Published online Jan 26, 2026. doi: 10.4330/wjc.v18.i1.111954
Revised: August 4, 2025
Accepted: November 24, 2025
Published online: January 26, 2026
Processing time: 185 Days and 13.6 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) has rapidly become the leading cause of chronic liver disease and cirrhosis worldwide, driven by the global surge in metabolic disorders such as obesity, diabetes, hypertension, and dyslipidemia. In parallel, heart failure with preserved ejection fraction (HF
Core Tip: The ever-changing terminology of metabolic dysfunction-associated steatotic liver disease (MASLD) to encompass metabolic dysfunction truly reflects its systemic impact beyond the liver. Cardiovascular diseases remain the leading cause of MASLD-related morbidity and mortality. Amongst the various cardiovascular diseases linked to MASLD, heart failure with preserved ejection fraction (HFpEF) is most closely related to MASLD, sharing a pathophysiologic foundation with MASLD and rooted in common metabolic risk factors. This review explores the intertwined mechanisms linking MASLD and HFpEF, highlighting their clinical convergence and therapeutic relevance.
- Citation: Brar AS, Khanna T, Sohal A, Hatwal J, Sharma V, Singh C, Batta A, Chandra P, Mohan B. Metabolic dysfunction-associated steatotic liver disease and heart failure with preserved ejection fraction: A state-of-the-art review. World J Cardiol 2026; 18(1): 111954
- URL: https://www.wjgnet.com/1949-8462/full/v18/i1/111954.htm
- DOI: https://dx.doi.org/10.4330/wjc.v18.i1.111954
Metabolic dysfunction-associated steatotic liver disease (MASLD) has been on the rise and has now become one of the leading causes of liver transplantation in the United States. The new nomenclature requires at least one of the five metabolic risk factors along with liver steatosis to diagnose MASLD and metabolic-associated steatohepatitis (MASH). These include diabetes, hypertension, hypertriglyceridemia, and low HDL levels[1,2]. The shared risk factors that can predispose patients to MASLD and MASH have been implicated in the development of cardiovascular disease (CVD). As a result, there have been studies aiming to estimate whether MASLD is a risk factor for CVD development. In a study by Simon et al[3], the risk of developing cardiovascular outcomes was significantly higher among patients with MASLD. The risk was independent of the common cardiometabolic risk factors shared by MASLD and CVD. Studies by Mantovani et al[4] and Simon et al[3] reported that MASLD was associated with an increased risk of developing CVD, independent of common cardiometabolic risk factors. In both studies, the risk was progressively higher in patients with non-cirrhotic fibrosis and cirrhosis[4]. CVD has also been reported to be the leading cause of mortality among patients with MASLD. A systematic review by Younossi et al[5] reported the all-cause mortality to be 12.6 per 1000 person-years. In their analysis, cardiac-specific mortality was noted to be 4.2 per 1000 person-years, while chronic liver disease-specific mortality was 0.92 per 1000 persons.
The common CVDs noted among patients with MASH are atherosclerosis, cardiomyopathy, arrhythmias, and heart failure, especially heart failure with preserved ejection[5]. There is a well-established bidirectional relationship between liver disease and heart failure. Heart failure can lead to congestive hepatopathy, while the presence of advanced fibrosis can result in cirrhotic cardiomyopathy. A recent 7-year cohort study found that MASLD was associated with diastolic dysfunction independent of other risk factors. A meta-analysis has also supported these findings[6]. Studies have also reported diastolic dysfunction to be related to the degree of liver fibrosis[7,8]. As there is new emerging evidence that suggests that the presence of MASLD/MASH, even without advanced fibrosis, can increase the risk of developing heart failure with preserved ejection fraction (HFpEF), our article aims to review the current epidemiology, pathogenesis, cellular mechanisms, as well as the impact of various therapies on MASLD on HFpEF.
Female sex hormones are well known to not only protect against CVD in pre-menopausal females but also contribute to improved adipose tissue function and preventing its systemic deposition.
Prior longitudinal studies and a meta-analysis have found that cardiac dysfunction consistent with HFpEF is commonly seen in MASLD patients[9]. Independent associations between MASLD and structural cardiac changes have been observed, including left atrial (LA) size, left ventricular hypertrophy (LVH), and strain[10-12]. Furthermore, biological sex plays a role in the association of MASLD and HFpEF due to the protective role of estrogen in CVD and difference in ectopic adipose deposition trends[13]. Echocardiography in MASLD patients has shown impaired LV relaxation and diastolic dysfunction. Doppler imaging in these patients has shown lower E wave and e’ velocity, decreased E/a ratio, and increased E/e.’ Overall, this suggests that MASLD patients tend to have higher LV filling pressure even after adjusting for comorbidities such as hypertension, diabetes, and obesity[14]. However, another longitudinal study found that the association of MASLD with LV hypertrophy (LVH) was not significant after controlling for obesity[11]. A magnetic resonance imaging (MRI)-based study tested for correlates of diastolic dysfunction and found that it was associated with hepatic steatosis and visceral obesity despite not showing a significant relation with myocardial triglycerides[15].
Multiple studies have aimed to establish the relationship between MASLD and heart failure. A recent meta-analysis by Qiu et al[16] examining 6 cohort studies containing 10979967 participants reported patients with MASLD at a 36% higher risk of incident HF than patients without MASLD. The increased risk was also noted among patients with mild steatosis. Regarding the risk of HFpEF among patients with MASLD, a study by Fudim et al[17] evaluating 870535 Medicare beneficiaries reported the presence of MASLD to be associated with a significantly increased risk of developing new onset HF. Their study reported a higher risk of developing HFpEF compared to the risk of HFrEF.
Single-center studies provide information regarding the prevalence of fatty liver among patients with HFpEF. In a study by Miller et al[18] evaluating 181 patients with HFpEF, 49 (27%) patients had MASLD. Twelve patients had imaging results consistent with cirrhosis. Notably, only 96 of the patients had abdominal imaging in the study, and among these patients, the prevalence of MASLD and cirrhosis was 50% and 11.5%, respectively. When including patients who had imaging or NFS scores consistent with cirrhosis, the prevalence was noted to be 48.6%. Their study also indicated that patients with fatty liver on imaging had more severe heart failure. Another survey by Wegermann et al[19] indicated that among 89 patients with biopsy-proven MASLD, the prevalence of HFpEF and diastolic dysfunction was 41.9% and 47.2%, respectively. A study by Minhas et al[20] utilizing United States national hospitalization data noted that nonalcoholic fatty liver disease (NAFLD)'s prevalence was 2.3% among patients with HFpEF. This study might underestimate the prevalence as it was based on International Classification of Diseases-10 codes, and information regarding more granular data was missing. Despite this limitation, this study is of interest because MASLD was associated with an increased risk of in-hospital complications among patients with heart failure with preserved ejection fraction[20]. Studies have established that the presence and extent of fibrosis in MASLD correlates with the prognosis of HFpEF[9,19,21,22]. More severe fibrosis, i.e., F3-F4, has shown associations with worse cardiovascular outcomes[23]. Worse diastolic function and greater LA diameter have been observed in MASLD patients with advanced fibrosis[18]. The association has remained true for patients hospitalized with decompensated HFpEF[24]. The significant effect of MASH on HFpEF prognosis supports the conjecture that the two diseases might progress via similar pathogenetic pathways. Thus, it is essential to understand the shared risk factors and the underlying pathophysiological mechanisms responsible for developing these conditions.
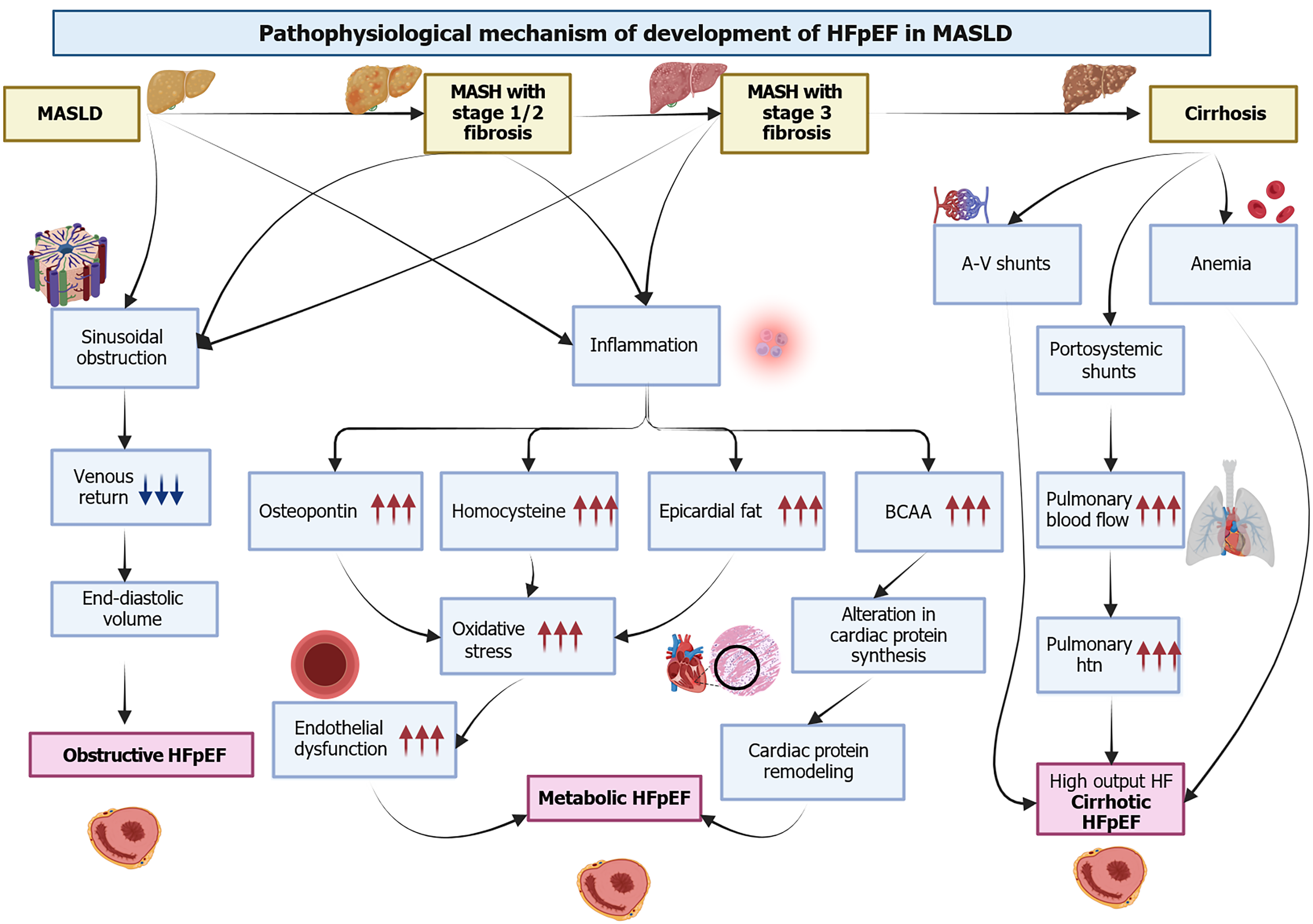
HFpEF is characterized by diastolic and systolic reserve abnormalities, pulmonary hypertension, endothelial dysfunction, and reserve failure[25]. Initially, HFpEF was studied as a unified syndrome; however, due to the differences in the underlying mechanisms, several subtypes of this disease have since been discussed. A previous study by Salah et al[9] reported that three significant phenotypes among patients with MASLD include obstructive, metabolic, and cirrhosis HFpEF phenotypes. Understanding these phenotypes is beneficial, as this can provide insight into the various ways by which MASLD/MASH can predispose to HFpEF development. Figure 1 highlights the pathophysiological mechanism of HFpEF development in MASLD.
The liver is a major metabolic organ; about 25% of blood volume passes through the liver before reaching the heart. In the early stages of MASLD, there is an increased resistance in the hepatic sinusoids, which may impede venous return. As fibrosis progresses, the resistance in the hepatic sinusoids may increase, further impeding the venous return[26]. Fibrosis may reduce preload, which impairs the peak oxygen consumption observed among patients with MASLD[27].
Several metabolic and inflammatory abnormalities, such as insulin resistance (IR), visceral adiposity, endothelial dysfunction, and dyslipidemia, have been linked to the development of HFpEF and MASLD. It has been hypothesized that increased inflammation in patients with MASLD may predispose them to the development of metabolic HFpEF[28]. As the history of the disease progresses from steatosis to inflammation and advanced fibrosis, studies have noted an increase in the production of increased systemic proinflammatory markers, such as interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), and chemokine ligand 3[29]. The increase in systemic inflammation can result in endothelial dysfunction, thus predisposing the patient to HFpEF[30].
Osteopontin has been suggested to play an essential role in inflammation, extracellular deposition, and fibrosis. Studies have reported osteopontin to be increased in MASLD, especially in those with advanced fibrosis[31]. It has been hypothesized that increased osteopontin may directly or indirectly increase the risk of HFpEF. A study reported that increased osteopontin levels among patients with HFpEF might predispose them to higher severity of HF symptoms[32]. Other markers of inflammation, such as asymmetrical dimethylarginine and homocysteine levels, are increased in patients with MASLD. These markers can enhance oxidative stress and platelet dysfunction[33].
Circulating branched-chain amino acid (BCAA) levels have also been increased among patients with MASLD. Increased BCAA exposure may activate cardiac protein synthesis, resulting in cardiac structural changes[34]. The presence of MASLD can also result in cardiac metabolic remodeling, which may even precede the structural remodeling seen in the advanced stages of the disease[35]. A previous study of 55 individuals noted a correlation between the presence of fat in the liver and myocardial IR[36]. Another study reported increased epicardial fat among patients with hepatic steatosis[35].
An additional HFpEF phenotype has been described among patients with advanced cirrhosis. Portal hypertension, a complication of cirrhosis, leads to the development of portosystemic shunts[36,37]. These shunts may increase pulmonary flow and result in the transit of vasoactive mediators from splanchnic circulation to the pulmonary system. This may result in the remodeling of pulmonary vasculature and pulmonary hypertension[38]. It has been suggested that micro-shunts may develop earlier during MASLD, leading to hemodynamic changes[39].
Patients with cirrhosis can also develop A-V shunts in the pulmonary and peripheral circulation[39]. The hemodyna
The five prominent risk factors that play a crucial role in the pathogenesis of both MASLD and HFpEF are obesity (visceral adiposity), diabetes mellitus, hypertension, hyperlipidemia, and obstructive sleep apnea (OSA) (Figure 2).
Type 2 diabetes mellitus (T2DM) increases both the risk and the morbidity associated with HFpEF and diabetic cardiomyopathy, conditions characterized by myocardial alterations that occur independently of hypertension and coronary artery disease. This association is well established[25,41-43]. The effects on the myocardium include hypertrophy, stiffness, and interstitial fibrosis, leading to diastolic compromise[44,45]. It is reported that up to 90% of T2DM patients develop MASLD, and MASLD is thrice as common in people with T2DM than those without[46]. Furthermore, T2DM is associated with obesity, indicative of a positive energy balance that takes a considerable toll on the liver, the main organ responsible for metabolism[46]. Fatty acid deposition in the liver does not end with steatosis; the high levels of circulating FAs also induce a pro-inflammatory state. Intermediates generated during lipid metabolism, such as ceramides and diacylglycerols (DAG), are lipotoxic and activate Kuppfer cells, which produce cytokines including IL-6 and TNF-α. These signals further recruit stellate cells, promoting fibrosis and perpetuating a vicious cycle between fatty liver disease and T2DM[47]. Adiponectin reduces Toll-like receptor four signaling, reduces the production of TNF-α, monocyte chemotactic protein-1, and IL-6, and increases the generation of anti-inflammatory IL-10, thereby suppressing inflammatory pathways[48].
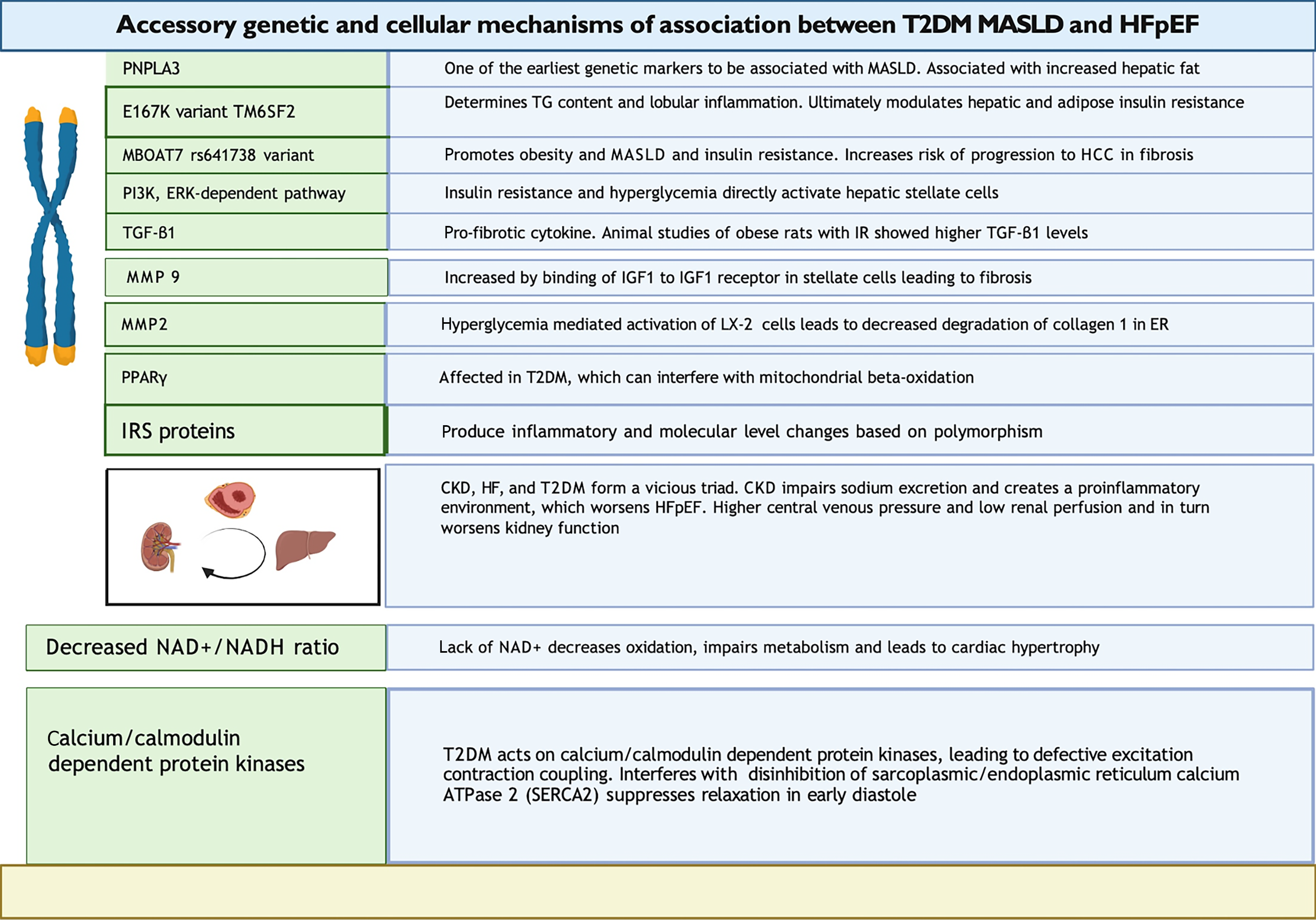
Various mechanisms have been proposed to explain the pathophysiology of HFpEF in T2DM. IR in T2DM impairs endothelial nitric oxide (NO) generation. Reduced NO and lower cGMP-PKG signaling activity impair vasodilation and make arteries and cardiomyocytes stiffer[49]. HFpEF patients are commonly plagued by endothelial dysfunction. Vascular stiffness impairs diastolic reserve, contributing to ventricular vascular uncoupling, which is almost ubiquitous in HFpEF[50]. Arterial stiffness affects preload and afterload, destabilizing stroke volume and blood pressure in HFpEF patients. Hyperglycemia in T2DM leads to the deposition of advanced glycation end-products (AGEs), resulting in concentric LV remodeling, wall stiffness, and slow LV relaxation[25,46]. AGEs promote inflammatory damage resulting in cell death[51]. Inappropriate protein glycosylation in the myocardium leads to interstitial fibrosis, worse compliance, and HFpEF[52]. The ensuing lipid toxicity, TG deposition, microvascular damage, endothelial injury, and coronary circulatory compromise culminate in diastolic function[43,53]. Epicardial fat deposition puts mechanical stress on the pericardium, causing diastolic dysfunction[52]. Similarly, elevated lipid levels affect the liver. T2DM patients exhibit IR, leading to increased peripheral fat breakdown and entry of free fatty acids into the liver[54]. Paradoxically, lipogenesis can also increase IR, conceivably attributable to ER stress and mammalian target of rapamycin (mTOR) activation. Downstream lipogenesis enzymes are activated, increasing fatty acid production[55]. While fatty acid synthesis is increased, beta-oxidation is impaired due to IR, culminating in increased steatosis[48]. Figure 3 highlights the critical accessory genetic and cellular association mechanisms between T2DM, MASLD, and HFpEF.
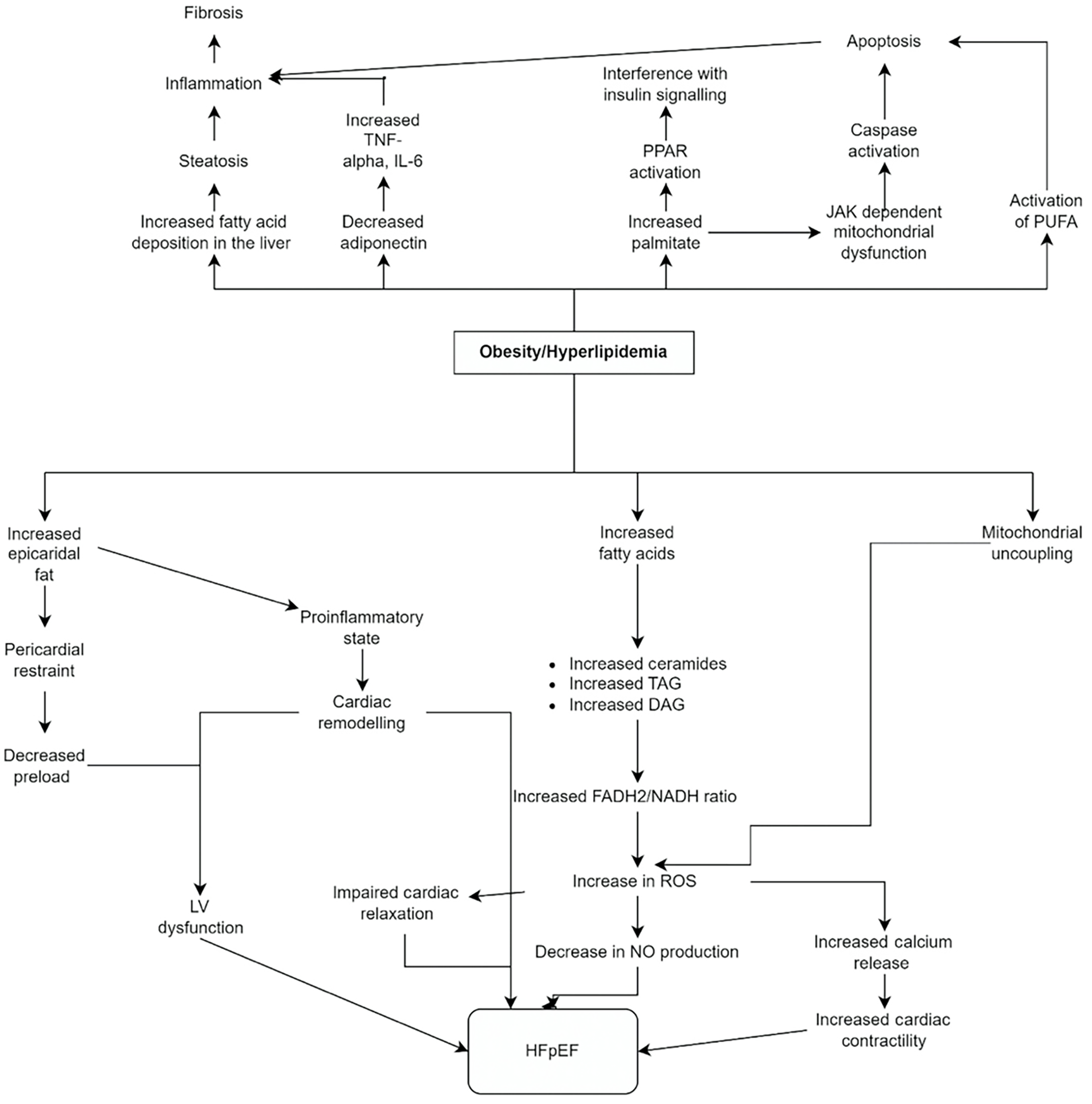
Obesity contributes to both HFpEF and MASLD. Obesity-driven increase in de novo lipogenesis propagates HFpEF primarily by creating a pro-inflammatory state and deposition of epicardial fat[52,56-58]. Obesity and weight gain propagate diastolic dysfunction attributed to reduced systolic reserve, and visceral adipose tissue exhibits a firmer linkage with concentric remodeling[59,60]. The role of epicardial adipose tissue, which is strongly correlated with body mass index (BMI), in HFpEF has been well studied and involves both mechanical and inflammatory effects[61]. Epicardial fat increases pulmonary capillary wedge pressure (PCWP) at rest by uncoupling LV filling pressure (PCWP) and LV pre-load (end-diastolic volume)[62]. It raises intracavitary pressure, underestimating brain natriuretic peptide (BNP) levels, and contributes to counterproductive interactions between the heart and pericardium, creating a diagnostic conundrum[63]. Additionally, epicardial fat contributes to inflammation by increasing the secretion of IL-1β, IL-6, and TNF-α, contributing to cardiac muscle mitochondrial dysfunction[64]. Meanwhile, anti-inflammatory cytokines like adiponectin are reduced in obesity. Higher levels of epicardial adipose tissue in HFpEF have been associated with worse hemodynamics, poor right ventricle-pulmonary artery coupling, LV fibrosis, decreased exercise capacity, and higher hospitalization and mortality rates[61]. Lipotoxicity does not spare the myocardium. As fatty acids increase in circulation, they are increasingly taken up by the myocardium. This leads to higher levels of long-chain fatty acyl CoA intracellularly and higher synthesis of ceramides, triacylglycerol, and DAG[65], When the heart relies more on adipose than glucose to fulfill its energy needs, it shifts towards increased Flavin adenine dinucleotide hydride-2/nicotinamide adenine dinucleotide hydrogen ratio, less efficient energy generation, and greater production of reactive oxygen radicals[56]. While the energy production using fat is less efficient, the energy demand of the adipose-riddled myocardium is higher. This is proposed to contribute to HFpEF because impaired ATP metabolism initially affects the sarcoplasmic reticular calcium ATPase to lower cytosolic Ca2+ during diastole, thereby interfering with relaxation[57,66].
Similarly, obesity contributes to the development of hepatic steatosis, as well as its progression to hepatitis and cirrhosis[67]. Furthermore, hepatic steatosis has been demonstrated as a risk factor for ischemic heart disease in mouse models[68]. It is theorized that when the level of circulating free fatty acids is high enough to overwhelm adipose tissue, hepatocytes must take over and store the excessive triglycerides[69]. If the patient has concurrent T2DM and IR, increased lipolysis further propagates this effect[69]. Both lipotoxicity and glucotoxicity contribute to obesity-related MASH. Insulin-resistant adipocytes release excessive free fatty acids, promoting inflammation and hepatic lipid deposition. Meanwhile, hepatocytes are unable to dispose of the accumulated fat, resulting in hepatic steatosis[70]. Lipid-laden hepatocytes initiate a pro-inflammatory arc, activating Kupffer, dendritic, and hepatic stellate cells. Neutrophils, T-lymphocytes, and macrophages further invade the liver[71]. The inflammatory cascade attempts to heal injured hepatocytes by recruiting stellate cells, activated endothelial cells, and myofibroblasts[72]. However, the injury tends to overwhelm the healing capacity, and the inflammatory process culminates in scarring and even neoplasia[73]. Additionally, animal models have demonstrated associations between cardiac steatosis and transcription factor Forkhead box protein O1,109, which has recently been designated a cellular mechanism involved in HFpEF[74].
Meanwhile, obesity disrupts the balanced adipokine profile seen in healthy individuals. Adipose hypertrophy promotes the production of pro-inflammatory cytokines[75]. While some adipokines, such as adiponectin and obestatin, have protective effects on the liver, others, including TNF-α, IL-6, chemerin, retinol-binding protein-4, and resistin, exacerbate steatosis and hepatitis[75]. Adiponectin exhibits anti-inflammatory effects by decreasing pro-inflammatory and increasing anti-inflammatory cytokines. Patients with hepatic steatosis show lower adiponectin levels than healthy controls, while those with steatohepatitis show even lower levels. Counterintuitively, patients who have progressed to cirrhosis display higher levels of adiponectin, which could represent impaired clearance or an attempt to salvage the hepatic injury[76,77]. Figure 4 depicts the vital pathophysiological mechanisms by which obesity/hyperlipidemia contributes to the risk of developing MASLD and HFpEF.
Another common risk factor between HFpEF and MASLD is hypertension. Hypertension is a well-known risk factor for HF development, including HFpEF[77]. The rise in blood pressure from an ideal baseline is proportional to LVH, even starting from pre-hypertensive range pressures[78]. In hypertensive patients, the LV must constantly pump against an increased afterload. In line with Laplace’s Law, the increased work causes LV to undergo pathological hypertrophy and fibrosis, contributing significantly to diastolic dysfunction. Hypertensive patients who develop LVH are at high risk of developing HF in the future. A study demonstrated that every 1% increase in LV mass above baseline was associated with a 1% higher risk of HF[78].
MASLD has been reported to be present in 30% of hypertensive patients[79,80]. Recent studies have suggested a bidirectional relationship between the two. While the presence and value of hypertension have been shown to predict MASLD, the presence of MASLD can also serve as a predictor for hypertension[81]. Additionally, concomitant hypertension is associated with greater odds of MASLD progression to fibrosis[82]. Even in normotensive patients, higher BP can predict hepatic fibrosis[83]. A cohort study of Nagasaki nuclear bomb survivors found that hypertension predicted the development of MASLD over time[84]. A study in China found that hypertension was a significant factor in predicting MASLD, though less than obesity and dyslipidemia[84]. One of the explanations for this correlation is based on the Renin Angiotensin Activating System (RAAS). Animal models have helped confirm the role of RAAS in developing both hepatic fibrosis and hypertension[85]. Specific mutations in angiotensinogen and its receptors have been associated with MASLD[82].
Lipotoxicity has undoubtedly been implicated in HFpEF pathogenesis[86]. In HFpEF, increased fatty acid oxidation leads to greater cardiac uptake of fatty acids and myocardial lipid deposition[87,88]. A cardiac MRI study found 2.3 times higher lipid content in the myocardium compared to controls[89]. Inactivation of adipose triglyceride lipase also reduces lipolysis, increases lipid deposits in the heart, and worsens diastolic function[65]. Excessive acyl CoA synthase activity increases triglyceride accumulation and is associated with concentric LVH[65]. Toxicity is likely a consequence of DAGs and ceramides than triglycerides. Activating DAG kinases that decrease DAG levels has been associated with improved cardiac function[90]. Inhibiting ceramide synthesis has also led to improvements in cardiac activity, as seen in imaging[91]. In mice that lack malonyl-CoA decarboxylase, a fatty diet did not lead to cardiac dysfunction, possibly due to low levels of acylcarnitines, i.e. toxic lipid intermediaries[92].
DAGs and ceramides upregulate NADPH oxidase and form reactive oxygen species (ROS). One of the mechanisms of ROS-induced damage is NO depletion. In HFpEF, myocardial tissue shows increased nitrotyrosine staining, indicating diversion of NO toward peroxynitrite formation. The resulting decrease in NO availability likely contributes to diastolic dysfunction[93]. ROS also acts on the ryanodine receptor to increase calcium release, which could contribute to increased passive cardiac muscle tension[94]. ROS can also activate pro-fibrotic pathways such as NF-κB and MAPK[95]. Lipotoxic effects on the heart can induce mitochondrial abnormalities, including mitochondrial uncoupling[96]. Overall, multimodal pathogenesis links hyperlipidemia and HFpEF.
Higher triglyceride levels are also associated with MASLD, even in the non-obese population[97]. As in obesity, high amounts of circulating lipids tend to get deposited in hepatocytes[98]. Fat deposition makes the liver more vulnerable to inflammation-mediated injury and impairs its regenerative capacity. The effect of increased hepatocyte exposure to fatty acids has been evaluated in in vitro models. Hepatocytes exposed to high levels of palmitate and oleate show increased expression of FAT/CD36 and fatty acid transport protein MRI-2, leading to intracellular accumulation of DAG and ceramides[99]. Moreover, oleate has been associated with steatosis and palmitate has been associated with apoptosis in in vitro studies[100]. Lipophosphatidylcholine (LPC) is a lipid subgroup that induces leukocyte chemotaxis. Both patients and animal models of MASLD have shown high LPC levels. LPC is theorized to promote apoptosis by activating pathways involving transcription factor CCAAT/enhancer-binding homologous protein. This upregulates pro-apoptotic proteins such as p53 upregulated modulator of apoptosis. Ceramide levels are also high in MASLD patients. They interact with TNF-α and increase ROS generation, resulting in inflammation and apoptosis[101]. They have also been associated with IR[102].
Studies on the mechanisms of hepatocyte apoptosis in lipotoxicity have implicated both intrinsic and extrinsic pathways. Intrinsic pathways are activated by endoplasmic reticulum (ER) stress, ROS, and altered mitochondrial permeability. ER stress is involved in the steatosis to hepatitis progression[100]. XBP1 and eIF2α are ER stress-sensing pathways that also regulate lipid metabolism. The change from steatosis to MASH involves upregulating pro-apoptotic genes and downregulating ER stress pathway genes. The Fas ligand initiates extrinsic pathways[103].
MASLD pathogenesis in OSA largely centers around chronic intermittent hypoxia (CIH)[104]. The stress of CIH leads to the release of ROS and inflammatory mediators, which contribute to hepatic inflammation and fibrosis[105]. One factor that mediates this effect is HIF-1α. HIF-1α is increased in OSA and MASLD through oxidative stress and mitochondrial injury[106,107]. HIF-1α influences the release of lysyl oxidase (LOX), which plays a role in extracellular matrix protein cross-linking and promotes fibrosis[108]. This hypothesis is supported by the finding that LOX levels are higher in patients with hepatic fibrosis[109]. In animal models, HIF-1α deletion has been associated with reduced expression of genes promoting lipogenesis and reduced fibrosis[110]. HIF-2 is also implicated and results in dysregulated lipid metabolism, which leads to severe hepatic steatosis[111]. Generally, prolyl hydroxylase domain (PHD) enzymes degrade HIFs. Hypoxia results in decreased PHD activity, enabling excessive HIF activity and consequent liver injury[112]. The use of HIF-1α inhibitors has been observed to improve liver fibrosis[113]. Oxidative injury damages DNA, lipids, and proteins. The resultant inflammation triggers Kupffer cells and stellate cells to promote fibrosis. Hypoxia also activates NF-κB, increasing TNF-α and IL-6 to trigger hepatic injury[114]. The hypoxia from OSA purportedly alters intestinal mucosal permeability, upregulates TLR-4 expression in the liver, and affects the gut microbiome, all of which play a role in MASLD[115]. OSA impacts sleep quality, and prior studies have linked low sleep duration with MASLD[116]. This is partially explained by the role of circadian rhythms in regulating insulin and lipid metabolism, the disturbance of which could promote inflammation and liver disease[117].
Endothelial dysfunction also serves as a connecting link between OSA and HFpEF. OSA patients show reduced nitrite concentrations in plasma and impaired vasodilation. ROS activate NF-κB to increase the production of C-reactive protein, IL-6, IL-8, TNF-α, and vascular and intracellular adhesion molecules, which cause endothelial injury, atherosclerosis, and HF. Inflammation also generates transforming growth factor-beta (TGF-β), which lays down the fibrotic matrix and further impairs LV diastolic function[118]. ROS generation in OSA contributes to cell injury and death[119]. A systematic review by Polecka et al[120] found that HFpEF patients with concomitant OSA showed higher BNP levels than those without OSA[120]. Moreover, BNP levels decrease in OSA patients upon positive airway pressure treatment[121]. The severity of acute intermittent hypoxia in OSA also correlates with diastolic compromise in HFpEF[121]. Diastolic compromise, measured by isovolumetric relaxation time, is associated with more severe sleep-disordered breathing. Echocardiographic assessment and LA volume index have also been used to confirm the association between OSA and diastolic dysfunction[122]. Moderate and severe OSA show a more reliable association with poor diastolic function than mild OSA[123]. Like BNP levels, echocardiography has been used to assess improved diastolic dysfunction after treatment with continuous positive airway pressure in OSA patients. Overall, OSA and HFpEF share a bidirectional relationship, and treatment of OSA can improve HFpEF[124].
Lifestyle interventions play a crucial role in the management of HFpEF and MFLD. These interventions incorporate calorie restriction, graded physical therapy, and dietary modification. Weight loss significantly impacts IR, visceral obesity, inflammation, hypertension, and cardiovascular risk[125-128].
Weight loss leads to decreased hepatic IR and FFA levels, and an 8% weight loss is sufficient to reverse hepatic IR, while sustained weight loss exceeding 10% reverses fibrosis and portal inflammation[129-132]. BMI correlates with HFpEF incidence in a dose-dependent manner[133]. The benefits of weight loss in HFpEF are attributed to improved myocardial function, microvasculature, chronic inflammation reversal, and hemodynamic status enhancement[134].
The current guidelines from American College of Cardiology, American Heart Association and European Society of Cardiology recommend calorie restriction in combination with 150 minutes of aerobic activity per week[125-127]. This regimen has been shown to significantly improve markers of metabolic health, such as blood pressure, hemoglobin A1c, and insulin levels[135]. However, maintaining long-term weight loss requires higher levels of physical activity (200-300 minutes/week) as the body responds by lowering its metabolic rate to regain weight. This highlights the importance of bariatric surgery (BS)[132,136-140].
The latest European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD) and the European Association for the Study of Obesity (EASO) joint evidence-based guidelines recommend a Mediterranean diet for its high of monounsaturated fatty acid and polyunsaturated fatty acid content[127,141,142]. While both the Dietary Approaches to Stop Hypertension diet and the Mediterranean diet demonstrate efficacy in reducing hypertension, the latter exhibits superior anti-inflammatory properties and reverses the underlying mechanisms of obesity, whereas the former has a greater effect on lowering blood pressure[143-145]. Furthermore, the Mediterranean diet discourages the consumption of processed or high fructose foods and sugary drinks, which contribute to the reduction of AGEs and lower central adiposity[146-150]. A high-protein diet may benefit MASLD due to its anorexigenic properties[151-153]. However, it is crucial to consider the protein source, as red meat consumption increases cardiovascular risk[153].
Physical activity benefits blood pressure independent of weight loss through vasculature modulation and sympathetic activity attenuation[138,154,155]. In conclusion, exercise in combination with a hypocaloric diet improved peak oxygen consumption, yet only exercise has been linked with enhanced quality of life[156-158]. These findings align with current statements by American College of Cardiology[159].
BS may facilitate calorie restriction with a goal of 7%-10% body weight reduction in line with current guidelines[160]. These include Roux-en-Y gastric bypass and Laparoscopic adjustable gastric banding[161]. In obese patients, BS is correlated with more significant weight loss and reversal rates of T2DM compared with nonsurgical therapy[162]. Furthermore, a greater decrease in glycosylated hemoglobin and a favorable metabolic profile was observed. The beneficial impact of BS in metabolic disease and MASLD is attributed to their effects on dietary patterns, alteration in stomach functioning, gut absorption metrics, gut hormone release, intestinal microbial biodiversity, and bile acid secretion. Accordingly, weight loss attributed to BS has been associated with resolution and improved liver fat content, steatohepatitis, and fibrosis[163]. Resolution/improvement has been seen in 30%-40% of all liver fibrosis patients, highlighting its success[164,165]. Interestingly, it is also associated with a decreased risk of hepatocellular carcinoma (HCC)[166]. BS decreases the risk of major adverse cardiac events and causes mortality in patients with MASLD and obesity[167].
Furthermore, BS is robustly aligned with improved cardiac functioning and relaxation due to improvements in hemodynamics, inflammation, and a favorable metabolic profile128. This is attributed to improved diastolic function and cardiac geometry. As a result, markers implicated in HFpEF, such as LA diameter, LV mass index, and LV mass, improve in patients undergoing BS[168].
Metformin is a biguanide widely used in T2DM patients because of its safety and efficacy, and it is often prescribed to overweight patients[169]. Metformin is usually the initial therapy in diabetic patients and benefits patients with stage B HFpEF through reversal of diastolic dysfunction, as its use is closely correlated with decreased LV mass index and improved ejection fraction[170,171].
While EASL-EASO-EASD and American Association for the Study of Liver Diseases (AASLD) do not recommend the use of metformin in MASLD, there are still benefits to its use in this population in terms of its effects on determinants such as obesity, T2DM, and dyslipidemia[127,172]. Metformin use demonstrates a significant decrease in liver enzymes and displays a modest, nonsignificant association with reduced steatosis or lobular inflammation, irrespective of diabetic status. However, it has no effect on fibrosis[173-177].
Metformin use has a protective effect on HFpEF incidence and progression through its action on skeletal muscles, liver, gut, heart, and vasculature[178]. Its protective effect on the heart is attributed to improved glycemic control, protection against hyperinsulinemia, and reduction of oxidative stress by halting titin-mediated cardiac hypertrophy and action at the eNOS pathway[179-184]. In conclusion, using metformin before the development of MASLD does not confer any protective effects. Still, it may serve as an adjunct to lifestyle modification early in the disease course[185]. In HFpEF, the addition of metformin to ACEi and Beta blockers in patients with HFpEF was associated with decreased mortality[186].
Peroxisome proliferator activated receptor (PPAR) agonists such as fibrates and thiazolidinediones are among the most extensively studied in MASLD. They selectively augment the transcriptional activity of nuclear receptors engaged in glucose and lipid metabolism in various tissues to mitigate inflammation, fibrosis, and atherosclerosis[187,188]. These agonists are further classified into PPAR-alpha, PPAR beta/delta, and PPAR gamma. PPAR alpha inhibits hepatic steatosis by promoting fatty acid oxidation in the liver and NF-kB inhibition-mediated anti-inflammation, while sup
Current EASL-EASO-EASD and AASLD guidelines recommend the use of PPAR-gamma agonist Pioglitazone yet concerning side effects, such as weight gain and fluid retention, have been obstacles in its widespread use[127,209,210]. Consistent with these efforts, newer agents such as lobeglitazone, saroglitazar, elafibranor, and lanifibranor are currently under development.
SGLT-2i acts by attenuating glucose absorption in pre-renal tubules, preventing hyperglycemia. Furthermore, it inhibits glucose uptake in hepatocytes, which is robustly associated with antiproliferative activity across multiple hepatocellular lines[211]. Finally, in vitro studies have demonstrated that the pleiotropic nature of these agents enables modulation of glucose and cholesterol metabolism, suppression of inflammation, weight loss, and reversal of steatosis and fibrosis[212-214].
AASLD recommends using SGLT2i for NAFLD-associated cardiometabolic diseases[172]. Drugs such as dapagliflozin, empagliflozin, and canagliflozin have been shown to decrease liver fat and are routinely used as antidiabetic agents[215]. SGLT2i plays a significant role in inducing peripheral insulin sensitivity, upregulating beta cell mass, and reducing oxidative stress[216-219]. SGLT2i provides more significant weight loss benefits and anti-hypertensive effects than metformin and dipeptidyle peptidase 4 (DPP-4) inhibitors[220]. Furthermore, glycemic control, weight loss, and anti-hypertensive benefits are additive when combined with Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), leading to decreased risk of macrovascular and microvascular complications[221,222].
SGLT2i has also been reported to have beneficial effects on OSA, diabetes, blood pressure, arrhythmias, renal damage, and anemia in patients with HFpEF[223]. SGLT-2i impedes the pathophysiological impact of diabetes on HFpEF development and reduces mortality[224]. It exerts these effects by attenuating ER stress through upregulation of sirtuin-1, preventing myocardial injury by inhibiting Na-H exchange in the heart and kidneys, and reducing epicardial fat, thereby protecting against myocardial fibrosis and microcirculatory damage[224]. In addition to the benefits above, improved vascular physiology and pressure reduction confer protection against the risk of atrial arrhythmias by preventing atrial remodeling[225].
In conclusion, SGLT-2 use is tightly linked with decreased cardiovascular mortality, improved quality of life, and improved exercise capacity in patients with HFpEF[226-228]. The pleiotropic nature of SGLT-2i makes them attractive options in the potentiation of metabolic disease. It illustrates their inextricable link with a reduction in cardiovascular and non-cardiovascular mortality in patients with T2DM[229].
DPP-4 inhibitors, GLP-1 RA, and, more recently, dual GLP-1/glucose-dependent insulinotropic polypeptide RA tirzepa
Liraglutide and semaglutide have both been approved for the management of weight loss in obese patients, irrespective of diabetic status[231]. GLP-1 RA use has demonstrated better glycemic control, weight loss, and a favorable metabolic profile compared to insulin treatment, highlighting their role as effective anti-diabetic agents[232,233]. Tirzepatide was approved after the most recent EASL-EASO-EASD guidelines were published. It has demonstrated superiority to GLP-1 RAs in terms of glycemic control and weight loss and holds potential as a viable alternative in the near future[234]. Induction of insulin sensitivity is accredited to enhanced glucose-mediated insulin secretion at the pancreas, mitigation of postprandial hyperglycemia through retardation of gastric emptying, and lowered glycosylated hemoglobin with a net weight loss[235]. Peripherally, GLP-1 agonists cause early satiety through their action at the brainstem and hypothalamus, induce adipocyte apoptosis, decrease adipokine and cytokine production, and promote glucose uptake by adipocytes and skeletal muscles, further reducing IR[236].
In conjunction with the aforementioned metabolic benefits, the direct action of incretin mimetics at the liver mitigates MASLD by indirectly modulating response to energy and nutrients. In trials, GLP-1 RAs have been robustly linked with reduced visceral and liver fat, irrespective of diabetic status. Another analysis noted a more significant reduction in steatosis associated with GLP-1 RA compared with other anti-diabetic agents, including SGLT-2i and PPAR agonists[237-239].
The role of GLP-1 RA in reversing fibrosis is indirect and attributed to its anti-inflammatory and atheroprotective effects. At a cellular level, these agents inhibit de novo lipogenesis by inhibiting regulators of the carbohydrate element binding protein (ChREPB) transcription factor, stearoyl-CoA desaturase-1, and activation of AMPK. Interestingly, this pathway also hampers cardiac hypertrophy in mouse models[240]. Downstream, this results in decreased TG synthesis, hepatic gluconeogenesis, and VLDL production while promoting insulin signaling and glycogen synthesis in the liver[241]. Therefore, in MASLD patients, GLP-1 RA reduces cardiovascular events and mortality comparable to SGLT-2i and superior to other anti-diabetic agents, including metformin[242]. At the cellular level, its cardio-protective effect corre
Statins act on the rate-limiting step in cholesterol synthesis by inhibiting HMG-CoA reductase and lowering serum low-density lipoprotein (LDL)-C levels and triglycerides[247,248]. These agents facilitate LDL clearance through modulation of protein kinase-A and potentiation of ChREPB and carnitine palmitoyltransferase[1]. Furthermore, these agents counter steatosis through modulation of PPAR-gamma and PPAR-alpha, alteration of adiponectin signaling, and impeding peripheral lipolysis[249-254]. Finally, statins retard fibrosis through alteration of the sonic hedgehog pathway. In summary, statins favor fatty acid oxidation, interfere with de novo lipogenesis, and prevent the progression of MASLD[255]. Furthermore, statins directly impact lipid profile and endothelial dysfunction and exert antioxidant, anti-inflammatory, and immunomodulatory effects[256].
Statins have been shown to prevent myocardial hypertrophy, fibrosis, and LVH as they mitigate microvascular dysfunction, lower coronary atherosclerosis, and improve the lipid profile[248]. Their pleiotropic action highlights their importance in improving cardiovascular outcomes. Statins prevent intimal thickening, cell replication, and recruitment of leukocytes recruitment of immune cells by inhibiting LFA-1 and ICAM, resulting in significant downregulation of GTPase synthesis[257,258]. Their favorable effect on metabolic profile further contributes to atheroprotection, as NO is essential for vasodilation, platelet aggregation, vascular smooth muscle proliferation, and immune cell recruitment[259-261]. As a result, statins are established as agents that improve cardiovascular outcomes through primary and secondary prevention.
In MASLD, the benefit of RAAS inhibition is attributed to the modulation of cardiometabolic risk factors leading to improved metabolic profile and weight loss[262-264]. Furthermore, ACEi use in MASLD patients is correlated with reduced progression to cancer and cirrhosis[265]. In experimental models, ACE-I and ARB have inhibited cellular processes linked with AT-II that favor lipogenesis, adipocyte growth, adipokine release, and proinflammatory cytokine release and interfere with insulin receptor-PI3K signaling[264,266-271]. In addition, vasodilation improves pancreatic blood supply, enhances insulin secretion, improves glucose metabolism, and facilitates glucose delivery and uptake by peripheral insulin-sensitive tissue[272,273].
The anti-fibrotic effect of ACE-I and ARB is attributed to their modulation of AT-II and renin. Renin enhances the release of TGF-beta1, PAI-1, fibronectin, and collagen, while AT-II increases hepatic stellate cell activation, migration, and concentration. AT-II also converts hepatic stellate cells to myofibroblasts. Simultaneously, it upregulates tissue inhibitors of metalloproteinase-1 release that secretes and incorporates collagen into the extracellular matrix. In addition, ACE-I and ARB inhibit ROS generation and decrease cytokine levels that further oppose fibrosis, lipogenesis, and IR[274-278].
MRAs, ARNi, and ARB are class 2b recommendations for treating HFpEF associated with decreased hospitalization, specifically in patients with EFs on the lower end of the spectrum.
These agents impede RAAS and mitigate arterial hypertension by altering vascular tone, enhancing sympathetic activity, and activating other downstream hormones[273,279,280].
Beta-blockers (BB) use has been traditionally limited in NAFLD due to its negative effect on glucose and lipid metabolism[281]. Traditionally, non-selective BB use has been associated with improved survival in HCC due to its role in attenuating vascular remodeling and portal hypertension and ultimately delaying decompensation[282]. On the other hand, BB use has been associated with lower exercise capacity in HFpEF. However, newer BB, such as nebivolol and carvedilol, have improved cardiometabolic risk factors, insulin sensitization, and metabolic profile[283,284]. This effect is attributed to their ability to peripherally mitigate afterload, lower glucose levels, and inhibit obesity-mediated hypertension[285].
Several novel treatments for MASLD are being developed and tested in Phase 2 or 3 clinical trials. These drugs may affect HFpEF by modifying shared risk factors that contribute to the development of both conditions. Lanifibranor, a pan-PPAR agonist, is undergoing Phase-3 trials[286]. Saroglitazar acts as an agonist on PPAR/α/γ receptors, and its efficacy in improving fibrosis is being evaluated in a 4-arm randomized trial[287]. Resmetirom is a thyroid hormone receptor β agonist that helps regulate lipid metabolism in the liver. It effectively reduced liver fat content in a Phase-2 trial and was recently approved in March 2024 for the management of NASH with F2 and F3 fibrosis[288]. VK-2809 is an alternative THR β agonist associated with lower hepatic fat deposition being studied in Phase-2 trials[289]. Semaglutide is undergoing Phase-3 trials to evaluate its ability to resolve MASLD and improve fibrosis. Aldafermin and Pegbelfermin are FGF 21 analogs that are being tested in Phase-2 trials, as FGF also play critical roles in metabolizing glucose, lipids, and bile acids. Pegbelfermin efficiently reduced hepatic fat and is now being tested in patients with Stage-3 fibrosis[290]. Galectins are proteins involved in inflammation and fibrosis. The Galectin-3 inhibitor Belapectin is being tested in adults with MASLD-associated cirrhosis and portal hypertension. It has reduced the hepatic venous pressure gradient in patients without varices and is thus being tested in Phase-3 trials[291].
Stem cell therapy is based on recent evidence that indicates a complex interplay between various cardiometabolic risk factors and their impact on genetics. Mesenchymal stem cells are unique in their ability to differentiate into distinct cell types and may serve to repair damaged tissue. These cells enhance tissue regeneration through various growth factors and amplify the ability of the body to generate stem cells[292]. Furthermore, as a testament to their pluripotency, MSCs alter their phenotype based on the inflammatory cytokine encountered and alter macrophages to aid in tissue repair[292-295]. MSC therapy improved LV ejection fraction in patients after myocardial infarction and has been shown to mount a favorable immune response in ischemic cardiomyopathy, emphasizing its potential as a breakthrough therapy in the future[296-298]. Nevertheless, clinical trial data have been varied, and these drugs have not yet been tested in HFpEF or MASLD populations. Figures 5 and 6 highlight the critical chains of interactions between HFpEF and MASLD, which serve as pharmacological targets, and the currently available drugs, which reduce the burden of MASLD and HFpEF. Table 1 highlights the most recent mechanistic evidence linking disease progression in MASLD and HFpEF.
| Ref. | Mechanistic link | Model |
| Nasiri-Ansari et al[212], 2021 | Empagliflozin attenuates MASLD progression in ApoE-/- mice by promoting hepatic autophagy, reducing ER stress, and inhibiting hepatocyte apoptosis | ApoE-/- mice |
| Li et al[214], 2021 | Dapagliflozin alleviates hepatic steatosis and protects against liver inflammation and fibrosis by reducing lipid accumulation, oxidative stress, and inflammatory responses | Mouse (DIO/MASLD model) |
| Wang et al[54], 2021 | FGF1ΔHBS prevents diabetic cardiomyopathy by maintaining mitochondrial homeostasis and reducing oxidative stress via AMPK/Nur77 suppression | Diabetic mice |
| Schiattarella et al[67], 2019 | iNOS-driven nitrosative stress leads to coronary microvascular inflammation and rarefaction, driving concentric remodeling and diastolic dysfunction in HFpEF | ZSF1 rat model |
| Hopf et al[182], 2018 | Insulin resistance impairs titin phosphorylation via reduced PKG/PKA signaling, increasing cardiomyocyte stiffness and promoting HFpEF in diabetes | Human cardiomyocytes |
| Slater et al[183], 2019 | Metformin improves diastolic function in an HFpEFlike mouse model by lowering titinbased passive stiffness and enhancing titin compliance via PKG-N2BA isoform shift | HFpEF mouse model |
| Zhao et al[110], 2019 | Digoxin inhibits PKM2 and thereby suppresses HIF-1α and NLRP3 inflammasome activation, attenuating steatohepatitis and associated inflammation | Mouse (MASH model) |
MASLD has emerged as the leading cause of chronic liver disease and cirrhosis worldwide in the last 3 decades. The exponential increase in global MASLD prevalence is closely related to the sharp rise in metabolic disease prevalence, including diabetes, hypertension, obesity, and dyslipidemia, in both developed and developing countries. In addition, the same period has seen increases in the incidence of heart failure with preserved ejection fraction. Shared risk factors on the background of metabolic dysfunction have made a common ground for further exploring the link between MASLD and CVD, particularly HfpEF. The consistent pool of evidence supports the higher risk of CVD in MASLD, as well as a bidirectional relationship between MASLD and HfpEF. Furthermore, the extent of fibrosis in MASLD has been shown to correlate with HFpEF prognosis and progression.
| 1. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1459] [Article Influence: 364.8] [Reference Citation Analysis (1)] |
| 2. | Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, Yong JN, Xiao J, Lee CW, Chan M, Chew NW, Xuan Tan EX, Siddiqui MS, Huang D, Noureddin M, Sanyal AJ, Muthiah MD. An Observational Data Meta-analysis on the Differences in Prevalence and Risk Factors Between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21:619-629.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 161] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 3. | Simon TG, Ebrahimi F, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Incident cardiac arrhythmias associated with metabolic dysfunction-associated steatotic liver disease: a nationwide histology cohort study. Cardiovasc Diabetol. 2023;22:343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 4. | Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 426] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1995] [Article Influence: 665.0] [Reference Citation Analysis (3)] |
| 6. | Wijarnpreecha K, Lou S, Panjawatanan P, Cheungpasitporn W, Pungpapong S, Lukens FJ, Ungprasert P. Association between diastolic cardiac dysfunction and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Jung JY, Park SK, Ryoo JH, Oh CM, Kang JG, Lee JH, Choi JM. Effect of non-alcoholic fatty liver disease on left ventricular diastolic function and geometry in the Korean general population. Hepatol Res. 2017;47:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Chung GE, Lee JH, Lee H, Kim MK, Yim JY, Choi SY, Kim YJ, Yoon JH, Kim D. Nonalcoholic fatty liver disease and advanced fibrosis are associated with left ventricular diastolic dysfunction. Atherosclerosis. 2018;272:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Salah HM, Pandey A, Soloveva A, Abdelmalek MF, Diehl AM, Moylan CA, Wegermann K, Rao VN, Hernandez AF, Tedford RJ, Parikh KS, Mentz RJ, McGarrah RW, Fudim M. Relationship of Nonalcoholic Fatty Liver Disease and Heart Failure With Preserved Ejection Fraction. JACC Basic Transl Sci. 2021;6:918-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Erratum: Nonalcoholic Steatohepatitis is Associated with Cardiac Remodeling and Dysfunction. Obesity (Silver Spring). 2017;25:1639-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, Shah SJ, Lima JAC, Lloyd-Jones DM. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9:e014279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 640] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 13. | Batta A, Hatwal J. Excess cardiovascular mortality in men with non-alcoholic fatty liver disease: A cause for concern! World J Cardiol. 2024;16:380-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (3)] |
| 14. | Pacana T, Cazanave S, Verdianelli A, Patel V, Min HK, Mirshahi F, Quinlivan E, Sanyal AJ. Dysregulated Hepatic Methionine Metabolism Drives Homocysteine Elevation in Diet-Induced Nonalcoholic Fatty Liver Disease. PLoS One. 2015;10:e0136822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Oh A, Okazaki R, Sam F, Valero-Muñoz M. Heart Failure With Preserved Ejection Fraction and Adipose Tissue: A Story of Two Tales. Front Cardiovasc Med. 2019;6:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Qiu M, Li J, Hao S, Zheng H, Zhang X, Zhu H, Zhu X, Hu Y, Cai X, Huang Y. Non-alcoholic fatty liver disease is associated with a worse prognosis in patients with heart failure: A pool analysis. Front Endocrinol (Lausanne). 2023;14:1167608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Fudim M, Zhong L, Patel KV, Khera R, Abdelmalek MF, Diehl AM, McGarrah RW, Molinger J, Moylan CA, Rao VN, Wegermann K, Neeland IJ, Halm EA, Das SR, Pandey A. Nonalcoholic Fatty Liver Disease and Risk of Heart Failure Among Medicare Beneficiaries. J Am Heart Assoc. 2021;10:e021654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Miller A, McNamara J, Hummel SL, Konerman MC, Tincopa MA. Prevalence and staging of non-alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. Sci Rep. 2020;10:12440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Wegermann K, Fudim M, Henao R, Howe CF, McGarrah R, Guy C, Abdelmalek MF, Diehl AM, Moylan CA. Serum Metabolites Are Associated With HFpEF in Biopsy-Proven Nonalcoholic Fatty Liver Disease. J Am Heart Assoc. 2023;12:e029873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Minhas AMK, Bhopalwala HM, Dewaswala N, Salah HM, Khan MS, Shahid I, Biegus J, Lopes RD, Pandey A, Fudim M. Association of Non-Alcoholic Fatty Liver Disease With in-Hospital Outcomes in Primary Heart Failure Hospitalizations With Reduced or Preserved Ejection Fraction. Curr Probl Cardiol. 2023;48:101199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 21. | Takahashi T, Watanabe T, Shishido T, Watanabe K, Sugai T, Toshima T, Kinoshita D, Yokoyama M, Tamura H, Nishiyama S, Arimoto T, Takahashi H, Yamanaka T, Miyamoto T, Kubota I. The impact of non-alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels. 2018;33:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Itier R, Guillaume M, Ricci JE, Roubille F, Delarche N, Picard F, Galinier M, Roncalli J. Non-alcoholic fatty liver disease and heart failure with preserved ejection fraction: from pathophysiology to practical issues. ESC Heart Fail. 2021;8:789-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Petta S, Argano C, Colomba D, Cammà C, Di Marco V, Cabibi D, Tuttolomondo A, Marchesini G, Pinto A, Licata G, Craxì A. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018;5:262-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Abudureyimu M, Luo X, Wang X, Sowers JR, Wang W, Ge J, Ren J, Zhang Y. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: from pathophysiology to therapeutics. J Mol Cell Biol. 2022;14:mjac028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Fudim M, Sobotka PA, Dunlap ME. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail. 2021;14:e007308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Austin P, Gerber L, Paik JM, Price JK, Escheik C, Younossi ZM. Aerobic capacity and exercise performance in nonalcoholic fatty liver disease. J Sports Med Phys Fitness. 2019;59:1376-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin Chim Acta. 2019;496:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 29. | Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65:425-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 30. | Mokotedi L, Michel FS, Mogane C, Gomes M, Woodiwiss AJ, Norton GR, Millen AME. Associations of inflammatory markers with impaired left ventricular diastolic and systolic function in collagen-induced arthritis. PLoS One. 2020;15:e0230657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Bruha R, Vitek L, Smid V. Osteopontin - A potential biomarker of advanced liver disease. Ann Hepatol. 2020;19:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Coculescu BI, Manole G, Dincă GV, Coculescu EC, Berteanu C, Stocheci CM. Osteopontin - a biomarker of disease, but also of stage stratification of the functional myocardial contractile deficit by chronic ischaemic heart disease. J Enzyme Inhib Med Chem. 2019;34:783-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:948-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 34. | White PJ, Newgard CB. Branched-chain amino acids in disease. Science. 2019;363:582-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 35. | Perseghin G, Lattuada G, De Cobelli F, Esposito A, Belloni E, Ntali G, Ragogna F, Canu T, Scifo P, Del Maschio A, Luzi L. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology. 2008;47:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Lautamäki R, Borra R, Iozzo P, Komu M, Lehtimäki T, Salmi M, Jalkanen S, Airaksinen KE, Knuuti J, Parkkola R, Nuutila P. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291:E282-E290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Nardelli S, Riggio O, Gioia S, Puzzono M, Pelle G, Ridola L. Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects. World J Gastroenterol. 2020;26:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (3)] |
| 38. | Koulava A, Sannani A, Levine A, Gupta CA, Khanal S, Frishman W, Bodin R, Wolf DC, Aronow WS, Lanier GM. Diagnosis, Treatment, and Management of Orthotopic Liver Transplant Candidates With Portopulmonary Hypertension. Cardiol Rev. 2018;26:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Fernández-Rodriguez CM, Prieto J, Zozaya JM, Quiroga J, Guitián R. Arteriovenous shunting, hemodynamic changes, and renal sodium retention in liver cirrhosis. Gastroenterology. 1993;104:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 41. | Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, Orchard TJ, Rolka DB, Imperatore G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:359-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 42. | Park JJ. Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes. Diabetes Metab J. 2021;45:146-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 43. | Ren J, Wu NN, Wang S, Sowers JR, Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. 2021;101:1745-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 44. | Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17:585-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (35)] |
| 45. | Patel N, Ju C, Macon C, Thadani U, Schulte PJ, Hernandez AF, Bhatt DL, Butler J, Yancy CW, Fonarow GC. Temporal Trends of Digoxin Use in Patients Hospitalized With Heart Failure: Analysis From the American Heart Association Get With The Guidelines-Heart Failure Registry. JACC Heart Fail. 2016;4:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 563] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 47. | Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 48. | Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 49. | Kashiwagi A, Araki S, Maegawa H. Sodium-glucose cotransporter 2 inhibitors represent a paradigm shift in the prevention of heart failure in type 2 diabetes patients. J Diabetes Investig. 2021;12:6-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Ananthram MG, Gottlieb SS. Renal Dysfunction and Heart Failure with Preserved Ejection Fraction. Heart Fail Clin. 2021;17:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Wold LE, Ceylan-Isik AF, Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin. 2005;26:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Ayton SL, Gulsin GS, McCann GP, Moss AJ. Epicardial adipose tissue in obesity-related cardiac dysfunction. Heart. 2022;108:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133:2459-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 800] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 54. | Wang D, Yin Y, Wang S, Zhao T, Gong F, Zhao Y, Wang B, Huang Y, Cheng Z, Zhu G, Wang Z, Wang Y, Ren J, Liang G, Li X, Huang Z. FGF1(ΔHBS) prevents diabetic cardiomyopathy by maintaining mitochondrial homeostasis and reducing oxidative stress via AMPK/Nur77 suppression. Signal Transduct Target Ther. 2021;6:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 55. | Pyun JH, Kim HJ, Jeong MH, Ahn BY, Vuong TA, Lee DI, Choi S, Koo SH, Cho H, Kang JS. Cardiac specific PRMT1 ablation causes heart failure through CaMKII dysregulation. Nat Commun. 2018;9:5107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 56. | Clemenza F, Citarrella R, Patti A, Rizzo M. Obesity and HFpEF. J Clin Med. 2022;11:3858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 58. | Westcott F, Dearlove DJ, Hodson L. Hepatic fatty acid and glucose handling in metabolic disease: Potential impact on cardiovascular disease risk. Atherosclerosis. 2024;394:117237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014;2:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. Eur J Heart Fail. 2016;18:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020;8:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 62. | Borlaug BA, Reddy YNV. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019;7:574-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 63. | Obokata M, Reddy YNV, Melenovsky V, Sorimachi H, Jarolim P, Borlaug BA. Uncoupling between intravascular and distending pressures leads to underestimation of circulatory congestion in obesity. Eur J Heart Fail. 2022;24:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 64. | Kitzman DW, Upadhya B, Vasu S. What the dead can teach the living: systemic nature of heart failure with preserved ejection fraction. Circulation. 2015;131:522-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 611] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 66. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 885] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 67. | Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 688] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 68. | Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, Tybjærg-Hansen A. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 69. | Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 70. | Polyzos SA, Mantzoros CS. Leptin in health and disease: facts and expectations at its twentieth anniversary. Metabolism. 2015;64:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, Hwang JP, Barranco-Fragoso B, Cordova-Gallardo J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int J Mol Sci. 2018;19:2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 72. | Nati M, Haddad D, Birkenfeld AL, Koch CA, Chavakis T, Chatzigeorgiou A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev Endocr Metab Disord. 2016;17:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 73. | Papadimitriou K, Mousiolis AC, Mintziori G, Tarenidou C, Polyzos SA, Goulis DG. Hypogonadism and nonalcoholic fatty liver disease. Endocrine. 2024;86:28-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Wells RG, Schwabe RF. Origin and function of myofibroblasts in the liver. Semin Liver Dis. 2015;35:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Polyzos SA, Kountouras J, Anastasilakis AD, Triantafyllou GΑ, Mantzoros CS. Activin A and follistatin in patients with nonalcoholic fatty liver disease. Metabolism. 2016;65:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 77. | Slivnick J, Lampert BC. Hypertension and Heart Failure. Heart Fail Clin. 2019;15:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 78. | Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285-2292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 79. | Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 2018;68:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 570] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 80. | Xie J, Huang H, Liu Z, Li Y, Yu C, Xu L, Xu C. The associations between modifiable risk factors and nonalcoholic fatty liver disease: A comprehensive Mendelian randomization study. Hepatology. 2023;77:949-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 81. | Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 82. | Yoneda M, Hotta K, Nozaki Y, Endo H, Uchiyama T, Mawatari H, Iida H, Kato S, Fujita K, Takahashi H, Kirikoshi H, Kobayashi N, Inamori M, Abe Y, Kubota K, Saito S, Maeyama S, Wada K, Nakajima A. Association between angiotensin II type 1 receptor polymorphisms and the occurrence of nonalcoholic fatty liver disease. Liver Int. 2009;29:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Liu J, Lv H, Wang J, Zhu Q, Chen G, Jiang Y, Zhao K, Shao L, Shi J, Pan X. Blood pressure stratification for predicting liver fibrosis risk in metabolic dysfunction associated fatty liver disease. Ann Hepatol. 2023;28:100892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 84. | Tsuneto A, Hida A, Sera N, Imaizumi M, Ichimaru S, Nakashima E, Seto S, Maemura K, Akahoshi M. Fatty liver incidence and predictive variables. Hypertens Res. 2010;33:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 312] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 86. | Leggat J, Bidault G, Vidal-Puig A. Lipotoxicity: a driver of heart failure with preserved ejection fraction? Clin Sci (Lond). 2021;135:2265-2283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 87. | Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 888] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 88. | Hammer S, van der Meer RW, Lamb HJ, Schär M, de Roos A, Smit JW, Romijn JA. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab. 2008;93:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | Mahmod M, Pal N, Rayner J, Holloway C, Raman B, Dass S, Levelt E, Ariga R, Ferreira V, Banerjee R, Schneider JE, Rodgers C, Francis JM, Karamitsos TD, Frenneaux M, Ashrafian H, Neubauer S, Rider O. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2018;20:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 90. | Liu L, Yu S, Khan RS, Homma S, Schulze PC, Blaner WS, Yin Y, Goldberg IJ. Diacylglycerol acyl transferase 1 overexpression detoxifies cardiac lipids in PPARγ transgenic mice. J Lipid Res. 2012;53:1482-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 341] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 92. | Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes. 2009;58:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 93. | van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 94. | Donoso P, Sanchez G, Bull R, Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci (Landmark Ed). 2011;16:553-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Maulik SK, Kumar S. Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods. 2012;22:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 399] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 97. | Tilg H, Moschen AR. Evolving therapies for non-alcoholic steatohepatitis. Expert Opin Drug Discov. 2014;9:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1486] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 99. | Chabowski A, Żendzian-Piotrowska M, Konstantynowicz K, Pankiewicz W, Mikłosz A, Łukaszuk B, Górski J. Fatty acid transporters involved in the palmitate and oleate induced insulin resistance in primary rat hepatocytes. Acta Physiol (Oxf). 2013;207:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 445] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 101. | Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2012;23:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 102. | Konstantynowicz-Nowicka K, Harasim E, Baranowski M, Chabowski A. New evidence for the role of ceramide in the development of hepatic insulin resistance. PLoS One. 2015;10:e0116858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 802] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 104. | Mesarwi OA, Loomba R, Malhotra A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am J Respir Crit Care Med. 2019;199:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 105. | Gupta N, Agrawal S, Goel AD, Ish P, Chakrabarti S, Suri JC. Profile of sleep disordered breathing in heart failure with preserved ejection fraction. Monaldi Arch Chest Dis. 2020;90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Asfari MM, Niyazi F, Lopez R, Dasarathy S, McCullough AJ. The association of nonalcoholic steatohepatitis and obstructive sleep apnea. Eur J Gastroenterol Hepatol. 2017;29:1380-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Petta S, Marrone O, Torres D, Buttacavoli M, Cammà C, Di Marco V, Licata A, Lo Bue A, Parrinello G, Pinto A, Salvaggio A, Tuttolomondo A, Craxì A, Bonsignore MR. Obstructive Sleep Apnea Is Associated with Liver Damage and Atherosclerosis in Patients with Non-Alcoholic Fatty Liver Disease. PLoS One. 2015;10:e0142210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Tang H, Lv F, Zhang P, Liu J, Mao J. The impact of obstructive sleep apnea on nonalcoholic fatty liver disease. Front Endocrinol (Lausanne). 2023;14:1254459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 109. | Wang X, de Carvalho Ribeiro M, Iracheta-Vellve A, Lowe P, Ambade A, Satishchandran A, Bukong T, Catalano D, Kodys K, Szabo G. Macrophage-Specific Hypoxia-Inducible Factor-1α Contributes to Impaired Autophagic Flux in Nonalcoholic Steatohepatitis. Hepatology. 2019;69:545-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 110. | Zhao P, Han SN, Arumugam S, Yousaf MN, Qin Y, Jiang JX, Torok NJ, Chen Y, Mankash MS, Liu J, Li J, Iwakiri Y, Ouyang X. Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am J Physiol Gastrointest Liver Physiol. 2019;317:G387-G397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Liu X, Huang K, Niu Z, Mei D, Zhang B. Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin Pharmacol Toxicol. 2019;124:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, Torbenson MS, Pedrosa RP, Lorenzi-Filho G, Steele KE, Schweitzer MA, Magnuson TH, Lidor AO, Schwartz AR, Polotsky VY. Lysyl Oxidase as a Serum Biomarker of Liver Fibrosis in Patients with Severe Obesity and Obstructive Sleep Apnea. Sleep. 2015;38:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G582-G592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 114. | Chen LD, Wu RH, Huang YZ, Chen MX, Zeng AM, Zhuo GF, Xu FS, Liao R, Lin QC. The role of ferroptosis in chronic intermittent hypoxia-induced liver injury in rats. Sleep Breath. 2020;24:1767-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 115. | Barceló A, Barbé F, de la Peña M, Vila M, Pérez G, Piérola J, Durán J, Agustí AG. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J. 2006;27:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 116. | Kumar D, Dwivedi DK, Lahkar M, Jangra A. Hepatoprotective potential of 7,8-Dihydroxyflavone against alcohol and high-fat diet induced liver toxicity via attenuation of oxido-nitrosative stress and NF-κB activation. Pharmacol Rep. 2019;71:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 117. | Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 118. | Parikh MP, Gupta NM, McCullough AJ. Obstructive Sleep Apnea and the Liver. Clin Liver Dis. 2019;23:363-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 119. | Barros D, García-Río F. Obstructive sleep apnea and dyslipidemia: from animal models to clinical evidence. Sleep. 2019;42:zsy236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 120. | Polecka A, Olszewska N, Danielski Ł, Olszewska E. Association between Obstructive Sleep Apnea and Heart Failure in Adults-A Systematic Review. J Clin Med. 2023;12:6139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 121. | Kim D, Kim HJ, Kushida CA, Heo NY, Ahmed A, Kim WR. Short Sleep Duration Is Associated With Abnormal Serum Aminotransferase Activities and Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2018;16:588-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 122. | Sanderson JE, Fang F, Lu M, Ma CY, Wei YX. Obstructive sleep apnoea, intermittent hypoxia and heart failure with a preserved ejection fraction. Heart. 2021;107:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 123. | Gillani SW, Ghayedi N, Roosta P, Seddigh P, Nasiri O. Effect of Metformin on Lipid Profiles of Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Controlled Trials. J Pharm Bioallied Sci. 2021;13:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 124. | Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E; Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 125. | Correction to: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2020;141:e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 126. | Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3739] [Cited by in RCA: 3955] [Article Influence: 791.0] [Reference Citation Analysis (0)] |
| 127. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3281] [Article Influence: 328.1] [Reference Citation Analysis (6)] |
| 128. | Sorimachi H, Obokata M, Omote K, Reddy YNV, Takahashi N, Koepp KE, Ng ACT, Rider OJ, Borlaug BA. Long-Term Changes in Cardiac Structure and Function Following Bariatric Surgery. J Am Coll Cardiol. 2022;80:1501-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 129. | You T, Wang X, Yang R, Lyles MF, Gong D, Nicklas BJ. Effect of exercise training intensity on adipose tissue hormone sensitive lipase gene expression in obese women under weight loss. J Sport Health Sci. 2012;1:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345-32353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1004] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 131. | Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 665] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 132. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1774] [Article Influence: 161.3] [Reference Citation Analysis (3)] |
| 133. | Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 588] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 134. | Reddy YNV, Anantha-Narayanan M, Obokata M, Koepp KE, Erwin P, Carter RE, Borlaug BA. Hemodynamic Effects of Weight Loss in Obesity: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2019;7:678-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 135. | Semmler G, Datz C, Reiberger T, Trauner M. Diet and exercise in NAFLD/NASH: Beyond the obvious. Liver Int. 2021;41:2249-2268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 136. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1008] [Article Influence: 63.0] [Reference Citation Analysis (1)] |
| 137. | Glass LM, Dickson RC, Anderson JC, Suriawinata AA, Putra J, Berk BS, Toor A. Total body weight loss of ≥ 10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 138. | Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog Cardiovasc Dis. 2018;61:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 324] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 139. | Effects of Weight Loss and Sodium Reduction Intervention on Blood Pressure and Hypertension Incidence in Overweight People With High-Normal Blood Pressure. Arch Intern Med. 1997;157:657. [RCA] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 563] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 140. | Brar A, Kalra S, Garg A, Karki B, Chirumamilla Y, Belbase S, Maini S. S2067 Impact of Bariatric Surgery on Cardiovascular Outcomes in Obese Patients With HFpEF: An NRD Propensity Score-Matched Analysis. Am J Gastroenterol. 2024;119:S1476-S1478. [DOI] [Full Text] |
| 141. | Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 142. | Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr. 1998;67:577S-582S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 251] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 143. | Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A, Das A, Hartley L, Stranges S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019;3:CD009825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 144. | Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 145. | Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 146. | Ribeiro PVM, Tavares JF, Costa MAC, Mattar JB, Alfenas RCG. Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutr Rev. 2019;77:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 147. | Zelber-Sagi S, Salomone F, Kolodkin-Gal I, Erez N, Buch A, Yeshua H, Webb M, Halpern Z, Shibolet O. Protective role of soluble receptor for advanced glycation end-products in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2017;49:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 148. | Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A, Emoto M, Yamamoto Y, Yamamoto H, Nishizawa Y, Kurajoh M, Yamamoto T, Inaba M. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes. 2013;62:478-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 149. | Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, Risérus U. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (3)] |
| 150. | Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Häring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 652] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 151. | De Chiara F, Ureta Checcllo C, Ramón Azcón J. High Protein Diet and Metabolic Plasticity in Non-Alcoholic Fatty Liver Disease: Myths and Truths. Nutrients. 2019;11:2985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 152. | McIver CM, Wycherley TP, Clifton PM. MTOR signaling and ubiquitin-proteosome gene expression in the preservation of fat free mass following high protein, calorie restricted weight loss. Nutr Metab (Lond). 2012;9:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 153. | Ropelle ER, Pauli JR, Fernandes MF, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JA, Franchini KG, Velloso LA, Saad MJ, Carvalheira JB. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes. 2008;57:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 154. | Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA, Turer AT. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 155. | El Meouchy P, Wahoud M, Allam S, Chedid R, Karam W, Karam S. Hypertension Related to Obesity: Pathogenesis, Characteristics and Factors for Control. Int J Mol Sci. 2022;23:12305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 156. | Walters GWM, Yeo JL, Bilak JM, Pepper C, Gulsin GS, Freeman SC, Gray LJ, McCANN GP, Brady EM. The Effectiveness of Lifestyle Interventions in Heart Failure With Preserved Ejection Fraction: A Systematic Review and Network Meta-Analysis. J Card Fail. 2024;30:994-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 157. | Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 702] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 158. | Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 159. | Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J Am Coll Cardiol. 2023;81:1810-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 393] [Reference Citation Analysis (0)] |
| 160. | Chauhan M, Singh K, Thuluvath PJ. Bariatric Surgery in NAFLD. Dig Dis Sci. 2022;67:408-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 161. | de Brito E Silva MB, Tustumi F, de Miranda Neto AA, Dantas ACB, Santo MA, Cecconello I. Gastric Bypass Compared with Sleeve Gastrectomy for Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-analysis. Obes Surg. 2021;31:2762-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 162. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 983] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 163. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 164. | Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 165. | Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1040-1060.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 166. | Ramai D, Singh J, Lester J, Khan SR, Chandan S, Tartaglia N, Ambrosi A, Serviddio G, Facciorusso A. Systematic review with meta-analysis: bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 167. | Krishnan A, Hadi Y, Alqahtani SA, Woreta TA, Fang W, Abunnaja S, Szoka N, Tabone LE, Thakkar S, Singh S. Cardiovascular Outcomes and Mortality After Bariatric Surgery in Patients With Nonalcoholic Fatty Liver Disease and Obesity. JAMA Netw Open. 2023;6:e237188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 168. | Lascaris B, Pouwels S, Houthuizen P, Dekker LR, Nienhuijs SW, Bouwman RA, Buise MP. Cardiac structure and function before and after bariatric surgery: a clinical overview. Clin Obes. 2018;8:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 169. | Piera-Mardemootoo C, Lambert P, Faillie JL. Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: A systematic review and meta-analysis of placebo-controlled randomized trials. Therapie. 2021;76:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 170. | Andersson C, Søgaard P, Hoffmann S, Hansen PR, Vaag A, Major-Pedersen A, Hansen TF, Bech J, Køber L, Torp-Pedersen C, Gislason GH. Metformin is associated with improved left ventricular diastolic function measured by tissue Doppler imaging in patients with diabetes. Eur J Endocrinol. 2010;163:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 171. | Kamel AM, Sabry N, Farid S. Effect of metformin on left ventricular mass and functional parameters in non-diabetic patients: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. 2022;22:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 172. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 676] [Article Influence: 169.0] [Reference Citation Analysis (1)] |
| 173. | Said A, Akhter A. Meta-Analysis of Randomized Controlled Trials of Pharmacologic Agents in Non-alcoholic Steatohepatitis. Ann Hepatol. 2017;16:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 174. | Huang Y, Wang X, Yan C, Li C, Zhang L, Zhang L, Liang E, Liu T, Mao J. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Medicine (Baltimore). 2022;101:e31437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 175. | Jalali M, Rahimlou M, Mahmoodi M, Moosavian SP, Symonds ME, Jalali R, Zare M, Imanieh MH, Stasi C. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2020;159:104799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 176. | Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti-hyperglycaemic drugs in patients with non-alcoholic fatty liver disease with or without diabetes: An updated systematic review of randomized controlled trials. Diabetes Metab. 2020;46:427-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 177. | Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 178. | Gu J, Yin ZF, Zhang JF, Wang CQ. Association between long-term prescription of metformin and the progression of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and hypertension. Int J Cardiol. 2020;306:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 179. | Leung M, Wong VW, Hudson M, Leung DY. Impact of Improved Glycemic Control on Cardiac Function in Type 2 Diabetes Mellitus. Circ Cardiovasc Imaging. 2016;9:e003643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 180. | Yin M, van der Horst IC, van Melle JP, Qian C, van Gilst WH, Silljé HH, de Boer RA. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H459-H468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 181. | Wang XF, Zhang JY, Li L, Zhao XY, Tao HL, Zhang L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol. 2011;38:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 182. | Hopf AE, Andresen C, Kötter S, Isić M, Ulrich K, Sahin S, Bongardt S, Röll W, Drove F, Scheerer N, Vandekerckhove L, De Keulenaer GW, Hamdani N, Linke WA, Krüger M. Diabetes-Induced Cardiomyocyte Passive Stiffening Is Caused by Impaired Insulin-Dependent Titin Modification and Can Be Modulated by Neuregulin-1. Circ Res. 2018;123:342-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 183. | Slater RE, Strom JG, Methawasin M, Liss M, Gotthardt M, Sweitzer N, Granzier HL. Metformin improves diastolic function in an HFpEF-like mouse model by increasing titin compliance. J Gen Physiol. 2019;151:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 184. | Cittadini A, Napoli R, Monti MG, Rea D, Longobardi S, Netti PA, Walser M, Samà M, Aimaretti G, Isgaard J, Saccà L. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 185. | Huang KH, Lee CH, Cheng YD, Gau SY, Tsai TH, Chung NJ, Lee CY. Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: A real-world cohort study. Front Endocrinol (Lausanne). 2022;13:1027484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 186. | Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. 2020;19:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 187. | Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang H, Lu X. PPAR-γ signaling in nonalcoholic fatty liver disease: Pathogenesis and therapeutic targets. Pharmacol Ther. 2023;245:108391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 192] [Reference Citation Analysis (0)] |
| 188. | Qiu YY, Zhang J, Zeng FY, Zhu YZ. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol Res. 2023;192:106786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 160] [Reference Citation Analysis (0)] |
| 189. | Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, Sidey J, Shi H, Aqel BA, McClain CJ, Prough RA. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta. 2016;1859:1083-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 190. | Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol. 2008;28:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 191. | Veiga FMS, Graus-Nunes F, Rachid TL, Barreto AB, Mandarim-de-Lacerda CA, Souza-Mello V. Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): Hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie. 2017;140:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 192. | Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 313] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 193. | Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 751] [Article Influence: 37.6] [Reference Citation Analysis (6)] |
| 194. | Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1032] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 195. | Zarei M, Barroso E, Palomer X, Dai J, Rada P, Quesada-López T, Escolà-Gil JC, Cedó L, Zali MR, Molaei M, Dabiri R, Vázquez S, Pujol E, Valverde ÁM, Villarroya F, Liu Y, Wahli W, Vázquez-Carrera M. Hepatic regulation of VLDL receptor by PPARβ/δ and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab. 2018;8:117-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 196. | Gastaldelli A, Sabatini S, Carli F, Gaggini M, Bril F, Belfort-DeAguiar R, Positano V, Barb D, Kadiyala S, Harrison S, Cusi K. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improvement of steatohepatitis in patients with NASH. Liver Int. 2021;41:2659-2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 197. | Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci U S A. 1998;95:14751-14756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 198. | Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91:254S-257S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 199. | Armoni M, Kritz N, Harel C, Bar-Yoseph F, Chen H, Quon MJ, Karnieli E. Peroxisome proliferator-activated receptor-gamma represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J Biol Chem. 2003;278:30614-30623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 200. | Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 201. | Li XY, Ji PX, Ni XX, Chen YX, Sheng L, Lian M, Guo CJ, Hua J. Regulation of PPAR-γ activity in lipid-laden hepatocytes affects macrophage polarization and inflammation in nonalcoholic fatty liver disease. World J Hepatol. 2022;14:1365-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 202. | Esposito K, Ciotola M, Carleo D, Schisano B, Saccomanno F, Sasso FC, Cozzolino D, Assaloni R, Merante D, Ceriello A, Giugliano D. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 203. | Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 922] [Article Influence: 153.7] [Reference Citation Analysis (1)] |
| 204. | Vandewalle B, Moerman E, Lefebvre B, Defrance F, Gmyr V, Lukowiak B, Kerr Conte J, Pattou F. PPARgamma-dependent and -independent effects of rosiglitazone on lipotoxic human pancreatic islets. Biochem Biophys Res Commun. 2008;366:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 205. | Miao M, Wang X, Liu T, Li YJ, Yu WQ, Yang TM, Guo SD. Targeting PPARs for therapy of atherosclerosis: A review. Int J Biol Macromol. 2023;242:125008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 206. | Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 207. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 2202] [Article Influence: 244.7] [Reference Citation Analysis (0)] |
| 208. | Lange NF, Graf V, Caussy C, Dufour JF. PPAR-Targeted Therapies in the Treatment of Non-Alcoholic Fatty Liver Disease in Diabetic Patients. Int J Mol Sci. 2022;23:4305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 209. | Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf. 2010;9:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 210. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1573] [Article Influence: 524.3] [Reference Citation Analysis (1)] |
| 211. | Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriya K, Namisaki T, Yoshiji H. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142:1712-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 212. | Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE((-/-)) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int J Mol Sci. 2021;22:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 213. | Han T, Fan Y, Gao J, Fatima M, Zhang Y, Ding Y, Bai L, Wang C. Sodium glucose cotransporter 2 inhibitor dapagliflozin depressed adiposity and ameliorated hepatic steatosis in high-fat diet induced obese mice. Adipocyte. 2021;10:446-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 214. | Li L, Li Q, Huang W, Han Y, Tan H, An M, Xiang Q, Zhou R, Yang L, Cheng Y. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway. Front Pharmacol. 2021;12:589273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 215. | Cusi K. Time to Include Nonalcoholic Steatohepatitis in the Management of Patients With Type 2 Diabetes. Diabetes Care. 2020;43:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 216. | Yaribeygi H, Sathyapalan T, Maleki M, Jamialahmadi T, Sahebkar A. Molecular mechanisms by which SGLT2 inhibitors can induce insulin sensitivity in diabetic milieu: A mechanistic review. Life Sci. 2020;240:117090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 217. | Fakhrolmobasheri M, Abhari AP, Manshaee B, Heidarpour M, Shafie D, Mohammadbeigi E, Mozafari AM, Mazaheri-Tehrani S. Effect of sodium-glucose cotransporter 2 inhibitors on insulin resistance; a systematic review and meta-analysis. Acta Diabetol. 2023;60:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 218. | Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, Moeckel GW, Samuel VT, Whaley JM, Shulman GI, Kibbey RG. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60:890-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 219. | Osorio H, Coronel I, Arellano A, Pacheco U, Bautista R, Franco M, Escalante B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev. 2012;2012:542042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 220. | Pinto LC, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL. Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: a systematic review and meta-analysis. Diabetol Metab Syndr. 2015;7:A58. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 221. | Zhou Y, Geng Z, Wang X, Huang Y, Shen L, Wang Y. Meta-analysis on the efficacy and safety of SGLT2 inhibitors and incretin based agents combination therapy vs. SGLT2i alone or add-on to metformin in type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 222. | Kunutsor SK, Zaccardi F, Balasubramanian VG, Gillies CL, Aroda VR, Seidu S, Davies MJ, Khunti K. Glycaemic control and macrovascular and microvascular outcomes in type 2 diabetes: Systematic review and meta-analysis of cardiovascular outcome trials of novel glucose-lowering agents. Diabetes Obes Metab. 2024;26:1837-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 223. | Ostrominski JW, Vaduganathan M. Chapter 2: Clinical and Mechanistic Potential of Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors in Heart Failure with Preserved Ejection Fraction. Am J Med. 2024;137:S9-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 224. | Packer M. Differential Pathophysiological Mechanisms in Heart Failure With a Reduced or Preserved Ejection Fraction in Diabetes. JACC Heart Fail. 2021;9:535-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 225. | Nishinarita R, Niwano S, Niwano H, Nakamura H, Saito D, Sato T, Matsuura G, Arakawa Y, Kobayashi S, Shirakawa Y, Horiguchi A, Ishizue N, Igarashi T, Yoshizawa T, Oikawa J, Hara Y, Katsumura T, Kishihara J, Satoh A, Fukaya H, Sakagami H, Ako J. Canagliflozin Suppresses Atrial Remodeling in a Canine Atrial Fibrillation Model. J Am Heart Assoc. 2021;10:e017483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 226. | Jaiswal A, Jaiswal V, Ang SP, Hanif M, Vadhera A, Agrawal V, Kumar T, Nair AM, Borra V, Garimella V, Ishak A, Wajid Z, Song D, Attia AM, Huang H, Aguilera Alvarez VH, Shrestha AB, Biswas M. SGLT2 inhibitors among patients with heart failure with preserved ejection fraction: A meta-analysis of randomised controlled trials. Medicine (Baltimore). 2023;102:e34693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 227. | Gao M, Bhatia K, Kapoor A, Badimon J, Pinney SP, Mancini DM, Santos-Gallego CG, Lala A. SGLT2 Inhibitors, Functional Capacity, and Quality of Life in Patients With Heart Failure: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2024;7:e245135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 228. | Treewaree S, Kulthamrongsri N, Owattanapanich W, Krittayaphong R. Is it time for class I recommendation for sodium-glucose cotransporter-2 inhibitors in heart failure with mildly reduced or preserved ejection fraction?: An updated systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:1046194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 229. | Banerjee M, Pal R, Maisnam I, Mukhopadhyay S. GLP-1 receptor agonists, SGLT2 inhibitors and noncardiovascular mortality in type 2 diabetes: Insights from a meta-analysis. Diabetes Metab Syndr. 2024;18:102943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 230. | Aroda VR, Henry RR, Han J, Huang W, DeYoung MB, Darsow T, Hoogwerf BJ. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247-1258.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 231. | Jensterle M, Rizzo M, Haluzík M, Janež A. Efficacy of GLP-1 RA Approved for Weight Management in Patients With or Without Diabetes: A Narrative Review. Adv Ther. 2022;39:2452-2467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 232. | Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 233. | Nauck MA, Mirna AEA, Quast DR. Meta-analysis of head-to-head clinical trials comparing incretin-based glucose-lowering medications and basal insulin: An update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide. Diabetes Obes Metab. 2023;25:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 234. | Caruso I, Di Gioia L, Di Molfetta S, Cignarelli A, Palmer SC, Natale P, Strippoli GFM, Perrini S, Natalicchio A, Laviola L, Giorgino F. Glucometabolic outcomes of GLP-1 receptor agonist-based therapies in patients with type 2 diabetes: a systematic review and network meta-analysis. EClinicalMedicine. 2023;64:102181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 235. | Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 236. | Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473-4488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 729] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 237. | Liao C, Liang X, Zhang X, Li Y. The effects of GLP-1 receptor agonists on visceral fat and liver ectopic fat in an adult population with or without diabetes and nonalcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One. 2023;18:e0289616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 238. | Kongmalai T, Srinonprasert V, Anothaisintawee T, Kongmalai P, McKay G, Attia J, Thakkinstian A. New anti-diabetic agents for the treatment of non-alcoholic fatty liver disease: a systematic review and network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2023;14:1182037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 239. | Gu Y, Sun L, Zhang W, Kong T, Zhou R, He Y, Deng C, Yang L, Kong J, Chen Y, Shi J, Hu Y. Comparative efficacy of 5 sodium-glucose cotransporter protein-2 (SGLT-2) inhibitor and 4 glucagon-like peptide-1 (GLP-1) receptor agonist drugs in non-alcoholic fatty liver disease: A GRADE-assessed systematic review and network meta-analysis of randomized controlled trials. Front Pharmacol. 2023;14:1102792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 240. | Mells JE, Fu PP, Sharma S, Olson D, Cheng L, Handy JA, Saxena NK, Sorescu D, Anania FA. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225-G235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 241. | Luque MA, González N, Márquez L, Acitores A, Redondo A, Morales M, Valverde I, Villanueva-Peñacarrillo ML. Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human myocytes. J Endocrinol. 2002;173:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 242. | Krishnan A, Schneider CV, Hadi Y, Mukherjee D, AlShehri B, Alqahtani SA. Cardiovascular and mortality outcomes with GLP-1 receptor agonists vs other glucose-lowering drugs in individuals with NAFLD and type 2 diabetes: a large population-based matched cohort study. Diabetologia. 2024;67:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 243. | Ye X, Li J, Liu Z, Sun X, Wei D, Song L, Wu C. Peptide mediated therapy in fibrosis: Mechanisms, advances and prospects. Biomed Pharmacother. 2023;157:113978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 244. | Huixing L, Di F, Daoquan P. Effect of Glucagon-like Peptide-1 Receptor Agonists on Prognosis of Heart Failure and Cardiac Function: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin Ther. 2023;45:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 245. | Siddiqui HF, Waqas SA, Batool RM, Salim H, Minhas AMK, Hasni SF, Alsaid A, Sannino A, Afzal AM, Khan MS. The effect of GLP-1 receptor agonists on cardiac remodeling in heart failure patients with preserved and reduced ejection fraction: a systematic review and meta-analysis. Heart Fail Rev. 2025;30:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 246. | Zhang Y, Yang D, Jia Q, Yan J, An F. The effect of glucagon-like peptide-1 receptor agonists on cardiac function and structure in patients with or without type 2 diabetes mellitus: An updated systematic review and meta-analysis. Diabetes Obes Metab. 2024;26:2401-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 247. | National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation. 1994;89:1333-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 490] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 248. | Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1136] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 249. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 1777] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 250. | Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 251. | Yano M, Matsumura T, Senokuchi T, Ishii N, Murata Y, Taketa K, Motoshima H, Taguchi T, Sonoda K, Kukidome D, Takuwa Y, Kawada T, Brownlee M, Nishikawa T, Araki E. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res. 2007;100:1442-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 252. | Sanguino E, Roglans N, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Atorvastatin reverses age-related reduction in rat hepatic PPARalpha and HNF-4. Br J Pharmacol. 2005;145:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 253. | Lonardo A, Lombardini S, Ricchi M, Scaglioni F, Loria P. Review article: hepatic steatosis and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 254. | Sugiyama S, Fukushima H, Kugiyama K, Maruyoshi H, Kojima S, Funahashi T, Sakamoto T, Horibata Y, Watanabe K, Koga H, Sugamura K, Otsuka F, Shimomura I, Ogawa H. Pravastatin improved glucose metabolism associated with increasing plasma adiponectin in patients with impaired glucose tolerance and coronary artery disease. Atherosclerosis. 2007;194:e43-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 255. | Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 256. | Jasińska M, Owczarek J, Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59:483-499. [PubMed] |
| 257. | Chen Z, Fukutomi T, Zago AC, Ehlers R, Detmers PA, Wright SD, Rogers C, Simon DI. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 258. | Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1). J Biol Chem. 1999;274:21926-21931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 290] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 259. | Aoki I, Aoki N, Kawano K, Shimoyama K, Maki A, Homori M, Yanagisawa A, Yamamoto M, Kawai Y, Ishikawa K. Platelet-dependent thrombin generation in patients with hyperlipidemia. J Am Coll Cardiol. 1997;30:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 260. | Haramaki N, Ikeda H, Takenaka K, Katoh A, Sugano R, Yamagishi S, Matsuoka H, Imaizumi T. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. 2007;27:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 261. | Ali FY, Armstrong PC, Dhanji AR, Tucker AT, Paul-Clark MJ, Mitchell JA, Warner TD. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009;29:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 262. | Li G, Peng Y, Chen Z, Li H, Liu D, Ye X. Bidirectional Association between Hypertension and NAFLD: A Systematic Review and Meta-Analysis of Observational Studies. Int J Endocrinol. 2022;2022:8463640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 263. | Li Y, Xu H, Wu W, Ye J, Fang D, Shi D, Li L. Clinical application of angiotensin receptor blockers in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Oncotarget. 2018;9:24155-24167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 264. | Thethi T, Kamiyama M, Kobori H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep. 2012;14:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 265. | Zhang X, Wong GL, Yip TC, Cheung JTK, Tse YK, Hui VW, Lin H, Lai JC, Chan HL, Kong AP, Wong VW. Risk of liver-related events by age and diabetes duration in patients with diabetes and nonalcoholic fatty liver disease. Hepatology. 2022;76:1409-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 266. | Yvan-Charvet L, Quignard-Boulangé A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011;79:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 267. | Folli F, Saad MJ, Velloso L, Hansen H, Carandente O, Feener EP, Kahn CR. Crosstalk between insulin and angiotensin II signalling systems. Exp Clin Endocrinol Diabetes. 1999;107:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 268. | Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137-35146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 269. | Awad K, Zaki MM, Mohammed M, Lewek J, Lavie CJ, Banach M; Lipid and Blood Pressure Meta-analysis Collaboration Group. Effect of the Renin-Angiotensin System Inhibitors on Inflammatory Markers: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Mayo Clin Proc. 2022;97:1808-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 270. | Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 271. | Weisinger RS, Begg DP, Jois M. Antagonists of the renin-angiotensin system and the prevention of obesity. Curr Opin Investig Drugs. 2009;10:1069-1077. [PubMed] |
| 272. | Rong X, Li Y, Ebihara K, Zhao M, Naowaboot J, Kusakabe T, Kuwahara K, Murray M, Nakao K. Angiotensin II type 1 receptor-independent beneficial effects of telmisartan on dietary-induced obesity, insulin resistance and fatty liver in mice. Diabetologia. 2010;53:1727-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 273. | Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol. 2012;4:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 274. | Munshi MK, Uddin MN, Glaser SS. The role of the renin-angiotensin system in liver fibrosis. Exp Biol Med (Maywood). 2011;236:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 275. | Grace JA, Herath CB, Mak KY, Burrell LM, Angus PW. Update on new aspects of the renin-angiotensin system in liver disease: clinical implications and new therapeutic options. Clin Sci (Lond). 2012;123:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 276. | Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond). 2007;113:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 277. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 278. | Lugo-Baruqui A, Muñoz-Valle JF, Arévalo-Gallegos S, Armendáriz-Borunda J. Role of angiotensin II in liver fibrosis-induced portal hypertension and therapeutic implications. Hepatol Res. 2010;40:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 279. | Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 280. | Navar LG. Physiology: hemodynamics, endothelial function, renin-angiotensin-aldosterone system, sympathetic nervous system. J Am Soc Hypertens. 2014;8:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 281. | Wernhart S, Papathanasiou M, Rassaf T, Luedike P. The controversial role of beta-blockers in heart failure with preserved ejection fraction. Pharmacol Ther. 2023;243:108356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 282. | Cromer M, Wilcox CM, Shoreibah M. Beta-blockers and cirrhosis: Striking the right balance. Am J Med Sci. 2024;367:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 283. | Gammone MA, Efthymakis K, D'Orazio N. Effect of Third-Generation Beta Blockers on Weight Loss in a Population of Overweight-Obese Subjects in a Controlled Dietary Regimen. J Nutr Metab. 2021;2021:5767306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 284. | Toblli JE, DiGennaro F, Giani JF, Dominici FP. Nebivolol: impact on cardiac and endothelial function and clinical utility. Vasc Health Risk Manag. 2012;8:151-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 285. | Fonseca VA. Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin. 2010;26:615-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 286. | Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, Loomba R, Harrison SA, Balabanska R, Mateva L, Lanthier N, Alkhouri N, Moreno C, Schattenberg JM, Stefanova-Petrova D, Vonghia L, Rouzier R, Guillaume M, Hodge A, Romero-Gómez M, Huot-Marchand P, Baudin M, Richard MP, Abitbol JL, Broqua P, Junien JL, Abdelmalek MF; NATIVE Study Group. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021;385:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 536] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 287. | Sinakos E, Liava C, Loomba R. Emerging advances in the pharmacologic treatment of nonalcoholic steatohepatitis and related cirrhosis. Ann Gastroenterol. 2022;35:213-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 288. | Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, Moussa SE. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 582] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 289. | Loomba R, Neutel J, Mohseni R, Bernard D, Severance R, Dao M, Saini S, Margaritescu C, Homer K, Tran B, Mancini M, Masamune H, Lian B. LBP-20-VK2809, a Novel Liver-Directed Thyroid Receptor Beta Agonist, Significantly Reduces Liver Fat with Both Low and High Doses in Patients with Non-Alcoholic Fatty Liver Disease: A Phase 2 Randomized, Placebo-Controlled Trial. J Hepat. 2019;70:e150-e151. [DOI] [Full Text] |
| 290. | Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, Christian R. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 435] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 291. | Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, Noureddin M, Pyko M, Shiffman M, Sanyal A, Allgood A, Shlevin H, Horton R, Zomer E, Irish W, Goodman Z, Harrison SA, Traber PG; Belapectin (GR-MD-02) Study Investigators. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334-1345.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 292. | Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 293. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 974] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 294. | Alcayaga-Miranda F, Cuenca J, Khoury M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front Immunol. 2017;8:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 295. | Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, Ghogomu ET, Coyle D, Clifford T, Tugwell P, Wells GA. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386:258-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 296. | Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro-Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 297. | Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res. 2015;117:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 298. | Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moyé LA, Simari RD; Cardiovascular Cell Therapy Research Network (CCTRN). Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/