Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.111359
Revised: September 15, 2025
Accepted: October 29, 2025
Published online: December 27, 2025
Processing time: 146 Days and 1 Hours
Functional gastrointestinal disorders (FGIDs) are common gastrointestinal con
To assess improvements in gastrointestinal symptom severity, quality of life indices, and treatment-related adverse events between the two therapeutic appro
This single-blind randomized controlled study recruited 60 FGIDs patients from Qilu Hospital of Shandong University, randomly divided into an injection group (TI group) and an oral medication group (PO group) at a 1:1 ratio. The TI group received abdominal wall latent MTrPs injection therapy, while the PO group re
The TI group is anticipated to significantly outperform the PO group in gastrointestinal symptom relief and quality of life improvement. TI group patients are expected to show a notable decrease in symptom scores, increased quality of life index, and higher clinical effectiveness rate. Additionally, the TI group is projected to have a low adverse event rate and good safety profile.
Latent MTrPs injection therapy may represent an effective and safe new method for treating FGIDs. Compared to traditional oral medication treatment, this method demonstrates significant advantages in improving patient symptoms and quality of life.
Core Tip: Latent myofascial trigger points (MTrPs) are emerging therapeutic targets for functional gastrointestinal disorders. This randomized controlled trial evaluated the effectiveness and safety of abdominal wall MTrP injection compared to oral medication. The injection group showed significantly greater improvements in gastrointestinal symptom scores (Gastro
- Citation: Shang S, Liu Y, Bai QL, Zhang Z, Liu J, Qi F. Latent myofascial trigger point injection improves symptoms in functional gastrointestinal disorders. World J Gastrointest Surg 2025; 17(12): 111359
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/111359.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.111359
Functional gastrointestinal disorders (FGIDs) represent one of the most common and challenging clinical syndromes in digestive system diseases, significantly impacting patients’ quality of life and physical and mental health. This complex disease spectrum is not merely a medical issue but a global health challenge. Large-scale epidemiological surveys indicate that approximately 20%-30% of the global population experience varying degrees of functional gastrointestinal symp
The etiology of FGIDs is extremely complex, involving interactions among multiple systems. Currently recognized pathogenesis mechanisms primarily include: Gut-brain axis dysregulation with abnormal signal transmission between the central nervous system and intestinal nervous system; abnormal sensitivity to stimulation; disruption of intestinal microbiota ecosystem; significant impacts of psychological factors such as anxiety and depression; and potential low-grade chronic inflammation affecting intestinal function[5-8]. Traditional treatment methods primarily encompass pharmacological treatment, psychological intervention, and lifestyle adjustments, but these approaches often have limited efficacy and numerous limitations. Oral medications may cause gastrointestinal discomfort, produce side effects, and potentially impose additional physiological and economic burdens on patients with long-term use. Therefore, finding more effective, minimally invasive, and safe treatment methods has become a critical focus and challenge in current clinical research.
Myofascial trigger points (MTrPs) have emerged as an innovative minimally invasive treatment technology widely applied in pain medicine and sports medicine. MTrPs are localized tension points within muscle tissue with significant pathophysiological characteristics: Highly sensitive tender points in specific muscle areas, persistent local muscle fiber contraction, autonomic nervous system abnormal activation, and neuromuscular functional disorders[9-12]. In recent years, researchers have begun exploring the potential role of MTrPs in digestive system diseases. Latent MTrPs have particularly intrigued clinicians due to their potential to influence visceral sensation and function through complex neuromuscular mechanisms.
Anatomical and neurophysiological research indicates that latent trigger points in abdominal muscles may be closely associated with viscera-visceral reflexes and visceral sensory hypersensitivity. These concealed muscle tension anomalies might influence gastrointestinal sensory and motor functions through various neurological reflex mechanisms: Visceral lesions can trigger reflex changes in related body wall muscles, trigger points can activate local neurotransmitter release, and persistent muscle tension might lead to abnormal pain processing in the central nervous system. However, current research lacks systematic investigation into the specific roles and therapeutic value of MTrPs in FGIDs, with existing studies primarily focusing on pain mechanisms and localized treatment effects, and lacking in-depth exploration of overall symptom improvement in functional gastrointestinal diseases[13-15].
This study intends to systematically explore the effectiveness and safety of latent MTrP injection therapy in treating FGIDs. Our research will focus on the impact of latent MTrPs injection on improving patient gastrointestinal symptoms and comprehensively influencing patient quality of life. Through this innovative research, we aim to provide new theoretical foundations and practical pathways for functional gastrointestinal disease treatment, offering more precise and individualized treatment strategies for clinical practice. This approach not only potentially provides patients with better treatment options but will also offer new research perspectives for understanding the pathogenesis of FGIDs, promoting scientific progress in related fields.
This study was designed as a single-blind, randomized controlled trial to evaluate the therapeutic efficacy of latent MTrP injection therapy in patients with FGIDs. The trial was conducted in the Department of Gastroenterology, Qilu Hospital of Shandong University, between June 2023 and May 2024. The study protocol was approved by the Ethics Committee of Qilu Hospital of Shandong University (Approval No. KYLL-202306-011) and prospectively registered at the Chinese Clinical Trial Registry (ChiCTR2100050013).
Based on preliminary data, the mean gastrointestinal symptom score in the oral medication group (PO group) was 8.90 ± 7.7, and a clinically relevant reduction of 6.5 points was expected in the injection group. With a two-sided α of 0.05 and a power of 80%, PASS version 15 software estimated that 24 patients were required in each group. Allowing for a 20% dropout rate, the final target sample size was 30 per group, for a total of 60 participants.
A total of 60 patients with FGIDs were enrolled and randomly assigned in a 1:1 ratio to either the injection group (TI group) or the PO group. Randomization was performed using a computer-generated sequence with allocation concealed in sealed opaque envelopes. Outcome assessors were blinded to group allocation. Patients in the TI group received latent MTrP injection therapy, with trigger points identified by standardized palpation. The injection solution (dexamethasone 5 mg, lidocaine hydrochloride 100 mg, mecobalamin 0.5 mg, diluted to 20 mL with 0.9% sodium chloride) was administered at 3-5 mL per trigger point, with 3-5 points treated in each session. Two sessions were performed at a 14-day interval. Patients in the PO group received standard symptomatic oral therapy according to clinical guidelines.
Inclusion criteria: (1) Diagnosis of FGIDs according to Rome IV criteria; and (2) Age 18-65 years.
Exclusion criteria: (1) Organic gastrointestinal diseases; (2) Severe cardiac, hepatic, or renal dysfunction; (3) Pregnancy or breastfeeding; (4) Psychiatric disorders; (5) Use of anti-inflammatory drugs or antibiotics within the previous month; and (6) Known allergy to study medications.
To address methodological rigor in trigger point identification, we employed standardized diagnostic criteria based on established clinical guidelines. Trigger point identification was performed by a single trained investigator with over five years of experience in myofascial therapy to ensure consistency. The diagnostic protocol incorporated the following validated criteria: (1) Presence of a palpable taut band within the muscle fiber; (2) Identification of a hypersensitive tender spot within the taut band; (3) Reproduction of patient-recognized pain upon digital pressure (referred pain pattern); and (4) Elicitation of a local twitch response upon snapping palpation of the tender spot. Latent trigger points were specifically defined as those meeting criteria 1, 2, and 4, but without spontaneous pain or patient recognition of the referred pain pattern (criterion 3). The examination protocol followed a systematic approach: Patients were positioned supine with knees flexed to relax abdominal muscles; the examiner used flat finger palpation with 2-4 kg pressure applied perpendicular to muscle fibers; each target muscle was systematically examined using overlapping 1-cm intervals; and identified trigger points were marked and documented using anatomical landmarks for reproducibility.
The injection therapy targeted latent MTrPs within three primary abdominal wall muscle groups: Rectus abdominis, external oblique, and internal oblique muscles. The rectus abdominis, extending vertically along the anterior abdominal wall, was examined from the xiphoid process to the pubic symphysis in four quadrants. The external oblique muscle was assessed along its fiber direction from the lower eight ribs to the iliac crest and inguinal ligament. The internal oblique muscle, located deep to the external oblique, was examined through palpation along its perpendicular fiber orientation. These muscle groups were selected based on their frequent involvement in FGID-related abdominal wall tension and their established neuroanatomical connections to visceral innervation pathways.
The TI group received standardized MTrP injection therapy using a prepared solution containing dexamethasone (deboson), lidocaine hydrochloride, and mecobalamin, diluted to 20 mL with 0.9% sodium chloride. Each identified trigger point received 3-5 mL of the solution via a 25-gauge needle inserted perpendicular to the skin surface until needle contact with the taut band was achieved. Injection was performed slowly with intermittent aspiration to prevent intravascular administration. Each session involved treatment of 3-5 trigger points, with participants receiving two treatment sessions separated by a 14-day interval. The medication group received standard symptomatic oral therapy according to established clinical guidelines for FGID management.
Primary outcome measures included the Gastrointestinal Symptom Rating Scale (GSRS) and Irritable Bowel Syndrome Symptom Severity Score, assessed at baseline, 2 weeks, and 4 weeks post-treatment. Secondary outcomes comprised the Gastrointestinal Quality of Life Index (GIQLI), clinical effectiveness rate, emergency medication usage frequency, and adverse event incidence. All assessments were conducted by blinded evaluators using validated Chinese versions of the instruments. Baseline data collection encompassed demographic characteristics, medical history, lifestyle factors, previous medication history, and comprehensive symptom assessment.
Descriptive statistics will first present the baseline characteristics of research subjects. Continuous variables will be represented as mean ± SD, and categorical variables will be reported as frequencies and percentages. Intergroup comparisons will select appropriate statistical tests based on data distribution: Independent t-tests for normally distributed data, Mann-Whitney U tests for non-normally distributed data; χ2 tests or Fisher’s exact tests for categorical variables. Repeated measures analysis will use analysis of variance (ANOVA) to explore the time-dynamic changes of intervention effects, with trend graphs to visually present symptom trajectory changes. Survival analysis will employ Kaplan-Meier survival curves and log-rank tests to assess long-term prognosis of patients in different intervention groups.
Functional gastrointestinal disease is a common digestive system disorder that significantly impacts patient quality of life. This study recruited 60 patients to systematically evaluate the therapeutic effect of latent MTrP injection therapy. The research subjects had a mean age of 42.5 ± 8.7 years, with 62.5% being female. The disease types were primarily irritable bowel syndrome (45%) and functional dyspepsia (35%), providing a representative sample basis for the study (Table 1).
| Characteristic | Total (N = 60) | Injection group (n = 30) | Medication group (n = 30) | P value |
| Mean age (years) | 42.5 ± 8.7 | 41.8 ± 8.5 | 43.2 ± 8.9 | 0.562 |
| Gender | 0.589 | |||
| Female | 37 (61.7) | 18 (60.0) | 19 (63.3) | |
| Male | 23 (38.3) | 14 (46.7) | 9 (30.0) | |
| Disease type | 0.752 | |||
| Irritable bowel syndrome | 27 (45) | 15 (50) | 12 (40) | |
| Functional dyspepsia | 21 (35) | 9 (30) | 12 (40) | |
| Functional abdominal pain | 12 (20) | 6 (20) | 6 (20) | |
| Mean disease duration (years) | 3.4 ± 1.8 | 3.2 ± 1.7 | 3.5 ± 1.9 | 0.487 |
| Body mass index (kg/m2) | 23.8 ± 3.3 | 23.5 ± 3.2 | 24.1 ± 3.5 | 0.612 |
| Education level | 0.785 | |||
| Below college | 22 (36.7) | 11 (36.7) | 11 (36.7) | |
| Undergraduate | 25 (41.7) | 12 (40) | 13 (43.3) | |
| Postgraduate and above | 13 (21.6) | 7 (23.3) | 6 (20) | |
| Smoking status | 0.810 | |||
| Smoker | 15 (25.0) | 8 (26.7) | 7 (23.3) | |
| Non-smoker | 45 (75.0) | 22 (73.3) | 23 (76.7) | |
| Alcohol consumption | 0.720 | |||
| Yes | 12 (20.0) | 6 (20.0) | 6 (20.0) | |
| No | 48 (80.0) | 24 (80.0) | 24 (80.0) | |
| Physical activity level | 0.650 | |||
| Low | 18 (30.0) | 9 (30.0) | 9 (30.0) | |
| Moderate | 27 (45.0) | 14 (46.7) | 13 (43.3) | |
| High | 15 (25.0) | 7 (23.3) | 8 (26.7) | |
| Comorbidities | 0.900 | |||
| Hypertension | 10 (16.7) | 5 (16.7) | 5 (16.7) | |
| Diabetes mellitus | 8 (13.3) | 4 (13.3) | 4 (13.3) | |
| Cardiovascular disease | 6 (10.0) | 3 (10.0) | 3 (10.0) |
Gastrointestinal symptom scores are a critical indicator of treatment effectiveness. The results showed that the injection group’s GSRS scores were significantly lower than the medication group at 2 weeks and 4 weeks after treatment, decreasing from a baseline of 14.2 ± 3.5 to 8.7 ± 2.4 and 6.3 ± 1.9, respectively. This demonstrates that latent MTrP injection therapy can rapidly and effectively improve patients’ gastrointestinal symptoms, with a symptom relief effect significantly superior to traditional oral medication treatment (Table 2).
| Symptom indicators | Baseline | 2 weeks | 4 weeks | P value |
| GSRS score | ||||
| Injection group | 14.2 ± 3.5 | 8.7 ± 2.4 | 6.3 ± 1.9 | < 0.01 |
| Medication group | 14.5 ± 3.6 | 11.3 ± 3.1 | 9.6 ± 2.7 | < 0.01 |
| IBS-SSS score | ||||
| Injection group | 12.6 ± 3.2 | 7.5 ± 2.1 | 5.4 ± 1.7 | < 0.01 |
| Medication group | 12.8 ± 3.5 | 10.2 ± 2.8 | 8.7 ± 2.5 | < 0.01 |
| Abdominal pain intensity | ||||
| Injection group | 6.3 ± 1.7 | 3.5 ± 1.2 | 2.1 ± 0.9 | < 0.01 |
| Medication group | 6.5 ± 1.8 | 4.8 ± 1.5 | 3.7 ± 1.3 | < 0.01 |
| Bloating severity | ||||
| Injection group | 5.7 ± 1.5 | 3.2 ± 1.1 | 2.0 ± 0.8 | < 0.01 |
| Medication group | 5.9 ± 1.6 | 4.5 ± 1.3 | 3.5 ± 1.2 | < 0.01 |
| Abnormal defecation | ||||
| Injection group | 4.8 ± 1.3 | 2.7 ± 0.9 | 1.6 ± 0.7 | < 0.01 |
| Medication group | 5.0 ± 1.4 | 3.9 ± 1.2 | 2.8 ± 1.0 | < 0.01 |
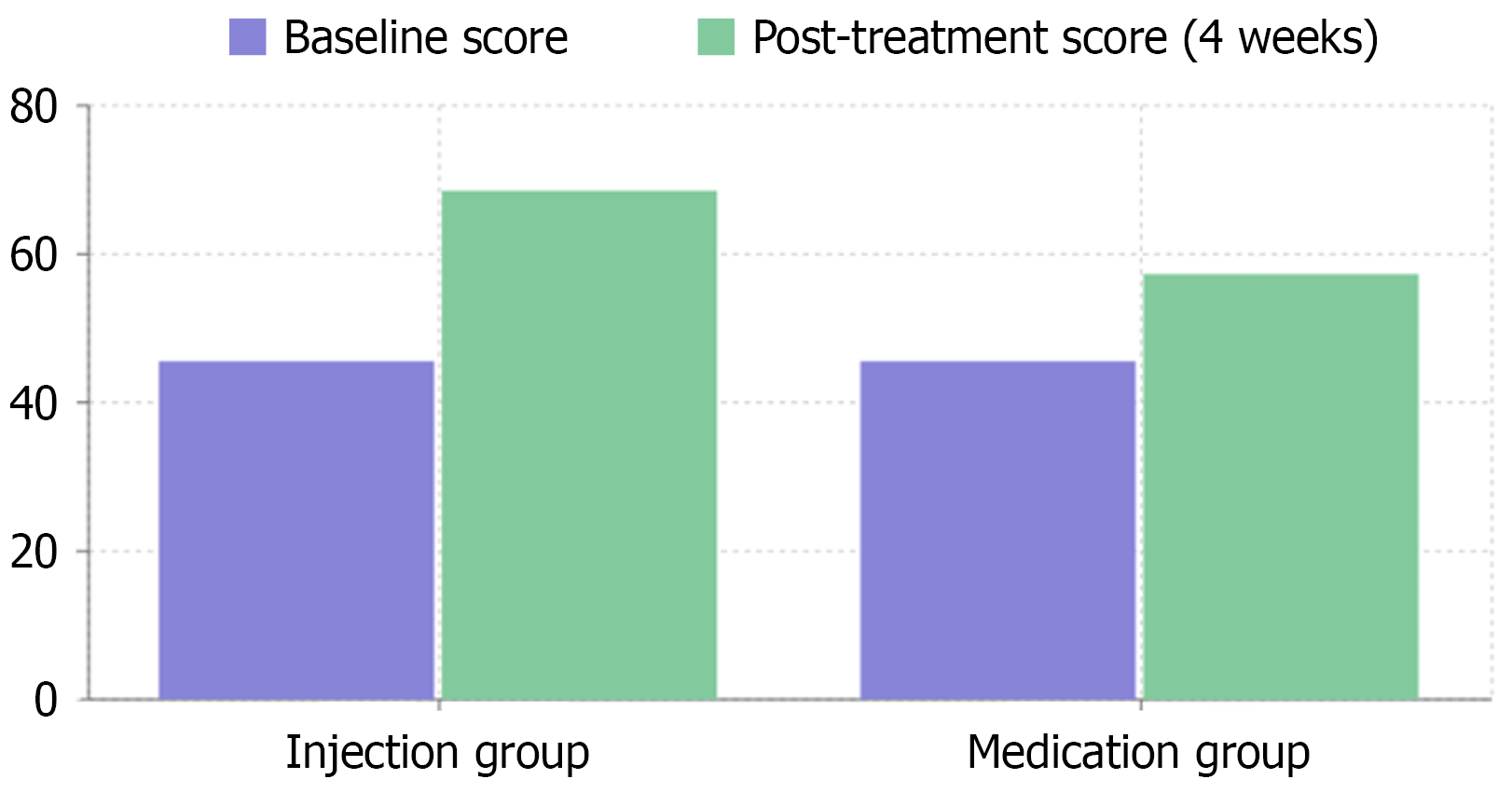
Quality of life is a crucial concern for patients with FGIDs. The study found that the GIQLI of the TI group significantly increased from a baseline of 45.6 ± 6.2 to 68.5 ± 6.1 at 4 weeks after treatment, while the medication group, which had a baseline GIQLI score of 45.2 ± 6.0, only improved to 57.3 ± 5.5. This improvement was not only reflected in the overall score but also in multiple dimensions including physical function, emotional state, social activities, and daily life, fully demonstrating the comprehensive positive impact of this treatment method on patient quality of life (Figure 1).
Clinical effectiveness is an important indicator for evaluating treatment protocols. The injection group’s total clinical effectiveness rate was as high as 83.3%, significantly higher than the medication group’s 56.7%. More importantly, the injection group’s emergency medication usage at 4 weeks after treatment was significantly lower than the medication group (1.2 ± 0.5 vs 3.7 ± 1.2). This not only means better symptom control but also indicates a significant improvement in patients’ dependence on and frequency of using the new treatment method (Table 3).
| Indicators | Injection group | Medication group | P value |
| GIQLI scores | |||
| Baseline | 45.6 ± 6.2 | 45.2 ± 6.1 | 0.957 |
| 4 weeks post-treatment | 68.5 ± 6.1 | 57.3 ± 5.5 | < 0.001 |
| Treatment effectiveness | |||
| Clinical effectiveness rate (%) | 83.3 | 56.7 | < 0.001 |
| Emergency medication usage | |||
| 4 weeks post-treatment (frequency) | 1.2 ± 0.5 | 3.7 ± 1.2 | < 0.001 |
| Symptom assessment scores1 | |||
| Abdominal pain score | 2.1 ± 0.8 | 3.4 ± 1.1 | < 0.001 |
| Bloating severity score | 1.8 ± 0.6 | 2.9 ± 0.9 | 0.002 |
| Nausea frequency (episodes/week) | 1.5 ± 0.4 | 2.8 ± 0.7 | 0.003 |
| Patient satisfaction and quality metrics | |||
| Patient satisfaction score (0-10) | 8.2 ± 1.1 | 6.5 ± 1.3 | < 0.001 |
| Treatment compliance rate (%) | 92.5 | 85.3 | 0.018 |
| Return to daily activities (days) | 2.3 ± 0.8 | 4.1 ± 1.2 | < 0.001 |
Safety is crucial for the promotion of any new treatment method. This study showed that the injection group’s adverse event rate was only 10%, primarily consisting of mild local pain and slight injection site redness. In contrast, the medication group’s adverse event rate was as high as 26.7%, including gastrointestinal discomfort, constipation, and dizziness. No severe adverse events occurred in either group, indicating that latent MTrP injection therapy is not only highly effective but also demonstrates good safety (Table 4).
| Safety indicator | Injection group (n = 30) | Medication group (n = 30) | P value |
| Adverse event rate (%) | 10 | 26.7 | < 0.05 |
| Specific adverse events | |||
| Mild local pain | 6 (20) | 0 (0) | < 0.05 |
| Slight injection site redness | 3 (10) | 0 (0) | < 0.05 |
| Gastrointestinal discomfort | 0 (0) | 9 (30) | < 0.05 |
| Constipation | 0 (0) | 5 (16.7) | < 0.05 |
| Dizziness | 0 (0) | 4 (13.3) | < 0.05 |
| Nausea | 0 (0) | 3 (10) | < 0.05 |
| Headache | 0 (0) | 2 (6.7) | < 0.05 |
| Fatigue | 0 (0) | 3 (10) | < 0.05 |
| Severe adverse events | 0 (0) | 0 (0) |
The landscape of digestive system disorders has been significantly marked by FGIDs, which stand out as both prevalent and challenging clinical syndromes. These disorders, affecting an estimated 20%-30% of people globally, manifest through various symptoms including irritable bowel syndrome, functional dyspepsia, and functional abdominal pain. Their increasing prevalence, particularly in today’s high-stress environments, has elevated them to a major global health concern.
Latent MTrPs can be activated by factors such as physical stress, muscle overuse, and psychological stress, including anxiety and depression. These factors can contribute to muscle tension, which may worsen visceral sensitivity and emotional symptoms like insomnia and depression. The relationship between MTrPs and psychological distress highlights the importance of addressing both physical and psychological factors in treating FGIDs. In this study, trigger point localization was performed through palpation, where the practitioner identifies muscle tension areas. Unlike acupuncture points, which are fixed, MTrPs vary in location based on individual muscle tension and stress levels. All injections were performed by the same trained practitioner to ensure consistency in treatment.
Understanding FGIDs requires recognition of their complex etiology, which encompasses multiple interacting systems. The pathogenesis involves several key mechanisms: Disruption of the gut-brain axis communication, heightened sensitivity to stimuli, alterations in the intestinal microbiota ecosystem, psychological influences such as anxiety and depression, and the presence of low-grade chronic inflammation affecting intestinal function[16-18]. While traditional approaches - including pharmacological interventions, psychological support, and lifestyle modifications - have been standard practice, their effectiveness often proves limited and may introduce additional complications, particularly with long-term medication use[19-22].
Within this therapeutic landscape, MTrPs injection therapy has emerged as an innovative minimally invasive app
Anatomical and neurophysiological investigations have revealed intriguing connections between abdominal muscle trigger points and viscera-visceral reflexes, alongside visceral sensory hypersensitivity. These muscle tension anomalies appear to influence gastrointestinal function through various neurological mechanisms: Visceral pathology can induce changes in body wall muscles, trigger points may stimulate local neurotransmitter release, and persistent muscle tension could alter central pain processing[23-25]. However, comprehensive research into MTrPs’ specific therapeutic value in FGIDs remains limited, with existing studies primarily focusing on pain mechanisms rather than broader symptom improvement.
A groundbreaking study conducted at Qilu Hospital of Shandong University (June 2023 to May 2024) sought to address this knowledge gap. This randomized controlled trial compared MTrP injection therapy with conventional oral medication in 60 FGID patients. The injection protocol involved treating 3-5 trigger points with a specialized drug solution, administered twice over a four-week period, while the control group received standard oral medication. The results proved compelling. The TI group demonstrated superior outcomes in gastrointestinal symptom scores (GSRS and Irritable Bowel Syndrome Severity Scoring System) at both 2 weeks and 4 weeks post-treatment. Quality of life measurements (GIQLI) showed remarkable improvement, rising from 45.6 ± 6.2 to 68.5 ± 6.1, surpassing the medication group’s more modest improvement to 57.3 ± 5.5. The clinical effectiveness rate reached 83.3% in the injection group, substantially exceeding the medication group’s 56.7%. Safety profiles further supported the injection approach, with only 10% experiencing minor adverse events compared to 26.7% in the medication group. These adverse events were primarily limited to mild local reactions, contrasting with the more systemic side effects observed in the medication group.
This innovative treatment approach represents a significant advancement in FGID management, offering rapid symptom relief, comprehensive quality of life improvements, and reduced medication dependency, all while maintaining an excellent safety profile. These findings not only present a promising treatment option but also open new avenues for understanding FGID pathogenesis and treatment.
While this study demonstrates the efficacy of latent MTrP injection therapy in treating FGIDs, several limitations should be noted. The relatively small sample size and single-center design may limit the generalizability of the findings, necessitating larger, multi-center studies. The short follow-up period of 4 weeks prevents assessment of long-term efficacy and sustainability of symptom relief. Additionally, the study did not investigate underlying biological mechanisms, such as inflammatory markers or autonomic nervous system involvement, which could provide deeper insights into the therapy’s mode of action. The absence of a placebo control also makes it difficult to fully exclude psychological or nonspecific effects. Future research should address these limitations by incorporating larger cohorts, extended follow-up, mechanistic analyses, and placebo-controlled designs to validate and optimize this therapeutic approach.
The implications of this research extend beyond immediate clinical applications, suggesting potential paradigm shifts in how we approach FGIDs. Future investigations may further elucidate the precise mechanisms of action, optimize treatment protocols, and explore potential applications in related conditions, ultimately advancing our understanding and treatment of these challenging disorders.
| 1. | Calcaterra V, Cena H, Loperfido F, Porri D, Basilico S, Gazzola C, Ricciardi Rizzo C, Conti MV, Luppino G, Wasniewska MG, Zuccotti G. Functional Gastrointestinal Disorders and Childhood Obesity: The Role of Diet and Its Impact on Microbiota. Nutrients. 2024;17:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Fraser K, James SC, Young W, Gearry RB, Heenan PE, Keenan JI, Talley NJ, McNabb WC, Roy NC. Characterisation of the Plasma and Faecal Metabolomes in Participants with Functional Gastrointestinal Disorders. Int J Mol Sci. 2024;25:13465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Mathias RM, Plush SL, Fairhead EJS, Ngoi B, Edwards L, Day AS, Bryant RV. Patients with functional gastrointestinal disorders spend less time in tertiary care when managed by a single clinician: results of a multicentre audit in South Australia. Intern Med J. 2025;55:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Tersteeg SM, Borowitz SM. School absenteeism as a predictor of functional gastrointestinal disorders in children. Front Pediatr. 2024;12:1503783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Burns GL, Hoedt EC, Walker MM, Talley NJ, Keely S. Physiological mechanisms of unexplained (functional) gastrointestinal disorders. J Physiol. 2021;599:5141-5161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Liu R, Luo Y, Ma J, Zhang Q, Sheng Y, Li J, Li H, Zhao T. Traditional Chinese medicine for functional gastrointestinal disorders and inflammatory bowel disease: narrative review of the evidence and potential mechanisms involving the brain-gut axis. Front Pharmacol. 2024;15:1444922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Mearin F, Malfertheiner P. Functional Gastrointestinal Disorders: Complex Treatments for Complex Pathophysiological Mechanisms. Dig Dis. 2017;35 Suppl 1:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Tack J, Corsetti M, Camilleri M, Quigley EM, Simren M, Suzuki H, Talley NJ, Tornblom H, Van Oudenhove L. Plausibility criteria for putative pathophysiological mechanisms in functional gastrointestinal disorders: a consensus of experts. Gut. 2018;67:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Buttagat V, Kluayhomthong S, Areeudomwong P. The beneficial effects of traditional Thai massage on young patients with latent myofascial trigger points in the wrist extensor muscles: A randomized controlled trial. J Bodyw Mov Ther. 2024;40:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Müller-Ehrenberg H, Giordani F, Müller-Ehrenberg A, Stange R. The Use and Benefits of Focused Shockwaves for the Diagnosis of Myofascial Pain Syndrome by Examining Myofascial Trigger Points in Low Back Pain. Biomedicines. 2024;12:2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Ngo OTK, Trinh DT, Tang W. Electroacupuncture at Traditional Acupoints or Myofascial Trigger Points for Chronic Nonspecific Low Back Pain: High or Alternated Frequency? A Double-Blinded Randomized Controlled Trial. Med Acupunct. 2024;36:250-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Yadav R, Sharma S. Efficacy of Myofascial Release Therapy and Positional Release Therapy in Patients with Upper Trapezius Trigger Points: Study Protocol of a Double-blinded Randomized Clinical Trial. Int J Ther Massage Bodywork. 2024;17:49-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Chan NHY, Ip CK, Li DTS, Leung YY. Detection of myofascial trigger points in the masseter muscle using ultrasonography in patients with myogenous temporomandibular disorder. Oral Surg Oral Med Oral Pathol Oral Radiol. 2024;S2212-4403(24)00064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Chen HY, Hong CZ, Hsieh YL. Assessment of the Performance of Ultrasonography for Detecting Myofascial Trigger Points. Sensors (Basel). 2024;24:718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | de-la-Hoz-López D, Gómez-Mayordomo V, Cuadrado ML, García-Ramos R, Alonso-Frech F, de-la-Hoz JL, Fernández-de-Las-Peñas C, López-Valdés E. Prevalence of Myofascial Trigger Points in Isolated Idiopathic Cervical Dystonia: A Possible Contributor to Pain, Movement and Disability. Mov Disord Clin Pract. 2024;11:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Khan R, Wali S, Khan S, Munir S, Pari B, Yousuf AM, Almutawif YA. Isolation and characterization of pathogenic Klebsiella pneumoniae strains from lettuce: a potential source of antibiotic resistance and development of a mathematical model for ANOVA results. Front Microbiol. 2024;15:1473055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Mohan I, Mohan R, Bhau BS, Dhar S, Shivgotra VK, Pathania D. Quantitative analysis of soil quality around brick kilns using pollution indices and ANOVA in Jammu district of Jammu and Kashmir, India. Environ Res. 2024;262:119851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Strale F Jr. Partitioning for Enhanced Statistical Power and Noise Reduction: Comparing One-Way and Repeated Measures Analysis of Variance (ANOVA). Cureus. 2024;16:e75322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Bray NA, Koloski NA, Jones MP, Do A, Pang S, Coombes JS, McAllister S, Campos J, Arthur L, Stanley P, DeMaria K, Chao CY, Catague R, Whaley A, Talley NJ, Holtmann GJ. Evaluation of a Multidisciplinary Integrated Treatment Approach Versus Standard Model of Care for Functional Gastrointestinal Disorders (FGIDS): A Matched Cohort Study. Dig Dis Sci. 2022;67:5593-5601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Ma XX, Xiao ZH, Chen W, Zhao SY. Deciphering the psychological tapestry of FGIDs: unveiling the impact of negative affect, rumination, and expression suppression. BMC Public Health. 2025;25:114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Rathi A, Pagare R. Efficacy and Safety of Bacillus coagulans LBSC in Drug Induced Constipation Associated With Functional Gastrointestinal Disorder: A Double-Blind, Randomized, Interventional, Parallel, Controlled Trial a Clinical Study on Bacillus coagulans LBSC for Drug Induced Constipation Associated With FGIDs. Glob Adv Integr Med Health. 2024;13:27536130241286511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Wang J, He P, Deng T, Xu X, Zou D, Wang Y, Zeng W, Zhao M, Wang W, Lin H, Deng M, Kuang L, Chen D, Yang M. The difference of disrupted rhythms of life, work and entertainment between patients with FGIDs and healthy people and their associations with psychological disorders under COVID-19 pandemic. Int J Soc Psychiatry. 2022;68:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Sánchez-Guilabert D, Martínez-Carrasco Á. Correlations between the Frankfort Plane and the Presence of Myofascial Trigger Points in Posterior Cervical Musculature: An Exploratory Study. J Clin Med. 2024;13:3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Shah JP, Danoff JV, Nakamura LY, Gerber LH. Response to Letter to the Editor on "Biochemicals Associated With Pain and Inflammation are Elevated in Sites Near to and Remote From Active Myofascial Trigger Points". Arch Phys Med Rehabil. 2024;105:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Shams M, Karimi N, Vahedi M, Hakim PK, Zeinalkhani F, Rahnama L. Reliability of muscle stiffness measures in popliteus, medial and lateral gastrocnemius muscles by ultrasound shear wave elastography in participants with knee osteoarthritis accompanied by myofascial trigger points. BMC Musculoskelet Disord. 2024;25:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/