Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.112729
Revised: September 6, 2025
Accepted: October 11, 2025
Published online: November 27, 2025
Processing time: 112 Days and 21 Hours
Peroral endoscopic esophageal myotomy (POEM) is an innovative, minimally invasive endoscopic technique that has been widely adopted and recognized for the clinical management of achalasia because of its advantages of minimal trauma and rapid recovery. Nevertheless, clinical data have indicated that approximately 67% of patients experience esophageal pain after POEM. This high prevalence of pain not only affects patients’ post-POEM recovery experience and quality of life but also presents challenges to its clinical implementation. Therefore, it is urgently necessary to explore effective intervention strategies.
To accurately determine the incidence of post-POEM pain and to comprehensively investigate the potential risk factors for the development of post-POEM pain.
In this study, 123 patients who were clinically diagnosed with achalasia and who underwent POEM were included. Baseline demographic characteristics, post-POEM numerical rating scale (NRS) pain scores, and anesthesia/surgery-related parameters were systematically collected. Patients were categorized into a pain group and a non-pain group on the basis of whether the NRS score exceeded 4 at 12 hours post-POEM. Univariate and multivariate logistic regression analyses were conducted to identify independent risk factors associated with post-POEM pain.
On the basis of the predefined inclusion and exclusion criteria, 123 eligible patients were enrolled. After adjusting for confounding factors, stepwise multivariate logistic regression analysis revealed that the preoperative Eckardt score [odds ratio (OR) = 1.317, 95% confidence interval (95%CI): 0.992-1.748, P = 0.057] and preoperative anxiety status (OR = 5.195, 95%CI: 1.691-15.959, P = 0.004) were independent risk factors for post-POEM pain. Our multifactor model exhibited robust predictive ability for postoperative pain following POEM, with an area under the receiver operating characteristic curve of 0.760 (95%CI: 0.661–0.859).
Patients with achalasia who underwent POEM presented a high prevalence of post-POEM pain, which was moderate or severe in 26.8% of these patients. After adjusting for confounding factors, multivariate analysis revealed that preoperative anxiety and a higher Eckardt score were independent risk factors for post-POEM pain.
Core Tip: This study revealed that the incidence of postoperative pain in patients with achalasia who underwent peroral endoscopic esophageal myotomy (POEM) significantly increased, with 26.8% of patients experiencing at least moderate-intensity pain (numerical rating scale score ≥ 4). Furthermore, preoperative anxiety and a high Eckardt score were independent risk factors for post-POEM pain. These findings suggest that incorporating clinical symptom assessment, psychological status screening, and optimized analgesic strategies into preoperative anesthesia management is crucial for preventing and relieving postoperative pain.
- Citation: Yin JW, Zhang WQ, Xu H, Wen TT, Song SW. Risk factors for esophageal pain after peroral endoscopic myotomy under general anesthesia: A retrospective study. World J Gastrointest Surg 2025; 17(11): 112729
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/112729.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.112729
Achalasia is a spastic esophageal motility disorder that is characterized by impaired inhibitory motor neuron function and leads to incomplete relaxation of the lower esophageal sphincter as well as the absence of peristaltic contractions. The clinical manifestations of achalasia include dysphagia, gastroesophageal reflux, esophageal pain, weight loss, and anemia[1]. These symptoms can be quantitatively assessed using the Eckardt score (0-3 points assigned to the severity of each symptom, with a total score ranging from 0 to 12 points)[2].
Owing to the rapid advancements in endoscopic technology and associated equipment, peroral endoscopic esophageal myotomy (POEM) has been established as a safe, effective, and minimally invasive treatment for achalasia as well as other esophageal motility disorders. Compared with laparoscopic Heller myotomy, POEM is significantly less invasive, which has paradoxically led to underappreciation of post-POEM pain management among clinicians. Clinical studies have revealed a striking 67% incidence of post-POEM esophageal pain, with 10% of cases classified as severe pain[3]. This pain significantly impedes recovery and quality of life. Furthermore, post-POEM pain can induce eating dysfunction and is accompanied by negative emotional experiences, which can increase the risk of post-POEM complications[4-6].
Although postoperative pain after POEM has been reported previous research, there is currently an unmet need for large-sample, rigorous studies that explore patient-specific and procedure-specific risk factors underlying this pain—information critical for optimizing clinical pain management.
Therefore, accurate identification of risk factors for post-POEM esophageal pain is critically important for early prevention and clinical intervention.
This retrospective real-world study aimed to identify risk factors for postoperative pain among patients who underwent POEM under general anesthesia. The study protocol was approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine ([2025B] IIT Ethics Approval No. 0707) and was registered with the Chinese Clinical Trial Registry (https://www.chictr.org.cn/Registration number: ChiCTR2500104753). All the data were anonymized to protect patient privacy.
This study enrolled 123 patients who underwent POEM at the Endoscopy Centre of the First Affiliated Hospital, Zhejiang University School of Medicine between August 2022 and May 2025. Patients with a diagnosis of achalasia, an Eckardt score > 3, and scheduled for elective POEM[7] were screened.
Inclusion criteria: (1) No gender restriction, aged 18 to 80 years; (2) American Society of Anesthesiologists physical status classification of I-II; and (3) Availability of complete anesthesia records and surgical reports.
Exclusion criteria: (1) Presence of esophagogastric varices, active gastrointestinal malignancy, pregnancy, or allergy to opioid drugs such as morphine; and (2) Intraoperative complications affecting post-POEM pain assessment, including but not limited to esophageal perforation, subcutaneous emphysema, pneumothorax, mediastinal emphysema, pneumoperitoneum, and pleural effusion[8].
The numerical rating scale (NRS) is a subjective assessment tool that allows patients to self-report their pain intensity using numerical values. It is widely used in clinical settings because of its simplicity and ease of administration. The NRS ranges from 0 to 10, where 0 represents "no pain" and 10 represents the "worst pain imaginable"[9]. Patients are asked to select a number that best corresponds to their current pain level. The scale can be administered verbally, visually, or electronically, making it adaptable to various clinical scenarios.
The effectiveness and validity of the NRS have been extensively studied. Research has demonstrated that the NRS has good to excellent test-retest reliability (intraclass correlation coefficient 0.75-0.94) in patients with low back pain, suggesting that measurements are consistent over time when no clinical change has occurred[10]. Compared with other pain assessment tools, such as the visual analog scale or the faces pain scale, the NRS is generally preferred by clinicians and patients because it is easier to administer and understand[9].
The NRS divides pain into three distinct categories based on severity: (1) Mild pain (scores 1-3): Pain that is noticeable but does not significantly interfere with daily activities or sleep; (2) Moderate pain (scores 4-6): Pain that is strong enough to disrupt normal activities and may cause sleep disturbances; and (3) Severe pain (scores 7-10): Pain that makes concentration difficult, severely limits daily activities, and often prevents sleep.
Patients were categorized into a pain group and a non-pain group according to whether their NRS pain score was at least 4 at 12 hours post-POEM. Clinical studies have provided robust evidence regarding postoperative chest pain characteristics following POEM. Specifically, 67% of patients developed chest pain within the first postoperative day in one study, with 10% of these patients experiencing severe pain. Consistently, research has demonstrated that chest pain after POEM is most intense within the initial 24 hours post-procedure, and the pain peak typically occurs within the first 12 hours[5].
From a physiological standpoint, the 12-hour post-operative time point aligns with the peak concentration of inflammatory mediators released during surgical tissue manipulation. The POEM procedure inherently involves the creation of a submucosal tunnel and the performance of esophageal myotomy, both of which induce a local inflammatory response. This inflammatory process typically peaks between 12 hours and 16 hours post-operatively, directly contributing to heightened pain perception in patients during this period[5,11].
Furthermore, from a logistical perspective, 12 hours often coincides with shift changes or routine clinical rounds, facilitating consistent documentation and interdisciplinary communication about pain management. It also precedes typical discharge planning for patients with uncomplicated POEM (often within 24-48 hours), ensuring that pain is controlled before the patient transitions to oral analgesics.
Clinical demographic data, perioperative anesthetic parameters, and surgical parameters were collected. The following data were extracted from the anesthesia information management system (Do Care, Medical System Company): Occurrence of post-POEM pain, age, sex, body mass index, preoperative anxiety status (by reviewing preoperative nursing assessment records and clinical medical records, patients were categorized into the anxiety group if their records explicitly documented terms such as "anxiety" or "nervousness", or if they had received preoperative sedative medications; the remaining patients were assigned to the nonanxiety group), preoperative comorbidities, operation duration, anesthesia duration, intraoperative use of opioid drugs, anesthesia maintenance methods, lifestyle habits (such as smoking and alcohol consumption), preoperative Eckardt score, length of esophageal myotomy, and distance from the endpoint of esophageal myotomy to the cardia.
Patients were instructed to maintain a clear liquid diet for 48 hours prior to POEM and to completely abstain from any oral intake after midnight on the day of surgery. Prophylactic intravenous antibiotic administration and proton pump inhibitor therapy were initiated on the day of surgery and continued throughout the post-POEM hospital stay[8]. Anticoagulant or antiplatelet therapy was managed in accordance with the current guidelines of the British Society of Gastroenterology and the European Society of Gastrointestinal Endoscopy for patients undergoing endoscopic procedures while on antithrombotic therapy[12].
All procedures were conducted at the endoscopy center of our hospital, with all patients receiving general anesthesia, endotracheal intubation, and positive pressure ventilation. Patients were permitted to consume carbohydrate-rich beverages on the day prior to surgery but were mandated to fast for 12 hours preoperatively (with strict prohibition of any food or liquid intake). Upon arrival at the operating theater, continuous monitoring of electrocardiogram, noninvasive blood pressure, and pulse oxygen saturation was immediately initiated. Following positioning in the left lateral decubitus position, esophagogastroduodenoscopy was performed under topical lidocaine anesthesia to prophylactically clear residual food or fluid from the dilated esophagus and mitigate the risk of aspiration.
After the operation, once the patients were awake they were transferred to the post-anesthesia care unit and, following a 1-hour observation period, were moved to the ward. If a patient’s NRS pain score reached 4 or higher at 12 hours after POEM, 100 mg of tramadol was intramuscularly administered for pain control.
Our institution strictly adheres to the clinical practice guidelines for POEM established by the American Gastroenterological Association[13]. Consistent with these recommendations, POEM was performed for achalasia types I to III, unresolved esophagogastric junction outflow obstruction, and/or spastic esophageal disorders.
All POEM procedures were performed by experienced endoscopists, each of whom independently performed at least 50 POEM procedures. General anesthesia was administered, and carbon dioxide (CO2) insufflation was maintained throughout the procedure. A high-definition gastroscope (GIF 290, Olympus Corporation, Tokyo, Japan) fitted with a transparent cap (Olympus Corporation) was used. Approximately 10-13 cm proximal to the gastroesophageal junction (GEJ), a submucosal bleb was created by injection of a mixture of methylene blue and normal saline. A 2-cm longitudinal mucosal incision was made using an ERBE Endocut Q 3:1:1 current (with a triangular-tip knife, Olympus, or a HybridKnife, ERBE Elektromedizin GmbH, Tübingen, Germany). Under continuous CO2 insufflation, a submucosal tunnel was meticulously dissected, extending 3-4 cm beyond the GEJ into the gastric cardia. Selective myotomy was carried out according to preoperative manometric findings and achalasia subtype. Hemostasis and preemptive co
Key intraoperative steps potentially influencing postoperative pain included precise hemostasis intraoperatively to minimize tissue trauma and edema, continuous utilization of CO2 insufflation throughout the procedure (to reduce the incidence of subcutaneous emphysema and pneumomediastinum), and secure closure of the mucosal incision (to decrease the risk of inflammation).
Perioperative relevant information of all patients meeting the inclusion criteria was collected via standardized clinical questionnaires. The data are expressed as numerical values and percentages, and continuous data are expressed as the mean ± SD, while the χ2 test was used to assess between-group differences in distribution. Univariate regression analysis was conducted to explore the associations between each factor and post-POEM pain, and multivariate stepwise logistic regression analysis was used to identify independent risk factors for post-POEM pain. A P value < 0.1 was considered to indicate statistical significance (two-tailed test). All the statistical analyses were performed using SPSS 23.0 statistical software (IBM Corporation, Armonk, NY, United States) and R Studio version 1.4 software (University of Auckland, New Zealand).
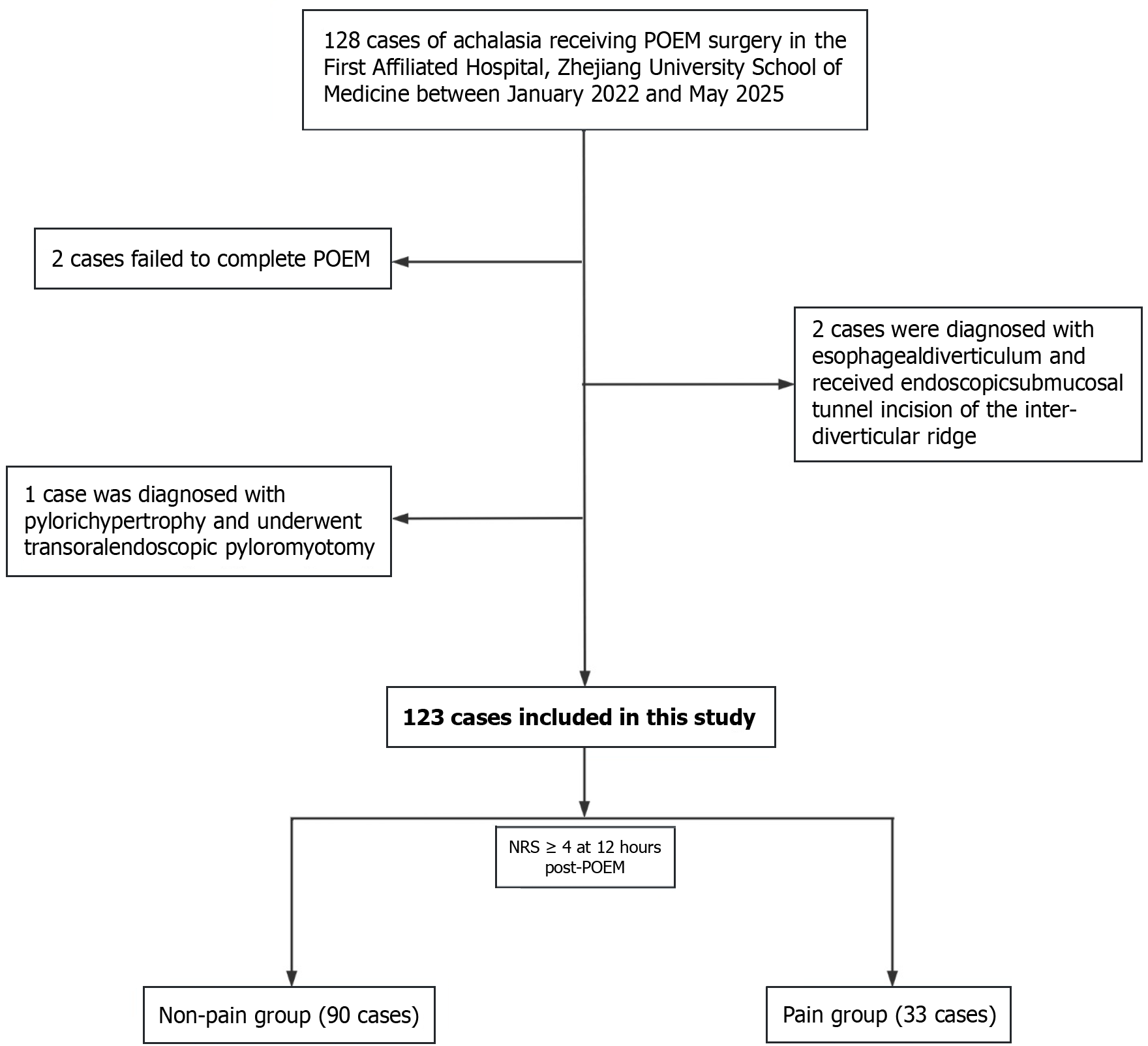
The flow diagram for this clinical study is shown in Figure 1. A total of 123 patients were included and were stratified into a non-pain group (90 patients) and a pain group (33 patients) on the basis of the occurrence of post-POEM pain. The baseline demographics and perioperative characteristics of the two groups are presented in Table 1. In the non-pain group, the average preoperative Eckardt score was 5.3 ± 1.7. The pain group had a significantly greater average preoperative Eckardt score (6.2 ± 1.7 (Z = 0.643, P = 0.008). In the pain group, twenty-eight (84.8%) patients had preoperative anxiety (P = 0.001).
| No pain (n = 90) | Pain (n = 33) | χ2/Z | P value | |
| Sex | 0.534 | |||
| Male | 34 (37.8) | 15 (45.5) | ||
| Female | 56 (62.2) | 18 (54.5) | ||
| Age (years) | 49 ± 17 | 46 ± 15 | 16.311 | 0.453 |
| BMI (kg/m2) | 21.0 ± 3.3 | 20.4 ± 3.6 | 0.002 | 0.343 |
| Preoperative anxiety status | 44 (48.9) | 28 (84.8) | 12.864 | 0.001 |
| History of pain | 13 (14.4) | 5 (15.2) | 1.000 | |
| Unhealthy lifestyle habits | 9 (10.0) | 4 (12.1) | 0.746 | |
| Eckardt score | 5.3 ± 1.7 | 6.2 ± 1.7 | 0.643 | 0.008 |
| Tunnel length (cm) | 12.4 ± 1.2 | 12.6 ± 1.3 | 0.036 | 0.558 |
| Muscle incision length (cm) | 7.6 ± 1.7 | 7.5 ± 1.7 | 0.003 | 0.712 |
| Distance from the myotomy endpoint to the cardia (cm) | 2.4 ± 0.6 | 2.6 ± 0.5 | 2.238 | 0.120 |
| Operative time (minute) | 85 ± 33 | 100 ± 42 | 3.259 | 0.042 |
| Anesthesia time (minute) | 100 ± 34 | 117 ± 42 | 1.949 | 0.025 |
| Inductive opioid medication | 25.8 ± 6.5 | 24.9 ± 7.1 | 1.438 | 0.233 |
| Anesthesia method | 0.840 | |||
| Total intravenous anesthesia | 41 (45.6) | 16 (48.5) | ||
| Intravenous-inhalational anesthesia | 49 (54.4) | 17 (51.5) | ||
| Anticholinergic drugs (mg) | 42 (46.7) | 13 (39.4) |
With respect to the surgical procedure, in the pain group, the mean surgery and anesthesia times were 100 ± 42 minutes (P = 0.042) and 117 ± 42 minutes (P = 0.025), respectively.
As shown in Table 2, univariate logistic regression analysis revealed that the preoperative Eckardt score (P = 0.01), preoperative anxiety status (P = 0.001), operative time (P = 0.048), and anesthesia time (P = 0.031) were potential risk factors for suboptimal pain control following POEM. To identify independent risk factors, multivariate logistic regression analysis was performed. Variables with a P value < 0.1 in the univariate analysis were included in the multivariate analysis. Stepwise logistic regression was used to mitigate the effects of confounding variables, thereby facilitating the construction of an optimally fitted model for predicting poor pain control risk.
| Univariate | Multivariate | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Sex (male) | 0.729 (0.325-1.633) | 0.442 | ||
| Age (years) | 0.991 (0.996-1.015) | 0.450 | ||
| BMI (kg/m2) | 0.942 (0.834-1.065) | 0.341 | ||
| Preoperative anxiety status | 5.855 (2.075-16.522) | 0.001 | 5.195 (1.691-15.959) | 0.004 |
| History of pain | 1.058 (0.346-3.237) | 0.922 | ||
| Unhealthy lifestyle habits | 1.241 (0.355-4.341) | 0.735 | ||
| Eckardt score | 1.363 (1.075-1.726) | 0.01 | 1.317 (0.992-1.748) | 0.057 |
| Tunnel length (cm) | 1.101 (0.778-1.559) | 0.586 | ||
| Muscle incision length (cm) | 0.957 (0.757-1.208) | 0.709 | ||
| Distance from the myotomy endpoint to the cardia (cm) | 1.816 (0.855-3.859) | 0.121 | ||
| Operative time (minute) | 1.011 (1.001-1.023) | 0.048 | ||
| Anesthesia time (minute) | 1.012 (0.988-1.008) | 0.031 | ||
| Intraoperative opioid consumption | 0.979 (0.922-1.041) | 0.504 | ||
| Anesthesia method | 0.889 (0.400-1.976) | 0.773 | ||
| Anticholinergic drugs (mg) | 0.571 (0.248-1.316) | 0.188 | ||
The final model revealed the preoperative Eckardt score and preoperative anxiety status as independent risk factors for post-POEM pain. A one-unit increase in the preoperative Eckardt score was associated with a 1.317-fold increase in the odds of suboptimal pain control [odds ratio (OR) = 1.317; 95% confidence interval (95%CI): 0.992-1.748; P = 0.057]. Patients who presented with preoperative anxiety had 5.195-fold greater odds of poor pain control (OR = 5.195; 95%CI: 1.691-15.959; P = 0.004).
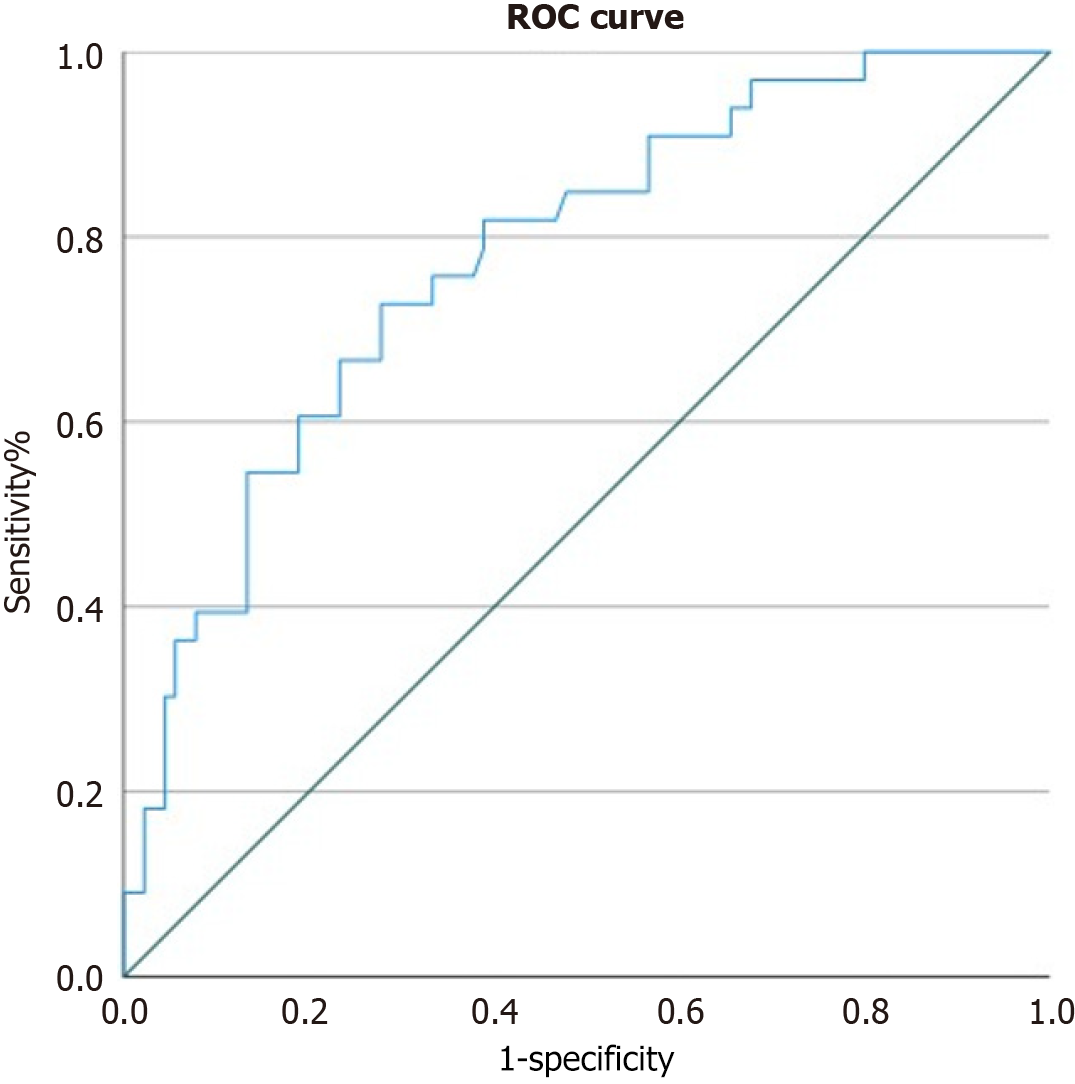
We constructed a receiver operating characteristic (ROC) curve, as shown in Figure 2, to illustrate and visualize the relationships between the potential risk factors and postoperative pain. The ROC curve reflected a good classification ability, with an area under the ROC curve value of 0.760 (95%CI: 0.661-0.859).
The safety, feasibility, and efficacy of POEM for the management of achalasia are well established. Despite its natural orifice approach, this procedure is commonly associated with mild-to-moderate acute post-POEM pain[8,9]. In this retrospective analysis involving 123 achalasia patients who underwent POEM at our institution, the preoperative Eckardt score and preoperative anxiety status were identified as risk factors for suboptimal post-POEM pain control.
Previous investigations into anesthetic management for POEM have focused predominantly on anesthesia-related complications, including intraoperative regurgitation and aspiration, subcutaneous emphysema, and carbon dioxide pneumoperitoneum[14-18], as well as comparative efficacy studies of post-POEM analgesic agents[15]. A study by Yurtlu and Aslan[17] indicated that patients required analgesia following POEM but failed to specify the details of analgesic administration. Xie et al[19] reported that compared with morphine, oxycodone exhibited superior efficacy in terms of managing post-POEM pain, which is characterized by distinct visceral pain features. Another retrospective analysis of 45 patients by Chen et al[20] revealed that the severity of post-POEM pain was primarily correlated with the baseline condition of patients with achalasia but neither addressed the impact of perioperative anesthetic factors nor clarified the perioperative analgesic protocols. In contrast, the present study represents a large-sample retrospective analysis integrating comprehensive anesthetic management data and detailed patient records, aiming to identify potential risk factors for post-POEM pain and to facilitate the development of personalized perioperative analgesic strategies.
Stepwise multivariate logistic regression analysis revealed that preoperative anxiety status was associated with post-POEM pain. Preoperative anxiety refers to a state of restlessness or tension in patients triggered by disease, hospitalization, anesthesia, surgery, or unspecified worries, which may be accompanied by physical manifestations such as palpitations, shortness of breath, diaphoresis, urinary frequency, abdominal pain, diarrhea, or sleep disturbances. The specific mechanisms underlying the impact of preoperative anxiety on post-POEM pain have yet to be fully elucidated. Preoperative anxiety increases not only the consumption of anesthetic and vasoactive drugs during surgery but also the demand for post-POEM analgesics and is positively correlated with both acute and chronic post-POEM pain[21-23].
Preoperative anxiety is a well-established modulator of postoperative pain perception. Patients with anxiety often exhibit heightened sensitivity to pain, a phenomenon particularly relevant in procedures such as POEM that involve the esophagus—an organ highly influenced by psychoneuroenterological mechanisms. Anxiety activates the stress-response system, leading to increased release of cortisol and catecholamines, which can lower pain thresholds and facilitate central sensitization[21]. Furthermore, anxiety amplifies the attention of the brain to visceral stimuli and disrupts descending inhibitory pain pathways. This may result in visceral hypersensitivity, a key pathophysiological feature of functional gastrointestinal disorders, causing patients to perceive normal postoperative visceral distension (e.g., from CO2 insufflation) and inflammatory stimuli as disproportionately painful[24].
In addition, a potential mechanistic pathway involves the activation of somatostatin-expressing neurons in the central amygdala during anxiety, which not only elicits anxiety-like behaviors but also concurrently induces significant mechanical allodynia, thus indicating partial overlap in their neurobiological substrates[25].
To operationalize these findings, preoperative anxiety screening (e.g., via validated tools such as the state-trait anxiety inventory[26] or the generalized anxiety disorder-7[27]) should be integrated into standardized POEM patient assessments. For individuals with moderate-to-severe anxiety, multimodal interventions are warranted, including (1) comprehensive preprocedural education and shared decision-making; (2) psychological support modalities (e.g., cognitive-behavioral therapy[28]; and (3) judicious pharmacologic anxiolysis with benzodiazepines when clinical indications are met.
Our analysis revealed that a higher preoperative Eckardt score was a significant predictor of increased pain following POEM. This association can be mechanistically explained through the interplay of disease severity, the extent of subsequent surgical trauma, and the resulting inflammatory response.
An elevated Eckardt score is a direct indicator of long-standing and severe achalasia. Chronic obstruction from a nonrelaxing lower esophageal sphincter forces the esophageal body to generate higher pressures to propel contents forward, leading to compensatory muscular hypertrophy, neuronal remodeling, and low-grade inflammatory changes within the esophageal wall. This pathophysiological remodeling creates an esophagus that is primed for a heightened response to injury[29]. During POEM, the creation of a submucosal tunnel and the myotomy itself constitute controlled surgical trauma. In patients with severe disease (high Eckardt score), the procedural challenges are often greater; a thicker muscular layer may necessitate more extensive dissection or a longer myotomy time, potentially leading to greater tissue manipulation and trauma. Furthermore, the preexisting state of neuronal dysfunction and sensitization in a severely diseased esophagus likely lowers the threshold for nociceptor activation following surgical stimulation.
The biological link between this increased tissue trauma and pain is the amplified local inflammatory response. Greater trauma triggers more pronounced release of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) and mediators (e.g., prostaglandins and bradykinin). This inflammatory cascade not only sensitizes peripheral nociceptors at the site of injury (peripheral sensitization) but can also lead to central sensitization, amplifying the perception of pain[5]. Consequently, patients with high Eckardt scores not only experience pain from the standard procedural insult but are also susceptible to an exaggerated pain state because of their preexisting esophageal condition and the consequently magnified postoperative inflammatory cascade. These findings underscore the importance of recognizing high preoperative Eckardt scores as a marker for patients who may require more aggressive, multimodal analgesic strategies during the perioperative period.
It is plausible that anxiety and severe achalasia act synergistically. Patients who have endured achalasia symptoms for a long time (as reflected by a high Eckardt score) may develop anticipatory anxiety regarding procedures, further lowering their pain threshold and creating a cycle of increased pain perception.
Despite our efforts to provide a comprehensive analysis of the factors influencing pain following POEM, this study had several limitations. First, this was a single-center, retrospective study, which may have introduced selection bias and restricted the generalizability of our results. Consequently, our findings require validation through multicenter prospective investigations. Second, although the NRS is a widely accepted tool for pain assessment, it remains inherently subjective and is influenced by individual differences in pain perception and reporting, potentially affecting the accuracy and consistency of the outcome measures. Third, the relatively modest sample size may have limited the statistical power to detect all relevant risk factors. Furthermore, although we adjusted for several potential confounders, unmeasured variables—such as subtle technical variations during surgery and differences in the extent of tissue trauma—may have influenced postoperative pain outcomes. Future studies with larger, prospective, multicenter designs and the incorporation of objective pain biomarkers are needed to validate and extend our conclusions.
Through systematic clinical data analysis, this study demonstrated that the incidence of postoperative pain in patients with achalasia who underwent POEM significantly increased, with 26.8% of patients experiencing at least moderate-intensity pain (NRS score ≥ 4). Furthermore, preoperative anxiety and a high Eckardt score were independent risk factors for post-POEM pain. These findings suggest that incorporating clinical symptom assessment, psychological status screening, and optimized analgesic strategies into preoperative anesthesia management is crucial for preventing and relieving postoperative pain. Prospective studies are needed to explore more preoperative anesthesia-related independent risk factors and optimize multimodal analgesic strategies.
| 1. | Bechara R, Woo M, Hookey L, Chung W, Grimes K, Ikeda H, Onimaru M, Sumi K, Nakamura J, Hata Y, Maruyama S, Gomi K, Shimamura Y, Inoue H. Peroral endoscopic myotomy (POEM) for complex achalasia and the POEM difficulty score. Dig Endosc. 2019;31:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Löser B, Recio Ariza O, Saugel B, Reuter DA, Zöllner C, Werner YB, Rösch T, Petzoldt M. Anesthesia for Patients Undergoing Peroral Endoscopic Myotomy Procedures: A Review of the Literature. Anesth Analg. 2020;130:1331-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA. 2015;313:1841-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 4. | Nabi Z, Reddy DN, Ramchandani M. Adverse events during and after per-oral endoscopic myotomy: prevention, diagnosis, and management. Gastrointest Endosc. 2018;87:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 5. | Kim RK, Kim JW, Angelotti T, Esquivel M, Tsui BC, Hwang JH. Magnesium and Esophageal Pain After Peroral Endoscopic Myotomy of the Esophagus: A Randomized, Double-Blind, Placebo-Controlled Trial. Anesth Analg. 2025;140:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Li QL, Chen WF, Zhou PH, Yao LQ, Xu MD, Hu JW, Cai MY, Zhang YQ, Qin WZ, Ren Z. Peroral endoscopic myotomy for the treatment of achalasia: a clinical comparative study of endoscopic full-thickness and circular muscle myotomy. J Am Coll Surg. 2013;217:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Cappell MS, Stavropoulos SN, Friedel D. Updated Systematic Review of Achalasia, with a Focus on POEM Therapy. Dig Dis Sci. 2020;65:38-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Misra L, Fukami N, Nikolic K, Trentman TL. Peroral endoscopic myotomy: procedural complications and pain management for the perioperative clinician. Med Devices (Auckl). 2017;10:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Thong ISK, Jensen MP, Miró J, Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;18:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 419] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 10. | Shafshak TS, Elnemr R. The Visual Analogue Scale Versus Numerical Rating Scale in Measuring Pain Severity and Predicting Disability in Low Back Pain. J Clin Rheumatol. 2021;27:282-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 11. | Lu J, Ji W, Sang C, Wang Z, Bo L. Postoperative Pain Management Strategies Following Peroral Endoscopic Myotomy (POEM): A Review. J Pain Res. 2025;18:2761-2773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Veitch AM, Radaelli F, Alikhan R, Dumonceau JM, Eaton D, Jerrome J, Lester W, Nylander D, Thoufeeq M, Vanbiervliet G, Wilkinson JR, van Hooft JE. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Endoscopy. 2021;53:947-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Yang D, Bechara R, Dunst CM, Konda VJA. AGA Clinical Practice Update on Advances in Per-Oral Endoscopic Myotomy (POEM) and Remaining Questions-What We Have Learned in the Past Decade: Expert Review. Gastroenterology. 2024;167:1483-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 14. | Löser B, Werner YB, Löser A, Rösch T, Petzoldt M. [Anesthesia in gastrointestinal endoscopy: peroral endoscopic myotomy]. Anaesthesist. 2019;68:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Sarkar S, Khanna P, Gunjan D. Anesthesia for Per-oral endoscopic myotomy (POEM) - not so poetic! J Anaesthesiol Clin Pharmacol. 2022;38:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Jayan N, Jacob JS, Mathew M, Mukkada RJ. Anesthesia for peroral endoscopic myotomy: A retrospective case series. J Anaesthesiol Clin Pharmacol. 2016;32:379-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Yurtlu DA, Aslan F. Challenges in Anesthesia Management for Peroral Endoscopic Myotomy: A Retrospective Analysis. Surg Laparosc Endosc Percutan Tech. 2021;31:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Nishihara Y, Yoshida T, Ooi M, Obata N, Izuta S, Mizobuchi S. Anesthetic management and associated complications of peroral endoscopic myotomy: A case series. World J Gastrointest Endosc. 2018;10:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Xie G, Li S, Zeng M, Zhou R, Song S, Chu L, Fang X. Oxycodone is superior to morphine for pain relief following peroral oesophageal myotomy: a prospective, randomized, controlled trial. Wideochir Inne Tech Maloinwazyjne. 2022;17:624-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Chen WN, Xu YL, Zhang XG. High Eckardt score and previous treatment were associated with poor postperoral endoscopic myotomy pain control: A retrospective study. World J Clin Cases. 2022;10:5655-5666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, Riva-Cambrin J. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9:e025091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 243] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 22. | Vasilopoulos T, Wardhan R, Rashidi P, Fillingim RB, Wallace MR, Crispen PL, Parvataneni HK, Prieto HA, Machuca TN, Hughes SJ, Murad GJA, Tighe PJ; Temporal Postoperative Pain Signatures (TEMPOS) Group. Patient and Procedural Determinants of Postoperative Pain Trajectories. Anesthesiology. 2021;134:421-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 24. | Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016;S0016-5085(16)00223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 1495] [Article Influence: 149.5] [Reference Citation Analysis (1)] |
| 25. | Chen WH, Lien CC, Chen CC. Neuronal basis for pain-like and anxiety-like behaviors in the central nucleus of the amygdala. Pain. 2022;163:e463-e475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Zsido AN, Teleki SA, Csokasi K, Rozsa S, Bandi SA. Development of the short version of the spielberger state-trait anxiety inventory. Psychiatry Res. 2020;291:113223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 27. | Hasan EM, Calma CL, Tudor A, Oancea C, Tudorache V, Petrache IA, Tudorache E, Papava I. Coping, Anxiety, and Pain Intensity in Patients Requiring Thoracic Surgery. J Pers Med. 2021;11:1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Chand SP, Kuckel DP, Huecker MR. Cognitive Behavior Therapy. 2023 May 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 29. | Savarino E, Bhatia S, Roman S, Sifrim D, Tack J, Thompson SK, Gyawali CP. Achalasia. Nat Rev Dis Primers. 2022;8:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (1)] |