©The Author(s) 2025.
World J Biol Chem. Dec 5, 2025; 16(4): 111831
Published online Dec 5, 2025. doi: 10.4331/wjbc.v16.i4.111831
Published online Dec 5, 2025. doi: 10.4331/wjbc.v16.i4.111831
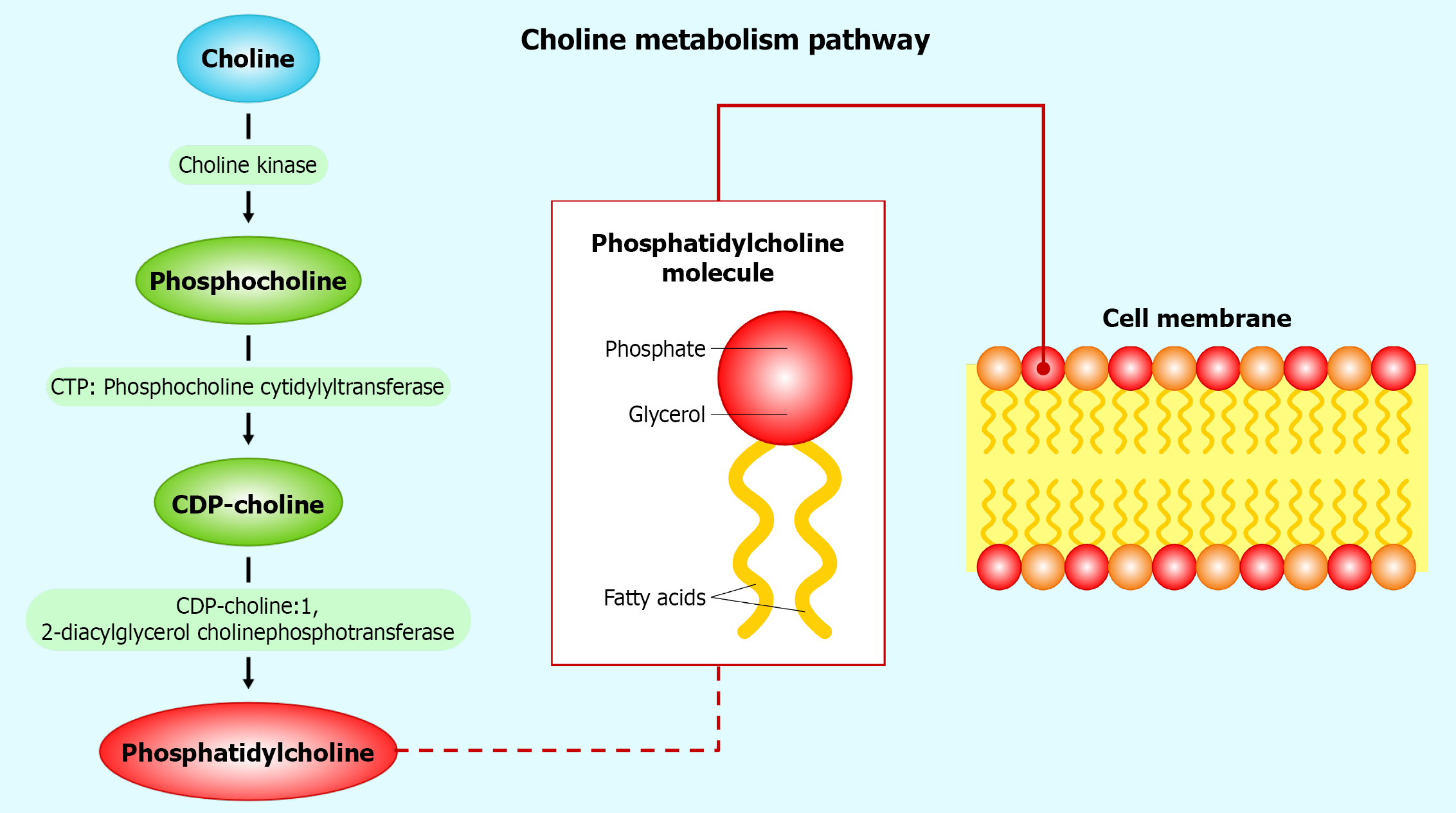
Figure 1 Choline’s conversion to phosphatidylcholine.
This figure illustrates the cytidine diphosphate (CDP)-choline pathway, a critical metabolic route converting choline into phosphatidylcholine (PC), the predominant phospholipid constituting over 50% of mammalian cell membranes. Choline is initially phosphorylated by choline kinase to form phosphocholine, which cytidine triphosphate: Phosphocholine cytidylyltransferase then transforms into CDP-choline; subsequently, CDP-choline: 1,2-diacylglycerol cholinephosphotransferase catalyzes its conversion into PC. The diagram highlights PC’s amphipathic structure—featuring a hydrophilic head and hydrophobic tails—essential for membrane fluidity and signal transduction. In hepatocytes, where this pathway is notably active, PC facilitates very-low-density lipoprotein assembly, exporting triglycerides to prevent lipid accumulation. CDP: Cytidine diphosphate; PC: Phosp
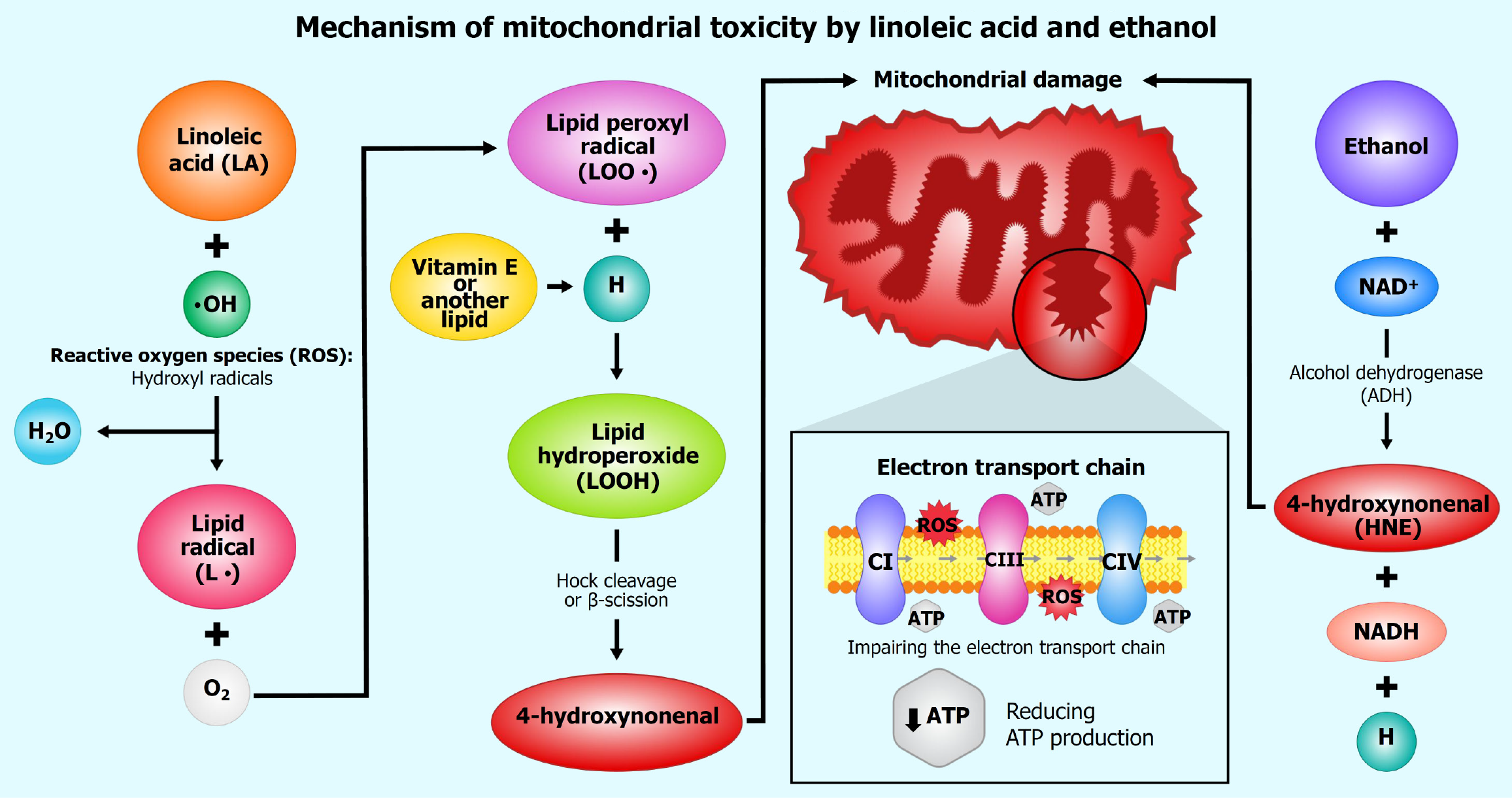
Figure 2 Mechanism of mitochondrial toxicity induced by linoleic acid and ethanol.
This schematic illustrates the synergistic mechanisms by which LA and ethanol induce mitochondrial toxicity, leading to cellular dysfunction. LA undergoes peroxidation to generate reactive oxygen species, such as hydroxyl radicals (OH), and lipid radicals (L). These reactive intermediates, in the presence of molecular oxygen (O2), further propagate the formation of lipid peroxyl radicals (LOO). Subsequently, lipid peroxides (LOOH) are produced, which can be exacerbated by hydrogen ions (H+). In this context, lipid peroxides may undergo Hock cleavage or β-scission, yielding secondary products like 4-hydroxynonenal, a highly reactive aldehyde known to impair mitochondrial membrane integrity. These stressors inhibit the electron transport chain, notably Complex III, reducing adenosine triphosphate synthesis. Vitamin E’s role in neutralizing lipid peroxyl radicals is also shown.
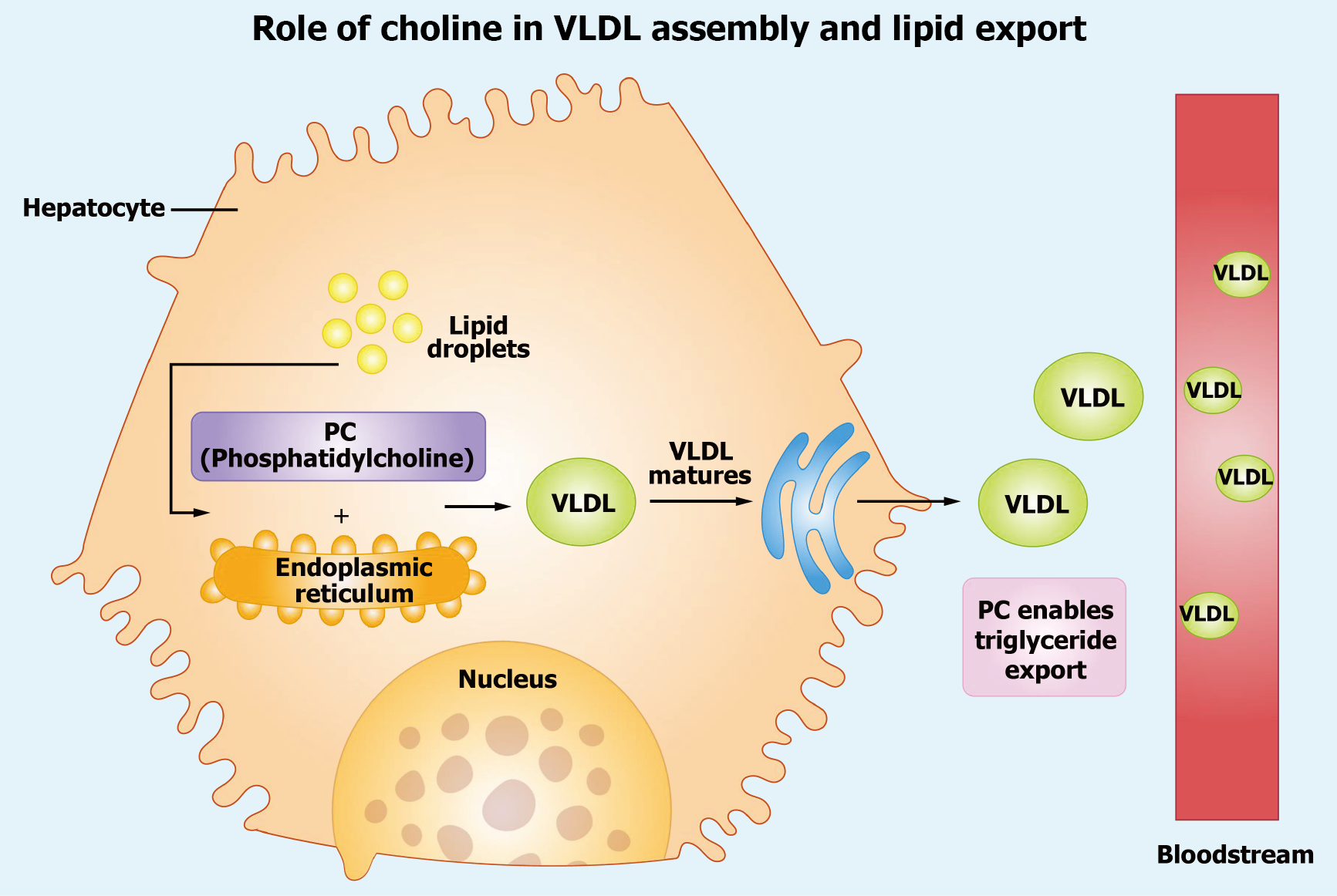
Figure 3 Role of choline in verylowdensity lipoprotein assembly and lipid export.
This schematic illustrates choline’s role, via phosphatidylcholine (PC), in verylowdensity lipoprotein (VLDL) assembly and lipid export within a hepatocyte. The hepatocyte, depicted with a jagged plasma membrane, contains a central nucleus (light orange) and an endoplasmic reticulum (ER, orange) where PC (purple box) facilitates initial VLDL assembly. Lipid droplets (yellow circles) contribute triglycerides to VLDL, which matures in the Golgi apparatus (light purple) before export into the bloodstream (red). Green VLDL particles, marked with "PC Enables Triglyceride Export", highlight PC’s role in lipid secretion. Arrows trace the pathway from ER to Golgi to bloodstream, emphasizing the sequential process of VLDL biogenesis and hepatic lipid clearance. VLDL: Verylowdensity lipoprotein; PC: Phosphatidylcholine.
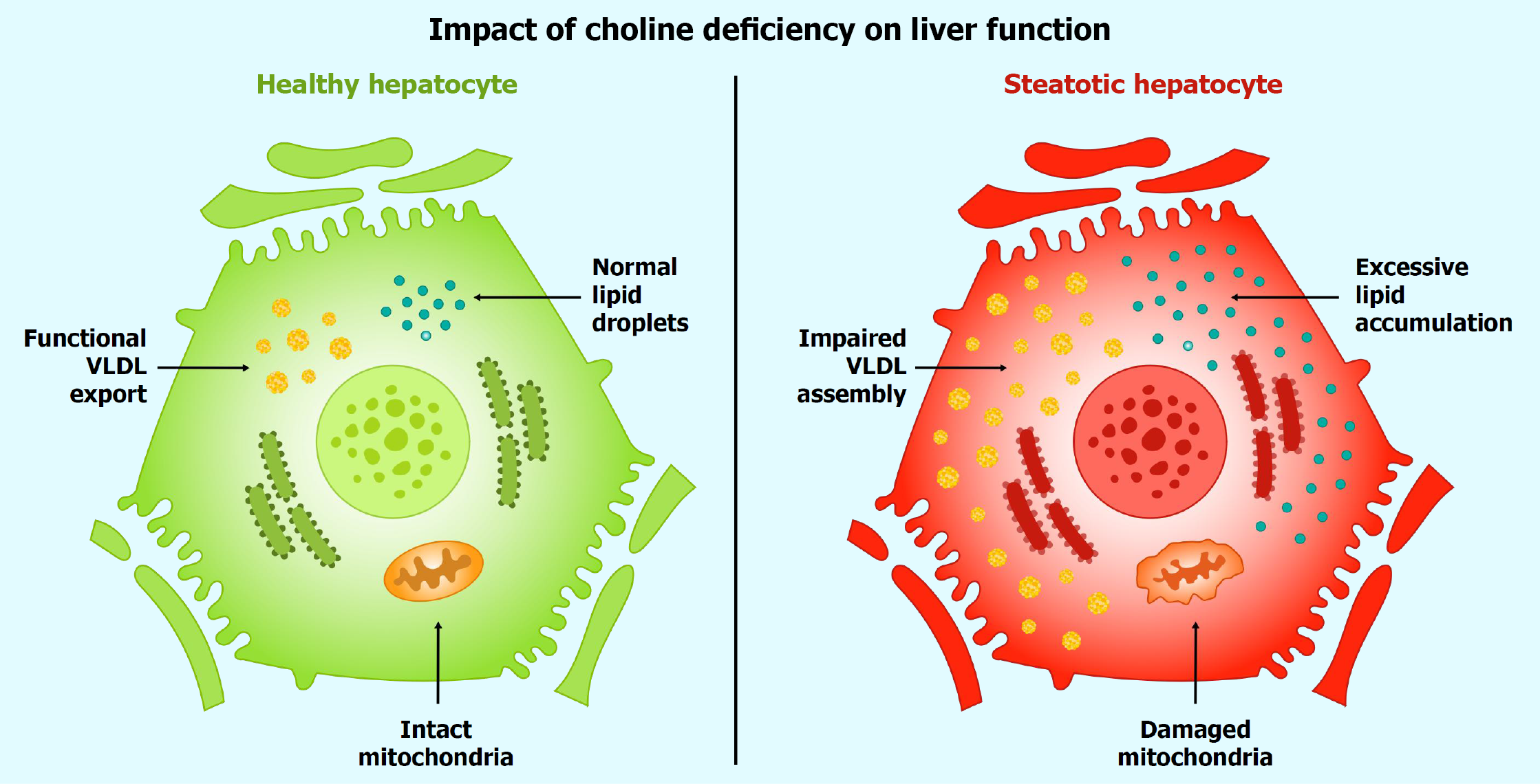
Figure 4 Impact of choline deficiency on liver function.
This figure juxtaposes a healthy hepatocyte (left, green) with a steatotic hepatocyte (right, red) to depict the consequences of choline deficiency. The healthy hepatocyte features a smooth plasma membrane, sparse lipid droplets (yellow, < 5% of cell volume), intact mitochondria (orange), and an outward arrow indicating robust verylowdensity lipoprotein (VLDL) export, reflecting efficient triglyceride clearance. In contrast, the steatotic hepatocyte exhibits a disrupted membrane, excessive lipid accumulation (dense yellow droplets exceeding 5% of liver weight), damaged mitochondria, and impaired VLDL assembly. This comparison underscores choline’s pivotal role in hepatic lipid homeostasis, where its deficiency precipitates steatosis—a condition historically linked to diets low in methyl donors. VLDL: Verylowdensity lipoprotein.
- Citation: Mercola J. Fatty liver reexamined choline and mitochondrial toxin amelioration. World J Biol Chem 2025; 16(4): 111831
- URL: https://www.wjgnet.com/1949-8454/full/v16/i4/111831.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v16.i4.111831