Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.111268
Revised: September 10, 2025
Accepted: October 17, 2025
Published online: December 27, 2025
Processing time: 146 Days and 1.2 Hours
Acute nonvariceal upper gastrointestinal bleeding (NVUGIB) is a life-threatening emergency. Endoscopic hemostasis and vascular interventional therapy are two major minimally invasive treatment strategies. Although these modalities are widely used, their comparative efficacy and safety across different patient populations and anatomical sites remain controversial.
To evaluate the clinical outcomes and safety of endoscopic hemostasis combined with vascular interventional therapy for NVUGIB.
A systematic search was done on PubMed, EMBASE, Cochrane Library, and Web of Science (from database establishment to April 2025). Randomized controlled trial (RCT) quality was assessed via Cochrane RoB 2.0, and observational studies via the Critical Appraisal Skills Program. RevMan 5.4 was used for quantitative analysis; fixed/random-effects models were chosen through I²-assessed heterogeneity. Publication bias was checked using funnel plots and sensitivity analysis via model switching.
Twenty-one studies (3 RCTs, 12 single-group studies, and 6 retrospective cohort studies) with good quality were included. For single-group data, combined therapy had risk differences of 0.70 (clinical success), 0.24 (mortality), and 0.22 (rebleeding; all P < 0.00001, I² = 0). Moreover, the ≥ 60-year rebleeding risk difference was 0.43. Reintervention was found to differ by approach (Z = 3.03, P = 0.002, inter-subgroup I² = 99%). In the RCT and cohort studies, combined vs standard therapy had similar initial hemostasis (Z = 0.04, P = 0.97) and mortality (Z = 1.56, P = 0.12) but lower rebleeding (Z = 3.26/P = 0.001; Z = 2.95/P = 0.03). Symmetrical funnel plots and robust sensitivity analysis showed no publication bias.
Combined endoscopic hemostasis and vascular interventional therapy for acute NVUGIB can significantly reduce rebleeding, without differences in mortality. Age and vascular interventional methods may influence the therapeutic efficacy.
Core Tip: This meta-analysis demonstrates that combining endoscopic hemostasis with vascular interventional therapy is effective for acute nonvariceal upper gastrointestinal bleeding, significantly reducing rebleeding rates versus standard therapy. While it shows no significant differences in initial hemostasis success or mortality, mortality findings require further validation. These results aid clinical treatment optimization, though subgroup effects related to age and intervention methods need deeper investigation.
- Citation: Zhou CJ, Sun H, Tang XH. Endoscopic hemostasis combined with vascular interventional therapy for acute nonvariceal upper gastrointestinal bleeding: A meta-analysis. World J Gastrointest Surg 2025; 17(12): 111268
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/111268.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.111268
Acute nonvariceal upper gastrointestinal bleeding (NVUGIB) is a life-threatening emergency in gastroenterology, characterized by high mortality rates that pose a severe threat to patient health. With the continuous advancement of endoscopic and vascular interventional techniques, endoscopic hemostasis and vascular interventional therapy have emerged as critical modalities for treating acute NVUGIB. However, controversy persists regarding the superiority of these methods in terms of clinical efficacy and safety. Epidemiological data indicate an increasing global incidence of NVUGIB. According to recent studies, the annual incidence of NVUGIB is approximately 54.4 cases per 100000 individuals, with an overall mortality rate as high as 13.5%[1]. Currently, endoscopic hemostasis is the first-line treatment for NVUGIB, owing to its minimally invasive nature and operational convenience[2]. Nevertheless, vascular interventional therapy has a unique therapeutic value for patients with failed endoscopic treatment, recurrent bleeding, or complex anatomical structures[3]. Most existing studies have focused on single-center, small-sample comparisons of the two treatment methods and lack a comprehensive, systematic evaluation. Although some studies have compared endoscopic hemostasis with vascular interventional therapy, their conclusions vary owing to factors such as sample size, study design, and baseline patient characteristics. Therefore, an urgent need exists to integrate relevant evidence through meta-analyses to provide reliable support for clinical decision-making.
We systematically collected high-quality research worldwide, applied strict inclusion and exclusion criteria, and used advanced meta-analytical methods to comprehensively evaluate the clinical efficacy and safety of endoscopic hemostasis and vascular interventional therapies for acute NVUGIB. This study aimed to systematically compare the two treatment methods in terms of hemostasis success, rebleeding, complications, and mortality rates, thereby clarifying their advantages and limitations in clinical application. Through a rigorous scientific meta-analysis, this study provides clinicians with more objective and comprehensive evidence-based medical insights for selecting treatment protocols for acute NVUGIB, optimizing treatment strategies, improving patient survival rates, and enhancing outcomes, thus offering significant guidance for its clinical management.
Computer-based searches were conducted in PubMed, Web of Science, EMBASE, and the Cochrane Library from inception to May 2025, focusing on English-language literature. For instance, the search strategy for PubMed was as follows: {[nonvariceal(Title/Abstract)] AND [upper gastrointestinal bleeding(Title/Abstract)] OR [acute(Title/Abstract)] AND [vascular intervention(Title/Abstract)] OR [endoscopy hemostasis(Title/Abstract)]}.
This strategy integrated keywords related to the disease (“nonvariceal”, “upper gastrointestinal bleeding”, “acute”) and treatment modalities (“vascular intervention”, “endoscopic hemostasis”), with systematic retrieval of titles and abstracts to ensure comprehensive capture of relevant studies. Similar search strategies were adopted for other databases using subject headings (e.g., Medical Subject Headings terms) and free-text keywords to maintain consistency and comparability across databases. This study has been registered in the International Prospective Register of Systematic Reviews (No. CRD420251077603).
Inclusion criteria: (1) Study design: Single-arm intervention studies, retrospective cohort studies, and randomized controlled trials (RCTs) in English; (2) Study population: Patients diagnosed with acute NVUGIB via gastroscopy, imaging, or other clinical examinations[4], regardless of age, sex, or race; (3) Interventions: Experimental group - endoscopic hemostasis (including injection hemostasis, thermal coagulation, and mechanical hemostasis) or vascular interventional therapy (e.g., transarterial embolization); control group - standard treatment[5] or conventional therapy (e.g., pharmacological hemostasis and conservative treatment); and (4) Outcome measures: Successful initial hemostasis rate, clinical success rate, rebleeding rate, mortality rate, reintervention rate, and complication rate (at least one of them).
Exclusion criteria: (1) Duplicate publications or overlapping datasets; (2) Studies with missing primary outcome data that cannot be supplemented by contacting the authors; (3) Studies that included patients with variceal upper gastrointestinal bleeding or comorbidities that severely affect outcomes (e.g., terminal cancer or multi-organ failure); (4) Abstract-only articles, conference abstracts, reviews, case reports, or lecture notes; and (5) Studies without full-text access or that failed quality assessment (high risk of bias in RCTs and severe methodological flaws in nonrandomized studies).
Literature screening and data extraction: Two independent reviewers strictly followed predefined inclusion and exclusion criteria to conduct literature screening, data extraction, and cross-verification. The screening process was performed in two stages. First, titles and abstracts were reviewed to exclude studies that did not meet the inclusion criteria or were duplicate publications. Second, the full texts of the remaining studies were evaluated to determine the final eligibility. The extracted key data included author information, publication year, study design, sample size, detailed interventions, follow-up duration, and outcome metrics. In cases of disagreement between the reviewers, a third reviewer was consulted to reach a consensus, ensuring the accuracy and reliability of literature screening and data extraction.
Literature quality assessment: To ensure the reliability of this meta-analysis, two reviewers independently evaluated the methodological quality of the included studies using targeted tools based on internationally recognized evidence-based medicine criteria. For RCTs, the Cochrane Risk of Bias tool (RoB 2.0)[6] was applied for systematic evaluation. This assessment covered dimensions including random sequence generation, allocation concealment, implementation of blinding, completeness of outcome data, selective reporting, and other potential sources of bias, aiming to comprehensively identify risks of bias in the study design and conduct. For single-group intervention studies and retrospective cohort studies, the Critical Appraisal Skills Program checklist[7] was used. The key evaluation criteria included sample representativeness, accuracy of intervention descriptions, objectivity of outcome measures, completeness of follow-up, and control of confounding factors to systematically assess the quality of the research evidence. After the two reviewers independently performed quality assessments, cross-verification was performed to ensure consistency. Discrepancies were initially resolved through discussion and, when necessary, adjudicated by a third reviewer. Finally, the quality assessment results were summarized by study type to provide a scientific basis for subsequent stratified meta-analyses.
The meta-analysis was performed using RevMan 5.4 software. Heterogeneity across studies was assessed using the I² statistic with the following criteria: Low heterogeneity was defined as P ≥ 0.10 and I² < 50%, warranting the use of the fixed-effect model. Significant heterogeneity was defined as P < 0.10 and I² ≥ 50%, requiring the random-effects model. In cases where P ≥ 0.10 but I² ≥ 50%, the I² statistic served as the primary determinant.
Sensitivity analysis was performed by switching between the fixed-effects model and the random-effects model. Specifically, the pooled effect sizes of the included studies were calculated based on the two models, and the magnitude of numerical change in pooled effect sizes and consistency of effect directions under different models were compared. If the difference in pooled effect sizes obtained from the two models was small and the effect directions were consistent, it indicated that the results of the meta-analysis were less affected by model selection and had good overall stability. In contrast, significant differences in effect sizes or changes in effect directions suggested poor result stability, requiring further analysis of the potential impact of heterogeneity sources on the conclusions.
Funnel plots were used to assess the publication bias of the included studies. The horizontal axis of the funnel plot represented the study effect sizes (e.g., mean difference, odds ratio), while the vertical axis represented the sample size (reflecting study precision). A symmetric “inverted funnel” shape of the funnel plot - where studies with effect sizes close to the true value were evenly distributed on both sides of the central line, with small-sample studies scattered at the bot
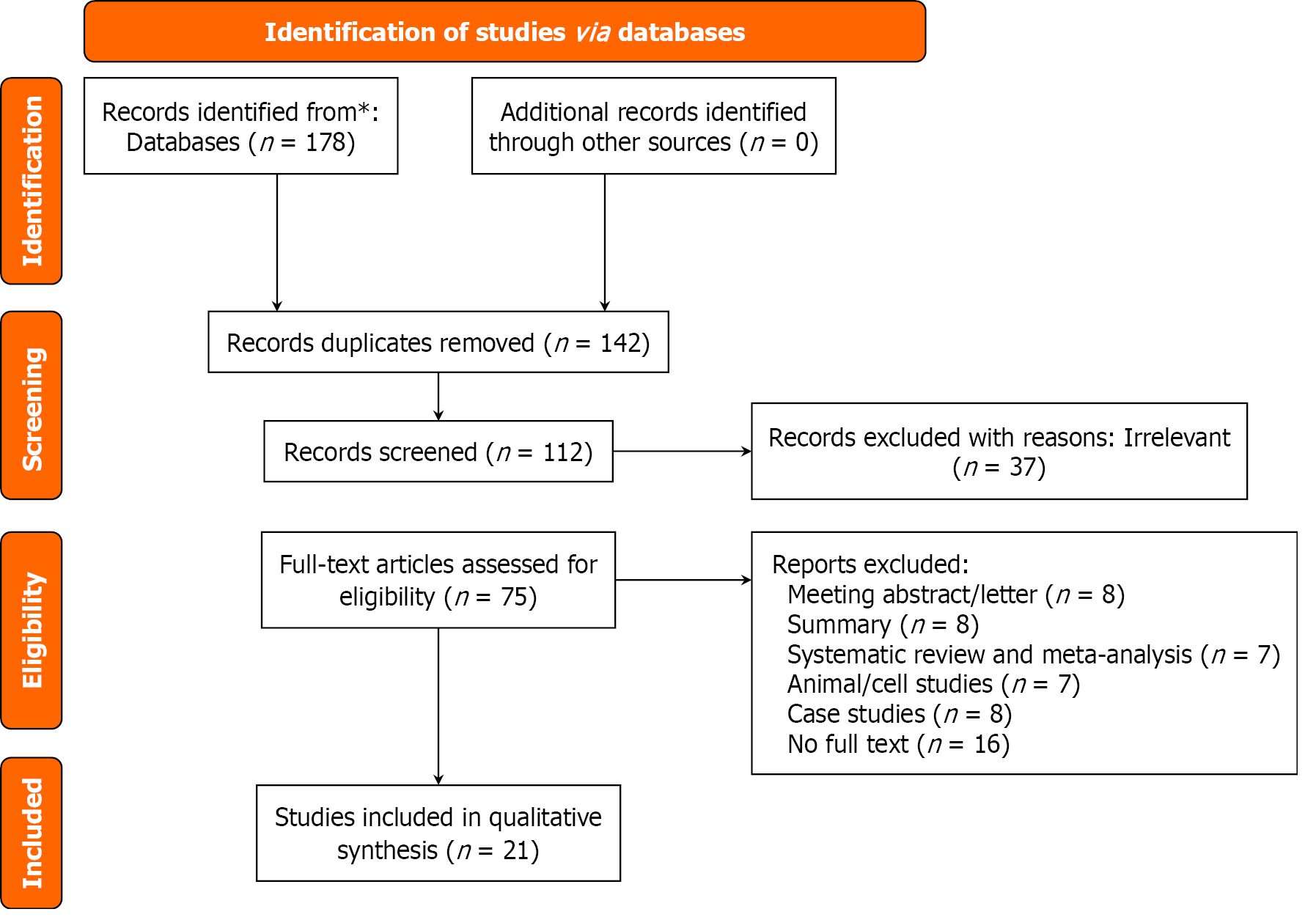
A total of 178 records were retrieved, with no additional studies identified from other sources. After the removal of 36 duplicate records, 37 irrelevant studies were excluded, leaving 75 full-text articles for evaluation. Ultimately, 21 studies[8-28] were included in the qualitative analysis. The literature screening process is illustrated in Figure 1.
The included studies were single-group intervention studies, retrospective cohort studies, and RCTs. The baseline characteristics are summarized in Table 1. All RCTs achieved a quality assessment score of ≥ 7 points. Most single-group intervention studies demonstrated “fairly good” performance in intervention description, objectivity of outcome measures, and completeness of follow-up, while sample representativeness and control of confounding bias were rated “average” - primarily in small-sample studies. Overall, the methodological quality of the included studies is acceptable. The quality assessment results are presented in Table 2.
| Ref. | Country | Study type1 | Participants | Male | Median age | Vascular intervention | Follow-up | Outcome2 |
| McGraw et al[8], 2023 | Pennsylvania | C | 269 | - | - | TAE | 6 years | (3), (4) |
| Lau et al[9], 2019 | Thailand | C | 241 | - | - | AE | 30 days | (4), (5) |
| Nykänen et al[10], 2017 | Finland | B | 85 | TAE | 30 days | (3), (4), (6) | ||
| Manta et al[11], 2013 | Italy | A | 30 | - | - | OTSC | 1-month | (3), (5) |
| Aina et al[12], 2001 | Canada | A | 132 | - | 63 | AE | 30 days | (2) |
| Arrayeh et al[13], 2012 | United States | B | 115 | 46 | 61 | AE | 30 days | (1) |
| Defreyne et al[14], 2001 | Belgium | A | 91 | AE | - | (1) | ||
| Dixon et al[15], 2013 | United Kingdom | B | 40 | 32 | 71 | AE | - | (1) |
| Holme et al[16], 2006 | Denmark | A | 40 | 21 | 70 | AE | - | (3), (4) |
| Kaminskis et al[17], 2019 | Latvia | B | 738 | 222 | 69 | AE | - | (3), (4), (5) |
| Laursen et al[18], 2014 | Denmark | C | 105 | - | - | STAE | 30 days | (3) |
| Muhammad et al[19], 2019 | Pakistan | A | 32 | 0.687 | 56 | TAE | 30 days | (2), (4) |
| Padia et al[20], 2009 | United States | A | 108 | 66 | 66 | Transcatheter embolization | 30 days | (2), (4), (5) |
| Poultsides et al[21], 2008 | United States | A | 57 | 38 | 65 | Embolization procedures | - | (2), (5), (6) |
| Song et al[22], 2011 | Korea | A | 16 | 11 | 59 | TAE | 4 weeks | (2), (3), (4), (5) |
| Spiliopoulos et al[23], 2018 | Italy | A | 44 | 44 | 74 | TAE | 3.5 years | (3), (4), (6) |
| Ephraim et al[24], 2022 | United States | A | 74 | - | - | TAE | 30 days | (2), (3), (4) |
| Ang et al[25], 2012 | Singapore | B | 93 | 64 | 67 | TAE | 30 days | (1), (4), (6) |
| Lee et al[26], 2015 | Korea | A | 66 | 42 | 60 | TAE or AE | 30 days | (3), (4) |
| Huang et al[27], 2014 | Taiwan, China | A | 49 | 31 | 67 | TAE | 30 days | (2), (3), (4), (5), (6) |
| Hur et al[28], 2017 | Korea | A | 152 | 109 | 66 | AE | 1-month | (2), (4), (6) |
| Ref. | Study type1 | Cochrane | CASP | ||||
| Representativeness of the sample | Clarity of intervention description | Objectivity of outcome measures | Completeness of follow-up | Control of confounding bias | |||
| McGraw et al[8], 2023 | C | 8 | - | - | - | - | - |
| Lau et al[9], 2019 | C | 7 | - | - | - | - | - |
| Nykänen et al[10], 2017 | B | - | Fairly good | Fairly good | Fairly good | Fairly good | Fairly good |
| Manta et al[11], 2013 | A | - | Average | Fairly good | Fairly good | Fairly good | Average |
| Aina et al[12], 2001 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Average |
| Arrayeh et al[13], 2012 | B | - | Fairly good | Fairly good | Fairly good | Fairly good | Average |
| Defreyne et al[14], 2001 | A | - | Fairly good | Fairly good | Fairly good | Average | Average |
| Dixon et al[15], 2013 | B | - | Average | Fairly good | Fairly good | Average | Fairly good |
| Holme et al[16], 2006 | A | - | Average | Fairly good | Fairly good | Average | Average |
| Kaminskis et al[17], 2019 | B | - | Fairly good | Fairly good | Fairly good | Average | Fairly good |
| Laursen et al[18], 2014 | C | 8 | - | - | - | Fairly good | - |
| Muhammad et al[19], 2019 | A | - | Average | Fairly good | Fairly good | Fairly good | Average |
| Padia et al[20], 2009 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Average |
| Poultsides et al[21], 2008 | A | - | Fairly good | Fairly good | Fairly good | Average | Fairly good |
| Song et al[22], 2011 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Fairly good |
| Spiliopoulos et al[23], 2018 | A | - | Average | Fairly good | Fairly good | Fairly good | Average |
| Ephraim et al[24], 2022 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Fairly good |
| Ang et al[25], 2012 | B | - | Fairly good | Fairly good | Fairly good | Fairly good | Average |
| Lee et al[26], 2015 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Fairly good |
| Huang et al[27], 2014 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Fairly good |
| Hur et al[28], 2017 | A | - | Fairly good | Fairly good | Fairly good | Fairly good | Fairly good |
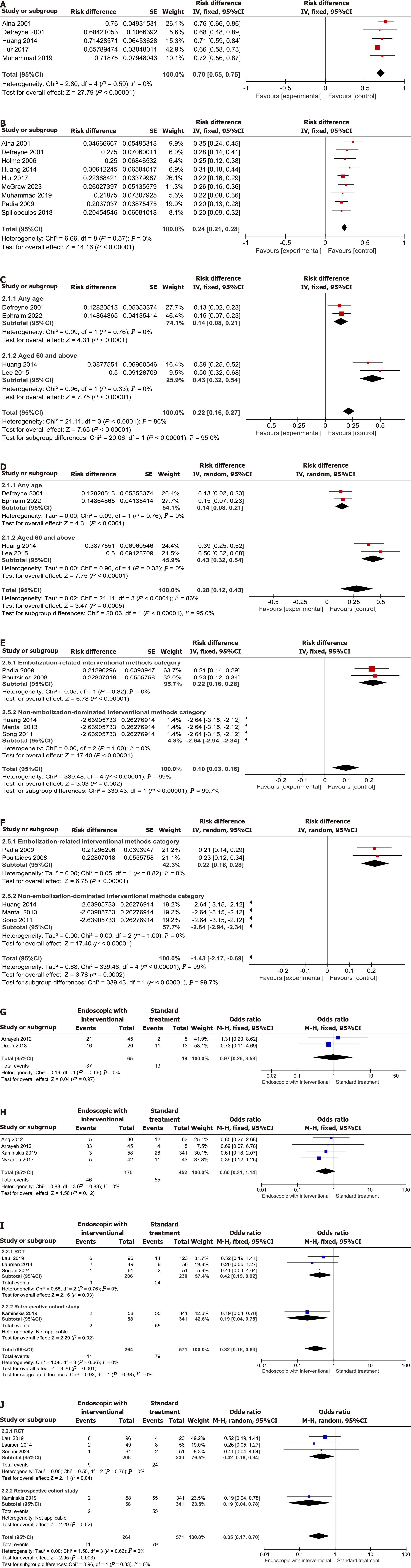
Single-group intervention studies: (1) Clinical success and mortality: Several studies[12,14,19,27,28] reported the clinical success rate. The heterogeneity was I² = 0 (P = 0.59), and the risk difference (RD) of clinical success before and after intervention was 0.70 [95% confidence interval (CI): 0.65-0.75] (P < 0.00001) (Figure 2A). Several studies[8,12,14,16,19,20,23,27,28] reported the mortality of endoscopic hemostasis combined with vascular interventional therapy. The heterogeneity was I² = 0 (P = 0.57), and the RD of mortality before and after intervention was 0.24 (95%CI: 0.21-0.28) (P < 0.00001) (Figure 2B); (2) Rebleeding: Two studies[14,24] reported the rebleeding rate in patients of all ages who underwent endoscopic hemostasis combined with vascular interventional therapy. Analyzed using a fixed-effects model, the RD of rebleeding before and after intervention was 0.14 (95%CI: 0.08-0.21). Two studies[26,27] reported the rebleeding rate in patients aged ≥ 60 years who received the same combined therapy. The RD of rebleeding before and after intervention was 0.43 (95%CI: 0.32-0.54). The overall rebleeding RD for all age groups was 0.22 (95%CI: 0.16-0.27) (Z = 7.75, P < 0.00001) (Figure 2C). After switching to a random-effects model, the overall rebleeding RD was 0.28 (95%CI: 0.12-0.43) (Z = 3.47, P < 0.00001) (Figure 2D). These results indicate that there are differences in the overall rebleeding rate between different effect models, and factors such as age may have an impact on the rebleeding rate; and (3) Reinterventions: Two studies[20,21] reported the reintervention rate in patients who underwent transcatheter embolization/embolization procedures. Analyzed using a fixed-effects model, the RD of reinterventions before and after intervention was 0.22 (95%CI: 0.16-0.28). Three studies[11,22,27] reported the reintervention rate in patients who received over-the-scope clip (OTSC)/endoscopic treatment/transcatheter arterial embolization. Analyzed using a fixed-effects model, the RD of reinterventions was -2.64 (95%CI: -2.94 to -2.34). The overall effect test showed a statistically significant difference (Z = 3.03, P = 0.002), suggesting a significant difference in the reintervention rate between different vascular interventional methods. This suggests that the choice of interventional method affects the probability of subsequent reinterventions. In addition, the subgroup heterogeneity test result (I² = 0) suggested no significant heterogeneity among the included studies within subgroups, while there was significant heterogeneity between the two subgroups (I² = 99%, P < 0.05), indicating that the heterogeneity may originate from the difference between embolization and non-embolization interventional methods (Figure 2E).
Analysis after switching to a random-effects model showed that the intra-subgroup analysis results were consistent with those of the aforementioned fixed-effects model, while the overall RD between subgroups was inconsistent with that of the fixed-effects model. When there is significant inter-subgroup heterogeneity (heterogeneity between embolization and non-embolization interventional methods), the fixed-effects model may produce bias due to the assumption of no substantial heterogeneity between studies. In contrast, the random-effects model better accounts for variations between studies; the stability and reliability of the results are more affected by heterogeneity, which also suggests that there are true differences in the effects of different interventional methods (Figure 2F).
RCTs and retrospective cohort studies: (1) Successful initial hemostasis and mortality: Two studies[13,15] reported the rate of successful initial hemostasis. There was no significant difference in the overall rate of successful initial hemostasis between the endoscopic hemostasis combined with vascular interventional therapy group and the standard treatment group (Z = 0.04, P = 0.97) (Figure 2G). Four retrospective cohort studies[10,13,17,25] reported that there was no significant difference in the overall mortality between the endoscopic hemostasis combined with vascular interventional therapy group and the standard treatment group (Z = 1.56, P = 0.12) (Figure 2H); (2) Rebleeding: Three RCTs[8,9,18] and one retrospective cohort study[17] reported that the overall rebleeding rate in the endoscopic hemostasis combined with vascular interventional therapy group was lower than that in the standard treatment group (Z = 3.26, P = 0.001). After switching to the random-effects model, the result was consistent with that of the aforementioned fixed-effects model (Z = 2.95, P = 0.03) (Figure 2I and J). This suggests that the regimen of endoscopic hemostasis combined with vascular interventional therapy has more advantages in reducing rebleeding and may be a better clinical treatment option; the study results are less affected by heterogeneity and, thus, relatively robust.
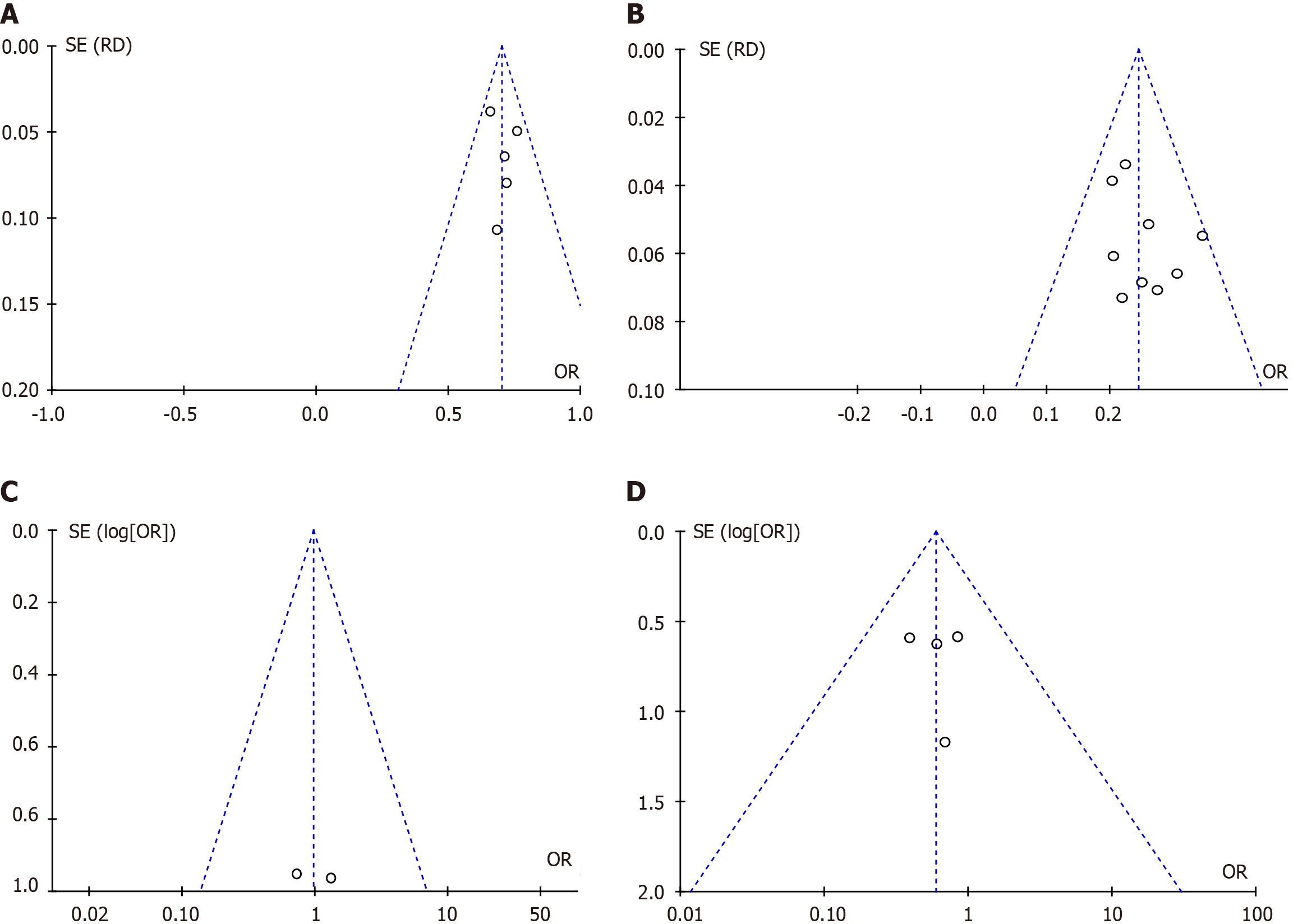
Publication bias assessment was performed for the extensively reported clinical success and mortality indicators from single-group intervention studies, as well as successful initial hemostasis and mortality indicators from RCTs or retrospective cohort studies. Funnel plots were used for visualization, and the results showed that the funnel plots of clinical success and mortality from single-group intervention studies, along with successful initial hemostasis and mortality from RCTs or retrospective cohort studies, were basically symmetrical (Figure 3). This indicates that there was no significant publication bias among the included studies, and the results are reliable.
This study demonstrated no significant difference in the initial hemostasis success rate between combined endoscopic-vascular interventional therapy and standard treatment, whereas the overall clinical success rate reached 70% (95%CI: 65%-75%). This discrepancy may be attributed to the definition of “clinical success”, which encompasses long-term efficacy (e.g., absence of rebleeding within 48-72 hours post-procedure), whereas initial hemostasis only reflects immediate effectiveness. A potential mechanism is that vascular interventional therapy (e.g., transarterial embolization) plays a critical role in controlling persistent or recurrent bleeding, particularly for bleeding sites inaccessible to endoscopy (e.g., branches of the short gastric artery) or patients with coagulopathy. Data from one study[29] showed that combined therapy achieved higher clinical success rates for Dieulafoy lesions than endoscopic therapy alone, supporting the complementary value of vascular intervention in complex bleeding cases. Heterogeneity in clinical success rates across the included studies may stem from differences in intervention timing; early combined therapy (within 24 hours of bleeding) appears superior to delayed intervention (> 48 hours)[30].
Single-group intervention studies showed that the rebleeding rate of the combined therapy group (endoscopic hemostasis combined with vascular interventional therapy) was 22%, and high heterogeneity might be mainly attributed to age. In RCTs and retrospective cohort studies, there was a significant difference in the rebleeding rate between the combined therapy and standard treatment groups (the former had a lower rebleeding rate). The lack of a control group in single-group studies may overestimate complication rates, as these studies often include patients with more complex conditions (e.g., advanced age and multi-organ failure). In controlled studies, the consistent finding of reduced rebleeding risk with combined therapy may be attributed to precise patient selection (e.g., screening high-risk patients based on the Forrest classification) and technological advancements (e.g., novel hemostatic clips combined with coil embolization)[31].
Single-group intervention studies reported a mortality rate of 24% (95%CI: 21%-28%), whereas retrospective cohort studies showed no significant difference between combined therapy and standard treatment. This discrepancy may arise from a case-mix bias in single-group studies (e.g., inclusion of more patients with end-stage liver disease or cancer), whereas cohort studies minimize confounding through baseline matching[32]. Mortality, a difficult endpoint, requires high-quality evidence to reach robust conclusions. In this study, only four cohort studies reported mortality data with small sample sizes, potentially leading to insufficient statistical power.
In the combined therapy group, the reintervention rate was significantly higher in the subgroup using embolization-related interventional methods than in the control group; in contrast, the reintervention rate was significantly lower in the subgroup adopting non-embolization-dominated interventional methods than in the control group. These findings indicate that the reintervention rate of combined therapy is closely associated with the application of embolization-related interventional methods - embolization-related interventions tend to increase the reintervention rate, while non-embolization-dominated interventions contribute to a reduction in this rate.
From the perspective of clinical practice and technical characteristics, the high reintervention rate of embolization-related interventions can also be attributed to their “non-reparative” effect on lesions. Specifically, these interventions only block blood flow without addressing the primary pathological lesions, such as ulcers or vascular malformations. When embolic materials (e.g., gelatin sponges) are absorbed or collateral circulation is established as a compensatory mechanism, recurrent bleeding is likely to occur, necessitating secondary interventions[33]. Additionally, embolization may damage surrounding normal blood vessels, leading to local tissue ischemia and necrosis, which further induces new bleeding or perforation and consequently increases the need for reinterventions.
On the other hand, the low reintervention rate of non-embolization-dominated interventions (e.g., OTSC) lies in their “precise lesion repair” capability. For instance, OTSCs directly occlude bleeding points, and tissue adhesives seal vascular breaches, thereby eliminating the causes of bleeding at the source[2]. Meanwhile, these interventions are confined to the mucosal layer, without affecting the main vascular trunks or collateral circulation, and thus, do not cause ischemic com
Immediate hemostasis and precise embolization: Endoscopic injection of epinephrine or placement of hemostatic clips under direct vision controls visible bleeding, whereas angiography localizes microvascular injuries (e.g., arterial branches < 2 mm in diameter) for superselective embolization using microcoils or gelatin sponges, minimizing damage to normal tissue[34].
Reducing hemodynamic instability risk: In patients with hypotension (systolic blood pressure < 90 mmHg), combination therapy shortens the hemostasis time and mitigates coagulopathy caused by repeated hemostasis failures[35]. Despite existing research exploring the clinical efficacy and safety of endoscopic and vascular interventional therapies for acute NVUGIB, several critical unresolved questions remain.
Optimal treatment sequence for bleeding at different anatomical sites (e.g., gastric fundus and duodenum): Universal evidence across subtypes is lacking.
Biomarker-based treatment stratification guidelines: Guidelines based on biomarkers such as platelet count and hemoglobin decline rate have not been established. The precise value of biomarkers in treatment selection - including threshold definitions and dynamic monitoring - remains unclear.
Treatment response and long-term prognosis in patients with low-risk forrest grade III: Differences in treatment response (e.g., necessity of combined therapy and preference for single-modality treatment) and their associations with long-term outcomes require further validation and refinement through large-sample studies.
Heterogeneity and bias risks: Despite efforts to control heterogeneity through sensitivity and subgroup analyses, significant heterogeneity persisted in certain outcomes (e.g., mortality), likely stemming from differences in study design (single-group vs controlled studies) and variability in interventions (different endoscopic techniques and interventional materials).
Insufficient high-quality evidence: Only three RCTs with small sample sizes were included, leading to inadequate statistical power for the subgroup analyses.
Lack of long-term follow-up data: Over 80% of studies had a follow-up period ≤ 30 days, precluding assessment of long-term outcomes (e.g., 1-year rebleeding rate, quality of life).
Potential impact of publication bias: Although Begg’s and Egger’s tests did not indicate a significant bias, the notable change in results after excluding studies at the funnel plot margins suggests a possible undetected small-sample bias.
Implications for individualized treatment: For patients with high risk (e.g., Forrest Ia and hemodynamic instability), early combined therapy may improve clinical success rates. For patients with low risk (e.g., Forrest IIc-III), primary endoscopic hemostasis is recommended to avoid overtreatment.
Importance of multidisciplinary collaboration: Establishing hemorrhage management teams comprising gastroenterologists, interventional radiologists, and intensivists to develop standardized protocols and reduce decision-making time is critical.
Future research directions: Large-scale multicenter RCTs are urgently required to address this issue: (1) Comparison between combined therapy and endoscopic/interventional monotherapy; (2) Development of artificial intelligence-based risk prediction models to guide treatment selection; and (3) Safety and efficacy of novel bioabsorbable embolization materials.
This meta-analysis demonstrated that combined endoscopic hemostasis and vascular interventional therapy for acute NVUGIB achieved high clinical success rates and reduced the risk of rebleeding compared with standard treatment. However, mortality outcomes are significantly influenced by study design, which limits their reliability. Current evidence supports the use of combination therapy in patients at high risk of bleeding; however, bias risks in single-group studies require cautious interpretation. Future research should include more high-quality RCTs and long-term follow-up data to clarify optimal treatment strategies and target populations.
| 1. | Kavitt RT, Gralnek IM. Ideal strategy for nonvariceal upper gastrointestinal bleeding. Curr Opin Gastroenterol. 2024;40:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Lau JYW, Li R, Tan CH, Sun XJ, Song HJ, Li L, Ji F, Wang BJ, Shi DT, Leung WK, Hartley I, Moss A, Yu KYY, Suen BY, Li P, Chan FKL. Comparison of Over-the-Scope Clips to Standard Endoscopic Treatment as the Initial Treatment in Patients With Bleeding From a Nonvariceal Upper Gastrointestinal Cause : A Randomized Controlled Trial. Ann Intern Med. 2023;176:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Ini’ C, Distefano G, Sanfilippo F, Castiglione DG, Falsaperla D, Giurazza F, Mosconi C, Tiralongo F, Foti PV, Palmucci S, Venturini M, Basile A. Embolization for acute nonvariceal bleeding of upper and lower gastrointestinal tract: a systematic review. CVIR Endovasc. 2023;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (1)] |
| 5. | Schmidt A, Gölder S, Goetz M, Meining A, Lau J, von Delius S, Escher M, Hoffmann A, Wiest R, Messmann H, Kratt T, Walter B, Bettinger D, Caca K. Over-the-Scope Clips Are More Effective Than Standard Endoscopic Therapy for Patients With Recurrent Bleeding of Peptic Ulcers. Gastroenterology. 2018;155:674-686.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Shah MP, Saleem S, Attar B, Cui C, Mutneja H. Hemospray Versus Conventional Therapy for Non-variceal Upper Gastrointestinal Bleeding: A Systematic Review and Meta-Analysis. Cureus. 2024;16:e55079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Yanting Z, Xv D, Long W, Wang J, Tang C, Feng M, Li X, Wang B, Zhong J. Experience and coping strategies of bowel dysfunction in postoperative patients with rectal cancer: a systematic review of qualitative evidence. PeerJ. 2023;11:e15037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | McGraw JR, Kiefer RM, Shah A, Clark TWI, Shlansky-Goldberg RD, Nadolski GJ, Hunt SJ, Gade TP. Outcomes of Transarterial Embolization for Acute Nonvariceal Upper Gastrointestinal Bleeding: Correlation with Periprocedural Endoscopy. J Vasc Interv Radiol. 2023;34:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Lau JYW, Pittayanon R, Wong KT, Pinjaroen N, Chiu PWY, Rerknimitr R, Holster IL, Kuipers EJ, Wu KC, Au KWL, Chan FKL, Sung JJY. Prophylactic angiographic embolisation after endoscopic control of bleeding to high-risk peptic ulcers: a randomised controlled trial. Gut. 2019;68:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Nykänen T, Peltola E, Kylänpää L, Udd M. Bleeding gastric and duodenal ulcers: case-control study comparing angioembolization and surgery. Scand J Gastroenterol. 2017;52:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Manta R, Galloro G, Mangiavillano B, Conigliaro R, Pasquale L, Arezzo A, Masci E, Bassotti G, Frazzoni M. Over-the-scope clip (OTSC) represents an effective endoscopic treatment for acute GI bleeding after failure of conventional techniques. Surg Endosc. 2013;27:3162-3164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, Soulez G. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Arrayeh E, Fidelman N, Gordon RL, LaBerge JM, Kerlan RK Jr, Klimov A, Bloom AI. Transcatheter arterial embolization for upper gastrointestinal nonvariceal hemorrhage: is empiric embolization warranted? Cardiovasc Intervent Radiol. 2012;35:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, Troisi R, Kunnen M. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Dixon S, Chan V, Shrivastava V, Anthony S, Uberoi R, Bratby M. Is there a role for empiric gastroduodenal artery embolization in the management of patients with active upper GI hemorrhage? Cardiovasc Intervent Radiol. 2013;36:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Holme JB, Nielsen DT, Funch-Jensen P, Mortensen FV. Transcatheter arterial embolization in patients with bleeding duodenal ulcer: an alternative to surgery. Acta Radiol. 2006;47:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Kaminskis A, Ivanova P, Kratovska A, Ponomarjova S, Ptašņuka M, Demičevs J, Demičeva R, Boka V, Pupelis G. Endoscopic hemostasis followed by preventive transarterial embolization in high-risk patients with bleeding peptic ulcer: 5-year experience. World J Emerg Surg. 2019;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Laursen SB, Hansen JM, Andersen PE, Schaffalitzky de Muckadell OB. Supplementary arteriel embolization an option in high-risk ulcer bleeding--a randomized study. Scand J Gastroenterol. 2014;49:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Muhammad A, Awais M, Sayani R, Saeed MA, Qamar S, Rehman A, Baloch NU. Empiric Transcatheter Arterial Embolization for Massive or Recurrent Gastrointestinal Bleeding: Ten-year Experience from a Single Tertiary Care Center. Cureus. 2019;11:e4228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Padia SA, Geisinger MA, Newman JS, Pierce G, Obuchowski NA, Sands MJ. Effectiveness of coil embolization in angiographically detectable versus non-detectable sources of upper gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Poultsides GA, Kim CJ, Orlando R 3rd, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008;143:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Song JS, Kwak HS, Chung GH. Nonvariceal upper gastrointestinal bleeding: the usefulness of rotational angiography after endoscopic marking with a metallic clip. Korean J Radiol. 2011;12:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Spiliopoulos S, Inchingolo R, Lucatelli P, Iezzi R, Diamantopoulos A, Posa A, Barry B, Ricci C, Cini M, Konstantos C, Palialexis K, Reppas L, Trikola A, Nardella M, Adam A, Brountzos E. Transcatheter Arterial Embolization for Bleeding Peptic Ulcers: A Multicenter Study. Cardiovasc Intervent Radiol. 2018;41:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Ephraim Joseph K, Devane AM, Abrams GA. Patient and endoscopic characteristics and clinical outcomes in subjects with non-variceal GI bleeding referred for transarterial embolization: a single-center experience. Abdom Radiol (NY). 2022;47:3883-3891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Ang D, Teo EK, Tan A, Ibrahim S, Tan PS, Ang TL, Fock KM. A comparison of surgery versus transcatheter angiographic embolization in the treatment of nonvariceal upper gastrointestinal bleeding uncontrolled by endoscopy. Eur J Gastroenterol Hepatol. 2012;24:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Lee HH, Park JM, Chun HJ, Oh JS, Ahn HJ, Choi MG. Transcatheter arterial embolization for endoscopically unmanageable non-variceal upper gastrointestinal bleeding. Scand J Gastroenterol. 2015;50:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Huang YS, Chang CC, Liou JM, Jaw FS, Liu KL. Transcatheter arterial embolization with N-butyl cyanoacrylate for nonvariceal upper gastrointestinal bleeding in hemodynamically unstable patients: results and predictors of clinical outcomes. J Vasc Interv Radiol. 2014;25:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Hur S, Jae HJ, Lee H, Lee M, Kim HC, Chung JW. Superselective Embolization for Arterial Upper Gastrointestinal Bleeding Using N-Butyl Cyanoacrylate: A Single-Center Experience in 152 Patients. J Vasc Interv Radiol. 2017;28:1673-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Rai P, Kumar P, Hoda US, Balankhe K. Endoscopic ultrasound-guided vascular interventions: A review (with videos). Indian J Gastroenterol. 2024;43:927-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Ozen C, Al-Hashimi M, Tornby Stender M, Thorlacius-Ussing O, Larsen AC. Transarterial embolization of gastroduodenal peptic ulcer bleeding: a single-center study of safety and efficacy. Langenbecks Arch Surg. 2025;410:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Li W, Wang J, Fu H, Liu J. Isolated sigmoid varicose vein rupture and hemorrhage: A case report. Medicine (Baltimore). 2022;101:e30024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Alali AA, Barkun AN. An update on the management of non-variceal upper gastrointestinal bleeding. Gastroenterol Rep (Oxf). 2023;11:goad011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (36)] |
| 33. | Loffroy R, Desmyttere AS, Mouillot T, Pellegrinelli J, Facy O, Drouilllard A, Falvo N, Charles PE, Bardou M, Midulla M, Aho-Gléglé S, Chevallier O. Ten-year experience with arterial embolization for peptic ulcer bleeding: N-butyl cyanoacrylate glue versus other embolic agents. Eur Radiol. 2021;31:3015-3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Kobayashi K, Inoue Y, Omiya K, Sato S, Kato T, Oba A, Ono Y, Sato T, Ito H, Matsueda K, Saiura A, Takahashi Y. Diagnosis and management of postpancreatectomy hemorrhage: A single-center experience of consecutive 1,096 pancreatoduodenectomies. Pancreatology. 2023;23:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 35. | Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, Saluja V, Mohan Agarwal P, Bihari C, Shasthry SM, Jindal A, Bhardwaj A, Kumar G, Sarin SK. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology. 2020;71:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/