Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.107301
Revised: June 30, 2025
Accepted: August 6, 2025
Published online: September 27, 2025
Processing time: 119 Days and 0.8 Hours
Preoperative prognosis assessment of hepatocellular carcinoma (HCC) is crucial, and pathologic grading is a key prognostic determinant that affects patient prog
To investigate the distinguishing capability of fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG PET/CT)-de
We retrospectively assessed the 18F-FDG PET/CT imaging datasets from 32 pa
Significant differences were observed in SUVmax, SUVmean, TLG, TNR, and TBR between Edmondson grade II and III HCC groups (P < 0.05), whereas MTV was not significantly different (P = 0.052). The maximum tumor diameter and Ki67 expression percentage significantly varied between the two groups (P < 0.05). SUVmax yielded the largest area under the receiver operating characteristic curve, measuring 0.853 (95% confidence interval: 0.709-0.997, P = 0.001). Using an optimal SUVmax cut-off of 10.95, the sensitivity and specificity for identifying Edmondson grade III HCC were 66.7% and 100%, respectively. Notably, significant positive correlations were identified in terms of SUVmax, SUVmean, TNR, TBR, and the percentage of Ki67 expression (P < 0.01). Conversely, MTV, TLG, and maximum tumor diameter exhibited no significant association with Ki67 expression (P > 0.05).
18F-FDG PET/CT-derived metabolic parameters, particularly SUVmax, SUVmean, TNR, TBR, and TLG, are valuable in differentiating Edmondson grade II and III HCC, with SUVmax showing the optimal differential diagnostic efficacy. TLG is a three-dimensional volumetric parameter that holds some differential diagnostic potential, but it fails to display a distinct advantage. Moreover, increased 18F-FDG uptake and Ki67 expression in tumor tissue correlate with poorer HCC prognoses, emphasizing their potential role in prognostic assessments.
Core Tip: Fluorine-18-fluorodeoxyglucose (18F-FDG)-positron emission tomography/computed tomography technology has established its indispensable position in numerous aspects of liver cancer diagnosis, staging, lymph node metastasis assessment, and prognosis prediction. The hypermetabolic nature of malignant cells, induced by accelerated glycolysis, increases 18F-FDG uptake. Results have revealed an intimate connection between 18F-FDG uptake and hepatocellular carcinoma differentiation status. This study assessed the diagnostic potential of conventional and three-dimensional metabolic 18F-FDG-positron emission tomography/computed tomography parameters in differentiating Edmondson grade II from grade III hepatocellular carcinoma.
- Citation: Niu XB, Li YP, Chao FF, Mei XL, Han XM, Wang RH. Diagnostic significance of fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography in differentiating Edmondson grade II and III hepatocellular carcinoma. World J Gastrointest Surg 2025; 17(9): 107301
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/107301.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.107301
Hepatocellular carcinoma (HCC), constituting approximately 90% of all primary liver malignancies, establishes itself as the predominant liver cancer type, with its continuously increasing global incidence[1]. The preoperative assessment of HCC prognosis holds crucial importance, with pathological grading serving as a pivotal prognostic determinant affecting patient outcomes[2,3]. Existing research has associated poorly differentiated tumors with an increased propensity for recurrence[4]. Consequently, accurately identifying the pathological grade before surgical intervention is crucial for optimizing treatment strategies and improving prognostic outcomes. Being a cutting-edge radiotracer imaging modality, positron emission tomography (PET)/computed tomography (CT) provides unique advantages by concurrently providing information about both the anatomical architecture of tissues and organs and the metabolic profiles of diverse substances. This technology has already established its indispensability in multiple aspects of liver cancer management, including diagnosis, staging, lymph node metastasis evaluation, and prognosis prediction[5,6]. Among various PET/CT radiotracers, fluorine-18-fluorodeoxyglucose (18F-FDG), which is a glucose analog, is most extensively used for tumor metabolic imaging. The hypermetabolic nature of malignant cells, driven by accelerated glycolysis, increases 18F-FDG uptake. Research findings indicate a strong correlation between 18F-FDG uptake degree in HCC and tumor differentiation status, with poorly differentiated tumors typically demonstrating increased maximum standardized uptake values (SUVmax)[5,7]. In recent years, three-dimensional (3D) metabolic parameters [e.g., total lesion glycolysis (TLG), metabolic tumor volume (MTV)] have gained attention for their tumor behavior prediction capacities. Multiple studies have corroborated their associations with microvascular invasion and prognosis in HCC, with increased MTV and TLG levels indicating increased risks of recurrence and extrahepatic metastasis[8,9]. However, the use of these parameters in distinguishing between different pathological grades of HCC remains underexplored. Originally, Ki67 was deemed an antigen discerned by a monoclonal antibody. However, in essence, Ki67 expression is present across every active cell cycle stage. It serves as a reliable biomarker reflecting cellular multiplication, with its expression elevation associated with greater tumor aggressiveness and malignancy[10]. Some studies have reported no significant differences in overall or tumor-free survival between Edmondson grade I vs grade II HCC or between grade III vs grade IV HCC, but patients with grade III HCC demonstrate markedly worse survival than grade I or II cases[11]. Despite these findings, limited research has focused specifically on differentiating Edmondson grades II and III HCC. This study assesses the diagnostic potential of conventional and 3D metabolic 18F-FDG PET/CT parameters in distinguishing between Edmondson grades II and III HCC. Further, it seeks to investigate the association between these imaging parameters and Ki67 expression, providing information about their combined use in prognostic assessment and therapeutic planning.
This retrospective study enrolled 32 histologically verified solitary HCC cases from March 2021 to April 2023. The patients, who received 18F-FDG PET/CT scans at the First Affiliated Hospital of Zhengzhou University, comprised 26 males and 6 females aged between 28 and 72 (mean: 53.3 ± 10.3) years. Inclusion criteria were patients who underwent 18F-FDG PET/CT examination in our hospital, which were later confirmed as HCC by surgical or biopsy pathological results, and had not received any antineoplastic therapies before the imaging examination. Exclusion criteria: (1) Those with a pathological grade of Edmondson I; (2) Those with active tumors originating from other organs; and (3) Those with incomplete clinical, imaging, and pathological data. Of these, 25 patients underwent curative surgical resection, whereas the remaining 7 underwent percutaneous biopsy. Patients were grouped based on the Edmondson pathological grading system as grades II (n = 17) and III (n = 15)[12]. The predominant grade was documented in cases where intratumorally heterogeneity in grading was observed. Immunohistochemical staining was conducted on all pathological specimens to quantify Ki67 expression levels.
A Siemens Biograph Truepoint 64 PET/CT system and a HM-20 medical cyclotron accelerator of the Sumitomo Group of Japan were used to acquire all PET/CT scans. Fasting for ≥ 6 hours was enforced before imaging, and blood glucose levels were strictly regulated at < 11.1 mmol/L. After ensuring that the patient was in a resting state, 18F-FDG (syn
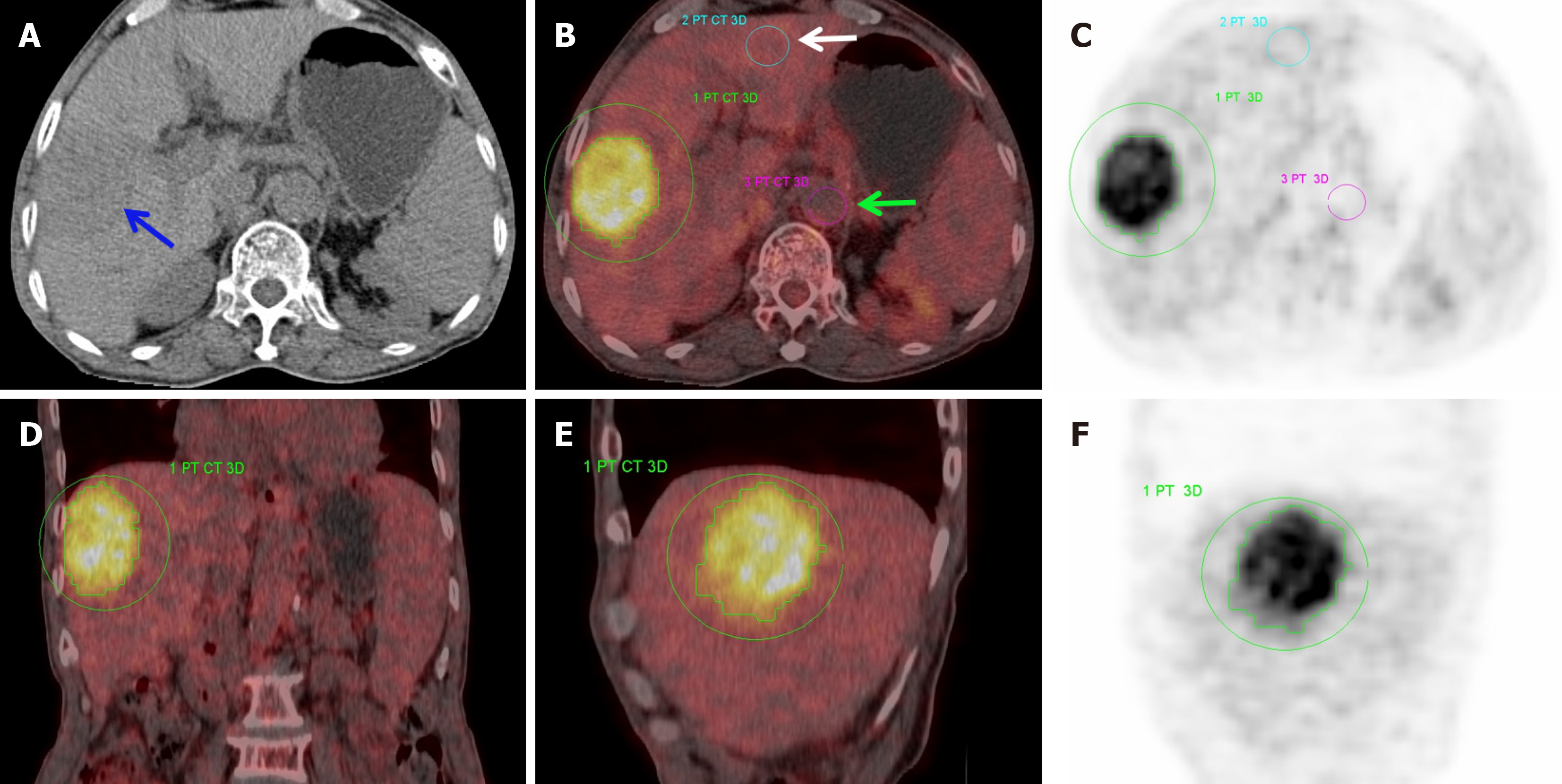
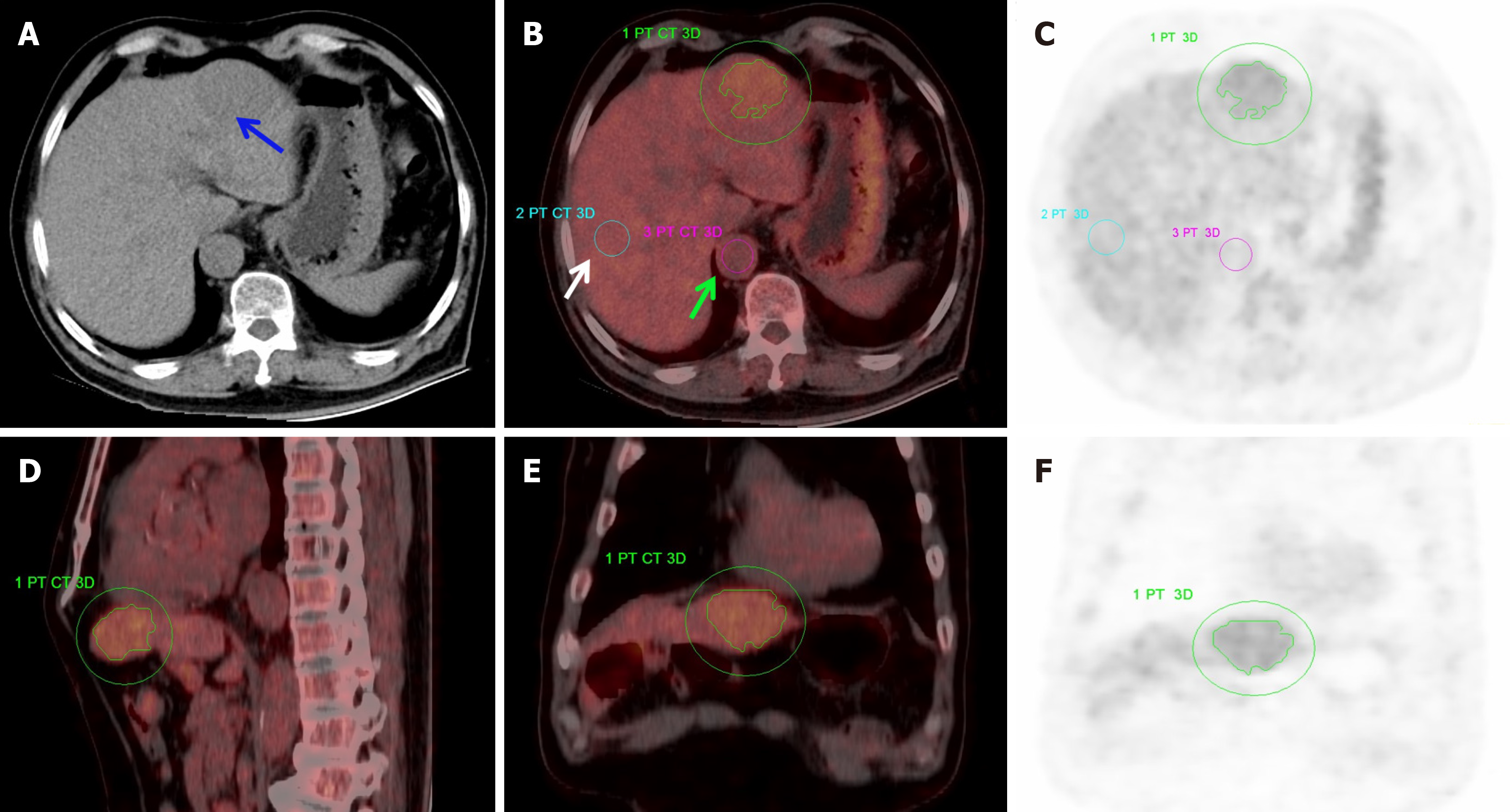
PET and CT datasets were co-registered and fused utilizing the Siemens Syngo TrueD workstation. Two highly experienced nuclear medicine physicians, blinded to clinical and pathological details, independently assessed the original and fused images to determine and localize tumor lesions. Using PET/CT images, the tumor margins were manually outlined to define a 3D volume of interest. Conventional metabolic metrics, such as the SUVmax and mean standardized uptake value (SUVmean), as well as volumetric parameters, including MTV and TLG, were then automatically calculated using the post-processing software. A region of interest measuring 1.5-2.0 cm² was delineated in the normal liver parenchyma of either the right or left lobe. Further, a region of interest of 1.0-1.5 cm² was placed on the abdominal aorta at the corresponding axial level of the lesion to serve as a blood pool reference. The tumor-to-normal background ratio (TNR) was computed as tumor SUVmax divided by normal liver SUVmax, whereas the tumor-to-blood pool ratio (TBR) was derived by dividing the tumor SUVmax by the SUVmax of the corresponding-level blood pool.
Statistical Package for the Social Sciences version 26.0 was used for data analysis. The Shapiro-Wilk test was applied for data distribution normality assessment. Continuous variables following and deviating from normal distribution were reported as mean ± SD and median (interquartile range), respectively. Group comparisons were performed using independent samples t-tests when variables followed a normal distribution and had equal variances; otherwise, the Mann-Whitney U test was employed. Receiver operating characteristic (ROC) curves were used to assess each parameter’s efficacy in distinguishing between Edmondson grades II and III HCC, with discriminative performance demonstrated by the area under the curve (AUC). The Youden’s index was used for optimal cut-off determination. We defined statistical significance as a P-value of < 0.05 (two-tailed). Associations between imaging parameters and Ki67 expression were assessed with Spearman’s rank correlation coefficients.
Statistically significant differences were determined between Edmondson grades II and III HCC groups for several metabolic parameters, including SUVmax, SUVmean, TLG, TNR, and TBR (P < 0.05; Figures 1 and 2). However, MTV differed insignificantly (P = 0.052). Of the study cohort, the median maximum tumor diameter (MTD) was 5.65 cm (interquartile range: 4.40-9.20 cm), and the median Ki67 expression level was 30% (interquartile range: 21.25%-60%). Both the MTD and the Ki67 expression percentage demonstrated marked differences between the Edmondson grade II and III HCC cases (P < 0.05; Table 1).
| Edmondson grade II (n = 17) | Edmondson grade III (n = 15) | Z | P value | |
| SUVmax | 6.43 (4.36-7.73) | 13.79 (9.04-17.99) | 3.399 | 0.001 |
| SUVmean | 3.24 (2.83-4.145) | 6.24 (3.99-6.83) | 3.040 | 0.002 |
| MTV | 31.23 (18.91-120.52) | 94.7 (41.9-280.0) | 1.945 | 0.052 |
| TLG | 107.3 (54.94-384.825) | 590.5 (208.9-1828.0) | 2.776 | 0.006 |
| TNR | 2.18 (1.405-2.605) | 4.76 (2.58-5.62) | 2.964 | 0.003 |
| TBR | 3.18 (2.015-4.18) | 6.27 (2.93-8.64) | 2.663 | 0.008 |
| Maximum tumor diameter (cm) | 5.4 (4.15-7.05) | 6.9 (4.8-11.3) | 1.984 | 0.047 |
| Ki67 expression percentage (mean ± SD) | 26.76% ± 13.22% | 52.3% ± 20.5% | 3.356 | 0.001 |
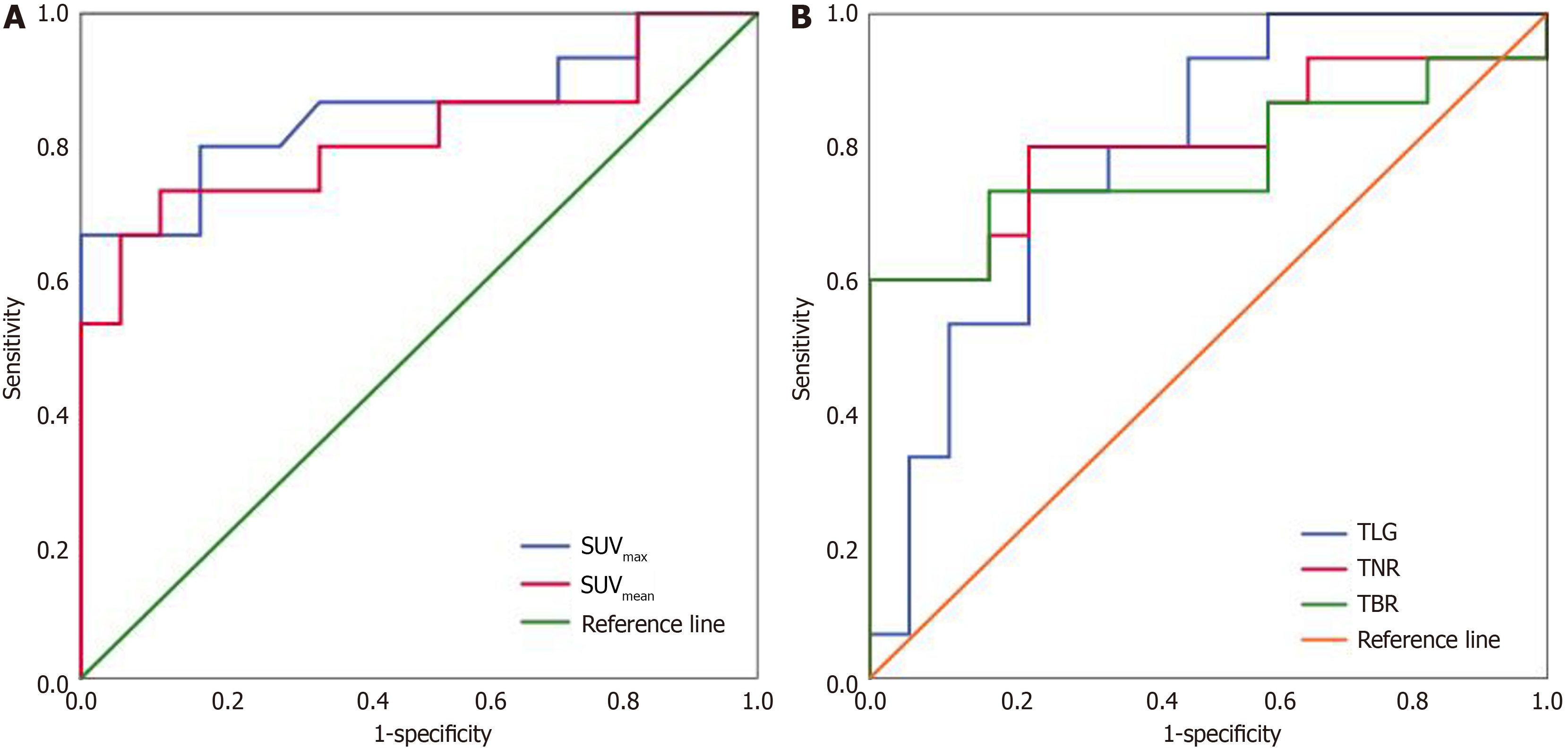
Among the assessed parameters, SUVmax demonstrated the highest discriminative ability, evidenced by an AUC of 0.853 [95% confidence interval (CI): 0.709-0.997, P = 0.001]. The Youden’s index-determined optimal SUVmax cut-off of 10.95 provided 66.7% sensitivity and 100% specificity for identifying Edmondson grade III HCC (Table 2, Figure 3).
| Cutoff | AUC (95%CI) | Sensitivity (%) | Specificity (%) | P value | |
| SUVmax | 10.95 | 0.853 (0.709-0.997) | 66.7 | 100 | 0.001 |
| SUVmean | 4.32 | 0.816 (0.655-0.976) | 73.3 | 88.2 | 0.002 |
| TLG | 248.1 | 0.788 (0.630-0.947) | 71.5 | 76.5 | 0.006 |
| TNR | 2.53 | 0.808 (0.643-0.973) | 80 | 76.5 | 0.003 |
| TBR | 4.46 | 0.776 (0.597-0.956) | 70.2 | 82.4 | 0.008 |
A significant positive correlation was identified between Ki67 expression percentage and several metabolic parameters, including SUVmax, SUVmean, TNR, and TBR, with Spearman’s rank correlation coefficient (rs) of 0.549, 0.658, 0.505, and 0.499, respectively (P = 0.001, < 0.001, 0.003, and 0.004, respectively). Conversely, Ki67 did not correlate strongly with MTV (rs = 0.042, P = 0.820), TLG (rs = 0.245, P = 0.176), or MTD (rs = 0.156, P = 0.394).
HCC represents a prevalent gastrointestinal malignancy in clinical settings, which is associated with high recurrence, metastasis, and mortality[13]. The metabolic behavior of HCC is closely associated with its malignant potential, as poorly differentiated tumors demonstrate reduced glucose-6-phosphatase activity, which decreases dephosphorylation and consequently increases 18F-FDG uptake[14]. This metabolic alteration enables 18F-FDG PET/CT to effectively assess tumor aggressiveness by quantifying the 18F-FDG uptake. Previous studies have consistently revealed that Edmondson grade I + II HCC demonstrates lower 18F-FDG PET/CT positivity rates, along with reduced SUVmax and TNR values, compared with Edmondson grade III + IV tumors[15]. However, limited research has specifically addressed the differentiation between Edmondson grades II and III HCC using 18F-FDG PET/CT. Herein, we analyzed data from patients with solitary HCC and revealed that Edmondson grade III tumors exhibited significantly higher SUVmax, SUVmean, TLG, TNR, and TBR levels compared with grade II tumors (P < 0.05). Notably, this study is among the first to identify TLG, which is a 3D volumetric parameter, as a potential discriminator between grade II and III HCC. However, MTV did not reach significant inter-group differences (P = 0.052). This finding may be affected by limited samples and the inclusion of a patient with grade II HCC presenting a massive tumor (MTD > 10 cm; MTV of 581). This outlier could potentially account for nonsignificant differences in MTV. Noteworthily, the pathological grading in this case was derived from biopsy, which may be subject to sampling bias due to tumor heterogeneity, potentially affecting the grading accuracy. These results align with the findings of Zhang et al[16], who conducted an in-depth 18F-FDG PET/CT-based investigation on moderately and poorly differentiated HCCs, revealing that poorly differentiated HCCs displayed higher SUVmax and TNR levels compared with moderately differentiated tumors. Further, they highlighted a positive correlation, wherein an increase in MTD was accompanied by corresponding SUVmax and TNR elevations. Our study did not perform subgroup analyses based on tumor size due to the limited cohort; however, we revealed a significantly larger MTD in patients with Edmondson grade III HCC compared with grade II cases (P < 0.05). To further assess the diagnostic efficacy of various parameters in distinguishing grade II from grade III HCC, we conducted a ROC curve analysis. Among all parameters assessed, SUVmax provided the strongest discriminative power (AUC = 0.853, 95%CI: 0.709-0.997). When SUVmax was set at an optimal cut-off value of 10.95, the sensitivity for identifying grade III HCC was 66.7%, whereas the specificity reached 100%. Interestingly, the diagnostic performance of 3D volumetric parameters, such as TLG, did not surpass that of conventional metabolic indices such as SUVmax and SUVmean. This indicates that traditional metabolic markers remain highly effective in differentiating HCC grades, whereas volumetric parameters provide additional insights.
The Ki67 protein expression level is intricately related to tumor cell proliferative activity, differentiation, aggressiveness, and patient outcomes[17,18]. Supporting this, Zhang et al[16] reported that HCC cases with high Ki67 expression demonstrated substantially increased SUVmax and TNR values compared with those having low expression, alongside a robust correlation between SUVmax and Ki67 expression levels. In line with these results, our study identified significant positive associations between the Ki67 expression percentage and metabolic parameters such as SUVmax, SUVmean, TNR, and TBR (P < 0.01). However, volumetric parameters, such as MTV or TLG, exhibited no meaningful correlations with Ki67 expression (P > 0.05). These observations reinforce the concept that increased 18F-FDG uptake in HCC indicates poorer tumor differentiation and heightened proliferative activity. The absence of a correlation between MTV/TLG and Ki67 expression may imply that HCCs with low Ki67 expression remain present as large-sized lesions, leading to substantial MTV and TLG. To gain a more comprehensive understanding of this complex association, future investigations that involve multi-center collaborations and large-scale sample sizes are warranted for further exploration.
Our studies highlighted the clinical use of 18F-FDG PET/CT in guiding treatment strategy formulation for HCC, as well as its valuable role in staging and treatment response assessments in certain cancers. A preliminary retrospective study in France demonstrated that 18F-FDG PET/CT detected new HCC lesions in 21% (26/122) of cases, which prompted tumor stage reclassification in 11% (14/122) and treatment plan alteration in 14% (17/122)[18]. Another study revealed that combined 18F-FDG and 18F-fluorocholine (dual tracer) PET-CT revealed new lesions in 14% (39/273) of patients, 12% (32/273) of patients with Barcelona Clinic Liver Cancer advanced stage, and 8% (21/273) of patients had treatment changes[19].
Several limitations should be acknowledged in this study. The first limitation is the single-center design and modest cohort size. Second, pathological data for seven cases were obtained via biopsy, which may introduce sampling bias due to tumor heterogeneity. Further, previous evidence has indicated a potential connection between 18F-FDG PET/CT metabolic measurements and HCC differentiation levels[20]. In hyperdifferentiated HCC, the high gluconeogenesis rate may lead to 18F-FDG PET/CT uptake levels comparable to those of unaffected liver tissue. Therefore, certain hyperdifferentiated HCC lesions can present with low metabolic burden despite high tumor burden on PET, possibly impacting clinical assessments. The accuracy of pathological grading and Ki67 expression quantification could be affected by the specific region of the tumor sampled, thereby potentially affecting the reliability of the results.
The differentiation between Edmondson grade II and grade III HCC involved 18F-FDG PET/CT-derived metabolic parameters, including SUVmax, SUVmean, TNR, TBR, and TLG. Among these, SUVmax appeared as the most effective diagnostic parameter. Meanwhile, TLG, which is a 3D volumetric metric, demonstrated some discriminative capacity. However, it did not surpass the performance of conventional metabolic indices. Importantly, the strong positive correlations between SUVmax, SUVmean, TNR, TBR, and Ki67 expression levels indicate that higher 18F-FDG uptake reflects increased Ki67 expression and greater proliferative activity, which are associated with poorer clinical outcomes in patients with HCC. These results highlight how 18F-FDG PET/CT can help in tailoring treatment strategies and prog
| 1. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1416] [Article Influence: 354.0] [Reference Citation Analysis (1)] |
| 2. | Zhang N, Yang X, Piao M, Xun Z, Wang Y, Ning C, Zhang X, Zhang L, Wang Y, Wang S, Chao J, Lu Z, Yang X, Wang H, Zhao H. Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma. Biomark Res. 2024;12:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 3. | Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK, Bailey HH, Méndez-Sánchez N, Yuen MF, Hwang JP. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2018;38:262-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Lv J, Yin H, Mao W, Shi H. Investigating the value of pre-treatment (18)F-FDG PET/CT in predicting the pathological characteristic of hepatocellular carcinoma and recurrence after liver transplantation. Abdom Radiol (NY). 2021;46:2490-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Zhang J, Jiang S, Li M, Xue H, Zhong X, Li S, Peng H, Liang J, Liu Z, Rao S, Chen H, Cao Z, Gong Y, Chen G, Zhang R, Zhang L. Head-to-head comparison of (18)F-FAPI and (18)F-FDG PET/CT in staging and therapeutic management of hepatocellular carcinoma. Cancer Imaging. 2023;23:106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 6. | Zhang ZY, Cheng HY. [Clinical application of positron emission tomography/computed tomography in hepatocellular carcinoma and related research advances]. Linchuang Gandanbing Zazhi. 2017;33:1365-1368. [DOI] [Full Text] |
| 7. | Sokmen BK, Inan N. 18 F-FDG PET/MRI of Primary Hepatic Malignancies: Differential Diagnosis and Histologic Grading. Curr Med Imaging. 2024;20:e080523216636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Wu F, Cao G, Lu J, Ye S, Tang X. Correlation between 18 F-FDG PET/CT metabolic parameters and microvascular invasion before liver transplantation in patients with hepatocellular carcinoma. Nucl Med Commun. 2024;45:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Jiang C, Ma G, Liu Q, Song S. The value of preoperative 18F-FDG PET metabolic and volumetric parameters in predicting microvascular invasion and postoperative recurrence of hepatocellular carcinoma. Nucl Med Commun. 2022;43:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Remnant L, Kochanova NY, Reid C, Cisneros-Soberanis F, Earnshaw WC. The intrinsically disorderly story of Ki-67. Open Biol. 2021;11:210120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Ning PG, Gao F, Hai JJ, Wu MH, Chen J, Zhu SC, Wang MY, Shi DP. [Prediction of pathological grading of hepatocellular carcinoma based on enhanced CT radiomics]. Zhongguo Yixue Yingxiang Jishu. 2020;36:1051-1056. [DOI] [Full Text] |
| 12. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 13. | Palen A, Grégoire E, Chopinet S, Borentain P, Gerolami R, Hardwigsen J. Liver transplantation for hepatocellular carcinoma after down staging with sorafenib: a monocentric case-matched series. J Gastrointestin Liver Dis. 2020;29:120-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Wang XY, Wang JD, Shi PD. [Relationship between maximum standardized uptake value of 18F-FDG PET/CT and clinicopathological features of hepatocellular carcinoma]. Gandanyi Waike Zazhi. 2019;31:144-148. [DOI] [Full Text] |
| 15. | Liu DJ, Feng YL, Yu FW, Shi HY, He XH, Yuan JW, Wen GH, Huang KM. [Comparative study of 18F-FDG PET and histopathology in hepatocellular carcinoma]. Zhonghua Heyixue Zazhi. 2008;28:307-310. [DOI] [Full Text] |
| 16. | Zhang YQ, Li BL, Yin W, Chen SG, Shi HC. [Correlations between maximum standardized uptake value in 18F-FDG PET/CT and clinical pathological characteristics of moderately to poorly differentiated hepatocellular carcinoma]. Zhongguo Linchuang Yixue. 2018;25:31-34. [DOI] [Full Text] |
| 17. | Lashen AG, Toss MS, Ghannam SF, Makhlouf S, Green A, Mongan NP, Rakha E. Expression, assessment and significance of Ki67 expression in breast cancer: an update. J Clin Pathol. 2023;76:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 18. | Kreipe H, Harbeck N, Christgen M. Clinical validity and clinical utility of Ki67 in early breast cancer. Ther Adv Med Oncol. 2022;14:17588359221122725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 19. | Chiu KWH, Chiang CL, Chan KSK, Hui Y, Ren J, Wei X, Ng KS, Lee HFV, Chia NH, Cheung TT, Chan S, Chan AC, Ng KCK, Seto WKW, Khong PL, Kong FM. Dual-tracer PET/CT in the management of hepatocellular carcinoma. JHEP Rep. 2024;6:101099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Albano D, Calabrò A, Dondi F, Bagnasco S, Tucci A, Bertagna F. The role of baseline 2-[(18) F]-FDG-PET/CT metrics and radiomics features in predicting primary gastric lymphoma diagnosis. Hematol Oncol. 2024;42:e3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/