Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.107297
Revised: April 7, 2025
Accepted: July 22, 2025
Published online: September 27, 2025
Processing time: 188 Days and 19.5 Hours
Gastric adenomyoma (GA) in children is a relatively rare condition, and currently, there is limited knowledge regarding its optimal diagnostic methods and treat
A 4-month-old boy was admitted to the hospital with a 2-month history of re
We report a case of GA in children, successfully treated with laparoscopic sur
Core Tip: The article reported one case of gastric adenomyoma in an infant who was primarily diagnosed with pyloric hypertrophy. We reviewed reported cases and found that gastric adenomyoma was frequently misdiagnosed as other diseases like duplication cyst. Presence of heterogeneous echo in ultrasound and cystic appearance in computed tomography were helpful for differentiating preoperatively. These findings could provide valuable reference for surgeons to improve the preoperative diagnostic accuracy.
- Citation: Zhang J, Sun S, Zheng S, Ma YY, Chen G. Gastric adenomyoma in children: A case report. World J Gastrointest Surg 2025; 17(9): 107297
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/107297.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.107297
Gastric adenomyoma (GA) is rare, with an especially low incidence in pediatric patients, making it a relatively unfamiliar entity in pediatric gastroenterology. Differentiating GA from lesions such as pyloric hypertrophy before surgery can be difficult because clinical manifestations and imaging features of GA often overlap with others. Therefore, GA can be easily misdiagnosed. This article reports a case of infantile GA and thoroughly examined the clinical course, diagnostic work-up, and treatment outcomes. It also provides a detailed literature review with the aim of improving the under
A 4-month-old boy was admitted to the hospital with a 2-month history of recurrent vomiting of gastric contents.
The symptoms of vomiting began 2 months ago.
There is no history of past illness.
There is no personal and family history.
He was in good nutritional status with a weight of 7.3 kg and a height of 66 cm. The child did not present with pallor or cyanosis of the skin and the abdomen was soft, with no palpable mass.
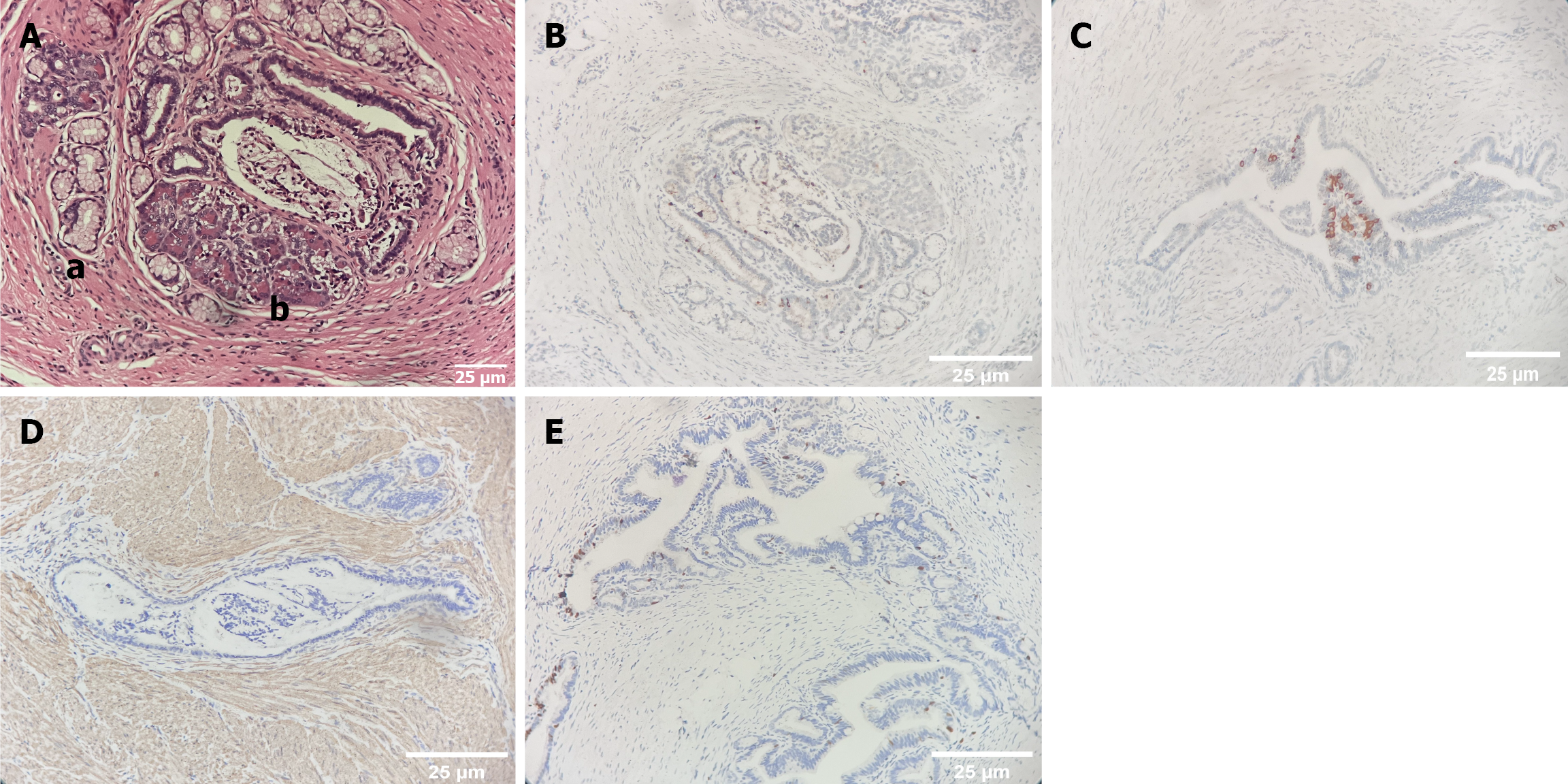
A mass approximately 2.8 cm × 2 cm × 1.5 cm in size and grayish-white in color was detected. An incision was made and a 1.2 cm × 1.2 cm × 0.7 cm cystic cavity was observed. The cystic wall was composed of smooth muscles lined with the gastric mucosal epithelium and a small amount of intestinal epithelium. Scattered glandular epithelium and a small amount of pancreatic tissue were observed in smooth muscle. Immunohistochemistry revealed positivity for gastrin, insulin, and smooth muscle actin, confirming the diagnosis of GA. In addition, the Ki-67 proliferation index was only 5%, indicating that the tumor had low malignant potential.
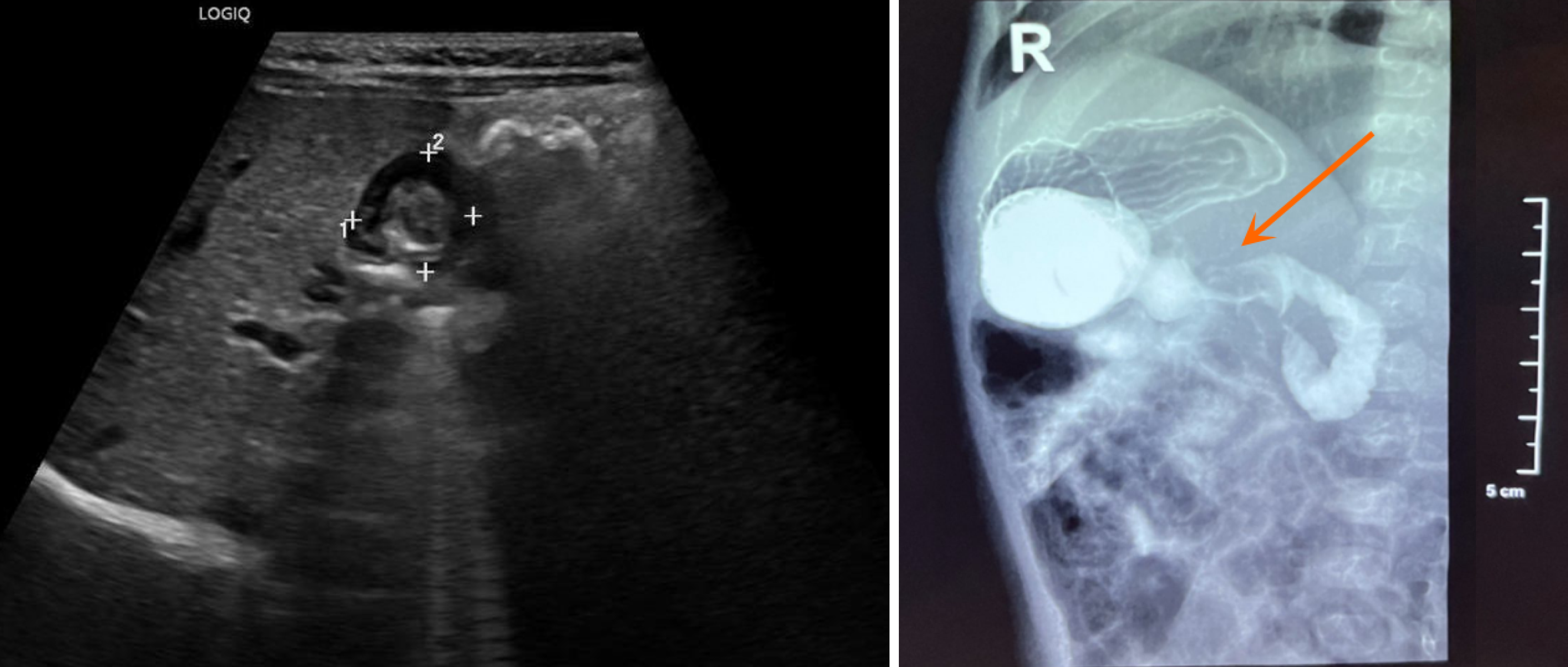
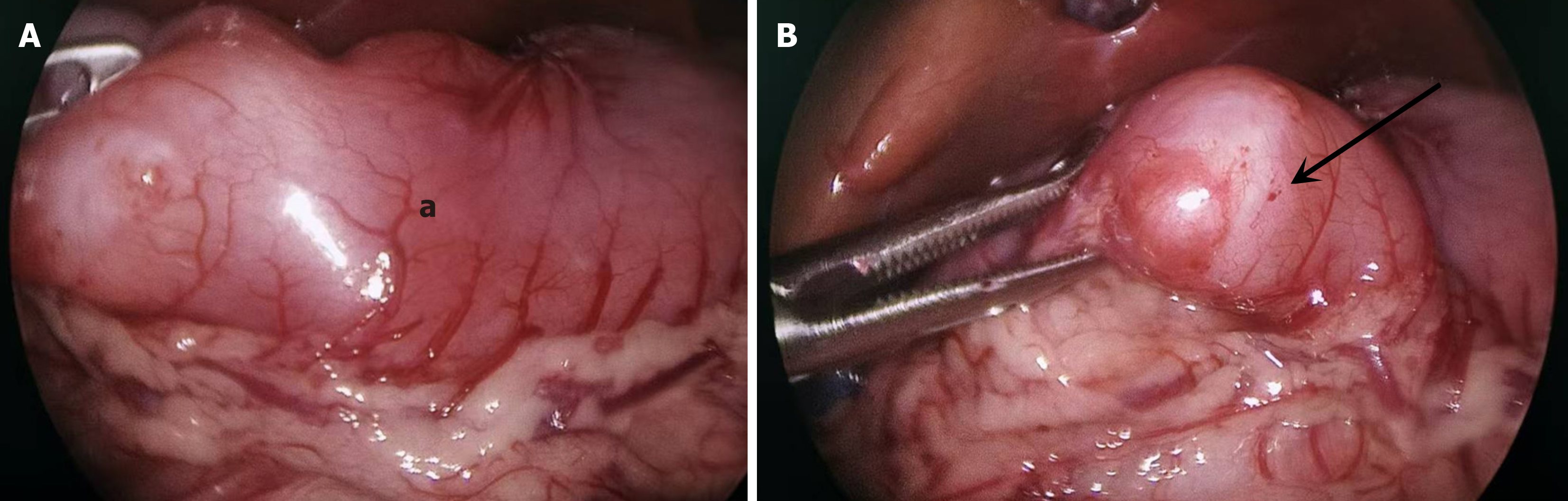
Ultrasound examination demonstrated that the pyloric canal muscle was 3.2 mm in thickness, 14.6 mm in diameter, and 17.6 mm in length. Upper gastrointestinal tract angiography (Figure 1) revealed elongation of the pyloric canal, with slow passage of barium. The preoperative diagnosis was congenital hypertrophic pyloric stenosis. During laparoscopic surgery, a mass was noted on the anterior wall of the pylorus and compression of the mass caused pyloric canal stenosis (Figure 2). Intraoperative frozen section examination suggested the possibility of GA.
The patient’s symptoms, as well as upper gastrointestinal tract angiography and ultrasound, initially suggested a diagnosis of congenital hypertrophic pylorus, which was invalidated during the operation when a mass was observed in the pyloric region. Postoperative pathological examination confirmed the presence of GA. Immunohistochemistry was positive for gastrin, insulin, and smooth muscle actin, confirming the diagnosis of GA. Additionally, the Ki-67 prolife
A pyloric canal resection and gastroduodenal anastomosis were performed.
The diet was gradually resumed 4 days postoperatively. The patient recovered well and was subsequently discharged from the hospital.
GA is a rare benign tumor composed of smooth muscle, duct, and glandular structures. However, controversy exists regarding the pathological nomenclature and classification of GA. Some studies suggest that GA should be classified as type III ectopic pancreas pathologically[1], given that the pathological characteristics of GA are similar to those of type III ectopic pancreas, including glands with clear capsules and corresponding duct structures. Most scholars believe that smooth muscle hyperplasia associated with GA is not a simple reactive change caused by epithelial displacement but a hamartoma containing incompletely differentiated pancreatic tissue components[2]. Therefore, GA is occasionally referred to as adenomyoma, myoepithelial hamartoma, adenomyoma hamartoma, adenomyomatous hamartoma, or adenomyosis. GA and type III ectopic pancreas have been reported to coexist[3].
Currently, some imaging findings can help distinguish GA from other lesions. For instance, GA is often localized to the pylorus on ultrasound and presents with uneven or nodular changes. In the present case reported in this article, ultrasonography revealed that the mass was biased toward the pylorus. The ultrasound image of congenital hypertrophic pyloric stenosis demonstrated a strong echo band (mucosal layer) in the center surrounded by a thickened, uniform, low-echo muscle layer. On computed tomography (CT) scans, GA is characterized by low-density cystic lesions with enhanced diaphragms composed of glandular or fibrous tissue, whereas congenital hypertrophic pyloric stenosis is characterized by soft tissue density around the pylorus[4]. If ectopic gastric mucosa in a gastric duplication is present, the cyst may exhibit an enhanced appearance. However, distinguishing GA from gastric stromal tumors using preoperative imaging is challenging. Endoscopic ultrasound examination allows doctors to view the internal structure of the mass clearly and determine its specific location[5]. Endoscopic ultrasound-guided fine-needle aspiration can be used for early histological diagnosis of masses larger than 2 cm in diameter before surgical resection[6,7]. However, due to the large diameter of the ultrasound endoscope, performing this operation on young infants can be challenging.
The radical cure for GA is surgical resection through the gastroduodenal anastomosis (Billroth I procedure). As lesions in children are usually small, gastroduodenal anastomosis can be performed in a single stage after complete resection of the tumor, which is beneficial for reducing the risk of recurrence. Endoscopic submucosal dissection (ESD) is a surgical technique that has garnered attention over the last two decades. A single-center retrospective study analyzed more than 500 patients who underwent ESD over 7 years and discovered that the postoperative pathology in 15 patients with a mean age of 46 years demonstrated GA[8]. Among them, 14 were located in the gastric submucosa and one in the superficial muscle layer of the gastric wall. The tumor diameter was less than 3 cm, and all tumors were successfully removed without obvious recurrence or metastasis within 1-5 years. We recommend that all patients undergo endoscopic examinations to evaluate the feasibility of ESD. Preoperative endoscopic ultrasound microprobe examination can aid in determining the location of lesions in the gastric wall. However, GA in children may involve the muscular or submucosal layer and due to the thin gastric wall, the adenomyoma tends to be relatively large. Currently, no reports focus on children undergoing ESD for the management of this condition.
GA is generally a benign lesion; however, some studies have suggested that it may be associated with tumors or malignant transformations. Although no cases of recurrence after resection have been reported, documented cases of GA coexisting with gastric adenocarcinoma are available[9-11]. Ng et al[12] reported on an eight-week-old infant with symptoms of intestinal obstruction accompanied by pseudomyxoma peritonei. Therefore, once a child is diagnosed with GA, the scope of the examination should be expanded appropriately.
To further explore patient characteristics, management considerations, and outcome in patients with GA, PubMed and Embase databases were searched using the search term “gastric adenomyoma” until December 2024. All article types published between 1960 and 2024 were included. The language or location was not limited, and each relevant text was thoroughly reviewed and examined. Cases were excluded from the analysis if the full paper was inaccessible, if the article was not a case report or case series, or if the article did not report on pediatric patients. Diagnosis of GA was based on pathological evidence. Relevant information, including author, year of publication, basic case information (number of cases and age), pathology, surgical conditions, and postoperative follow-up, was extracted from the literature. This study did not require approval from an ethics committee.
Nine relevant articles were included, encompassing seven female and two male patients, with a mean age of 34 months (1 week to 15 years). Moreover, the median age in the study was 4 months. Vomiting was the most frequently reported clinical manifestation in seven out of nine cases. One patient presented with the symptom of gastroesophageal reflux. Other non-specific symptoms included abdominal pain, fever, poor food intake, and weight loss. Preoperative physical examinations and laboratory tests revealed no abnormal results. Ultrasound was the principal diagnostic imaging modality and revealed the presence of a heterogeneous mass or cystic lesion within the pyloric wall in eight cases. Regarding upper gastrointestinal tract angiography, two patients exhibited gastric outlet obstruction, and one patient suggested gastroesophageal reflux. CT scans (2 out of 9) detected cystic masses in the pylorus, but in one case, the magnetic resonance findings demonstrated inflammatory myofibroblasts. Considering age, symptoms, and auxiliary examinations, the preoperative diagnoses were congenital pyloric hypertrophy (3/9), gastric duplication cyst (3/9), pyloric stenosis (2/9), and gastroesophageal reflux (1/9). Notably, none of the preoperative and postoperative diagnoses were consistent. Surgical exploration was performed in all nine cases. During surgery, masses with an average diameter of 19 mm were detected in the gastric pylorus. The surgical procedures adopted were partial pylorectomy in one case, distal gastrectomy combined with gastroduodenostomy in three cases, lesion resection (unspecified surgical details) in three cases, mass dissection and patch repair in one case, and pyloric junction resection in one case. Pathological exa
To date, no more than 60 cases of GA have been reported in children or adults[1]. The age of onset ranged from 1 week to 82 years, with the main age group being 40-60 years old[5]. Approximately 90% of GA cases occur in the gastric antrum[13]. The clinical manifestations of GA depend on the location, extent, and depth of the lesion infiltration. GA in adults usually presents with nonspecific clinical symptoms, including nausea, vomiting, abdominal pain, with occasional hematemesis, melena, and even secondary perforation peritonitis[14,15]. In pediatric cases, vomiting is the main clinical symptom (Table 1), which is often misdiagnosed as other conditions that may cause gastric outlet obstruction: Hyper
| Ref. | Gender | Age | Symptoms | Imaging | Preoperative diagnosis | Surgery |
| Moiseenko[9], 1964 | Female | 5 months old | Esophageal reflux | Barium swallow indicated esophageal reflux | Esophageal reflux | Nissen fundoplication and mass excision |
| Takeyama et al[16], 2007 | Female | 1 month old | Intermittent vomiting free of bile of one week | US demonstrated heterogeneous intramural mass of the pyloric region | Hypertrophic pyloric stenosis | Lesion excision |
| Min et al[2], 2012 | Female | 5 years old | Intermittent epigastric pain, vomiting, poor oral intake, irritability, and fever | US and CT demonstrated a thick-walled cystic lesion; UGI showed an extrinsic mass with a lobulated contour without evidence of perforation | Gastric duplication cyst | Distal gastrectomy with gastroduodenostomy |
| Castain and Rullier[10], 2012 | Female | 4 months old | Vomiting, weight loss, and abdominal pain | US showed a non-circumscribed heterogeneous mass of the pyloric wall with microcystic features | Hypertrophic pyloric stenosis | Pyloric junction removal |
| Rhim et al[17], 2013 | Male | 1 week old | Persistent vomiting | US showed asymmetrical submucosal thickening of the anterior wall of the pylorus and the lesion protruded into both luminal and serosal sides; UGI showed elongation and narrowing of the pyloric canal and shouldering of the antrum | Gastric outlet obstruction | Billroth I anastomosis |
| Aljahdali et al[11], 2012 | Male | 13 days old | Progressive nonbilious vomiting | US demonstrated a hyperechoic intramural pyloric mass | Pyloric stenosis | Mass dissection and Graham patch repair |
| Oviedo Gutiérrez et al[19], 2015 | Female | 49 days old | Nonbilious vomiting of 48 hours | US showed a nonobstructive nodular lesion in the anterior pyloric wall; MRI suggested a myofibroblastic tumor | Hypertrophic pyloric stenosis | Complete resection of the pyloric tumor |
| Arslan et al[20], 2018 | Female | 5 years old | Abdominal pain, poor oral intake, and fever | US showed an intramural hypoechoic heterogeneous nodular mass; CT revealed a cystic lesion | Gastric duplication cyst | Mass excision, double-layer transverse anastomosis |
| Kamrani et al[18], 2019 | Female | 15 years old | Nausea and vomiting over a period of two years | CT showed a circumferential prepyloric mass; EGD showed the distal antrum and proximal pylorus contained a frond-like circumferential nearly obstructing submucosal mass | Gastric duplication cyst | Distal gastrectomy with gastroduodenostomy |
Children with GA mainly present with vomiting symptoms similar to those in congenital hypertrophic pyloric stenosis or gastric duplication. Ultrasound and CT can detect low-density septal lesions in the pylorus, which are biased toward one side, and may be helpful in diagnosis. Currently, radical resection is the preferred treatment modality in children.
| 1. | Sakurai Y, Togasaki K, Nakamura Y, Fukuda H, Karaki H, Okaya T, Hirai F, Abe M, Sugano I. Gastric type III heterotopic pancreas presenting as adenomyoma in the antrum of the stomach: a case report. Clin J Gastroenterol. 2024;17:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Min SH, Kim HY, Kim SH, Jung SE, Park KW, Kim WS, Park SH. Gastric adenomyoma mimicking gastric duplication cyst in a 5-year-old girl. J Pediatr Surg. 2012;47:1019-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Erberich H, Handt S, Mittermayer C, Tietze L. Simultaneous appearance of an adenomyoma and pancreatic heterotopia of the stomach. Virchows Arch. 2000;436:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Maniatis V, Chatjilira T, Vardaki E, Kavadias S. Gastric adenomyoma: CT imaging. Eur J Radiol Extra. 2004;50:17-20. [DOI] [Full Text] |
| 5. | Duran Álvarez MA, Gómez López JR, Guerra Garijo T. Gastric Adenomyoma: The Unexpected Mimicker. GE Port J Gastroenterol. 2017;24:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (9)] |
| 7. | Jacobson BC, Bhatt A, Greer KB, Lee LS, Park WG, Sauer BG, Shami VM. ACG Clinical Guideline: Diagnosis and Management of Gastrointestinal Subepithelial Lesions. Am J Gastroenterol. 2023;118:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Wang S, Cao H, Zhang Y, Xu M, Chen X, Piao M, Wang B. Endoscopic submucosal dissection for gastric adenomyoma: A rare entity of 15 cases among 571 patients with gastric submucosal eminence lesions. Medicine (Baltimore). 2017;96:e6233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Moiseenko MD. [Adenomyoma of the Stomach Related to a Dystopic Rudiment of the Pancreas Associated with Gastric Ulcer (Observations on a Case)]. Vopr Onkol. 1964;10:93-95. [PubMed] |
| 10. | Castain C, Rullier A. Pyloric adenomyoma: a rare cause of gastric outlet obstruction in childhood. Diagn Histopathol. 2012;18:511-513. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Aljahdali A, Oviedo A, Blair GK. Gastric hamartoma of the pylorus in an infant. J Pediatr Surg. 2012;47:E29-E31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Ng WC, Yeoh SC, Joseph VT, Ong BH. Adenomyoma of the pylorus presenting as intestinal obstruction with pseudomyxoma peritonei--a case report. Ann Acad Med Singap. 1981;10:562-565. [PubMed] |
| 13. | Yie M, Jang KM, Kim MJ, Lee IJ, Yang DH, Jun S, Min K. Synchronous Ectopic Pancreases in the Cardia and Antrum of the Stomach: A Case Report. J Korean Soc Radiol. 2010;63:161. [DOI] [Full Text] |
| 14. | Zhu HN, Yu JP, Luo J, Jiang YH, Li JQ, Sun WY. Gastric adenomyoma presenting as melena: a case report and literature review. World J Gastroenterol. 2010;16:1934-1936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Kagawa S, Fujiwara T, Nishizaki M, Naomoto Y, Hiroshi I, Tanaka N. Adenomyoma of the stomach presenting as localized peritonitis. Dig Dis Sci. 2007;52:3184-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Takeyama J, Sato T, Tanaka H, Nio M. Adenomyoma of the stomach mimicking infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2007;42:E11-E12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Rhim JH, Kim WS, Choi YH, Cheon JE, Park SH. Radiological findings of gastric adenomyoma in a neonate presenting with gastric outlet obstruction. Pediatr Radiol. 2013;43:628-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kamrani K, Cutler J, Austin C, Hudacko R, Bhattacharyya N. Adenomyoma causing gastric outlet obstruction. J Pediatr Surg Case Rep. 2019;42:51-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Oviedo Gutiérrez M, Amat Valero S, Gómez Farpón A, Montalvo Ávalos C, Fernández García L, Lara Cárdenas DC, Barnes Marañón S, Granell Suárez C, Vega Mata N, López López AJ, González Guerrero M, Álvarez Muñoz V. [Infantile hypertrofic pyloric stenosis or gastric adenomyoma? Differential diagnosis of gastric outlet obstruction in children]. Cir Pediatr. 2015;28:153-155. [PubMed] |
| 20. | Arslan EE, Demir TA, Güney LH, Tepeoğlu M, Akıllı MS, Hiçsönmez A. A rare case of a gastric adenomyoma mimicking a gastric duplication cyst. Turk J Gastroenterol. 2018;29:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/