©The Author(s) 2026.
World J Cardiol. Jan 26, 2026; 18(1): 111882
Published online Jan 26, 2026. doi: 10.4330/wjc.v18.i1.111882
Published online Jan 26, 2026. doi: 10.4330/wjc.v18.i1.111882
Figure 1 The risk of future embolic events in patients with subclinical atrial fibrillation with a recent pacemaker implantation from the ASSERT study.
The upper panel depicts data from the original ASSERT study, where 10% patients developed Atrial high-rate episode (AHRE) within 3 months of a pacemaker implantation. The presence of AHRE was predictive of future risk of atrial fibrillation (AF) & ischemic events at 2.5-year follow-up. The lower panel stratifies patients based on subclinical AF duration and risk of future AF & ischemic events. AHRE: Atrial high-rate episode; SE: Systemic embolism; SCAF: Subclinical atrial fibrillation; FU: Follow up; HR: Hazard ratio.
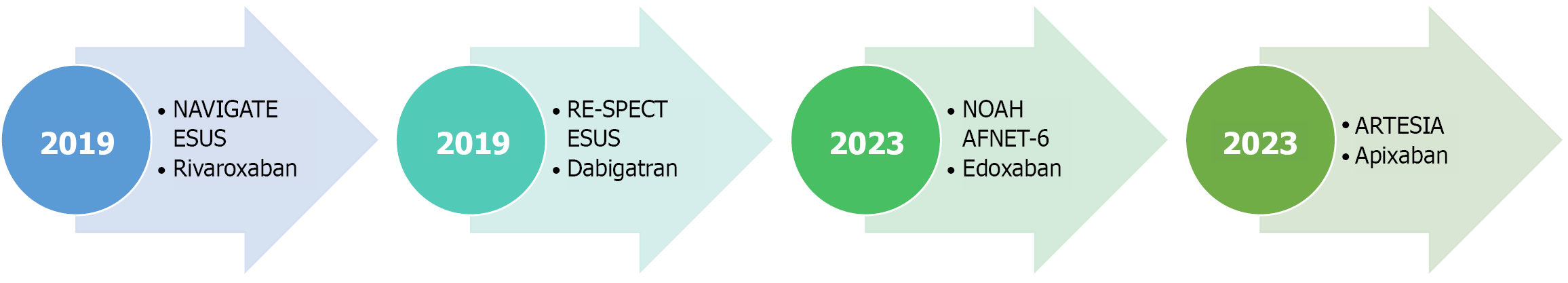
Figure 2 The four pivotal direct oral anticoagulant trials in patients with cryptogenic stroke & subclinical atrial fibrillation.
The NAVIGATE ESUS & RE-SPECT embolic stroke of unknown source evaluated the role of direct oral anticoagulant therapy in cryptogenic stroke, while NOAH-AFNET-6 & ARTESIA enrolled patients with subclinical atrial fibrillation. ESUS: Embolic stroke of unknown source.
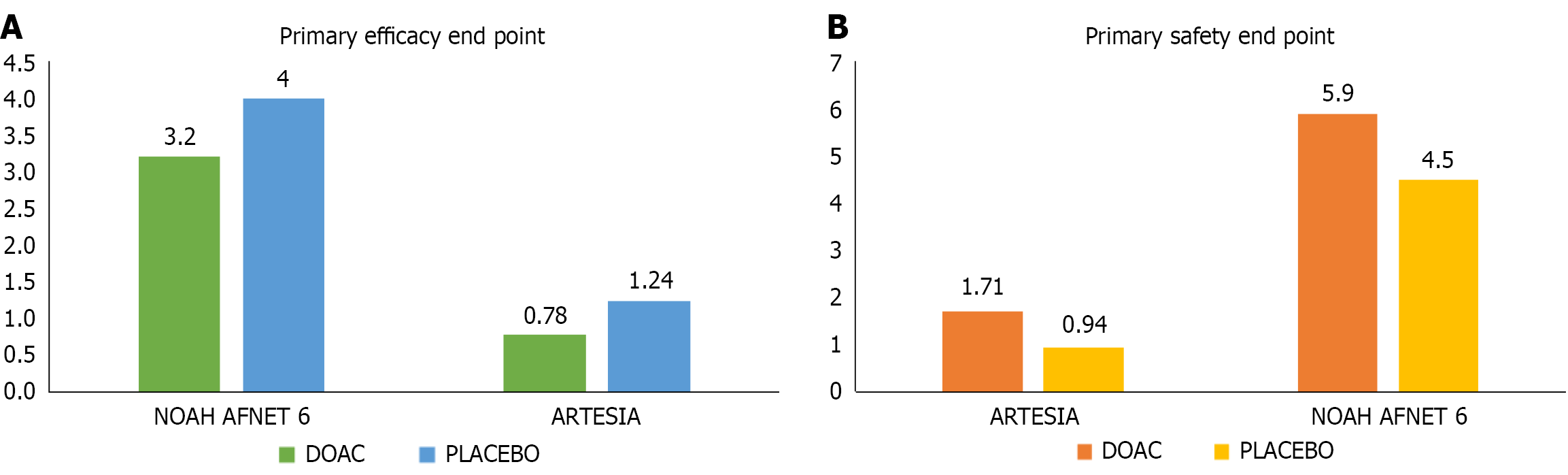
Figure 3 Comparison of results of two recently published large direct oral anticoagulant randomized controlled trials in patients with subclinical AF-NOAH-AFNET with edoxaban & ARTESIA with apixaban, respectively.
A: The primary efficacy outcome; B: The primary safety endpoint. The primary efficacy endpoint in the ARTESIA study was a composite of stroke/systemic embolism (SE), while in the NOAH AFNET-6 was cardiovascular death, stroke, or SE. The y axis represents the event rate measured in person-years. Similarly, major bleeding was the primary safety endpoint in ARTESIA, while in the NOAH-AFNET study, it was a combination of all-cause death or major bleeding. DOAC: Direct oral anticoagulant.
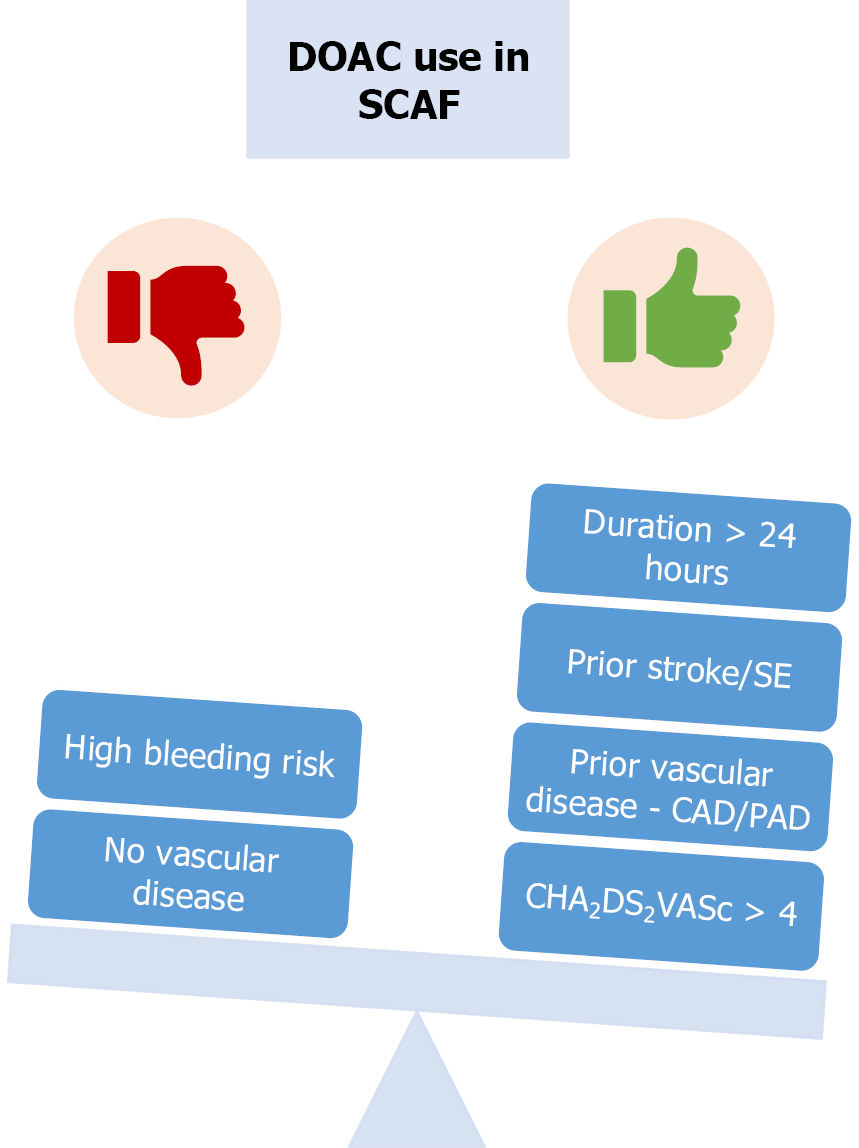
Figure 4 Flow chart regarding anticoagulation approach in subclinical atrial fibrillation.
The green colour indicated factors favouring direct oral anticoagulation (DOAC) use in subclinical atrial fibrillation, while the red colour represents factors arguing against DOAC therapy. The CHA2DS2-VASc score is used to predict the risk of ischemic stroke among patients with atrial fibrillation and ranges from 0 to 9, with higher scores indicating a greater risk of stroke. SCAF: Subclinical atrial fibrillation; CAD: Coronary artery disease; PAD: Peripheral artery disease; DOAC: Direct oral anticoagulation; CHA2DS2-VASc score: Congestive heart failure, hypertension, age ≥ 75 years (2 points), diabetes mellitus, prior stroke or TIA or thromboembolism (2 points), vascular disease, age 65-74 years, sex category.
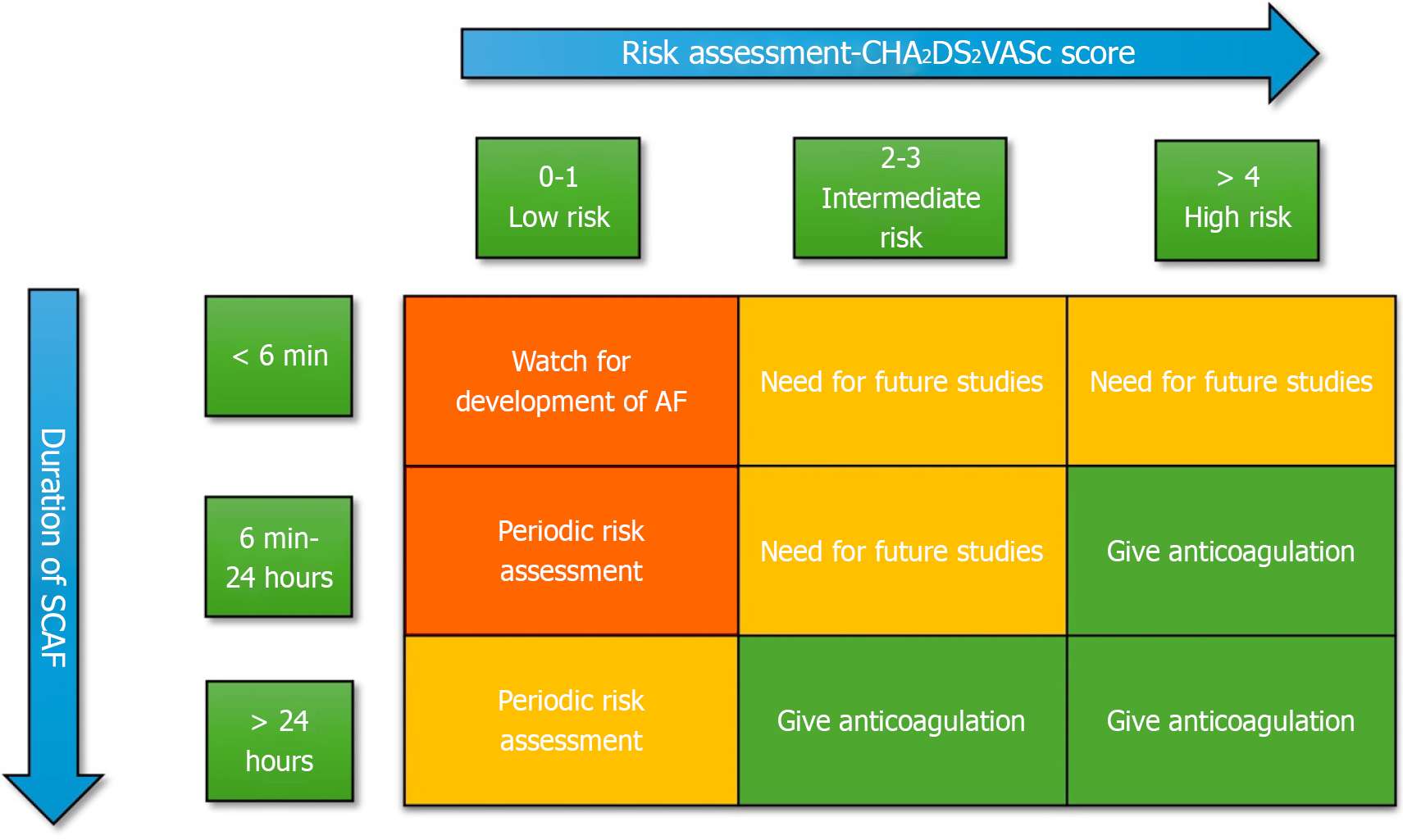
Figure 5 The contemporary 2023 ACC/AHA guideline on the use of oral anticoagulation in subclinical atrial fibrillation.
The orange zones indicate areas of low risk and obviate the need for oral anticoagulation. The green zones demarcate areas of high risk and need for oral anticoagulation. The yellow zones are a grey area and need further research.
- Citation: Pradhan A, Shah S, Vishwakarma P, Singh AK. Subclinical atrial fibrillation: Implications of recent trials for guideline updates? World J Cardiol 2026; 18(1): 111882
- URL: https://www.wjgnet.com/1949-8462/full/v18/i1/111882.htm
- DOI: https://dx.doi.org/10.4330/wjc.v18.i1.111882