Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110879
Revised: August 21, 2025
Accepted: September 15, 2025
Published online: November 27, 2025
Processing time: 126 Days and 3.2 Hours
Systemic immune-inflammation index (SII) combined with serum lactoferrin (LF) level can provide a reference for predicting the postoperative survival and pro
To evaluate the predictive value of SII combined with serum LF for postoperative survival in older patients with colon cancer.
This prospective study included 62 older patients [range, 65-85 years; average age (72.46 ± 6.02) years] with colon cancer who underwent radical surgery at our hospital between January 2023 and September 2024. Colon cancer was confirmed on postoperative pathology. All patients underwent peripheral blood, LF, and tumor marker tests and imaging examinations preoperatively. The ability to predict overall survival (OS) and disease-free survival (DFS) by dynamically monitoring the SII [platelet (PLT) count × neutrophil (NEU) count/lymphocyte (LYM) count] and LF levels in combination with postoperative follow-up data was assessed. SII, LF levels, and postoperative data were analyzed using receiver operating characteristic curves, univariate, and multivariate Cox regression ana
All patients were followed up conventionally postoperatively. There were no sig
This preliminary analysis suggests that the SII and LF levels may predict the survival and prognosis of older patients with colon cancer postoperatively, when assessing the risk of postoperative recurrence and complications. These two categories of indicators have good prognostic evaluation potential in clinical practice and can provide strong support for the development of individualized treatment strategies.
Core Tip: This study prospectively evaluated the predictive value of systemic immune-inflammation index (SII) combined with serum lactoferrin (LF) levels for survival outcomes in older patients with colon cancer undergoing radical surgery. Dynamic monitoring demonstrated that preoperative SII and LF levels were independent predictors of overall survival and disease-free survival. The findings suggest that SII and LF serve as simple and effective prognostic biomarkers, providing valuable guidance for individualized treatment strategies and postoperative recurrence risk assessment in elderly colon cancer patients.
- Citation: Zhu SS, Yang T, Cheng LL. Predictive value of systemic immune-inflammation index and serum lactoferrin for postoperative survival in older patients with colon cancer. World J Gastrointest Surg 2025; 17(11): 110879
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110879.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110879
With the accelerated aging of the global population, the incidence of colon cancer in older patients has increased annually, and it has become an important malignant tumor that threatens the lives and health of older patients[1]. According to existing statistical data, the incidence rate of colon cancer in older patients is higher than that in the young population, and older patients often have a variety of comorbidities, such as hypertension, diabetes, and cardiovascular disease. These factors make early diagnosis and treatment of colon cancer challenging in older patients[2]. In particular, the immune function of older patients generally decreases, and surgery is poorly tolerated, making postoperative recovery and long-term survival much worse than in young patients[3]. Therefore, for older patients with colon cancer, the prediction of postoperative recovery and prognosis is particularly important, and there is an urgent need to establish a simple and effective postoperative prognosis evaluation index for individualized treatment and accurate postoperative management[4]. Traditional tumor, node, metastasis (TNM) staging still plays an important role in the prognostic assessment of tumors; however, it has some limitations. Although TNM staging can be used to assess tumor size, me
For this prospective study, a total of 62 older patients with colon cancer who underwent radical surgery in our tumor surgery department between January 2023 and September 2024 were enrolled. All patients were confirmed to have colon cancer based on postoperative pathology. The patients' age ranged from 65 to 85 years old, and the average age was (72.46 ± 6.02) years old, including 36 males and 26 females. All patients completed relevant examinations, such as peri
Inclusion criteria: (1) Age 65 years or older with complete clinical data; (2) No severe infection or acute inflammation preoperatively; (3) Colon cancer diagnosis confirmed by postoperative pathology (TNM stage II-III); (4) No radiotherapy, chemotherapy or immunotherapy was received within 2 weeks preoperatively; and (5) The preoperative peripheral blood examination results were complete, including NEU, LYM, and PLT counts and LF level.
Exclusion criteria: (1) Patients with other malignant tumors (n = 3); (2) Preoperative severe infectious diseases (such as pulmonary infection or abdominal infection, n = 2); (3) The postoperative follow-up period was less than 6 months or the data was incomplete (n = 4); (4) The existence of autoimmune diseases or long-term use of immunosuppressive agents (such as hormone therapy lasting for more than 3 months, n = 2); and (5) Severe liver and kidney dysfunction preoperatively (n = 2). Ultimately, 62 patients met the inclusion criteria and were included in the statistical analysis. All patients received standard postoperative follow-up, and related outcome indicators, including survival and recurrence, were recorded for subsequent prognosis analysis.
Patient data included demographics (sex, age, body mass index, smoking status, alcohol consumption), comorbidities (including hypertension, diabetes, coronary heart disease, chronic obstructive pulmonary disease), treatments, tumor characteristics (site, size, TNM stage, differentiation, nerve or vascular invasion), laboratory tests (preoperative blood counts for SII calculation), serum LF level (ELISA within 3 days preoperatively), Eastern Cooperative Oncology Group performance status, Charlson Comorbidity Index, adjuvant chemotherapy details, and microsatellite instability status.
Routine blood tests and SII: Fasting venous blood 7 days preoperatively and 1 week, 1 month, and 3 months postoperatively. NEU, LYM, and PLT counts measured (Sysmex XN-1000) to calculate SII (PLT × NEU/LYM, ≥ 600 = high inflammation).
Serum LF: Five milliliters of fasting blood was collected at the same time points, and serum was extracted and tested by ELISA (BioTek ELx800), with 200 ng/mL as the risk threshold.
Liver and kidney function: Alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total bilirubin, creatinine, and blood urea nitrogen (BUN) levels were measured (AU5800) at the same time points. Abnormal values indicated malnutrition, liver dysfunction, or impaired metabolism.
Inflammatory and nutritional markers: C-reactive protein (CRP) level (immunoturbidimetry, > 10 mg/L = inflammation) and prealbumin (PAB) (immunodiffusion, < 150 mg/L = poor nutrition) were measured at the same time points.
Tumor markers: Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were measured (Roche Cobas e602 7 days preoperatively and at 1, 3, and 6 months postoperatively; elevated values indicated a risk of recur
All patients were followed from the 1st postoperative day until December 2024 or until death. Follow-up was conducted monthly via outpatient visits, phone visits, or electronic records, lasting at least 6 months. Endpoints included overall survival (OS) (time from surgery to death from any cause) and disease-free survival (DFS) (time to recurrence or metastasis, confirmed by imaging, tumor markers, or pathology, or death). Death was counted as an OS event, and recurrence or metastasis or marker elevation as a DFS event. Patients who were lost to follow-up for more than three cycles were excluded. Two researchers cross-checked the data to ensure accuracy.
Statistical analysis was performed with SPSS 25.0. Measurement data were expressed as mean ± SD or median (P25, P75), categorical data as frequency and percentage. Comparisons used t-test, χ² test, ANOVA, Mann-Whitney U, or Kruskal-Wallis H test as appropriate. Survival outcomes (OS, DFS, and post-recurrence survival) were analyzed at 18 months postoperatively using Kaplan-Meier and Cox proportional hazards models with stepwise selection (P < 0.05, entry, P > 0.10). Predictive ability of SII, LF, and other indicators was evaluated by area under the curve area under the curve (ROC) curves (Youden index). Variables were dichotomized and tested using logistic regression for independent predictors of 6-month survival, expressed as odds ratio (OR)/95%CI; Cox models reported hazard ratio (HR)/95%CI, including SII × LF interaction. Quality controls (QC) were applied (intra-assay coefficient of variability (CV) < 5%, inter-assay CV < 8%, Westgard rules, and National Institute of Standards and Technology-traceable controls). False discovery rate correction was used for longitudinal analyses; power was 78% to detect HR = 2.0 (α = 0.05, β = 0.2).
Baseline data show that the average patient age was (72.46 ± 6.02) years. No significant differences were found in sex; smoking history; drinking history; or the incidence of hypertension, diabetes, coronary heart disease, or chronic obstructive pulmonary disease (P > 0.05). Differences between the groups in TNM stage, degree of differentiation, and nerve or vessel invasion were not statistically significant (P > 0.05). Laboratory indicators such as NEU, LYM, and PLT count; SII; and LF levels were within the normal range, and there were no significant differences (P > 0.05) (Table 1).
| Project | Value or n (%) | χ²/t | P value |
| Age (years) | 72.46 ± 6.02 | - | - |
| Sex (male/female) | 36 (58.06)/26 (41.94) | 1.161 | 0.281 |
| BMI (kg/m²) | 22.53 ± 2.28 | - | - |
| Smoking history (yes/no) | 27 (43.55)/35 (56.45) | 0.968 | 0.325 |
| Drinking history (yes/no) | 23 (37.10)/39 (62.90) | 1.419 | 0.234 |
| Hypertension (yes/no) | 25 (40.32)/37 (59.68) | 2.258 | 0.133 |
| Diabetes (yes/no) | 15 (24.19)/47 (75.81) | 1.613 | 0.204 |
| Coronary heart disease (yes/no) | 8 (12.90)/54 (87.10) | 0.645 | 0.422 |
| COPD (yes/no) | 5 (8.06)/57 (91.94) | 0.218 | 0.641 |
| Tumor size (cm) | 4.73 ± 1.21 | - | - |
| TNM staging (II/III) | 26/36 | 0.95 | 0.331 |
| Degree of differentiation (high/medium/Low) | 13/34/15 | 2.323 | 0.313 |
| Neural/vascular invasion (yes/no) | 19 (30.65)/43 (69.35) | 1.774 | 0.183 |
| NEU (× 109/L) | 4.12 ± 1.08 | - | - |
| LYM (× 109/L) | 1.64 ± 0.42 | - | - |
| PLT (× 109/L) | 212.36 ± 54.27 | - | - |
| SII | 869.42 ± 211.57 | - | - |
| LF (μg/mL) | 3.89 ± 0.86 | - | - |
| ECOG performance status (0-1/≥ 2) | 48 (77.4%)/14 (22.6%) | Calculated | Calculated |
| Charlson comorbidity index | 3.2 ± 1.1 | - | - |
| Adjuvant chemotherapy | 51 (82.3%) | - | - |
| FOLFOX regimen | 33 (65% of chemo) | - | - |
| CAPOX regimen | 18 (35% of chemo) | - | - |
| MSI-H status | 9 (14.5%) | Calculated | Calculated |
From preoperatively to 3 months postoperatively, the NEU, LYM, and PLT counts and SII values of the patients gradually decreased; however, the differences were not significant (P > 0.05). The proportion of SII hyperreactive states gradually decreased, indicating gradual recovery of the postoperative immune response. The changes in the SII were stable at each time point and did not reach statistical significance (Table 2).
| Detection period | NEU (× 109/L) | LYM (× 109/L) | PLT (× 109/L) | SII | SII high/Low ratio (%) | t | P value |
| Preoperative 7 days | 4.12 ± 1.08 | 1.64 ± 0.42 | 212.36 ± 54.27 | 869.42 ± 211.57 | 85/15 | 1.422 | 0.234 |
| Postoperative 1 week | 3.95 ± 1.02 | 1.60 ± 0.40 | 210.58 ± 52.73 | 864.25 ± 215.48 | 83/17 | 0.876 | 0.412 |
| Postoperative 1 month | 3.80 ± 1.05 | 1.55 ± 0.38 | 208.12 ± 50.96 | 854.62 ± 206.93 | 81/19 | 1.242 | 0.302 |
| Postoperative 3 months | 3.60 ± 1.10 | 1.50 ± 0.35 | 205.37 ± 49.12 | 838.41 ± 198.74 | 80/20 | 1.061 | 0.375 |
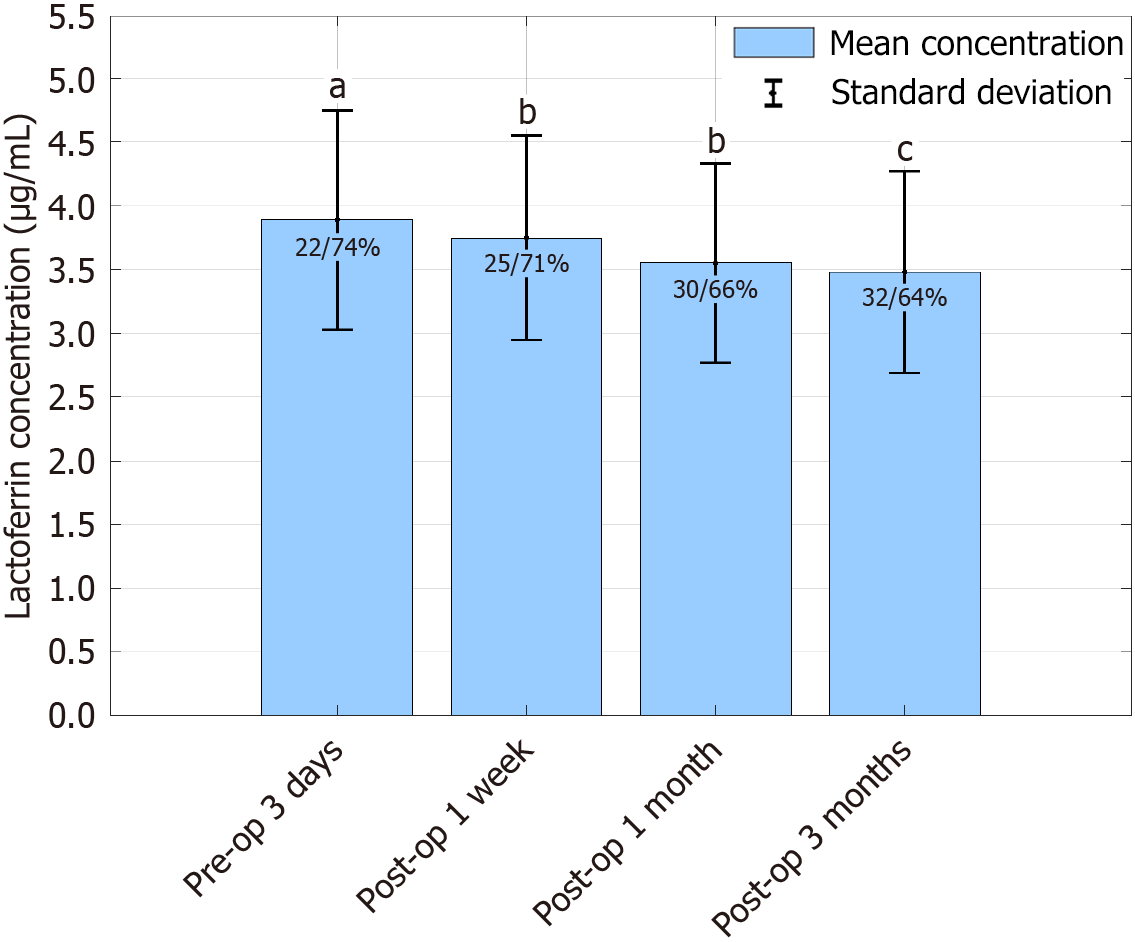
The LF level was (3.89 ± 0.86) μg/mL 3 days preoperatively and there was no significant difference (P > 0.05). One week, 1 month and 3 months postoperatively, the LF level decreased gradually, and all showed significant differences (P < 0.05), suggesting that immune function gradually weakened, and the proportion of low LF levels gradually increased (Table 3; Figure 1).
| Detection period | LF concentration (μg/mL) | Low/high ratio of LF (%) | χ2/t | P value |
| Preoperative 3 days | 3.89 ± 0.86 | 22/74 | 2.21 | 0.137 |
| Postoperative 1 week | 3.75 ± 0.80 | 25/71 | 5.432 | 0.03 |
| Postoperative 1 month | 3.55 ± 0.78 | 30/66 | 6.512 | 0.012 |
| Postoperative 3 months | 3.48 ± 0.79 | 32/64 | 7.234 | 0.008 |
ALT, AST, and BUN levels significantly increased 1 week postoperatively, suggesting that liver and kidney functions were affected. Liver and kidney functions were restored 1 month and 3 months postoperatively, but remained at a high level, and the differences were statistically significant (P < 0.05) (Table 4).
| Detection period | ALT (U/L) | AST (U/L) | ALB (g/L) | TBIL (μmol/L) | Cr (μmol/L) | BUN (mmol/L) | χ2/t | P value |
| Preoperative 1 week | 32.46 ± 4.21 | 28.72 ± 5.65 | 38.56 ± 6.89 | 12.34 ± 3.45 | 89.21 ± 10.34 | 4.91 ± 1.12 | 1.563 | 0.112 |
| Postoperative 1 week | 42.78 ± 7.13 | 40.13 ± 8.21 | 33.48 ± 5.67 | 16.45 ± 4.32 | 95.65 ± 12.56 | 5.34 ± 1.32 | 3.972 | 0.032 |
| Postoperative 1 month | 38.56 ± 5.02 | 35.97 ± 6.15 | 35.12 ± 4.76 | 14.02 ± 2.54 | 92.48 ± 9.87 | 5.12 ± 1.04 | 2.456 | 0.045 |
| Postoperative 3 months | 36.21 ± 6.01 | 33.84 ± 5.75 | 36.68 ± 5.12 | 13.21 ± 3.15 | 90.71 ± 11.21 | 4.87 ± 1.21 | 2.891 | 0.039 |
The CRP level significantly increased 1 week postoperatively, suggesting that the inflammatory response was enhanced. CRP levels decreased significantly 3 months postoperatively, indicating a decrease in inflammation. The PAB level decreased significantly 1 week postoperatively, reflecting insufficient postoperative nutritional reserve. However, the PAB increased 3 months after surgery (P < 0.05) (Table 5).
| Detection period | CRP (mg/L) | PAB (mg/L) | CRP low/high ratio (%) | PAB low/high ratio (%) | χ2/t | P value |
| Preoperative 3 days | 8.23 ± 2.45 | 160.23 ± 20.34 | 80.65/19.35 | 29.03/70.97 | 3.561 | 0.062 |
| Postoperative 1 week | 12.56 ± 3.15 | 145.87 ± 18.76 | 69.35/30.65 | 50.00/50.00 | 4.23 | 0.027 |
| Postoperative 1 month | 9.45 ± 2.89 | 150.12 ± 22.45 | 74.19/25.81 | 45.16/54.84 | 2.893 | 0.089 |
| Postoperative 3 months | 6.78 ± 1.87 | 155.34 ± 19.53 | 84.68/15.32 | 35.48/64.52 | 5.114 | 0.01 |
Six months postoperatively, CEA and CA19-9 levels increased significantly, with CEA (6.13 ± 1.52) ng/mL and CA19-9 (36.19 ± 12.78) U/mL, with positive percentages of 22.58% and 18.55%. Statistical analysis showed P > 0.05 6 months postoperatively, indicating an increased risk of recurrence (Table 6).
| Detection period | CEA (ng/mL) | CA19-9 (U/mL) | CEA positive proportion (%) | CA19-9 positive proportion (%) | χ2/t | P value |
| Preoperative 7 days | 4.72 ± 1.08 | 28.34 ± 10.47 | 14.52 | 9.68 | 2.324 | 0.13 |
| Postoperative 1 week | 5.38 ± 1.34 | 32.61 ± 11.32 | 18.38 | 12.9 | 3.126 | 0.075 |
| Postoperative 3 months | 4.96 ± 1.24 | 30.22 ± 9.88 | 15.32 | 11.29 | 2.812 | 0.097 |
| Postoperative 6 months | 6.13 ± 1.52 | 36.19 ± 12.78 | 22.58 | 18.55 | 4.214 | 0.04 |
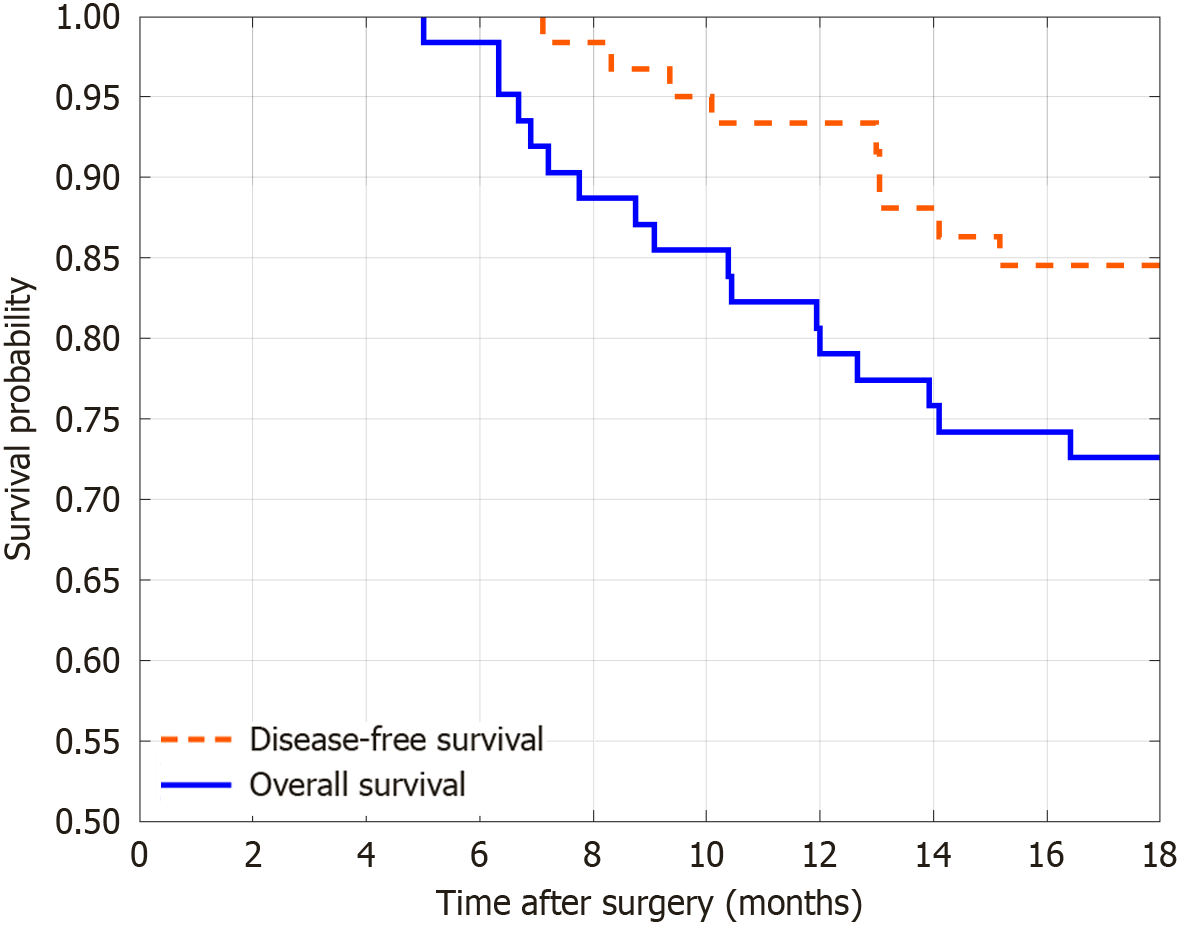
The follow-up showed a significant increase in OS and DFS 18 months postoperatively, indicating a correlation between longer follow-up time and the occurrence of endpoint events (Figure 2).
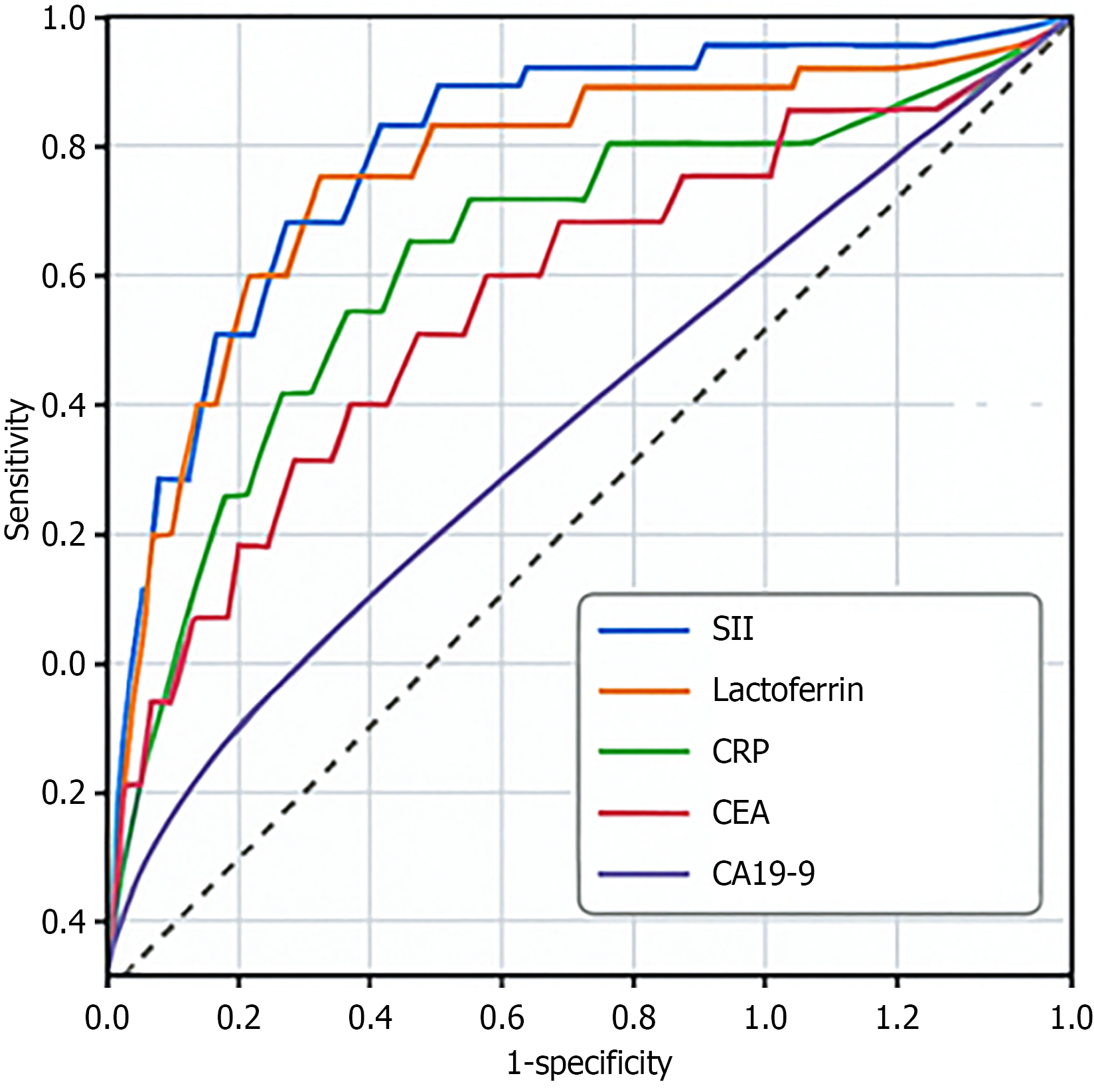
ROC analysis of the SII and levels of LF, CRP, CEA, and CA19-9 showed that the area under the curve (AUC) of the SII was 0.75, indicating a high predictive ability. The AUC for the LF and CRP levels were 0.72 and 0.74, respectively, both showing moderate predictive value. The AUC for CEA and CA19-9 levels were 0.73 and 0.72, respectively, both showing good diagnostic results with high sensitivity and specificity (Table 7; Figure 3). Based on ROC curve analysis, the optimal cutoff values were SII of 585 (sensitivity, 76%; specificity, 73%) and LF level less than 185 ng/mL (sensitivity, 72%; specificity, 68%). The fusion matrix for the SII (cut-off = 585) is listed in Table 8. A significant SII × LF interaction (HR = 1.92, 95%CI: 1.15-3.21, P = 0.012) was found.
| Indicator | Cut-off value | Sensitivity (%) | Specificity (%) | AUC | 95%CI | Youden's index |
| SII | 650 | 78 | 71 | 0.75 | 0.68-0.82 | 0.49 |
| LF | 200 | 70 | 65 | 0.72 | 0.65-0.79 | 0.35 |
| CRP | 10 | 66 | 72 | 0.74 | 0.68-0.80 | 0.38 |
| CEA | 5 | 80 | 60 | 0.73 | 0.67-0.79 | 0.4 |
| CA19-9 | 37 | 65 | 78 | 0.72 | 0.66-0.78 | 0.43 |
| Death | Survival | |
| High risk | 18 | 5 |
| Low risk | 7 | 32 |
| Overall accuracy: 80.6% |
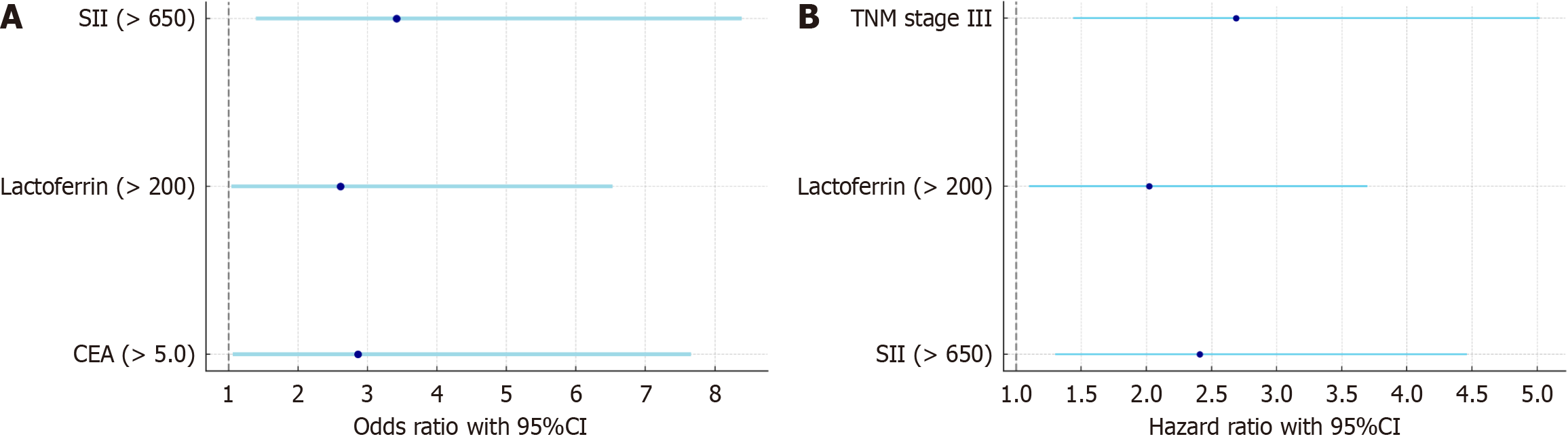
Logistic regression analysis showed that SII and LF and CEA levels were significant predictors of survival at 6 months postoperatively (P < 0.05), and SII had the highest predictive efficacy in multivariate analysis (OR = 3.42, 95%CI: 1.39-8.39) (Table 9; Figure 4A).
| Analysis type | Factor | β | SE | χ2 | P value | OR | 95%CI |
| Single factor | SII (> 650) | 1.35 | 0.48 | 7.85 | 0.005 | 3.85 | 1.52-9.69 |
| LF (> 200) | 1.12 | 0.49 | 5.33 | 0.021 | 3.07 | 1.26-7.46 | |
| CRP (> 10) | 0.95 | 0.52 | 3.24 | 0.072 | 2.59 | 0.95-7.04 | |
| CEA (> 5.0) | 1.27 | 0.54 | 5.61 | 0.018 | 3.55 | 1.19-10.46 | |
| CA19-9 (> 37) | 0.83 | 0.47 | 3.17 | 0.075 | 2.29 | 0.92-5.71 | |
| Constant | -1.05 | 0.45 | 5.444 | 0.02 | - | - | |
| Multiple factors | SII (> 650) | 1.23 | 0.48 | 6.5 | 0.011 | 3.42 | 1.39-8.39 |
| LF (> 200) | 0.96 | 0.49 | 4.12 | 0.043 | 2.61 | 1.04-6.53 | |
| CEA (> 5.0) | 1.05 | 0.51 | 4.25 | 0.039 | 2.86 | 1.06-7.66 | |
| Constant | -0.87 | 0.42 | 4.29 | 0.038 | - | - |
Univariate analysis showed that SII (> 650), LF level (> 200), CEA level (> 5.0), and TNM stage III were significantly associated with higher risk (P < 0.05). Multivariate analysis further confirmed the independence of SII, LF level, and TNM stage III in risk prediction, with both SII and LF level being high-risk factors (HR = 2.41 and 2.02). TNM stage III was also significantly associated with the risk (HR = 2.69) (Table 10; Figure 4B).
| Analysis type | Factor | β | SE | χ2 | P value | HR | 95%CI |
| Single factor | SII (> 650) | 0.88 | 0.29 | 9.21 | 0.002 | 2.41 | 1.35-4.29 |
| LF (> 200) | 0.7 | 0.28 | 6.25 | 0.012 | 2.02 | 1.17-3.49 | |
| CEA (> 5.0) | 0.66 | 0.3 | 4.85 | 0.028 | 1.93 | 1.07-3.47 | |
| TNM stage III | 1.02 | 0.29 | 12.39 | 0 | 2.78 | 1.52-5.07 | |
| Age ≥ 65 years old | 0.43 | 0.32 | 1.8 | 0.179 | 1.53 | 0.81-2.89 | |
| Multiple factors | SII (> 650) | 0.88 | 0.32 | 7.56 | 0.006 | 2.41 | 1.30-4.46 |
| LF (> 200) | 0.7 | 0.31 | 5.1 | 0.024 | 2.02 | 1.10-3.70 | |
| TNM stage III | 0.99 | 0.31 | 10.2 | 0.001 | 2.69 | 1.44-5.02 |
The detection methodology exhibited good performance with an intra-batch variation of 4.2% and an inter-batch variation of 7.6%. The recovery rate was within the range of 95%-105%, which met the experimental requirements (Table 11).
| Parameter | Value (%) |
| Intra-assay CV | 4.20 |
| Inter-assay CV | 7.60 |
| Recovery rate | 95-105 |
We found that both the SII and LF levels were significantly associated with the OS and DFS of patients with colon cancer, and both have high clinical predictive values. The SII, as an integrated inflammation-immunity index, is calculated with the NEU, PLT, and LYM counts and can reflect the immune inflammation state of patients with cancer more comprehensively. In this study, an increase in SII was associated with a decrease in OS and risk of recurrence. Inflammatory response plays a key role in the occurrence, development, and metastasis of colon cancer. In particular, in older patients, immune system function is generally decreased, and the continuous activation of the inflammatory microenvironment is more likely to induce poor biological behavior of the tumor[12]. Previous studies have also confirmed that SII has good prognostic prediction ability in a variety of digestive tract tumors, such as liver cancer, pancreatic cancer, and gastric cancer[13]. The mechanism may be that NEUs promote tumor angiogenesis and immune escape, PLTs helps tumor cell adhesion and metastasis, and a decrease in LYMs reflects the inhibition of antitumor immunity[14]. LF is a natural immune protein with multiple biological functions, such as regulation of iron metabolism, anti-inflammation, and anti-tumor[15]. This study found that the higher the LF level, the longer the survival of patients, and that its role in the postoperative recovery of patients with colon cancer deserves attention. On one hand, LF can slow the proliferation and invasion of tumor cells by inhibiting tumor-related inflammatory reactions; on the other hand, it can also enhance the immune function of T cells and NK cells and enhance the anti-cancer ability of the body[16]. In recent years, research on the role of LF in intestinal tumors has gradually increased, and some scholars have pointed out that it may be involved in the regulation of iron homeostasis, expression of inflammatory factors, and activation of immune cells in the tumor mic
Compared with traditional tumor markers such as CEA, CA19-9, and others, SII and LF have the advantages of simple detection, low cost, and sensitive dynamic changes, especially in the comprehensive management of older patients postoperatively, which potentially has wide application. Routine clinical assessment of postoperative recurrence risk and long-term survival of patients at this stage mostly depends on static indicators, such as TNM staging and histological classification. However, in our study, SII and LF levels, as continuous variables that can reflect the immune-inflammation-nutritional state during and early after surgery, are more individualized and forward-looking in nature[21]. The SII and LF level reflect the systemic response of the body to the tumor stress state from 2 different dimensions: Inflammation-immune response and nutrition-immune homeostasis, and their combination has a clear and clinical biological basis for predicting postoperative survival outcomes[22]. SII is composed of the NEU, PLT, and LYM counts, which comprehensively reflect the state of pro-inflammatory cell activation, coagulation system participation, and immune cell depletion. This signals high inflammation and high pro-tumor activity in the tumor microenvironment. As a multifunctional glycoprotein, LF not only regulates iron homeostasis, but also inhibits bacteria and inflammation. More studies have shown that LF can activate CD4+T cells and NK cells by inhibiting the expression of pro-inflammatory factors such as IL-6 and TNF-α, thereby enhancing the immune clearance of tumors, and has a potential anti-tumor mechanism. This mechanism is particularly significant in older patients with colon cancer[23]. Basic chronic inflammation and immune aging are common in older patients, and a low-reactivity but durable activation of the tumor microenvironment is easily formed. Increased SII is often accompanied by NEU-mediated matrix metalloproteinases release, upregulation of vascular endothelial growth factor, and tumor-associated macrophage recruitment, thus promoting tumor growth and metastasis. The decreased LF levels suggest that the antioxidant barrier was damaged and iron ions were overloaded, thus enhancing oxidative stress, DNA damage, and further undermining the immune recognition mechanism. Therefore, the combination of SII and LF can not only reveal the postoperative survival risk from the 2 Levels of inflammation-driven and immunonutrition depletion, but also capture the potential interaction mechanism between the 2, such as the negative regulatory effect of LF on the activation of SII NEUs[24]. This combined evaluation strategy not only increases the sensitivity and specificity of prognosis prediction but also provides a theoretical basis for target selection of postoperative immune and nutritional interventions in the future and a direction for individualized management. This proof-of-concept study has: (1) Limited power despite cross-validation; (2) Short follow-up (reframed as preliminary phase); and (3) A single-center design requiring multicenter validation with a 3-year follow-up.
Although this study revealed that the SII combined with serum LF level has a strong predictive value for the survival of older patients with colon cancer postoperatively, it still has certain limitations. First, because the sample size of the study was limited, there may have been selection and information bias, and the universality of the results still needs to be verified by a multicenter, large-sample prospective study. Second, the dynamic trends of LF level and SII and their relationship with postoperative recurrence and complications have not been explored in depth. Future research should be extended to patients in different populations and stages, and combined with molecular markers and tumor microenvironment factors to build a more accurate prognostic evaluation model. In terms of clinical promotion, the SII and LF detection methods are simple, inexpensive, repeatable, and suitable for routine application in primary hospitals and postoperative follow-up, with good practical potential. This study suggests that the combination of preoperative SII and LF level can be used as independent predictors of survival in older patients with colon cancer, providing a reliable basis for postoperative individualized management, risk stratification, and adjuvant treatment decision making, which has important clinical value.
| 1. | Wang QY, Zhong WT, Xiao Y, Lin GL, Lu JY, Xu L, Zhang GN, Du JF, Wu B. Pan-immune-inflammation value as a prognostic biomarker for colon cancer and its variation by primary tumor location. World J Gastroenterol. 2024;30:3823-3836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 2. | Millan M, Merino S, Caro A, Feliu F, Escuder J, Francesch T. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol. 2015;7:204-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (8)] |
| 3. | Cheng E, Shi Q, Shields AF, Nixon AB, Shergill AP, Ma C, Guthrie KA, Couture F, Kuebler P, Kumar P, Tan B, Krishnamurthi SS, Ng K, O'Reilly EM, Brown JC, Philip PA, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA. Association of Inflammatory Biomarkers With Survival Among Patients With Stage III Colon Cancer. JAMA Oncol. 2023;9:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Tian XP, Bu HM, Ma HY, Zhao M. Impact of dexmedetomidine-assisted anesthesia in elderly patients undergoing radical resection of colon cancer. World J Gastrointest Surg. 2024;16:2925-2933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Murray NP, Villalon R, Orrego S, Guzman E. Immune Dysfunction as Measured by the Systemic Immune-Inflammation Index is Associated with the Sub-Type of Minimal Residual Disease and Outcome in Stage II Colon Cancer Treated with Surgery alone. Asian Pac J Cancer Prev. 2021;22:2391-2397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Hou J, Zhao R, Cai T, Beaulieu-Jones B, Seyok T, Dahal K, Yuan Q, Xiong X, Bonzel CL, Fox C, Christiani DC, Jemielita T, Liao KP, Liaw KL, Cai T. Temporal Trends in Clinical Evidence of 5-Year Survival Within Electronic Health Records Among Patients With Early-Stage Colon Cancer Managed With Laparoscopy-Assisted Colectomy vs Open Colectomy. JAMA Netw Open. 2022;5:e2218371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (3)] |
| 7. | Daveri E, Vergani B, Lalli L, Ferrero G, Casiraghi E, Cova A, Zorza M, Huber V, Gariboldi M, Pasanisi P, Guarrera S, Morelli D, Arienti F, Vitellaro M, Corsetto PA, Rizzo AM, Stroscia M, Frati P, Lagano V, Cattaneo L, Sabella G, Leone BE, Milione M, Sorrentino L, Rivoltini L. Cancer-associated foam cells hamper protective T cell immunity and favor tumor progression in human colon carcinogenesis. J Immunother Cancer. 2024;12:e009720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Jan N, Hays RA, Oakland DN, Kumar P, Ramakrishnan G, Behm BW, Petri WA Jr, Marie C. Fecal Microbiota Transplantation Increases Colonic IL-25 and Dampens Tissue Inflammation in Patients with Recurrent Clostridioides difficile. mSphere. 2021;6:e0066921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Liao K, Zhu M, Guo L, Gao Z, Cheng J, Sun B, Qian Y, Lin B, Zhang J, Qian T, Jiang Y, Xu Y, Zhong Q, Wang X. Assessment of prognosis and responsiveness to immunotherapy in colorectal cancer patients based on the level of immune cell infiltration. Front Immunol. 2025;16:1514238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Almutairi AR, Slack M, Erstad BL, McBride A, Abraham I. Association of immune-checkpoint inhibitors and the risk of immune-related colitis among elderly patients with advanced melanoma: real-world evidence from the SEER-Medicare database. Ther Adv Drug Saf. 2021;12:2042098621991279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Vermandere K, Bostick RM, Tran HQ, Gewirtz AT, Barry EL, Rutherford RE, Seabrook ME, Fedirko V. Effects of Supplemental Calcium and Vitamin D on Circulating Biomarkers of Gut Barrier Function in Patients with Colon Adenoma: A Randomized Clinical Trial. Cancer Prev Res (Phila). 2021;14:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Kuo YH, You JF, Hung HY, Chin CC, Chiang JM, Chang CH. Number of negative lymph nodes with a positive impact on survival of stage III colon cancer; a retrospective observation study for right side and left side colon. BMC Cancer. 2022;22:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Öter S, Özkömeç A, Bozan MB, Yazar FM, Kale İT, Azak Bozan A, İşler A. Preoperative Delta Neutrophil Index, Platelet Lymphocyte Ratio and Immature Granulocyte Count for Differentiating Metastatic Colon Cancer from Non-Metastatic Colon Cancer: A Retrospective Study. Ann Ital Chir. 2024;95:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Li HX, Che L, Li Y, Wang TH, Min FD, Xu L, Wang M, Zheng ZX, Qu SN, Wang F, Tang W, Wei SJ, Sun YL, Zheng H, Yan T. Correlations between primary tumour location, biomarkers of inflammation and lung injury, and postoperative pulmonary complications in patients underwent laparoscopic colorectomy: a propensity score matched analysis of 300 patients. Front Immunol. 2025;16:1546167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Moussa MR, Fan N, Birk J, Provatas AA, Mehta P, Hatano Y, Chun OK, Darooghegi Mofrad M, Lotfi A, Aksenov A, Motta VN, Zenali M, Vaziri H, Grady JJ, Nakanishi M, Rosenberg DW. Systemic Inflammation and the Inflammatory Context of the Colonic Microenvironment Are Improved by Urolithin A. Cancer Prev Res (Phila). 2025;18:235-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Li H, Li C, Zhang B, Jiang H. Lactoferrin suppresses the progression of colon cancer under hyperglycemia by targeting WTAP/m(6)A/NT5DC3/HKDC1 axis. J Transl Med. 2023;21:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Ramírez-Sánchez DA, Canizalez-Román A, León-Sicairos N, Pérez Martínez G. The anticancer activity of bovine lactoferrin is reduced by deglycosylation and it follows a different pathway in cervix and colon cancer cells. Food Sci Nutr. 2024;12:3516-3528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Elmorshedy YM, Teleb M, Sallam MA, Elkhodairy KA, Bahey-El-Din M, Ghareeb DA, Abdulmalek SA, Abdel Monaim SAH, Bekhit AA, Elzoghby AO, Albericio F, Khattab SN. Engineered Microencapsulated Lactoferrin Nanoconjugates for Oral Targeted Treatment of Colon Cancer. Biomacromolecules. 2023;24:2149-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 19. | Wei YS, Feng K, Wu H. Regulation of the colon-targeted release rate of lactoferrin by constructing hydrophobic ethyl cellulose/pectin composite nanofibrous carrier and its effect on anti-colon cancer activity. Int J Biol Macromol. 2024;261:129466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Tanaka H, Gunasekaran S, Saleh DM, Alexander WT, Alexander DB, Ohara H, Tsuda H. Effects of oral bovine lactoferrin on a mouse model of inflammation associated colon cancer. Biochem Cell Biol. 2021;99:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Li H, Yao Q, Li C, Fan L, Wu H, Zheng N, Wang J. Lactoferrin Inhibits the Development of T2D-Induced Colon Tumors by Regulating the NT5DC3/PI3K/AKT/mTOR Signaling Pathway. Foods. 2022;11:3956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Raval H, Bhattacharya S, Bhirud D, Sangave PC, Gupta GL, Paraskar G, Jha M, Sharma S, Belemkar S, Kumar D, Maheshwari R. Fabrication of lactoferrin-chitosan-etoposide nanoparticles with melatonin via carbodiimide coupling: In-vitro & in-vivo evaluation for colon cancer. J Control Release. 2025;377:810-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 23. | Abd Elhamid AS, Heikal L, Ghareeb DA, Abdulmalek SA, Mady O, Teleb M, Khattab SN, El-Gizawy SA. Engineering Thermo/pH-Responsive Lactoferrin Nanostructured Microbeads for Oral Targeting of Colorectal Cancer. ACS Biomater Sci Eng. 2024;10:4985-5000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Liu C, Peng Q, Wei L, Li Z, Zhang X, Wu Y, Wang J, Zheng X, Wen Y, Zheng R, Yan Q, Ye Q, Ma J. Deficiency of Lactoferrin aggravates lipopolysaccharide-induced acute inflammation via recruitment macrophage in mice. Biometals. 2023;36:549-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |