Published online Jan 27, 2026. doi: 10.4240/wjgs.v18.i1.114309
Revised: October 22, 2025
Accepted: November 19, 2025
Published online: January 27, 2026
Processing time: 127 Days and 19.2 Hours
Postoperative recurrence remains a major challenge in gastric cancer mana
To evaluate postoperative serum CEA expression and its efficacy in predicting gastric cancer recurrence.
This retrospective study analyzed clinical data from 120 patients with primary gastric cancer treated between January 2022 and January 2023. Patients were cate
During 24 months of follow-up, 39 patients (32.50%) experienced recurrence. No significant baseline differences were observed between groups (P > 0.05). CEA, AFP, and CA19-9 levels at one week were comparable between groups (P > 0.05), whereas levels at three and six months were significantly higher in the recurrence group (P < 0.05). Logistic regression identified postoperative CEA and AFP as independent risk factors for recurrence (P < 0.05). Pearson correlation showed a negative association between CEA and AFP levels and recurrence interval (P < 0.05). ROC curve analysis demonstrated that combined CEA and AFP yielded an area under the curve of 0.826, with specificity of 94.36% and sensitivity of 90.50%, outperforming either marker alone (P < 0.05).
Dynamic postoperative monitoring of serum CEA and AFP enables early prediction and detection of gastric cancer recurrence.
Core Tip: Dynamic monitoring of carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) levels at three and six months after gastrectomy is crucial for postoperative management of gastric cancer. Elevated levels of these markers serve as independent risk factors for recurrence. Combined assessment of CEA and AFP enhances predictive accuracy, yielding an area under the curve of 0.826, and offers superior sensitivity and specificity compared with either marker alone. This combined approach facilitates earlier detection and timely intervention for recurrent gastric cancer.
- Citation: Duan XX, Yu X, Zhou L. Timeliness of postoperative serum carcinoembryonic antigen monitoring for predicting recurrence after gastric cancer surgery. World J Gastrointest Surg 2026; 18(1): 114309
- URL: https://www.wjgnet.com/1948-9366/full/v18/i1/114309.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v18.i1.114309
Gastric cancer is one of the most common malignant tumors of the digestive system and ranks first among digestive tract malignancies in China, with a high incidence among individuals aged 55-70 years[1]. In recent years, its incidence has shown an upward trend. The disease typically has an insidious onset and lacks specific clinical manifestations in the early stages. By the time it is clinically detected or diagnosed, most patients have already reached an advanced stage, posing a serious threat to their health and survival[2]. Gastric adenocarcinoma is the predominant pathological type, accounting for more than 95% of cases. Although gastric cancer mortality has declined in recent years, the average annual mortality rate remains relatively high[3,4]. Surgical resection remains the primary treatment for gastric cancer. However, clinical studies indicate that recurrence occurs in more than 50% of patients within two years after surgery, and the five-year survival rate remains around 30%[5]. Therefore, early detection of postoperative recurrence and timely intervention are essential for improving survival outcomes. Currently, serum tumor marker testing, such as carcinoembryonic antigen (CEA), is widely used to predict prognosis in patients with malignant tumors and has shown good clinical utility. However, the predictive value of dynamic postoperative monitoring for recurrence remains unclear. Thus, further investigation is needed to determine the role of these biomarkers in predicting recurrence after gastric cancer surgery. With ongoing advances in the combined detection of multiple serum tumor markers and their integration into clinical diagnosis, treatment, and prognostic evaluation, their clinical value continues to grow[6]. This study retrospectively analyzed the clinical data of 120 patients who underwent gastric cancer surgery to evaluate the predictive value of dynamic postoperative monitoring of serum CEA changes for recurrence, aiming to provide evidence to support its clinical application.
This retrospective study included 120 patients with primary gastric cancer who received treatment at our hospital between January 2022 and January 2023. The study was conducted in accordance with the principles of the Declaration of Helsinki. Patients were eligible for inclusion if they met the following criteria: (1) Their diagnosis was confirmed by imaging and histopathology consistent with gastric adenocarcinoma[7]; (2) They were between 18 and 75 years of age; (3) Their disease was classified as TNM stage I-IIIa; (4) They were suitable candidates for surgical resection with no residual tumor following surgery; (5) They had no history of gastrointestinal surgery; and (6) Their clinical and diagnostic data were complete. Patients were excluded if they had other concurrent organic lesions or malignant tumors, hematologic or immunologic disorders, or distant metastasis of gastric cancer. Those who died during the follow-up period were also excluded from the analysis. The cohort consisted of 68 men and 52 women aged 31-73 years (mean 59.37 ± 4.16 years). Pathological staging included stage I in 34 cases, stage II in 67 cases, and stage IIIa in 19 cases. Regarding differentiation, five tumors were well-differentiated, 46 were moderately differentiated, and 69 were poorly differentiated. The mean body mass index was 20.19 ± 0.62 kg/m2 (range 19.36-21.08 kg/m2).
Treatment: All enrolled patients underwent elective radical gastrectomy after diagnosis. Before surgery, neoadjuvant chemotherapy (NACT) was administered according to individualized clinical assessments and multidisciplinary team decisions. The main NACT regimens included the SOX regimen, consisting of S-1 (40-60 mg orally twice daily for 14 days) combined with oxaliplatin (130 mg/m2 administered intravenously on day 1), and the XELOX regimen, consisting of capecitabine (1000 mg/m2 orally twice daily for 14 days) combined with oxaliplatin (130 mg/m2 admini
Postoperative adjuvant chemotherapy was administered according to pathological stage and patient recovery, typically beginning within 4-8 weeks after surgery. The adjuvant regimens corresponded to the preoperative protocols, primarily consisting of SOX or XELOX, and were planned for a total of four to eight cycles.
CEA, alpha-fetoprotein, and carbohydrate antigen 19-9 testing: Peripheral venous blood samples (5 mL) were collected from all patients one week, three months, and six months after surgery. Samples were centrifuged at 4000 rpm for five minutes, and the supernatant was submitted for analysis. When immediate testing was not feasible, samples were stored at -80 °C and analyzed within one week. Levels of CEA, alpha-fetoprotein (AFP), and carbohydrate antigen 19-9 (CA19-9) were measured using chemiluminescence immunoassay on fully automated analyzers, following the protocols of the manufacturers. The normal reference ranges were as follows: CEA ≤ 5 ng/mL, CA19-9 ≤ 37 U/mL, and AFP < 30 ng/mL.
In this study, blood samples were also scheduled for collection at 12 months and 24 months postoperatively. For patients who experienced recurrence before a scheduled sampling time point, no further samples were collected. All laboratory testing was performed independently of clinical decision-making.
Follow-up: All patients were followed for 24 months after surgery, with evaluations conducted every three months. Follow-up was completed in January 2025 and consisted of regular outpatient visits that included abdominal ultrasound and gastroscopy, supplemented by positron emission tomography/computed tomography (PET/CT) scans when clinically indicated. The interval from postoperative discharge to documented recurrence was recorded for each patient.
Gastric cancer recurrence was determined based on the following criteria: (1) Imaging or PET/CT scans showing new masses within the primary surgical field; (2) Imaging or PET/CT scans revealing space-occupying lesions in other organs after exclusion of primary tumors; (3) Detection of metastatic lesions in the lungs, bones, or other organs without alternative etiology; (4) Confirmation of recurrence by gastroscopy and pathological biopsy; (5) Confirmation of recurrence through reoperation or exploratory surgery; and (6) Presence of uncontrollable hemorrhagic ascites or detection of cancer cells in ascitic fluid cytology[8]. The diagnosis of recurrence was established comprehensively based on clinical, imaging, and pathological findings. The specific diagnostic criteria and the number of patients meeting each criterion in the recurrence group (n = 39) are presented in Table 1. For patients who fulfilled multiple criteria, the most definitive diagnostic method, such as pathological confirmation, was used for classification.
| Criterion for recurrence | Number of cases | Percentage (%) |
| Imaging (CT or PET/CT) revealing new masses within the primary surgical field | 15 | 38.5 |
| Imaging (CT or PET/CT) detecting space-occupying lesions in other organs (distant metastasis), with primary tumors excluded | 12 | 30.8 |
| Confirmation of recurrence via gastroscopy and pathological biopsy (local recurrence) | 8 | 20.5 |
| Confirmation of recurrence through reoperation or exploratory surgery | 2 | 5.1 |
| Presence of uncontrollable hemorrhagic ascites, or detection of cancer cells in ascites cytology (peritoneal carcinomatosis) | 2 | 5.1 |
| Total | 39 | 100 |
All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States). The normality of continuous variables was assessed using the Shapiro-Wilk test. Quantitative data following a normal distribution are presented as mean ± SD, with intergroup comparisons conducted using independent-samples t-tests. Non-normally distributed data are presented as median (interquartile range), with intergroup comparisons performed using the Mann-Whitney U test. Categorical variables are expressed as counts and percentages, with intergroup comparisons conducted using the χ2 test or Fisher’s exact test, as appropriate. The exact statistical test used for each baseline characteristic is specified in Table 2. For the comparison of serial biomarker measurements, a two-way repeated-measures analysis of variance was used to evaluate the effects of group (recurrence vs non-recurrence), time, and their interaction on biomarker levels. This approach accounted for the family-wise error rate associated with multiple time-point com
| Items | Recurrence group | Non-recurrence group | t/χ2 | P value | |
| Sex | Male | 21 (53.85) | 47 (58.02) | 0.826 | 0.106 |
| Female | 18 (46.15) | 32 (41.98) | |||
| Age (years) | 59.48 ± 4.09 | 59.29 ± 4.11 | 0.883 | 0.217 | |
| Pathological staging | I | 12 (30.77) | 22 (27.16) | 0.907 | 0.114 |
| II | 20 (51.28) | 47 (58.02) | |||
| IIIa | 7 (17.95) | 12 (14.81) | |||
| Differentiation | Low differentiation | 23 (58.97) | 46 (56.79) | 0.746 | 0.083 |
| Medium differentiation | 15 (38.46) | 31 (38.27) | |||
| High differentiation | 1 (2.57) | 4 (4.94) | |||
| BMI (kg/m2) | 20.16 ± 0.68 | 20.23 ± 0.73 | 0.842 | 0.103 | |
At the final follow-up, 39 of the 120 patients (32.50%) experienced recurrence, whereas 81 patients (67.50%) remained recurrence-free. No significant differences were observed in baseline clinical characteristics between the two groups (P > 0.05), as shown in Table 2.
Serum CEA, AFP, and CA19-9 levels measured one week after surgery showed did not differ significantly between the recurrence and non-recurrence groups (P > 0.05). However, at three and six months postoperatively, levels of all three markers were significantly higher in the recurrence group compared with the non-recurrence group (P < 0.05), as shown in Table 3.
| Group | CEA (ng/mL) | CA19-9 (U/mL) | AFP (μg/L) | ||||||
| Post-1 week | Post-3 months | Post-6 months | Post-1 week | Post-3 months | Post-6 months | Post-1 week | Post-3 months | Post-6 months | |
| Recurrence group (n = 39) | 16.11 ± 3.18 | 25.23 ± 4.08a | 29.12 ± 4.26a,b | 26.34 ± 5.17 | 29.19 ± 5.08a | 35.27 ± 4.16a,b | 20.13 ± 3.27 | 24.29 ± 3.18a | 27.15 ± 3.41a,b |
| Non-recurrence group (n = 81) | 16.09 ± 3.22 | 19.12 ± 3.18a | 21.11 ± 4.17a,b | 26.24 ± 5.17 | 22.47 ± 5.15a | 26.08 ± 3.86a,b | 20.09 ± 3.23 | 22.21 ± 3.32a | 24.32 ± 3.41a |
| t value | 0.322 | 9.317 | 10.441 | 0.989 | 7.297 | 12.576 | 0.063 | 3.416 | 4.298 |
| P value | 0.064 | 0.000 | 0.000 | 0.103 | < 0.01 | 0.000 | 0.074 | 0.000 | 0.001 |
Multivariate logistic regression analysis identified postoperative changes in CEA and AFP levels as independent risk factors for recurrence in patients with gastric cancer (P < 0.05), as shown in Table 4.
| Independent variable | Coefficient of regression | SE | β | P value | OR (95%CI) |
| CEA | 0.427 | 0.221 | 0.315 | 0.001 | 1.318 (0.787-4.846) |
| AFP | 0.245 | 0.143 | 0.127 | 0.010 | 1.327 (1.304-2.132) |
| CA19-9 | 0.337 | 0.371 | 0.135 | 0.074 | 0.414 (0.126-0.627) |
The recurrence interval among patients ranged from five to 16 months, with a mean of 7.18 ± 1.83 months. Pearson correlation analysis demonstrated a significant negative correlation between serum CEA and AFP levels and the time to recurrence (P < 0.05), as shown in Table 5.
| Items | CEA | AFP | CA19-9 | |
| Time of postoperative recurrence | r value | -0.634 | -0.603 | -0.589 |
| P value | 0.001 | < 0.001 | 0.001 |
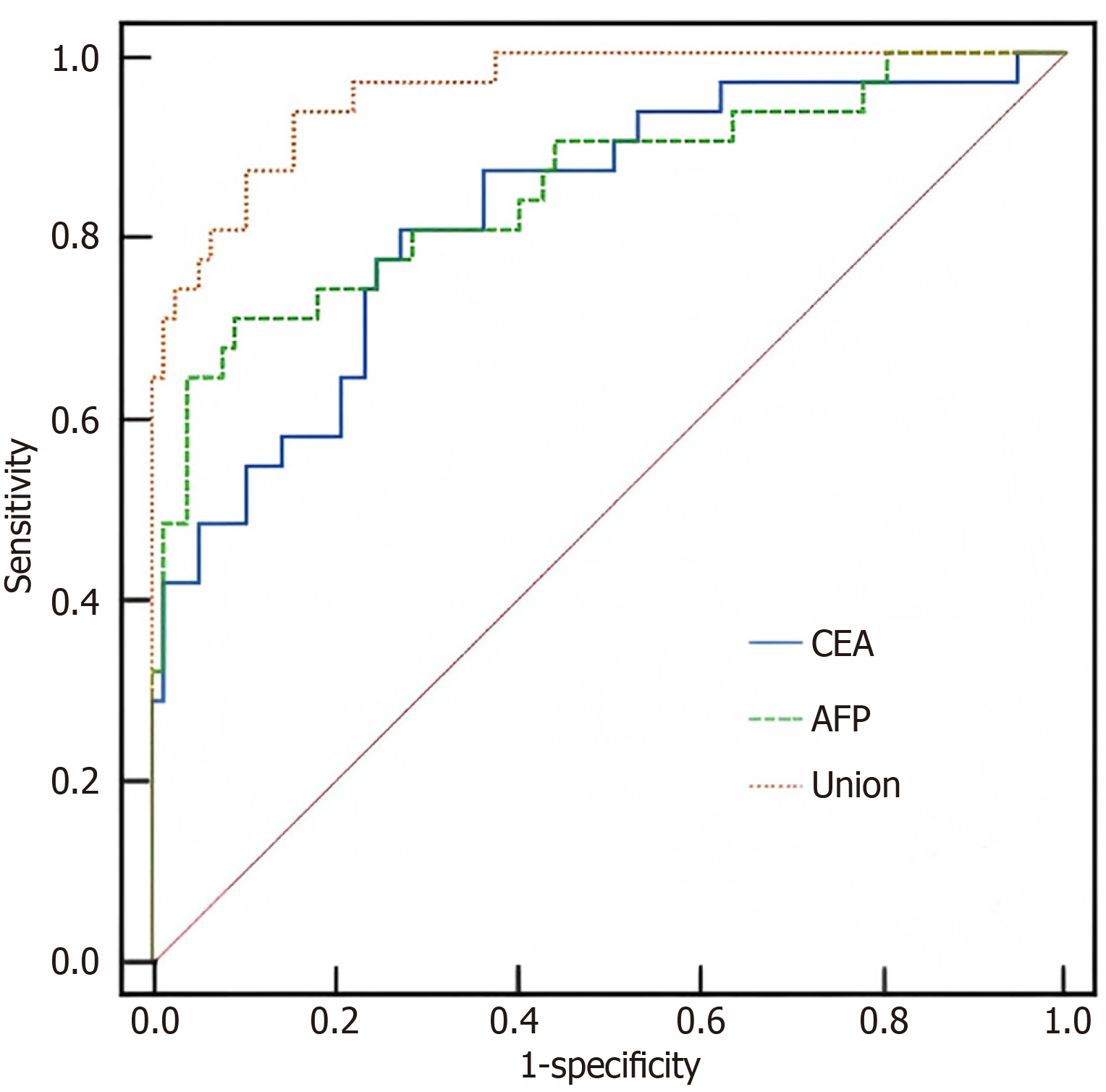
ROC curve analysis, using the six-month postoperative levels of serum CEA and AFP, showed that their combined measurement predicted recurrence with an AUC of 0.826, specificity of 94.36%, and sensitivity of 90.50%. The combined model demonstrated superior predictive accuracy compared with either marker alone (P < 0.05), as shown in Table 6 and Figure 1.
| Variable | AUC | 95%CI | Youden index | P value | Specificity (%) | Sensitivity (%) |
| CEA | 0.702 | 0.608-0.783 | 0.55 | 0.001 | 68.11 | 81.09 |
| AFP | 0.688 | 0.514-0.732 | 0.62 | 0.001 | 45.20 | 80.52 |
| Combination of the two | 0.826 | 0.702-0.878 | 0.69 | 0.001 | 94.36 | 90.50 |
Gastric cancer is a malignant tumor arising from the epithelial cells of the gastric mucosa, with major etiologic factors including genetic predisposition, dietary habits, and Helicobacter pylori infection[9,10]. Surgical resection remains the cornerstone of treatment, aiming to improve survival by removing the primary tumor and regional lymph nodes. Clinical studies have shown that radical surgery significantly prolongs survival in patients with gastric cancer. However, a substantial proportion of patients still experience postoperative recurrence, highlighting the need for targeted preventive and therapeutic strategies to further enhance treatment outcomes[11,12]. Integrating pathological characteristics and patterns of disease progression with serologic markers for early detection could markedly improve prognosis[13]. Although several studies have demonstrated the diagnostic value of tumor markers in gastric cancer, their role in monitoring postoperative recurrence remains to be fully validated through evidence-based research. Recurrence after NACT combined with minimally invasive radical surgery involves multiple biological mechanisms and is accompanied by changes in antibodies, cytokines, and other serum markers[14]. These tumor markers may originate either from the host response to malignant stimulation or directly from tumor cells through secretion or shedding of specific substances[15,16]. Dynamic monitoring of marker concentrations provides insight into tumor burden, disease progression, and the risk of metastasis or recurrence, thereby offering valuable guidance for clinical decision-making.
In the clinical diagnosis, treatment, and prognostic evaluation of malignant tumors of the digestive system, the detection of tumor markers such as CEA, AFP, and CA19-9 plays an important role. Among these, CEA is a glycoprotein commonly expressed on the membranes of malignant cells in digestive tract cancers, including gastric and colorectal cancers[17]. Studies have shown that CEA can also be detected during normal embryonic development of the digestive tract, suggesting a possible role in physiological processes[18]. In healthy individuals, serum CEA levels are extremely low but remain measurable using highly sensitive assays. As malignant tumors proliferate, CEA detaches from tumor cell membranes and enters the bloodstream, leading to elevated serum levels. Therefore, CEA serves as a valuable adjunctive marker in the diagnosis of gastrointestinal malignancies[19]. However, CEA lacks organ specificity, as mild elevations can also occur in benign conditions such as inflammatory bowel disease and pancreatitis. Clinical interpretation should therefore be made in conjunction with other diagnostic findings. AFP is a glycoprotein primarily synthesized by the fetal liver, yolk sac, and placenta, and its levels are typically very low in healthy adults. In malignant conditions such as hepatocellular carcinoma, AFP production is reactivated, resulting in significantly elevated serum concentrations. It also serves as a useful biomarker for the diagnosis and recurrence monitoring of gastric cancer[20]. CA19-9, a ganglioside derivative, is a high-molecular-weight carbohydrate antigen associated with adenocarcinoma cells of epithelial origin. Its serum levels are often markedly increased in patients with digestive system malignancies, including pancreatic, gastric, and colorectal cancers[21,22]. Although CA19-9 is commonly used in the assessment of gastric and colorectal cancers, its diagnostic sensitivity remains relatively limited[23]. This study employed dynamic monitoring of these tumor markers to provide early detection and prediction of postoperative recurrence in patients with gastric cancer.
This retrospective study analyzed 120 patients who underwent gastric cancer surgery. Among them, 39 patients experienced recurrence during follow-up, corresponding to a recurrence rate of 32.50%, which aligns with previously reported rates[24]. Comparison of postoperative serum tumor marker levels showed that patients in the recurrence group had significantly higher CEA, AFP, and CA19-9 levels at three and six months after surgery compared with the non-recurrence group, whereas no significant differences were observed one week preoperatively. These findings suggest that serum tumor markers have considerable clinical value in monitoring postoperative recurrence in gastric cancer. In particular, CEA and AFP exhibited more pronounced differences at three and six months, indicating that these two markers possess high sensitivity for the early detection of recurrence. Multivariate logistic regression analysis identified CEA and AFP as independent risk factors for postoperative recurrence. The regression coefficient for CEA was 0.427 (P = 0.014), with an odds ratio (OR) of 1.318, indicating a 31.8% increase in recurrence risk for each unit increase in CEA level. The regression coefficient for AFP was 0.245 (P = 0.001), with an OR of 1.327, suggesting a 32.7% increase in recurrence risk per unit rise in AFP level. Although CA19-9 showed an association with recurrence in univariate analysis, it did not reach statistical significance in multivariate analysis, possibly reflecting its greater susceptibility to confounding factors or a more complex biological role in gastric cancer recurrence[23]. Pearson correlation analysis demonstrated a negative correlation between CEA and AFP levels and the time to postoperative recurrence, indicating that higher concentrations of these markers were associated with earlier recurrence. Furthermore, ROC curve analysis revealed that the combined detection of CEA and AFP yielded an AUC of 0.826, which was significantly higher than that of either marker alone, indicating that combined testing could more effectively distinguish between patients with and without recurrence. The combined assay achieved specificity and sensitivity of 94.36% and 90.50%, respectively, both superior to those of single-marker analyses. These findings further support the clinical value of serum CEA and AFP as predictive biomarkers for postoperative recurrence in gastric cancer.
Despite the valuable insights gained, this study has several limitations that warrant consideration. First, as a single-center retrospective analysis, it is subject to potential selection bias, which may limit the generalizability of the findings. The sample size, particularly the number of recurrence events (n = 39), represents another constraint. Although mul
In summary, postoperative serum levels of CEA and AFP in patients with gastric cancer showed dynamic changes that were negatively correlated with recurrence timing. Continuous monitoring of these markers may enable earlier detection of recurrence and guide timely clinical intervention, thereby improving postoperative management and patient outcomes.
| 1. | Xu Y, Xia C, Wang J, Wu Y, Chen W. Divergent trends in the burden of esophageal, gastric, and liver cancers in China. J Natl Cancer Cent. 2025;5:306-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Okawa S, Charvat H. Age-standardized mortality-to-incidence ratio for stomach cancer in the world. Jpn J Clin Oncol. 2025;55:440-441. [PubMed] [DOI] [Full Text] |
| 3. | Kang D, Jeon HJ, Kim JH, Oh SI, Seong YS, Jang JY, Kim JW, Kim JS, Nam SJ, Bang CS, Choi HS. Enhancing Lymph Node Metastasis Risk Prediction in Early Gastric Cancer Through the Integration of Endoscopic Images and Real-World Data in a Multimodal AI Model. Cancers (Basel). 2025;17:869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Turkoglu E, Topal GA, Yıldırım S, Kınıkoglu O, Sarıyar Busery N, Kaya T, Yıldız HS, Turkoglu F, Tatar C, Sakin A, Isık D, Ay Ersoy S, Basoglu T, Odabas H, Turan N. The Association of Khorana Risk Score with Venous Thromboembolism and Overall Survival in Patients with Metastatic Gastric Cancer. Medicina (Kaunas). 2025;61:1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Chen Y, Shu J, Zhang Z, You Y, Yue S, Ji Q, Chen K, Liu Y, Duan B, Yu B, Kou S, Pang X, Wang W, Yang L, Zhao Z, Gao J. Dual-energy CT for predicting progression-free survival of locally advanced gastric cancer after gastrectomy: Insights into tumor angiogenesis. Eur J Surg Oncol. 2025;51:110017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Ren L, Liu J, Xu YY, Shi ZW. Untargeted metabolomics analysis of serum metabolic signatures as novel biomarkers for gastric carcinoma. World J Clin Oncol. 2025;16:108967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Brown LR, Soupashi M, Yule MS, Grossart CM, McMillan DC, Laird BJA, Wigmore SJ, Skipworth RJE. A Comparison of Established Diagnostic Criteria for Cachexia and Their Impacts on Prognostication in Patients with Oesophagogastric Cancer. Cancers (Basel). 2025;17:448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Shigeta K, Yamamoto Y, Shimoda T, Sugino T, Ono H. Early stage gastric cancer with unusual sarcomatous component and no recurrence after endoscopic submucosal dissection: a case report. Gastric Cancer. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Fan Z, He Z, Miao W, Huang R. Critical Analysis of Risk Factors and Machine-Learning-Based Gastric Cancer Risk Prediction Models: A Systematic Review. Process. 2023;11:2324. [DOI] [Full Text] |
| 10. | Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Ogimi M, Deguchi R, Teramura E, Ueda T, Morimachi M, Arase Y, Yamamoto M, Nishi T, Nabeshima K, Koyanagi K. A Case of Unresectable Gastric Cancer Successfully Treated with Conversion Surgery After Immunotherapy Including Treatment. Tokai J Exp Clin Med. 2025;50:70-74. [PubMed] |
| 12. | Eom BW, Han M, Yoon HM, Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Kim YW, Kim JW, Lee JH, Han SU, Ryu KW; Of The Korean Gastric Cancer Association TIC. National validation of laparoscopic approach for locally advanced gastric cancer: Comparison of a randomized controlled trial and real-world practice results. Chin J Cancer Res. 2024;36:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Tu H, Feng S, Chen L, Huang Y, Zhang J, Wu X. Contrast enhanced ultrasound combined with serology predicts hepatocellular carcinoma recurrence: a retrospective observation cohort study. Front Oncol. 2023;13:1154064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Guo J, Zheng L, Chen J, Lin W. Disparities of tumour markers in intraperitoneal drainage fluid between laparoscopic and open radical gastrectomy for gastric cancer. Wideochir Inne Tech Maloinwazyjne. 2024;19:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Zhang LK, Zheng HL, Zheng HH, Ma YB, Lin JX, Xu BB, Xue Z, Zheng ZW, Zheng CH, Huang CM, Xie JW. Effects of tumor marker regression load score on long-term prognosis of gastric cancer patients undergoing radical surgery after neoadjuvant chemotherapy. Eur J Surg Oncol. 2024;50:108367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Pang C, Ma Y, Shi W, Zi M, Chen J, Liang C, Li X, Liu Z, Du Y. Prognostic significance of serum tumor markers in various pathologic subtypes of gastric cancer. J Gastrointest Surg. 2024;28:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (6)] |
| 17. | Chen Y, Liu D, Wang Z, Lin Y, Jiang X, Liu J, Lian L. Machine learning-based dynamic CEA trajectory and prognosis in gastric cancer. BMC Cancer. 2025;25:1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Sharif ABM, Khan MMH, Rahman MM, Pervin MM, Bahar MM, Simon MAA, Akter A. Clinical Significance of Preoperative Serum CEA and CA 19-9 Level in Different Stages of Gastric Cancer. Mymensingh Med J. 2025;34:833-839. [PubMed] |
| 19. | Dai XR, Zhang MZ, Chen L, Guo XW, Li ZX, Yan KF, He QQ, Cheng HW. Diagnostic value of systemic immune-inflammation index and prognostic nutritional index combined with CEA in gastric cancer with lymph node metastasis. Front Endocrinol (Lausanne). 2025;16:1522349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Yamazawa S, Fukasawa-Hokazono M, Takase A, Kondo A, Matsubara J, Shinozaki-Ushiku A, Seto Y, Ushiku T. Immune evasion strategies in AFP-producing gastric carcinoma: characterized by HLA-G expression and HLA class I deficiency. Virchows Arch. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Luan F, Xu S, Chen K, Chen K, Kang M, Chen G, Chen J. Prognostic effect of CEA, AFP, CA19‑9 and CA242 for recurrence/metastasis of gastric cancer following radical gastrectomy. Mol Clin Oncol. 2025;22:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Deng L, Yin T, Li H, Wang X, Li J, Liu K, Long T, Wang Y, Cheng L. CEA, CA19-9, and CA72-4 in Gastric Cancer Diagnosis and Progression: a Chinese Retrospective Case-Control Study. Clin Lab. 2025;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Bąk M, Wojciech M, Pielech A, Holka S, Zawadzki M, Murawa D. The Advancement Stage of Gastric Cancer and the Levels of CEA and Ca19-9 in Serum and Peritoneal Lavage. Biomedicines. 2024;12:2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Hayasaka J, Kikuchi D, Nomura K, Odagiri H, Ochiai Y, Suzuki Y, Fukuma Y, Tanaka M, Yamashita S, Matsui A, Inoshita N, Kitagawa M, Hoteya S. Recurrence rate of intramucosal gastric cancer with positive vertical margin due to lesion damage during endoscopic submucosal dissection. Acta Gastroenterol Belg. 2021;84:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/