Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.112887
Revised: October 2, 2025
Accepted: October 29, 2025
Published online: December 27, 2025
Processing time: 139 Days and 14.9 Hours
A literature review revealed that intraductal papillary mucinous neoplasm of the biliary tract (IPMN-B) cases with characteristic clinical, imaging, endoscopic, and pathological features are exceedingly rare. Herein, we present a case of typical IPMN-B with malignant transformation that required 4 years for a definitive diagnosis, to enhance the understanding of this disease entity.
A 67-year-old male patient was referred to our hospital due to abdominal pain and jaundice. Four years before this admission, a cystic lesion and left hepatolithiasis were incidentally discovered. Laboratory tests revealed mild increases in serum white blood cell count, total bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma-glutamyl transferase. Tumour marker levels were within normal limits. Imaging findings demonstrated an enlargement of the cystic tumour from 41 mm to 63 mm, along with the deve
Heightened vigilance and recognition of IPMN-B are essential when “hepatic cysts” or “biliary stones” are inciden
Core Tip: Owing to the absence of specific clinical and imaging manifestations, intraductal papillary mucinous neoplasm of the biliary tract (IPMN-B) is frequently missed or misdiagnosed when incidentally discovered. Here, we present a case of IPMN-B that exhibited characteristic clinical, imaging, endoscopic, and pathological features and progressed to malignancy. This case illustrates the 4-year latent progression of IPMN-B from an initially 'benign' cystic lesion to a malignant neoplasm, emphasizing the importance of continuous monitoring for nonspecific imaging findings.
- Citation: Gu TY, Wang SH, Yang SY, Zhao SQ, Chen MJ, Li XP, Song ZW, Gu XX, Chen F. Intraductal papillary neoplasm of the biliary tract with typical clinicopathological, endoscopic features: A case report. World J Gastrointest Surg 2025; 17(12): 112887
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/112887.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.112887
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare tumour of the biliary system. In 2010, the World Health Organization officially classified IPNB as a distinct type of digestive system tumour, defining it as the growth of papillary tumours within and outside the bile ducts, typically with multifocal growth. IPNB can be divided into intraductal papillary mucinous neoplasm of the biliary tract (IPMN-B) and IPNB without mucin secretion (IPNB-NM) according to mucus secretion status, with the former accounting for 28%-37% of IPNB cases[1]. Owing to the absence of specific clinical and imaging manifestations, IPMN-B is frequently missed or misdiagnosed when incidentally discovered, often leading to missed treatment opportunities for optimal care. A literature review revealed that IPMN-B with typical clinical, imaging, endoscopic and pathological features is extremely rare. In this study, we report a case of characteristic IPMN-B that progressed to malignancy but required 4 years for a definitive diagnosis, intending to enhance the understanding of this disease entity.
A 67-year-old male patient was admitted to the hospital due to upper abdominal pain and jaundice.
Four years before his admission to the hospital, a cystic lesion and left hepatolithiasis were discovered by accident. After enhanced computed tomography (CT) and magnetic resonance (MR) imaging (MRI) were performed, surgical treatment was recommended, but the patient refused treatment and was lost to follow-up.
The patient had a history of hypertension, prostatic hyperplasia, and carotid plaque, which were all controlled with drugs, including 5 mg of amlodipine besylate daily, 2 mg of terazosin daily, 5 mg of rosuvastatin daily, and 100 mg of aspirin daily.
There was no family history of cancer or genetic diseases.
Specialist examination at admission showed stable vital signs, scleral icterus, mild tenderness in the upper abdomen, no rebound tenderness, no guarding, and negative percussion pain in the liver region.
At admission, a routine high-sensitivity blood test revealed a C-reactive protein concentration of 74.38 mg/L and a white blood cell count of 13.35 × 109/L. Liver function tests revealed a total bilirubin (TB) level of 175.3 μmol/L, an alanine aminotransferase (ALT) level of 184 U/L, an aspartate aminotransferase (AST) level of 135 U/L, an alkaline phosphatase (ALP) level of 724 U/L, and a gamma-glutamyl transferase (GGT) level of 156 U/L. The levels of tumour markers such as alpha-fetoprotein, des-gamma-carboxy prothrombin, carbohydrate antigen 19-9 (CA 19-9), and carcinoembryonic antigen (CEA) were within normal limits. Antibodies against hepatitis B surface antigen and hepatitis C virus were both negative.
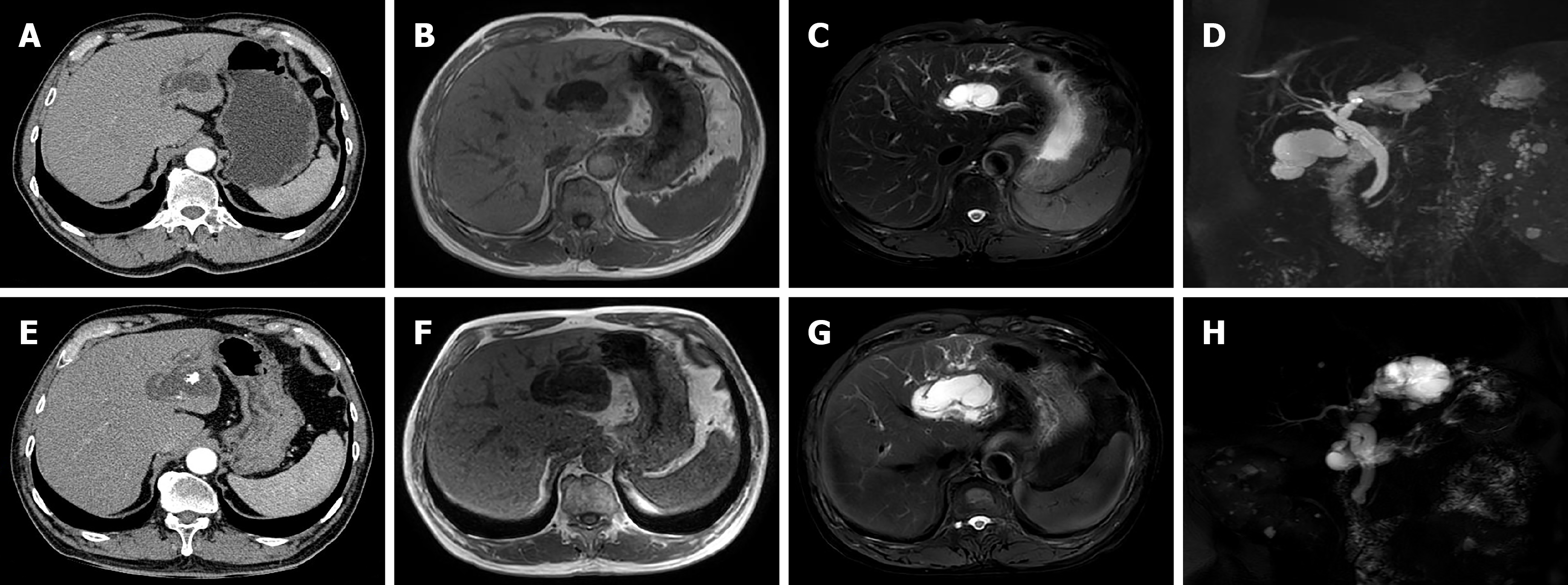
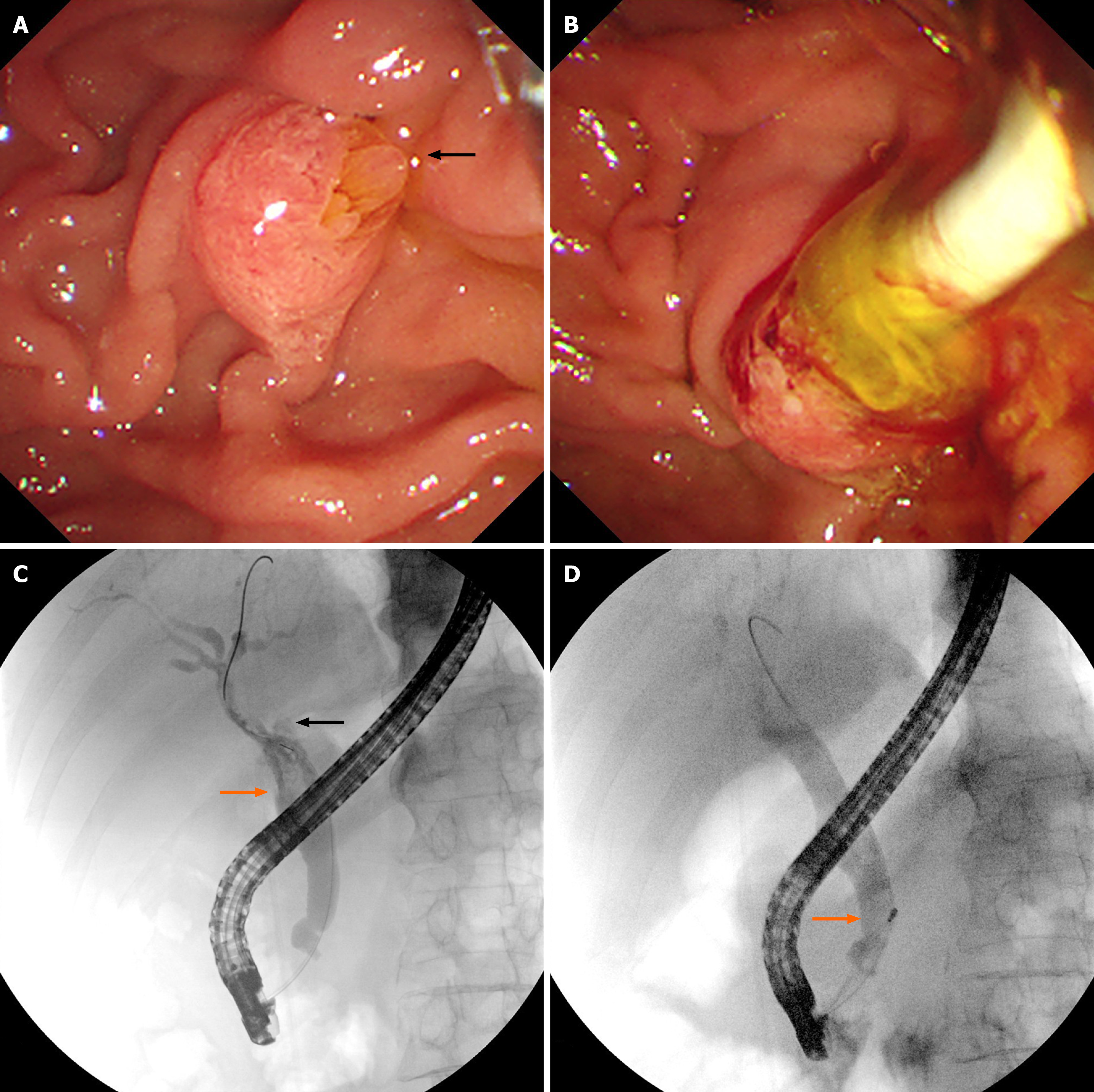
Abdominal ultrasound revealed dilatation of the common bile duct with multiple stones, dilatation of the left intrahepatic bile duct with multiple stones, adenomyosis of the gallbladder, and multiple crystals in the gallbladder. Enhanced CT images captured four years prior to admission revealed cystic dilatation of the left intrahepatic bile duct, with a size of approximately 41 mm × 22 mm, stones in the left intrahepatic bile duct, and adenomyosis at the fundus of the gallbladder (Figure 1A). MR images revealed dilatation of the left intrahepatic bile duct, local cystic dilatation, and stones in the left intrahepatic bile duct (Figure 1B-D). After admission, enhanced CT images captured in the arterial phase revealed cystic dilatation of the left hepatic duct, with a size of approximately 63 mm × 51 mm, numerous papillary nodules, stones in the left intrahepatic bile duct, and common bile duct dilatation (Figure 1E). MR images revealed cystic dilatation of the left hepatic duct with papillary nodules, stones in the left intrahepatic bile duct, common bile duct dilatation, and stones in the common bile duct (Figure 1F-H). Endoscopic retrograde cholangiopancreatography (ERCP) revealed that the duodenal papilla was enlarged and protruded into the intestinal cavity. However, there was no surface erosion and no visible tumour. The papillary orifice was significantly dilated, with a fish-mouth appearance. A large amount of translucent and jelly-like mucus was continuously oozing from the duodenal papilla. Cholangiography revealed significant dilatation of the common bile duct and multiple cloudy, irregular filling defects in the extrahepatic bile duct. During the clearing of the bile duct with the balloon catheter, the morphology and location of the filling defect in the bile duct were altered, and a large amount of translucent jelly-like mucus containing bile flowed out from the swollen duodenal papilla (Figure 2). The equipment we used was video duodenoscope (Olympus, JF-260V) and C-arm machine (GE HealthCare, OEC Elite). The wire-guided cannulation technique was selected. For this patient with IPMN-B, the papillary orifice was relatively large, making cannulation not particularly challenging. And during ERCP, a bile duct stent was placed to minimize post-ERCP cholangitis risk.
After a multidisciplinary team (MDT) discussion, the patient was diagnosed with IPMN-B, and surgery was recom
The patient was ultimately diagnosed with IPMN-B.
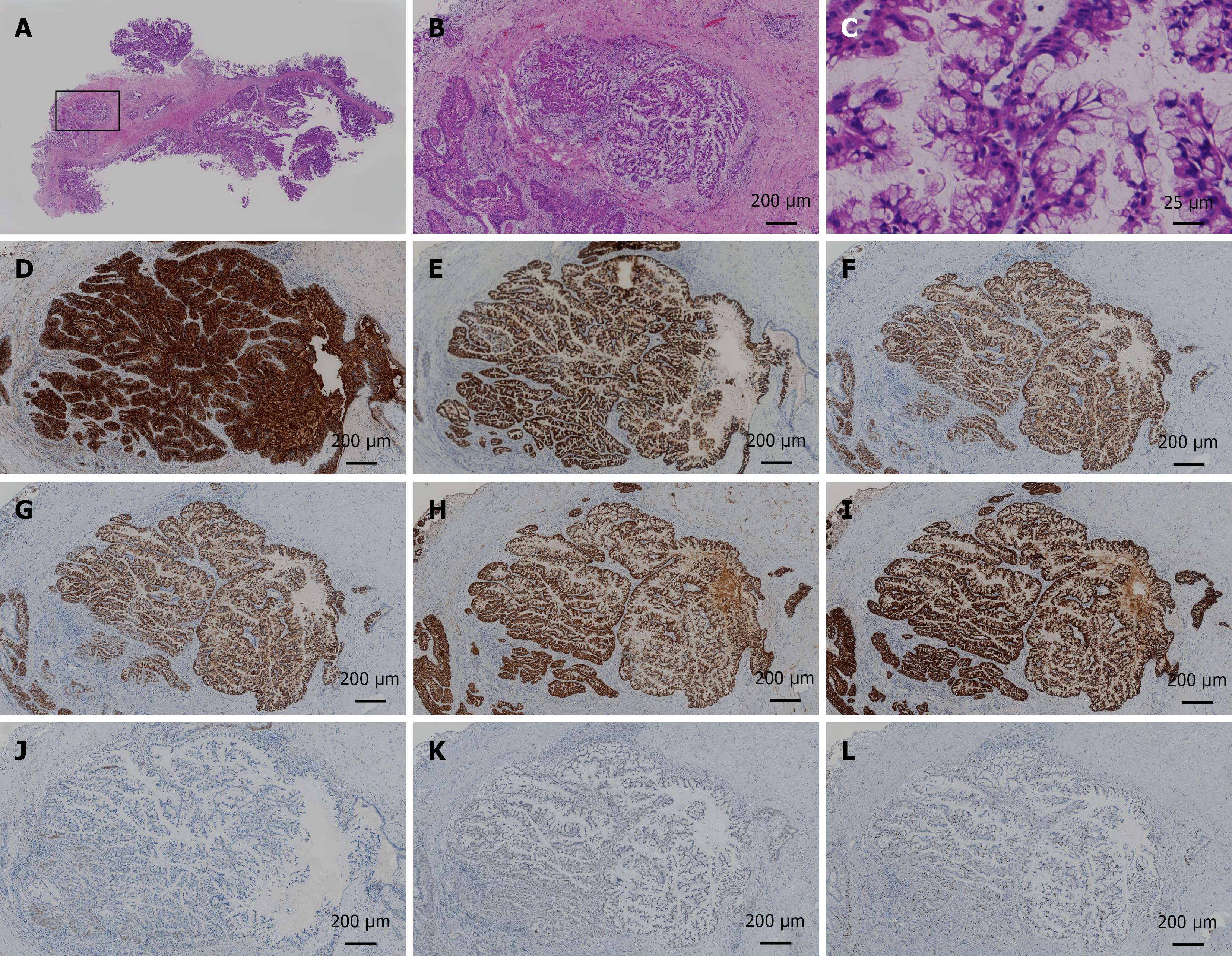
The patient underwent left hepatectomy and gallbladder resection. Intraoperative choledochoscopy revealed no lesions in the common bile duct or right hepatic duct. Rapid intraoperative pathological diagnosis revealed IPMN-B with high-grade intraepithelial neoplasia, and the surgical margin was negative. The operative time was 211 minutes. The surgical blood loss volume was 50 mL, and the operation went smoothly. Macroscopic examination of the resected liver sample revealed a cystic tumour that connected with the bile duct and contained a large amount of mucus, along with a granular tumour and stones (Figure 3). Postoperative pathological examination revealed that the tumour was IPMN-B with high-grade intraepithelial neoplasia and cancer, and the surgical margin was negative. The size was 5.0 cm × 3.0 cm × 2.5 cm, with high–moderate differentiation, nerve invasion (-), bile duct invasion (-), and vascular tumour thrombus (-). Immunohistochemical staining revealed that the tumour cells were positive for CK7, CK19, MUC1, MUC 2, MUC5AC, and MUC6; approximately 20% positive for Ki-67, negative for CK20, and wild-type for P53 (Figure 4).
The patient was discharged from the hospital 21 days after surgery. A follow-up examination was performed 9 months after surgery, and there was no recurrence or metastasis.
The pathogenesis of IPNB remains unclear. IPNB is likely to occur in East Asian countries such as China, Japan, and South Korea, and its occurrence is related to the prevalence of hepatic bile duct stones and Clonorchis sinensis infection in local areas. It also occurs sporadically in Western countries, indicating that genetic and environmental risk factors are also associated with its incidence[2-4]. Our IPMN-B patient had risk factors for hepatic bile duct stones. Some studies have shown that the clinical features, imaging and pathological manifestations of IPMN-B and IPNB-NM are completely different and that their diagnoses and treatments differ[1,5]. Moreover, IPMN-B is considered the biliary counterpart of IPMN-P because the two show striking similarities in terms of clinical, morphological, immune phenotype and biological findings[6-8]. Takanami et al[9] divided IPMN-B into ductal, cystic and intermediate types, which are similar to the main duct, branch ducts and mixed types of IPMN-P, respectively.
Patients with IPMN-B are typically asymptomatic initially. In the later stages, the most common presenting symptoms include abdominal pain, recurrent acute cholangitis, and obstructive jaundice. Recurrent cholangitis, resulting from the necrotic shedding of tumour tissue and recurrent bile duct obstruction due to mucin hypersecretion, represents a relatively frequent clinical manifestation. Our reported cases also exhibited these characteristic presentations.
In terms of liver function, IPMN-B patients may have elevated TB, ALT, AST, ALP, and GGT levels. Recurrent cholangitis causes repeated episodes of liver function abnormalities, which may hold diagnostic significance for IPMN-B. In terms of tumour markers, some studies report increased serum levels of CA 19-9 and CEA in 42%[10] and 25%[11] of patients, respectively. However, the levels of these markers may be confounded by concurrent biliary obstruction and cholangitis, limiting their diagnostic utility for IPMN-B. Consistent with this limitation, our patient presented with normal tumour marker levels, yet the final diagnosis was IPMN-B with associated malignancy.
Ultrasound examination, which is economical and convenient, is a suitable modality for tumour screening. It may detect bile duct dilatation, intrahepatic cystic masses, and intraluminal polypoid lesions suggestive of IPMN-B. However, these findings are frequently misinterpreted as “hepatic cysts” or “bile duct stones”. Consequently, further evaluation by CT and MRI is warranted for the identification of such abnormalities. Based on morphological criteria, the literature classifies IPNB into seven subtypes[5]: (1) Upstream-ductatic IPNB; (2) Typical IPNB; (3) Superficial-spreading IPNB; (4) No-mass-forming IPNB; (5) Intrahepatic-cystic IPNB; (6) Extrahepatic-cystic IPNB; and (7) Infiltrating IPNB. Both CT and MRI are noninvasive imaging techniques. These techniques can delineate the location and extent of bile duct stenosis or dilatation, the communication between cystic lesions and the biliary tree, and mural nodules. Notably, neither modality reliably detects mucin secretion. This limitation poses significant challenges in preoperative imaging assessment for IPMN-B. Our patient exhibited the characteristic type V, demonstrating simultaneous dilatation of both the proximal and distal bile duct—a finding of diagnostic importance. Nevertheless, insufficient radiologist familiarity with IPMN-B imaging features remains a barrier to accurate diagnosis.
Cholangiography enables dynamic observation, and its diagnostic significance is substantial, particularly during the initial contrast study, which has the highest diagnostic value. Both ERCP and percutaneous transhepatic cholangiography (PTC) can reveal the following findings: (1) Localized bile duct strictures; (2) Segmental or diffuse biliary duct dilation; (3) Intrahepatic cystic lesions communicating with the biliary tree; and (4) Multiple amorphous, cloud-like irregular filling defects within the bile duct[12]. ERCP offers an additional advantage over PTC by allowing direct endoscopic visualization of characteristic features, such as a fish mouth-shaped, enlarged duodenal papilla and the extrusion of translucent mucin[13]. In addition, cholangiography certainly has inherent limitations. It cannot reliably distinguish bile duct stones from benign tumours and fails to delineate the precise location of small tumours, the depth of tumour invasion, or the degree of lymph node involvement. These limitations can be effectively addressed by endoscopic ultrasound (EUS) and intraductal ultrasonography (IDUS). Furthermore, peroral cholangioscopy (POCS) enables direct visualization of biliary mucosal lesions, which provides the possibility to obtain preoperative cytological specimens and tissue samples[14]. This technique is able to diagnose early-stage, neoplastic bile duct lesions[15]. Choledochoscopy using narrow-band imaging may be helpful for the observation of fine mucosal structures, resulting in diagnosis of tumor spread in IPMN-B patients[16]. Confocal laser endomicroscopy enables in vivo histological evaluation using fluorescent pigment to diagnostically differentiate between benign and malignant biliary disease[17]. As these techniques become more widely available and the survival benefits are established. In our case, ERCP demonstrated characteristic findings of IPMN-B. A notable limitation was substantial mucin obstruction in the left hepatic duct, thereby precluding adequate visualization of the dilated duct and the intrahepatic cystic lesion communicating with it. Thereby, if conditions permit, the use of POCS in the future may help overcome this limitation.
When IPMN-B is diagnosed, surgical intervention is indicated as the sole radical curative approach. The selection of surgical modality depends on lesion location and extent, with options including hepatectomy, extrahepatic bile duct resection, liver transplantation, and pancreaticoduodenectomy. R0 resection rates reach 90% in reported cases[18]. Notably, IPMN-B originating from the biliary epithelium frequently manifests with multifocal or diffuse involvement, and the actual extent of the lesion usually exceeds the boundaries delineated by imaging modalities such as CT and MRI[19]. Therefore, systematic choledochoscopy was performed intraoperatively to exclude concomitant biliary tract lesions beyond the identified left hepatic duct involvement. For patients with unresectable tumour or prohibitive surgical risk, conservative management may be considered, such as ERCP-guided radiofrequency ablation[20], photodynamic therapy[21], and endoscopic retrograde biliary drainage[22].
With respect to the pathological classification of IPMN-B, based on morphological and mucin characteristics, some scholars have divided IPMN-B into four subtypes: Pancreaticobiliary type, intestinal type, gastric type and oncocytic type[2]. Similar to pancreatic cancer, IPMN-B and bile duct intraepithelial neoplasia (BilIN) are considered the two principal types of intraductal precursor lesions associated with the development of invasive cholangiocarcinoma[1,18]. Notably, our patient manifested all three pathological entities: IPMN-B, BilIN, and invasive carcinoma. This significant cytoarchitectural heterogeneity across different regions of an individual tumour is a key pathological feature of IPMN-B. Gross examination of the resected specimens revealed polypoid or villous tumour growth with associated intraductal mucin accumulation. Histologically, the intestinal subtype predominated and coexisted with other subtype components. Immunohistochemistry revealed that almost all the IPMN-B samples were positive for MUC2 expression and negative for P53 expression[1]. Additionally, MUC1 expression is rare, whereas MUC5AC and MUC6 are expressed in the majority of cases. An elevated Ki67 proliferation index is also observed. These characteristic pathological features were recapitulated in our patient. In terms of prognosis, the 5-year survival rates were 53.6% for all IPNB patients and 22.2% for those with invasive carcinoma[11]. The overall recurrence rate among IPNB patients is 13%-29%, increasing to 47%-62% in those with invasive carcinoma[18]. Compared with IPNB-NM, IPMN-B is associated with superior survival outcomes[1]. The factors associated with a poor prognosis include MUC1 overexpression, CK20 positivity, positive resection margins, lymph node metastasis, and involvement of multiple tumours[18,23].
Fortunately, with advances in imaging and endoscopic techniques (such as EUS, IDUS, and POCS), the early diagnosis rate of IPMN-B is expected to improve further in the future. Our ambitious goal is to control this disease before it becomes malignant, thereby improving outcomes. Furthermore, the development of a Kyotostyle classification system for IPMNB is considered to be of great potential value in the future. Such a framework could stratify patients according to risk and guide both follow-up strategies and surgical decision-making, thereby improving clinical management.
In summary, we report a case of IPMN-B with characteristic presentations and malignant transformation. While MDT management is increasingly recognized as essential for cancer patients, the initial diagnostic physician must have the ability to accurately differentiate IPMN-B from “hepatic cysts” or “biliary stones” before initiating MDT. Consequently, proficiency in diagnosing IPMN-B is essential for physicians across hepatobiliary surgery, gastroenterology, medical oncology, radiology, and pathology.
We sincerely appreciate the potential editors and reviewers for their succinct comments on improving this manuscript.
| 1. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K, Takano S, Kondo Y, Miyazaki M. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Kubota K, Jang JY, Nakanuma Y, Jang KT, Haruyama Y, Fukushima N, Furukawa T, Hong SM, Sakuraoka Y, Kim H, Matsumoto T, Lee KB, Zen Y, Kim J, Miyazaki M, Choi DW, Heo JS, Endo I, Hwang S, Nakamura M, Han HS, Uemoto S, Park SJ, Hong EK, Nanashima A, Kim DS, Kim JY, Ohta T, Kang KJ, Fukumoto T, Nah YW, Seo HI, Inui K, Yoon DS, Unno M. Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: a Japan-Korea collaborative study. J Hepatobiliary Pancreat Sci. 2020;27:581-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Mocchegiani F, Vincenzi P, Conte G, Nicolini D, Rossi R, Cacciaguerra AB, Vivarelli M. Intraductal papillary neoplasm of the bile duct: The new frontier of biliary pathology. World J Gastroenterol. 2023;29:5361-5373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Ying S, Ying M, Liang W, Wang Z, Wang Q, Chen F, Xiao W. Morphological classification of intraductal papillary neoplasm of the bile duct. Eur Radiol. 2018;28:1568-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Aoki Y, Mizuma M, Hata T, Aoki T, Omori Y, Ono Y, Mizukami Y, Unno M, Furukawa T. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J Pathol. 2020;251:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Nakanuma Y, Kakuda Y, Uesaka K. Characterization of Intraductal Papillary Neoplasm of the Bile Duct with Respect to the Histopathologic Similarities to Pancreatic Intraductal Papillary Mucinous Neoplasm. Gut Liver. 2019;13:617-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D'Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (2)] |
| 9. | Takanami K, Yamada T, Tsuda M, Takase K, Ishida K, Nakamura Y, Kanno A, Shimosegawa T, Unno M, Takahashi S. Intraductal papillary mucininous neoplasm of the bile ducts: multimodality assessment with pathologic correlation. Abdom Imaging. 2011;36:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Lee MH, Katabathina VS, Lubner MG, Shah HU, Prasad SR, Matkowskyj KA, Pickhardt PJ. Mucin-producing Cystic Hepatobiliary Neoplasms: Updated Nomenclature and Clinical, Pathologic, and Imaging Features. Radiographics. 2021;41:1592-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 11. | Krawczyk M, Ziarkiewicz-Wróblewska B, Podgórska J, Grzybowski J, Gierej B, Krawczyk P, Grąt M, Kornasiewicz O, Skalski M, Wróblewski T. Intraductal papillary neoplasm of the bile duct - A comprehensive review. Adv Med Sci. 2021;66:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Nakanuma Y, Uesaka K, Kakuda Y, Sugino T, Kubota K, Furukawa T, Fukumura Y, Isayama H, Terada T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J Clin Med. 2020;9:3991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Tsuyuguchi T, Sakai Y, Sugiyama H, Miyakawa K, Ishihara T, Ohtsuka M, Miyazaki M, Yokosuka O. Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct. J Hepatobiliary Pancreat Sci. 2010;17:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Anné F, Snauwaert C, Vandeputte L, Berrevoet F, Van Huysse J, Van Dorpe J, Hoorens A. The added value of peroral cholangioscopy to diagnose intraductal papillary neoplasm of the bile duct. Endoscopy. 2022;54:E759-E760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Shin IS, Moon JH, Lee YN, Kim HK, Lee TH, Yang JK, Cha SW, Cho YD, Park SH. Use of peroral cholangioscopy to screen for neoplastic bile duct lesions in patients with bile duct stones (with videos). Gastrointest Endosc. 2021;94:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T. Evaluation of peroral videocholangioscopy using narrow-band imaging for diagnosis of intraductal papillary neoplasm of the bile duct. Dig Endosc. 2009;21 Suppl 1:S103-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Koda H, Hara K, Nozomi O, Kuwahara T, Nobumasa M, Haba S, Akira M, Hajime I. High-Resolution Probe-Based Confocal Laser Endomicroscopy for Diagnosing Biliary Diseases. Clin Endosc. 2021;54:924-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Gordon-Weeks AN, Jones K, Harriss E, Smith A, Silva M. Systematic Review and Meta-analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates. Ann Surg. 2016;263:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Sakai Y, Ohtsuka M, Sugiyama H, Mikata R, Yasui S, Ohno I, Iino Y, Kato J, Tsuyuguchi T, Kato N. Current status of diagnosis and therapy for intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2021;27:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Natov NS, Horton LC, Hegde SR. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J Gastrointest Endosc. 2017;9:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Chi H, Yan X, Tong W, Tian Q. SpyGlass guided PDT for advanced intraductal papillary mucinous neoplasm of the bile tract: A case report and literature review. Photodiagnosis Photodyn Ther. 2024;46:104098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Tsuchida K, Yamagata M, Saifuku Y, Ichikawa D, Kanke K, Murohisa T, Tamano M, Iijima M, Nemoto Y, Shimoda W, Komori T, Fukui H, Ichikawa K, Sugaya H, Miyachi K, Fujimori T, Hiraishi H. Successful endoscopic procedures for intraductal papillary neoplasm of the bile duct: a case report. World J Gastroenterol. 2010;16:909-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Shi J, Wan X, Xie Y, Lin J, Long J, Xu W, Liang Z, Sang X, Zhao H. CK20 and lymph node involvement predict adverse outcome of malignant intraductal papillary neoplasm of the bile duct. Histol Histopathol. 2020;35:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/