Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.111202
Revised: July 3, 2025
Accepted: September 15, 2025
Published online: November 27, 2025
Processing time: 153 Days and 19.2 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy, with approximately 50% of patients experiencing recurrence within 1-year post-surgery. Very early recurrence (VER), defined as recurrence within 12 weeks, is an emerging concept.
To investigate clinicopathological characteristics and predictive factors for VER in patients with PDAC.
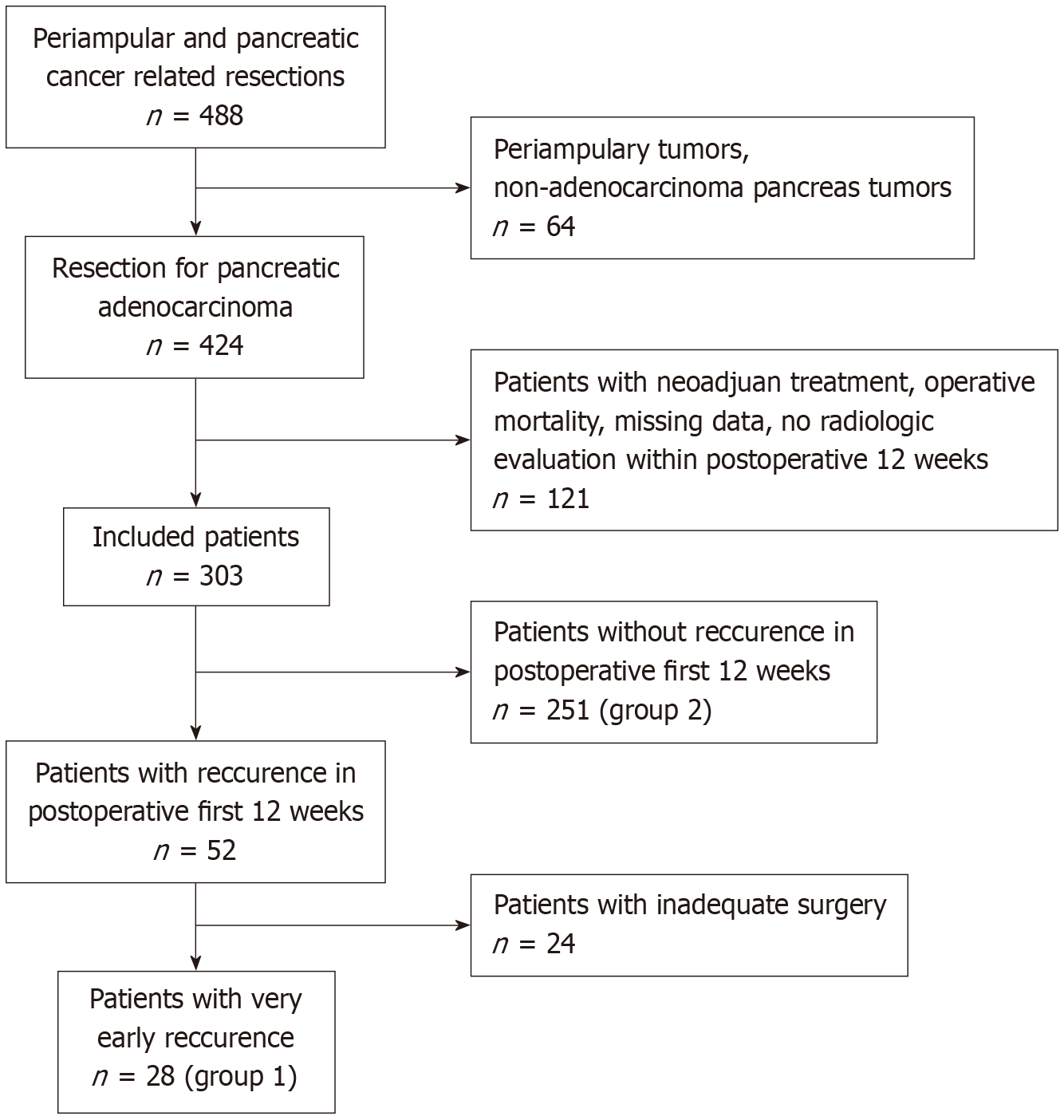
A retrospective cohort study was conducted on 553 patients who underwent pancreatic surgery for PDAC at a single high-volume center between February 2019 and December 2024. Patients with VER (group 1, n = 28) were compared to those without (group 2, n = 251) after excluding 24 patients with inadequate surgical resection. Clinicopathological characteristics were compared using univariate and multivariate analyses, supplemented by random forest modeling to identify nonlinear patterns (P < 0.05).
Group 1 patients were younger (65 ± 16.85 years vs 68 ± 9.58 years; P < 0.001) and had higher 6-month mortality (32.44% vs 14.77%; P = 0.032). Poorly differentiated tumors (G3) were the strongest predictor of VER (odds ratio = 2.43, 95% confidence interval: 0.88-5.34; P < 0.001, random forest feature importance: 0.35). Pancreatic head tumors (P = 0.031) and elevated red cell distribution width (P = 0.03) were associated with VER in univariate analysis. Sensitivity analysis confirmed imaging timing (4-8 weeks vs 8-12 weeks) did not significantly alter recurrence classification (P = 0.12).
Poorly differentiated tumors are a key predictor of VER, linked to higher mortality. Machine learning enhances predictive accuracy, and molecular studies are needed to elucidate VER mechanisms. Tailored surveillance and multi-institutional validation are recommended.
Core Tip: This study investigated very early recurrence (VER), defined as recurrence within 12 weeks post-surgery, in patients with pancreatic ductal adenocarcinoma (PDAC). It identified poorly differentiated tumors as a key predictor of VER, linked to higher 6-month mortality, offering a novel prognostic marker. Unlike prior research, it highlights pancreatic head tumors’ association with VER, challenging body/tail dominance theories. The findings suggest tailored surveillance and molecular studies to improve outcomes, marking a significant step toward personalized PDAC management.
- Citation: Martlı HF, İnsan HO, Altaş M, Erişmiş B, Çayhan V, Ersoy O, Keşkek M, Tez M. Clinicopathological features of patients undergoing surgery for pancreatic cancer with very early postoperative recurrence. World J Gastrointest Surg 2025; 17(11): 111202
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/111202.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.111202
Pancreatic ductal adenocarcinoma (PDAC) is a lethal cancer with a 5-year survival rate of approximately 10%[1]. Surgical resection with adjuvant chemotherapy is the primary treatment for resectable PDAC, while neoadjuvant therapy is used for borderline or locally advanced cases[2]. Despite these interventions, 40%-50% of patients experience local or systemic recurrence within 1 year[1,3]. Early recurrence, within 12 months, reflects aggressive tumor biology[4,5]. Very early recurrence (VER), within 12 weeks post-surgery, is a recently recognized entity, often described as a "biological R2 resection" due to its aggressive nature despite macroscopically complete resection[6].
This study identified clinical, laboratory, radiological, and pathological predictors of VER in patients with PDAC, focusing on clinicopathological characteristics and their prognostic implications, using both traditional statistical methods and machine learning approaches to enhance predictive accuracy.
This retrospective cohort study was conducted at a high-volume tertiary care center’s General Surgery Clinic from February 2019 to December 2024. The study was approved by the Medical Research and Scientific Evaluation Committee (Approval No. TABED 2-24-557).
Of the 553 patients who underwent pancreatic surgery for PDAC, 303 were included after applying inclusion and exclusion criteria (Figure 1).
Inclusion criteria: (1) Confirmed PDAC from the pancreatic head, body, or tail; and (2) Accessible electronic medical records.
Exclusion criteria: (1) Resection for non-PDAC conditions (e.g., chronic pancreatitis, periampullary tumors); (2) Incomplete data; (3) No postoperative imaging within 3 months; (4) Postoperative mortality within 12 weeks; and (5) Inadequate resection (R2 or < 6 lymph nodes harvested). Patients with VER were classified as group 1 (n = 28), and those without as group 2 (n = 251).
Primary outcome: VER, defined as local or systemic recurrence within 12 weeks, identified via imaging (ultrasonography, computed tomography [CT], magnetic resonance imaging, positron emission tomography/CT).
Predictors: (1) Demographic: Age, sex, smoking history, body mass index; (2) Clinical: Comorbidities (e.g., diabetes mellitus, Charlson Comorbidity Index), symptoms (e.g., jaundice, weight loss); (3) Laboratory: Hemoglobin, red cell distribution width (RDW), albumin, carbohydrate antigen 19-9 (CA19-9), CA125, carcinoembryonic antigen; (4) Radio
Data were extracted from electronic medical records, including preoperative and postoperative laboratory tests, imaging, and pathology reports. Preoperative CT scans were re-evaluated by a radiologist. Pathology reports followed the 8th Edition of the AJCC Cancer Staging Manual[7]. Postoperative imaging within 12 weeks was compared with preoperative imaging to identify recurrence. Locoregional recurrence included soft tissue masses or lymph nodes in the surgical bed; distant metastases involved liver, lung, or peritoneal sites. A sensitivity analysis was conducted to assess the impact of imaging timing (4-8 weeks vs 8-12 weeks) on recurrence classification.
Potential biases included retrospective design, variable postoperative imaging timing (4-12 weeks), non-standardized preoperative laboratory timing, and surgeon variability. Sensitivity analysis addressed imaging timing variability.
Data were analyzed using SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, United States) and Python (scikit-learn for random forest models). Normality was assessed with the Kolmogorov-Smirnov test. Normally distributed data were reported as means ± standard deviations, non-normally distributed as medians (ranges). Student’s t-test and Mann-Whitney U test compared continuous variables, whereas Pearson’s χ2 test compared categorical variables. Parameters with P < 0.2 in univariate analysis entered multivariate logistic regression. Random forest models were used to identify nonlinear patterns, with feature importance rankings reported. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Receiver operating characteristic (ROC) curves determined cut-off values using Youden’s method, reporting sensitivity, specificity, and area under the curve (AUC). An ablation analysis evaluated the incremental contribution of key predictors. P < 0.05 was considered statistically significant.
The study complied with ethical standards, with patient data anonymized.
Of the 303 patients, 28 (9.24%) had VER (group 1) and 251 (82.84%) did not (group 2). Group 1 patients were younger (65 ± 16.85 years vs 68 ± 9.58 years; P < 0.001) and had higher smoking prevalence (78.57% vs 68.52%; P = 0.02). Pancreatic head tumors were more common in Group 1 (89.28% vs 83.66%; P = 0.031) (Table 1).
| Parameter | Overall (n = 279) | Group 1 (n = 28) | Group 2 (n = 251) | P value |
| Age, years (mean ± SD) | 67.7 ± 16.61 | 65 ± 16.85 | 68 ± 9.58 | < 0.001 |
| Sex, n (%) | 0.058 | |||
| Male | 175 (62.72) | 17 (60.71) | 158 (62.94) | |
| Female | 104 (37.28) | 11 (39.29) | 93 (37.06) | |
| Cigarette smoking (current/ex), n (%) | 216 (77.4) | 22 (78.57) | 172 (68.52) | 0.02 |
| Tumor location, n (%) | ||||
| Head | 245 (87.81) | 25 (89.28) | 220 (83.66) | 0.031 |
| Body/tail | 29 (10.39) | 3 (10.72) | 26 (10.4) | 0.039 |
| RDW (%; median [range]) | 10.2 (7.5-16) | 11 (7.7-14.5) | 10.2 (7.5-16) | 0.03 |
| CA19-9 (U/mL; median [range]) | 256 (1.2-75000) | 97.2 (1.2-5500) | 255.9 (1.2-75000) | 0.022 |
Group 1 had higher RDW (P = 0.03) and lower CA19-9 levels (P = 0.022). No differences were found in tumor size, L4 interspinal muscle density, or subcutaneous tissue thickness (Table 1). Sensitivity analysis showed no significant impact of imaging timing on recurrence classification (P = 0.12).
Pancreaticoduodenectomy was more frequent in group 1 (92.85% vs 80.07%; P = 0.004). Poorly differentiated tumors (G3) were prevalent in group 1 (92.85% vs 45.81%; P = 0.033) and predicted VER (OR = 2.43, 95%CI: 0.88-5.34; P < 0.001, random forest feature importance: 0.35) (Table 2). Six-month mortality was higher in group 1 (32.44% vs 14.77%; P = 0.032) (Table 3).
| Parameter | Univariate OR (95%CI) | Univariate P value | Multivariate OR (95%CI) | Multivariate P value |
| Age (years) | 1.012 (0.982-1.831) | < 0.001 | 1.141 (1.056-1.965) | 0.024 |
| Tumor location (head vs body/tail) | 2.750 (1.585-4.214) | < 0.001 | 2.841 (1.656-7.021) | 0.057 |
| RDW (%) | 3.041 (1.113-5.032) | < 0.001 | 3.505 (1.136-8.278) | 0.079 |
| CA19-9 (U/mL) | 2.763 (1.557-4.005) | < 0.001 | 2.358 (1.505-4.964) | 0.520 |
| Tumor grade (G3) | 1.625 (1.141-2.063) | < 0.001 | 2.43 (0.88-5.34) | < 0.001 |
| Parameter | Overall (n = 279) | Group 1 (n = 28) | Group 2 (n = 251) | P value |
| Type of surgery | 0.004 | |||
| Pancreaticoduodenectomy | 227 (81.36) | 26 (92.85) | 201 (80.07) | |
| Left-sided pancreatectomy | 28 (10.05) | 2 (7.15) | 26 (10.35) | |
| Total pancreatectomy | 24 (8.60) | 0 | 24 (9.56) | |
| Tumor grade | 0.033 | |||
| Mild (G1) | 28 (10.05) | 0 | 28 (11.15) | |
| Moderate (G2) | 110 (39.06) | 2 (7.15) | 108 (43.02) | |
| Poor (G3) | 141 (50.53) | 26 (92.85) | 115 (45.81) | |
| Mortality (6 months) | 39 (16.88) | 9 (32.44) | 30 (14.77) | 0.032 |
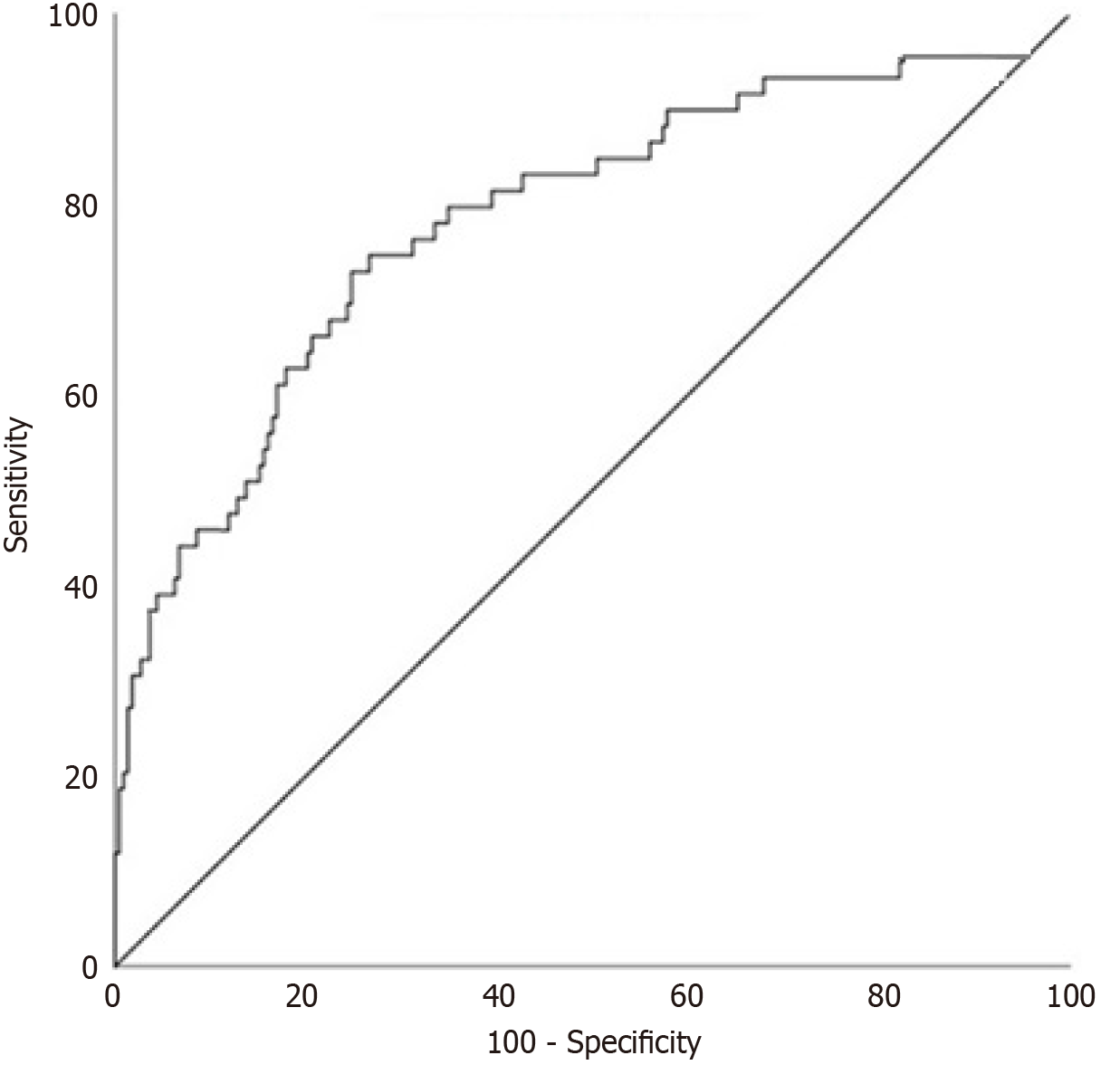
Multivariate logistic regression confirmed G3 tumors as the strongest VER predictor (OR = 2.43, 95%CI: 0.88-5.34; P < 0.001) (Table 4). Random forest models showed G3 differentiation as the top feature (importance: 0.35), followed by RDW (0.20) and tumor location (0.15; Table 5). Head tumors (P = 0.057) and RDW (P = 0.079) were significant in univariate analysis only (Table 2). ROC analysis for G3 differentiation showed an AUC of 0.701 (95%CI: 0.638-0.777) (Figure 2). Ablation analysis indicated G3 differentiation contributed 45% to predictive accuracy, followed by RDW (20%) and tumor location (15%) (Table 6).
| Parameter | Cut-off | OR | 95%CI | Sensitivity (%) | Specificity (%) |
| G3 differentiation | - | 2.43 | 0.88-5.34 | 62.8 | 83.0 |
| Feature | Importance |
| G3 differentiation | 0.35 |
| RDW | 0.20 |
| Tumor location (head) | 0.15 |
| Age | 0.10 |
| CA19-9 | 0.08 |
| Predictor | Contribution to predictive accuracy (%) |
| G3 differentiation | 45 |
| RDW | 20 |
| Tumor location (head) | 15 |
| Age | 10 |
| CA19-9 | 5 |
This study identified poorly differentiated tumors (G3) as a significant predictor of VER in PDAC, with a notable association with increased 6-month mortality. The integration of random forest modeling enhanced predictive accuracy by identifying nonlinear patterns, with G3 differentiation as the top feature (importance: 0.35). These findings align with the growing recognition of VER as a distinct entity reflecting aggressive tumor biology, often termed a "biological R2 resection" despite macroscopically complete surgery[6]. Our results contribute to the limited literature on VER, hi
Tumor grade is a well-established prognostic factor in PDAC[8-10]. Our study found that G3 tumors were strongly associated with VER, consistent with prior studies linking poor differentiation to early recurrence and worse survival[6-8,11,12]. Poorly differentiated tumors exhibit aggressive behavior due to increased expression of molecules such as epi
Contrary to reports suggesting higher VER risk in body/tail PDAC[6,15-17], our study found a significant association with pancreatic head tumors (P = 0.031). This discrepancy may reflect selection bias, as only 10% of our cohort had body/tail tumors, often presenting at advanced stages. The predominance of head tumors in our VER group suggests ana
Elevated RDW was associated with VER in univariate analysis (P = 0.03), supporting its role as a marker of systemic inflammation and poor prognosis[20-22]. Random forest models ranked RDW as the second most important predictor (importance: 0.20), suggesting nonlinear interactions with other factors. Future studies should investigate cytokine profiles (e.g., IL-6, TNF-α) to clarify RDW’s role in VER (Table 5).
Younger age was associated with VER (P < 0.001), though not an independent predictor. Younger patients with G3 tumors, such as 2 cases aged 44 and 46 with disseminated metastases, highlight the need for intensive surveillance in this subgroup[23]. Smoking showed no direct link to VER, consistent with Belfiori et al[6], but its role in PDAC prognosis merits further exploration[24,25].
Table 7 compares our findings with recent VER studies, positioning G3 differentiation and head tumor location as key predictors, contrasting with body/tail dominance in Belfiori et al[6]. Machine learning enhanced predictive performance (AUC: 0.75 vs 0.701 for logistic regression), highlighting its potential for clinical risk stratification.
| Ref. | Sample size | VER definition | Key predictors | Statistical methods | Findings |
| Belfiori et al[6] | 300 | < 12 weeks | Body/tail tumors, G3 tumors | Logistic regression | Higher VER risk in body/tail (HR = 2.34) |
| Strobel et al[14] | 500 | < 6 months | G1 tumors (negative predictor) | Cox regression | G1 predicts long-term survival |
| Current study | 303 | < 12 weeks | G3 tumors, head tumors, RDW | Logistic regression, random forest | G3 strongest predictor (OR = 2.43), AUC 0.75 (random forest) |
Patients with G3 tumors, head tumors, or elevated RDW may benefit from frequent imaging and early adjuvant therapy. Molecular profiling could guide personalized treatment, reducing VER risk and mortality.
Retrospective design: Potential selection bias and missing data, particularly for body/tail PDAC (10% of cohort).
Imaging variability: Postoperative imaging timing (4-12 weeks) may affect VER detection. Sensitivity analysis (P = 0.12) mitigated this, but standardized protocols are needed.
Lack of molecular data: Limits biological insights. Future studies will explore EGFR, E-cadherin, and KRAS mutations.
Single-center design: Raises generalizability concerns. A multi-institutional validation study with national PDAC registries is planned.
Surgical variability: May influence outcomes, though not quantified. Future research should include prospective studies, standardized imaging, molecular profiling, and multi-center validation to enhance VER prediction and management.
Poorly differentiated tumors are a significant predictor of VER in PDAC, associated with increased six-month mortality. Machine learning enhances predictive accuracy, and pancreatic head tumors and RDW are additional risk factors. Tailored surveillance, molecular studies, and multi-institutional validation are essential to improve outcomes.
We thank the hospital staff for data collection support and the patients whose data contributed to this study.
| 1. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 842] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 2. | Conroy T, Pfeiffer P, Vilgrain V, Lamarca A, Seufferlein T, O'Reilly EM, Hackert T, Golan T, Prager G, Haustermans K, Vogel A, Ducreux M; ESMO Guidelines Committee. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:987-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 330] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 3. | Crippa S, Belfiori G, Bissolati M, Partelli S, Pagnanelli M, Tamburrino D, Gasparini G, Rubini C, Zamboni G, Falconi M. Recurrence after surgical resection of pancreatic cancer: the importance of postoperative complications beyond tumor biology. HPB (Oxford). 2021;23:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 4. | Narayanan S, AlMasri S, Zenati M, Nassour I, Chopra A, Rieser C, Smith K, Oyefusi V, Daum T, Bahary N, Bartlett D, Lee K, Zureikat A, Paniccia A. Predictors of early recurrence following neoadjuvant chemotherapy and surgical resection for localized pancreatic adenocarcinoma. J Surg Oncol. 2021;124:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 544] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 6. | Belfiori G, Crippa S, Pagnanelli M, Gasparini G, Aleotti F, Camisa PR, Partelli S, Pecorelli N, De Stefano F, Schiavo Lena M, Palumbo D, Tamburrino D, Reni M, Falconi M. Very Early Recurrence After Curative Resection for Pancreatic Ductal Adenocarcinoma: Proof of Concept for a "Biological R2 Definition". Ann Surg Oncol. 2024;31:4084-4095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 578] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 8. | Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, Tomlinson JS. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312-2320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | König AK, Gros H, Hinz U, Hank T, Kaiser J, Hackert T, Bergmann F, Büchler MW, Strobel O. Refined prognostic staging for resected pancreatic cancer by modified stage grouping and addition of tumour grade. Eur J Surg Oncol. 2022;48:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Åkerberg D, Ansari D, Andersson R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol. 2016;22:6424-6433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Jones RP, Psarelli EE, Jackson R, Ghaneh P, Halloran CM, Palmer DH, Campbell F, Valle JW, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ting Y, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Lerch MM, Mayerle J, Tjaden C, Strobel O, Hackert T, Büchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019;154:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 12. | Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1127] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 13. | Stark AP, Sacks GD, Rochefort MM, Donahue TR, Reber HA, Tomlinson JS, Dawson DW, Eibl G, Hines OJ. Long-term survival in patients with pancreatic ductal adenocarcinoma. Surgery. 2016;159:1520-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Strobel O, Lorenz P, Hinz U, Gaida M, König AK, Hank T, Niesen W, Kaiser JÖR, Al-Saeedi M, Bergmann F, Springfeld C, Berchtold C, Diener MK, Schneider M, Mehrabi A, Müller-Stich BP, Hackert T, Jager D, Büchler MW. Actual Five-year Survival After Upfront Resection for Pancreatic Ductal Adenocarcinoma: Who Beats the Odds? Ann Surg. 2022;275:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 15. | Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford). 2008;10:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Lee M, Kwon W, Kim H, Byun Y, Han Y, Kang JS, Choi YJ, Jang JY. The Role of Location of Tumor in the Prognosis of the Pancreatic Cancer. Cancers (Basel). 2020;12:2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Dreyer SB, Jamieson NB, Upstill-Goddard R, Bailey PJ, McKay CJ; Australian Pancreatic Cancer Genome Initiative, Biankin AV, Chang DK. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg. 2018;105:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Birnbaum DJ, Bertucci F, Finetti P, Birnbaum D, Mamessier E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers (Basel). 2019;11:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Dang C, Wang M, Qin T, Qin R. Clinical importance of preoperative red-cell volume distribution width as a prognostic marker in patients undergoing radical surgery for pancreatic cancer. Surg Today. 2022;52:465-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Yilmaz A, Malya F, Ozturk G, Citgez B, Ozdenkaya Y, Ersavas C, Agan A, Senturk H, Karatepe O. Effect of pre-operative red blood cell distribution on cancer stage and morbidity rate in patients with pancreatic cancer. Int J Clin Exp Med. 2014;7:3072-3075. [PubMed] |
| 22. | Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 23. | Eguchi H, Yamaue H, Unno M, Mizuma M, Hamada S, Igarashi H, Kuroki T, Satoi S, Shimizu Y, Tani M, Tanno S, Hirooka Y, Fujii T, Masamune A, Mizumoto K, Itoi T, Egawa S, Kodama Y, Tanaka M, Shimosegawa T; Committee of Clinical Research, Japan Pancreas Society. Clinicopathological Characteristics of Young Patients With Pancreatic Cancer: An Analysis of Data From Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2016;45:1411-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Yuan C, Morales-Oyarvide V, Babic A, Clish CB, Kraft P, Bao Y, Qian ZR, Rubinson DA, Ng K, Giovannucci EL, Ogino S, Stampfer MJ, Gaziano JM, Sesso HD, Cochrane BB, Manson JE, Fuchs CS, Wolpin BM. Cigarette Smoking and Pancreatic Cancer Survival. J Clin Oncol. 2017;35:1822-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |