Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.112124
Revised: August 8, 2025
Accepted: September 17, 2025
Published online: November 27, 2025
Processing time: 130 Days and 19.5 Hours
This letter comments on a study on lipid metabolism, immunity, and lymph node metastasis in esophageal cancer, a clinically relevant topic given lipid-immune crosstalk in tumor progression, to be published by the World Journal of Gastroin
Core Tip: This letter raises concerns about a study investigating lipid metabolism and lymph node metastasis in esophageal cancer, focusing on three key issues: Insufficient data on lipid parameters [especially low-density lipoprotein (LDL) distribution] in the patient cohort, unclear rationale for selecting LDL-related genes and excluding other lipid metabolism pa
- Citation: Cui X, Liang Z. Concerns regarding lipid metabolism, immune regulation, and methodology in a study on esophageal cancer lymph node metastasis. World J Gastrointest Surg 2025; 17(11): 112124
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/112124.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.112124
We are writing this letter to comment on the study[1] exploring the relationship between lipid metabolism, immune regulation, and lymph node metastasis in esophageal cancer, to be published in the World Journal of Gastrointestinal Surgery.
This topic holds significant clinical and scientific relevance, as lipid metabolism and immune crosstalk have emerged as critical regulators of tumor progression and metastasis. However, upon careful review, we raise several concerns related to methodology, data interpretation, and supporting evidence that require further clarification to strengthen the study’s conclusions as follows.
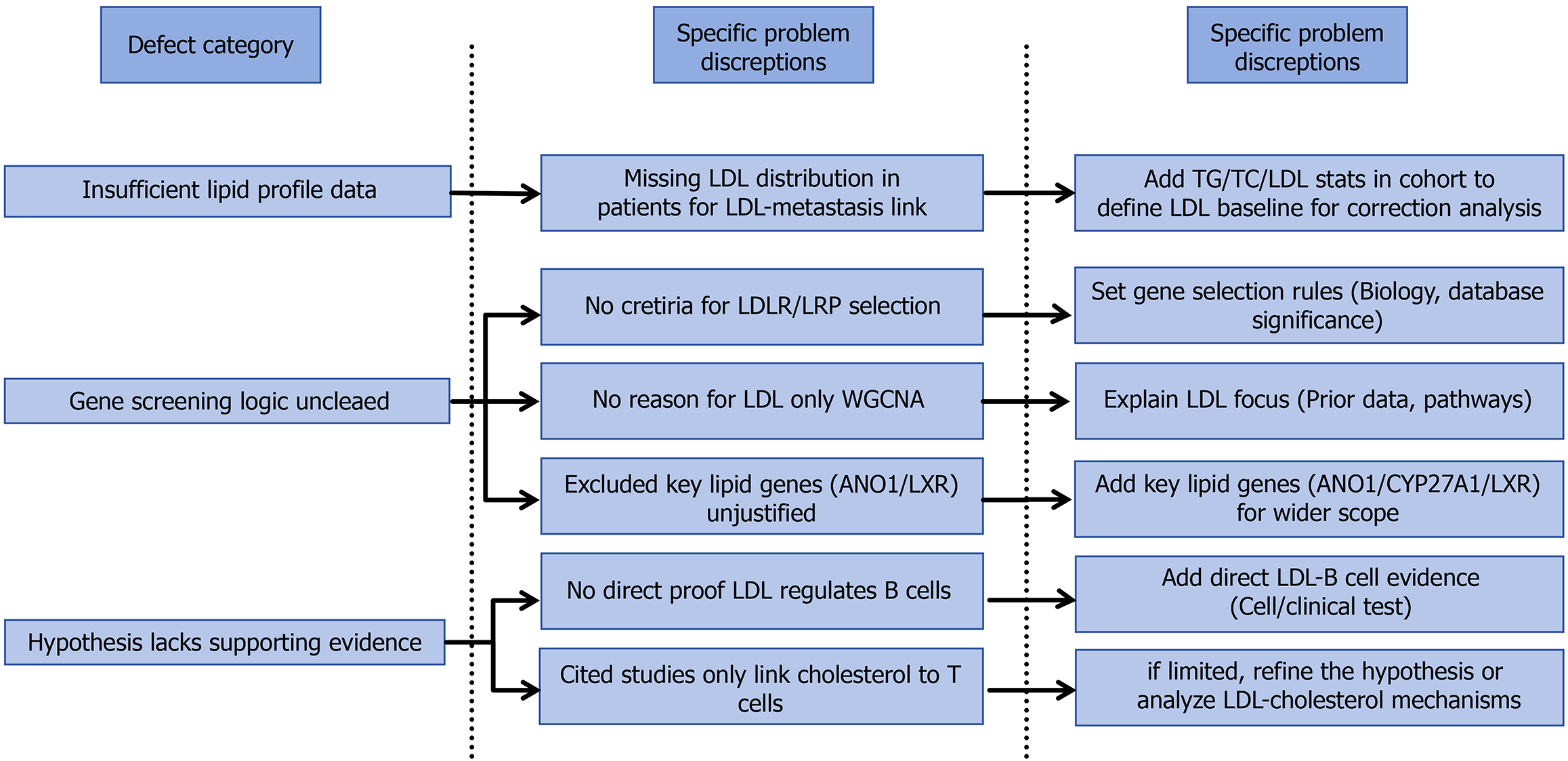
While the inclusion criteria explicitly list key lipid parameters [triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein, apolipoprotein-A1, apolipoprotein-B, lipoprotein (a) as variables to be examined, the study lacks a detailed description and analysis of these metrics in the enrolled patient cohort. Specifically, there is a notable absence of data on the LDL levels among the study population, which is central to the core hypothesis. This limits the ability to contextualize the observed associations between LDL and lymph node metastasis.
With regard to the methodology for gene screening, although the study focuses on LDL receptors and LDL receptor-related protein (LRP) family members (e.g., LRP6) identified via tumor gene databases, the process by which these specific receptors were selected is not adequately described. The criteria for prioritizing LDL receptors and LRP family members over other potential candidates remain unclear, making it difficult to assess the rigor of the screening strategy. Fur
In the discussion, the authors suggest a regulatory role of LDL in B lymphocytes, stating: “Although there is no su
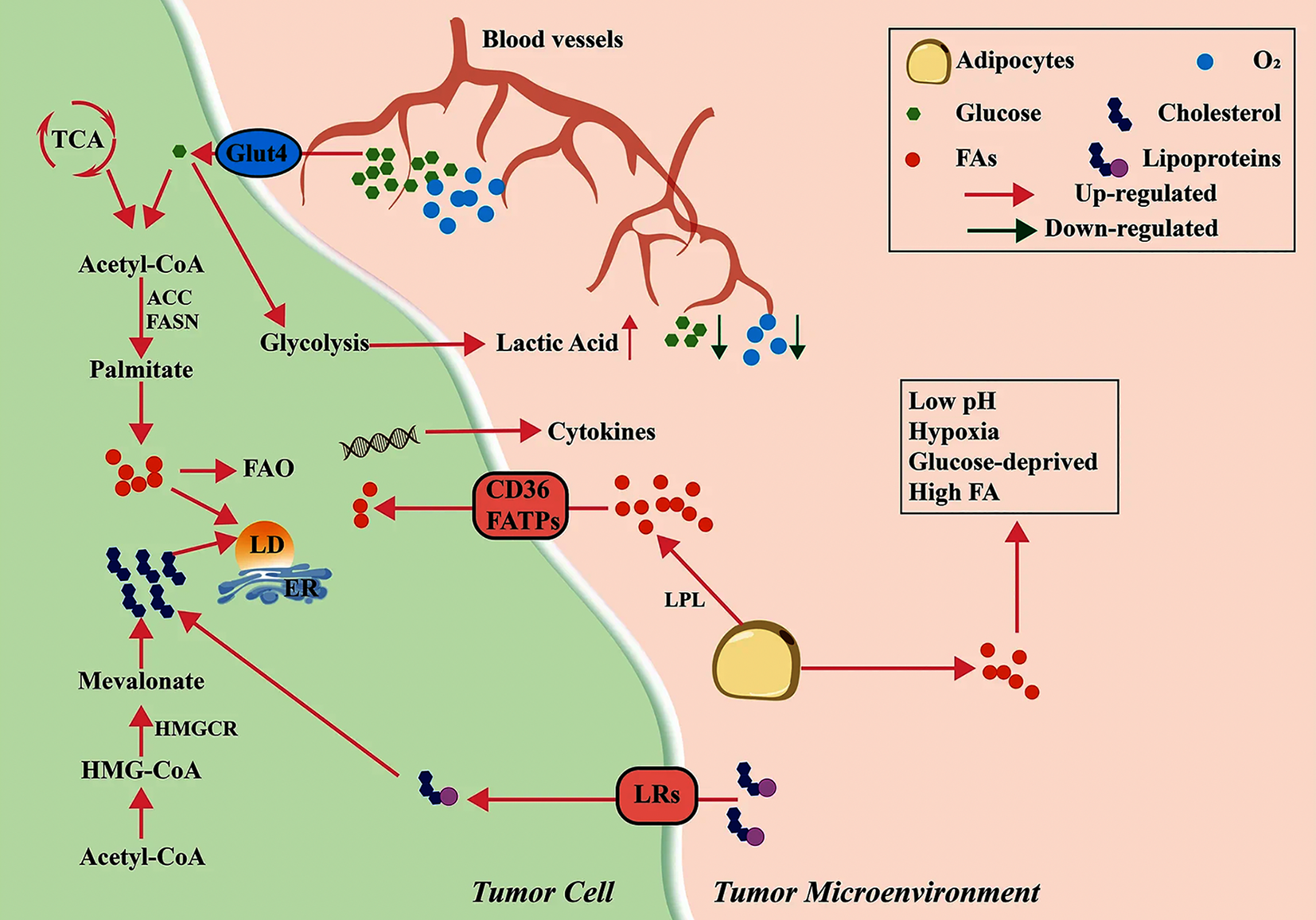
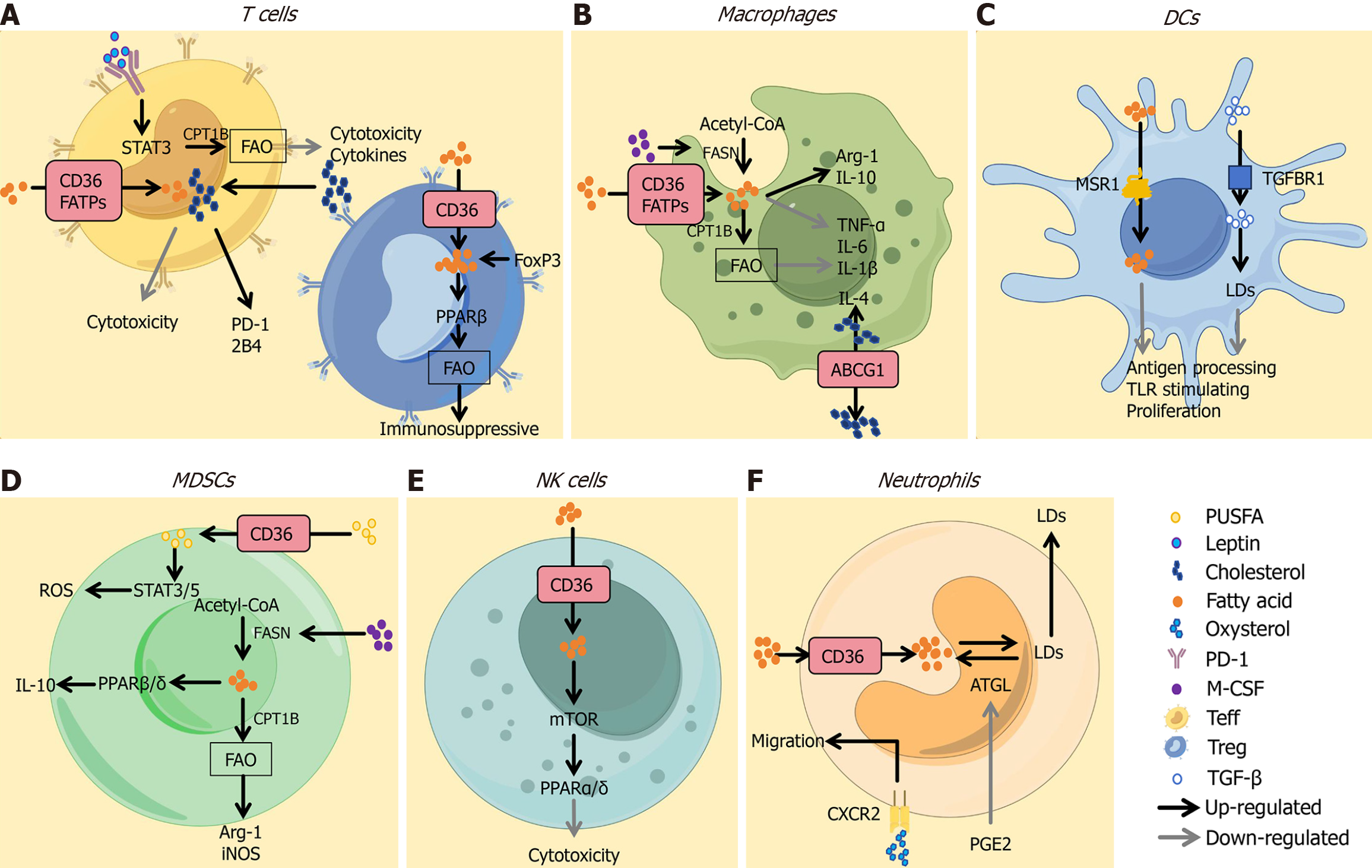
Recent advances in lipid metabolism research highlight its critical role in tumor cell metabolic reprogramming (Figure 1)[3]. Cholesterol and lipid biosynthesis are key drivers of tumor microenvironment remodeling. As demonstrated in the study by Ciavattone et al[4], fatty acids (FA) and cholesterol, as central lipid metabolites, enter T cells via transporters such as cluster of differentiation 36 or FA transport proteins, inducing effector T cell exhaustion, activating peroxisome proliferator-activated receptor beta and FA oxidation in regulatory T cells. Furthermore, studies by Li et al[5] and Dai et al[6] have shown that cholesterol may impair antigen presentation, suppressing Toll-like receptor signaling, inhibiting dendritic cell proliferation and modulating macrophage polarization. These processes underscore the complexity of lipid–immune crosstalk in tumors (Figure 2)[3].
The current literature review on B cell-lipid metabolism interactions appears insufficient. To reinforce the conclusions, we suggest that future studies should present detailed data on blood lipid levels (including LDL) in the 294 esophageal cancer patients and their correlation with pathological outcomes; clarify the rationale for selecting LDL-related genes over other lipid metabolism pathways; and provide more direct evidence for the proposed interplay between LDL and B lymphocytes. Addressing these points will enhance the robustness of the findings and their contribution to under
We are deeply grateful to Professor Chaoming Zhou and Professor Caiwen Duan for their guidance on this Letter. As authoritative experts in oncology and metabolomics, their insightful suggestions have significantly enhanced the academic rigor and professional depth of this manuscript.
| 1. | Xu XJ, Liu SW, Li JQ, He M, Wang H, Meng QJ. Effects of low-density lipoprotein cholesterol on lymph node metastasis after radical esophagectomy. World J Gastrointest Surg. 2025;17:106898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 2. | Deng CM, Zhang GG, Liu QW, Xu JJ, Liu ZC, Yang J, Xu TY, Li ZG, Zhang F, Li B. ANO1 Reprograms Cholesterol Metabolism and the Tumor Microenvironment to Promote Cancer Metastasis. Cancer Res. 2023;83:1851-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 3. | Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, Ping Y, Zhang Y. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol. 2021;14:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 4. | Ciavattone NG, Guan N, Farfel A, Stauff J, Desmond T, Viglianti BL, Scott PJ, Brooks AF, Luker GD. Evaluating immunotherapeutic outcomes in triple-negative breast cancer with a cholesterol radiotracer in mice. JCI Insight. 2024;9:e175320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Li G, Che X, Wang S, Liu D, Xie D, Jiang B, Zheng Z, Zheng X, Wu G. The role of cisplatin in modulating the tumor immune microenvironment and its combination therapy strategies: a new approach to enhance anti-tumor efficacy. Ann Med. 2025;57:2447403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 6. | Dai W, Ko JM, Yu VZ, Hou Z, Chow LK, Chung MKY, Islam KA, Ng BH, Wong CW, Leung KK, Chen C, Wong IYH, Law SY, Lo AW, Lam AK, Lung ML. Characterizing chromosome instability reveals its association with lipid-associated macrophages and clonal evolution of lymph node metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2025;628:217874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/