, Witsenburg M, Kappetein AP. Long term follow up after surgery in congenitally corrected transposition of the great arteries with a right ventricle in the systemic circulation. J Cardiothorac Surg. 2010;5:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Biliciler-Denktas G, Feldt RH, Connolly HM, Weaver AL, Puga FJ, Danielson GK. Early and late results of operations for defects associated with corrected transposition and other anomalies with atrioventricular discordance in a pediatric population. J Thorac Cardiovasc Surg. 2001;122:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Imamura M, Drummond-Webb JJ, Murphy DJ, Prieto LR, Latson LA, Flamm SD, Mee RB. Results of the double switch operation in the current era. Ann Thorac Surg. 2000;70:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Alghamdi AA, McCrindle BW, Van Arsdell GS. Physiologic versus anatomic repair of congenitally corrected transposition of the great arteries: meta-analysis of individual patient data. Ann Thorac Surg. 2006;81:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Sun J, Brizard C, Winlaw D, Alphonso N, d'Udekem Y, Eastaugh L, Marathe S, Bell D, Ayer J. Biventricular repair versus Fontan completion for patients with d- or l-transposition of the great arteries with ventricular septal defect and left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg. 2019;158:1158-1167.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Dennis M, Zannino D, du Plessis K, Bullock A, Disney PJS, Radford DJ, Hornung T, Grigg L, Cordina R, d'Udekem Y, Celermajer DS. Clinical Outcomes in Adolescents and Adults After the Fontan Procedure. J Am Coll Cardiol. 2018;71:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 41. | Chatterjee A, Bajaj NS, McMahon WS, Cribbs MG, White JS, Mukherjee A, Law MA. Transcatheter Pulmonary Valve Implantation: A Comprehensive Systematic Review and Meta-Analyses of Observational Studies. J Am Heart Assoc. 2017;6:e006432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Caughron H, Kim D, Kamioka N, Lerakis S, Yousef A, Maini A, Reginauld S, Sahu A, Shashidharan S, Jokhadar M, Rodriguez FH, Book WM, McConnell M, Block PC, Babaliaros V. Repeat Pulmonary Valve Replacement: Similar Intermediate-Term Outcomes With Surgical and Transcatheter Procedures. JACC Cardiovasc Interv. 2018;11:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Patel S, Shah D, Chintala K, Karpawich PP. Atrial baffle problems following the Mustard operation in children and young adults with dextro-transposition of the great arteries: the need for improved clinical detection in the current era. Congenit Heart Dis. 2011;6:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Bradley EA, Cai A, Cheatham SL, Chisolm J, Sisk T, Daniels CJ, Cheatham JP. Mustard baffle obstruction and leak - How successful are percutaneous interventions in adults? Prog Pediatr Cardiol. 2015;39:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Chatterjee A, Miller NJ, Cribbs MG, Law MA. Transcatheter Repair of Pulmonary Venous Baffle Stenosis. JACC Cardiovasc Interv. 2018;11:e129-e130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Emani SM, Beroukhim R, Zurakowski D, Pigula FA, Mayer JE, del Nido PJ, Geva T, Bacha EA. Outcomes after anatomic repair for d-transposition of the great arteries with left ventricular outflow tract obstruction. Circulation. 2009;120:S53-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American College of Cardiology; Society for Cardiovascular Angiography and Interventions.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao BL, Kharlamov AK, Teragawa H, Ueda H S-Editor: Liu M L-Editor: A P-Editor: Li JH