Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.109543
Revised: August 12, 2025
Accepted: October 10, 2025
Published online: November 27, 2025
Processing time: 144 Days and 2.8 Hours
Rectal cancer is a common malignant tumor of the digestive system, with older patients representing the predominantly affected population. Magnetic resonance imaging (MRI) has been widely applied in preoperative tumor assessment; how
To evaluate the value of HR-MRI combined with DCE scanning in the preope
This retrospective study included 148 consecutive older female patients with rectal cancer who were treated at our hospital between December 2020 and December 2024. Clinical data and HR-MRI and DCE scan findings were collected. Histopathological examination after surgical resection served as the gold stan
Among the 148 patients, the overall accuracy of T staging was 84.5%. Sensitivity for T1, T2, T3, and T4 staging was 75.00%, 62.50%, 89.47%, and 90.48%, respectively, whereas specificity was 100.00%, 94.35%, 79.25%, and 96.06%, respectively. T staging based on HR-MRI combined with DCE scanning showed good agreement with pathological staging (k = 0.8176, P < 0.001). For N staging, sensitivity and specificity were 54.88% and 84.85% for N0, 36.96% and 72.55% for N1, and 70.00% and 73.44% for N2, respectively; agreement with pathological N staging was poor (k = 0.259, P < 0.001).
HR-MRI combined with DCE scanning demonstrates high diagnostic accuracy for T staging of rectal cancer in older patients and can provide a theoretical basis for treatment planning. However, its diagnostic accuracy for N staging requires improvement.
Core Tip: This study evaluated the value of high-resolution magnetic resonance imaging combined with dynamic contrast-enhanced scanning for preoperative staging of rectal cancer in older patients. In a retrospective analysis of 148 patients (December 2020 to December 2024), postoperative histopathology served as the gold standard. T staging accuracy was 84.5%, with a sensitivity of 62.5%-90.5% and specificity of 79.3%-100% across T1-T4 stages, demonstrating excellent agreement with pathological staging (k = 0.817, P < 0.001). Conversely, N staging showed lower sensitivity (36.96%-70.0%) and specificity (72.55%-84.85%), with poor agreement (k = 0.259, P < 0.001). Overall, this combined imaging approach provides high diagnostic accuracy for T staging to guide treatment planning, although its performance in N staging requires improvement.
- Citation: Gao YL, Li HN, Wang Q, Shen W. Value of high-resolution magnetic resonance imaging and dynamic contrast-enhanced scanning in the preoperative diagnosis of rectal cancer in older patients. World J Gastrointest Surg 2025; 17(11): 109543
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/109543.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.109543
According to the International Agency for Research on Cancer, there were approximately 19.3 million new cancer cases and 10 million cancer-related deaths worldwide in 2020[1]. Of these, 19429789 new cases were reported, with breast, lung, colorectal, prostate, stomach, and liver cancers accounting for about 50% of the total burden[2]. In China, the incidence of colorectal cancer (CRC) has been increasing, making it the second most common malignant tumor and the fifth leading cause of cancer-related mortality[3]. CRC is one of the most prevalent malignancies of the digestive system, with rectal cancer comprising approximately 50%-60% of cases and low rectal cancer being particularly common[4]. Although the incidence of CRC has been declining in developed countries, it continues to increase in many developing nations, where it remains a frequent diagnosis, especially among individuals aged > 60 years[5,6]. This trend is closely associated with age-related physiological and pathological changes, including reduced intestinal mucosal repair, chronic inflammation, and impaired immune surveillance[7]. Older patients with rectal cancer often present with comorbidities such as car
In clinical practice, the accuracy of preoperative staging directly affects surgical protocol selection and adjuvant treatment decisions. In the traditional TNM staging system, the depth of primary tumor infiltration (T staging) and the status of regional lymph node metastasis (N staging) are key determinants for recommending neoadjuvant therapy and defining the extent of surgery[9]. According to the National Comprehensive Cancer Network and European Society for Medical Oncology guidelines, magnetic resonance imaging (MRI) is currently the preferred imaging modality for rectal cancer in clinical practice[10,11]. MRI is a rapid, easy, and noninvasive method commonly used for staging rectal cancer, predicting treatment response, and monitoring patients during follow-up. In particular, T2-weighted imaging (T2WI) provides clear visualization of the inner and outer layers of the colorectal wall, offering a reliable basis for staging CRC[12]. However, in older patients, conventional MRI often has limitations in delineating tumor boundaries due to age-related degenerative changes such as intestinal muscular atrophy and fat infiltration.
High-resolution MRI (HR-MRI), also known as MRI microscopy, provides sub-millimeter resolution with a high signal-to-noise ratio, offering advantages over standard MRI[13]. Dynamic contrast-enhanced MRI (DCE-MRI) allows noninvasive evaluation of tumor microvasculature and enables both quantitative and semiquantitative assessment of kinetic parameters related to perfusion and permeability[14,15]. Numerous studies have confirmed the effectiveness of DCE-MRI in evaluating therapeutic responses to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer[16-18]. However, its applicability in older patients remains controversial[19]. Age-related changes such as reduced vascular elasticity and slower contrast agent metabolism may alter enhancement patterns on DCE-MRI, while coexisting mesenteric arteriosclerosis may interfere with recognition of micro-lymph node perfusion features. These factors highlight the urgent need to validate the diagnostic performance of this technique in older patients.
Therefore, this study aimed to evaluate the diagnostic value of HR-MRI combined with DCE-MRI for preoperative T and N staging of rectal cancer in older patients, with the goal of providing a reference for selecting optimal imaging methods in the preoperative setting.
This retrospective study included 148 older patients with rectal cancer who were diagnosed via colonoscopy and rectoscopy at our hospital between December 2020 and December 2024. All patients underwent HR-MRI and DCE-MRI scanning as well as preoperative MRI followed by surgical treatment. T and N staging of rectal cancer were determined based on the 8th edition of the American Joint Committee on Cancer staging system for CRC as follows[20]. T staging: T1: Tumor invades the submucosa; T2: Tumor invades the muscularis propria but does not penetrate the serosa; T3: Tumor penetrates the serosa and extends into perirectal mesenteric fat; and T4: Tumor involves peritoneal structures or adjacent organs. N staging: N0: No regional lymph node metastasis; N1: Metastasis in 1-3 regional lymph nodes; N2: Metastasis in ≥ 4 perirectal lymph nodes.
Patients were included if they had pathologically confirmed rectal cancer via surgical biopsy, underwent 3.0T HR-MRI and DCE-MRI scans, received no prior treatment before MRI examination, had no history of contrast agent allergy, and had complete clinical data. Patients were excluded if they demonstrated poor compliance or significant MRI artifacts, underwent MRI examination but did not receive a pathological diagnosis at our hospital, or had incomplete clinical data.
Ethical approval for this study was obtained from the hospital’s Research Ethics Board. As this was a retrospective study, the Ethics Committee waived the requirement for informed consent.
Imaging was performed without bowel preparation but with adequate bladder filling. Patients were positioned supine, and gadopentetate dimeglumine (0.1 mmol/kg) was administered intravenously at a rate of 3.0 mL/second, followed by a 20 mL saline flush. HR-MRI included sagittal T2WI [repetition time (TR): 3000 ms, echo time (TE): 80 ms, field of view (FOV): 24 cm, matrix: 384 × 224, slice thickness/gap: 3.0 mm/0.5 mm, and number of excitations (NEX): 4], followed by axial and coronal T2WI (TR: 3000 ms, TE: 80 ms, FOV: 20 cm, matrix: 384 × 224, slice thickness/gap: 3.0 mm/0.5 mm, and NEX: 4). DCE-MRI was subsequently performed using a volumetric interpolated breath-hold examination sequence (TR: 3.0 ms, TE: 80 ms, FOV: 36 cm, and slice thickness/gap: 3.0 mm/0.5 mm), with 30 dynamic phases acquired (6 seconds/phase, 26 slices/phase).
For image analysis, two senior radiologists reached consensus on T staging using HR-MRI T2WI criteria: (1) T1: Mucosal or submucosal thickening with tumor signal intensity lower than that of the submucosa; (2) T2: Invasion of the muscularis propria with intact perirectal fat planes; (3) T3: Penetration of the muscularis propria with blurred perirectal fat boundaries; and (4) T4: Invasion of adjacent organs. DCE-MRI data were processed using dedicated software, with the arterial input function defined by a region of interest (ROI; 3-5 mm2) placed in a branch of the inferior mesenteric artery. Perfusion parameter maps were generated from the most enhanced portion of the lesion, and quantitative values were obtained from ROI measurements. All cases were pathologically confirmed after imaging.
(1) Consistency between preoperative staging of rectal cancer using HR-MRI combined with DCE-MRI and pathological staging, with histopathology serving as the gold standard; (2) Diagnostic efficacy of HR-MRI combined with DCE-MRI for preoperative T staging of rectal cancer (T1, T2, T3, and T4); and (3) Diagnostic efficacy of HR-MRI combined with DCE-MRI for preoperative N staging of rectal cancer (N0, N1, and N2).
All statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States). Measurement data are expressed as mean ± SD and were compared using the t-test. Categorical data were expressed as frequencies and percentages, with comparisons performed using the χ2 test. A P value < 0.05 was considered statistically significant. Consistency between preoperative staging and pathological results was assessed using the k statistic, where values of 0.01-0.39 indicated poor agreement, 0.40-0.74 indicated moderate agreement, and 0.75-1.00 indicated strong agreement. Inter-observer agreement between the two senior radiologists was evaluated using intraclass correlation coefficients.
In total, 148 older female patients with rectal cancer were included, with a mean age of 67.6 ± 7.9 years. Tumor location was upper rectum, middle rectum, and lower rectum in 24 (16.22%), 61 (41.22%), and 63 (42.57%) cases, respectively. Histopathological types included 142 cases of conventional adenocarcinoma (95.95%) and 6 cases of mucinous adenocarcinoma (4.05%). Gross pathological types included 122 ulcerative tumors (82.43%) and 26 exophytic tumors (17.57%). Details are presented in Table 1.
| Item | Number of cases | Percentage (%) |
| Total number | 148 | 100.00 |
| Sex | ||
| Female | 148 | 100.00 |
| Mean age (years) | 67.6 ± 7.9 | |
| Tumor location | ||
| Upper rectum | 24 | 16.22 |
| Middle rectum | 61 | 41.22 |
| Lower rectum | 63 | 42.57 |
| Histopathological type | ||
| Conventional adenocarcinoma | 142 | 95.95 |
| Mucinous adenocarcinoma | 6 | 4.05 |
| Gross pathological type | ||
| Ulcerative | 122 | 82.43 |
| Exophytic | 26 | 17.57 |
Pathological staging of the 148 patients showed 8 cases of T1, 24 cases of T2, 95 cases of T3, and 21 cases of T4 disease. The T staging results obtained using HR-MRI combined with DCE-MRI were generally consistent with pathological staging results (k = 0.8174, P < 0.001), as shown in Table 2.
| Pathological stage | Total | |||||
| T1 | T2 | T3 | T4 | |||
| MRI stage | T1 | 6 | 0 | 0 | 0 | 6 |
| T2 | 2 | 15 | 5 | 0 | 22 | |
| T3 | 0 | 9 | 85 | 2 | 96 | |
| T4 | 0 | 0 | 5 | 19 | 24 | |
| Total | 8 | 24 | 95 | 21 | 148 | |
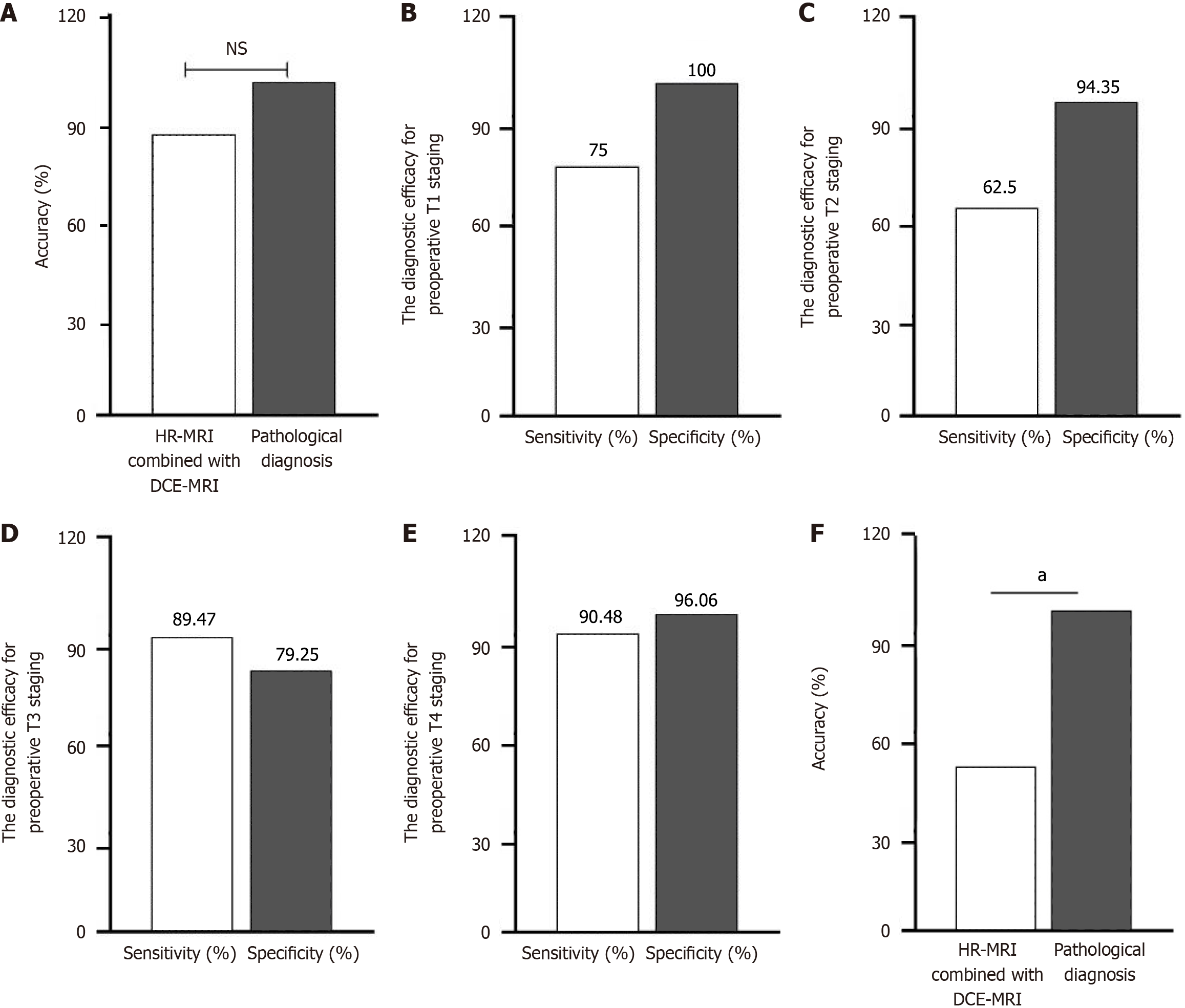
HR-MRI combined with DCE-MRI correctly identified the T stage in 125 of 148 patients, yielding an overall accuracy of 84.5%. Although this was lower than the 100% accuracy of pathological diagnosis, the difference was not statistically significant (P > 0.05), as shown in Figure 1A.
Among the 8 patients pathologically diagnosed with T1 stage, HR-MRI combined with DCE-MRI correctly identified 6 cases as T1. The sensitivity for T1 staging was 75.00% (6/8), and the specificity was 100.00% (140/140), as shown in Figure 1B.
Among the 24 patients pathologically diagnosed with T2 stage, HR-MRI combined with DCE-MRI correctly identified 15 cases. The sensitivity for T2 staging was 62.50% (15/24), and the specificity was 94.35% (117/124), as shown in Figure 1C.
Among the 95 patients pathologically diagnosed with T3 stage, HR-MRI combined with DCE-MRI correctly identified 85 cases. The sensitivity for T3 staging was 89.47% (85/95), and the specificity was 79.25% (42/53), as shown in Figure 1D.
Among the 21 patients pathologically diagnosed with T4 stage, HR-MRI combined with DCE-MRI correctly identified 19 cases. The sensitivity for T4 staging was 90.48% (19/21), and the specificity was 96.06% (122/127), as shown in Figure 1E.
Pathological staging of the 148 patients showed 82 cases of N0, 46 cases of N1, and 20 cases of N2 disease. The agreement between N staging results obtained using HR-MRI combined with DCE-MRI and pathological staging results was poor (k = 0.259, P < 0.001), as shown in Table 3.
| Pathological stage | Total | ||||
| N0 | N1 | N2 | |||
| MRI stage | N0 | 45 | 10 | 0 | 55 |
| N1 | 22 | 17 | 6 | 45 | |
| N2 | 15 | 19 | 14 | 48 | |
| Total | 82 | 46 | 20 | 148 | |
HR-MRI combined with DCE-MRI correctly identified the N stage in 76 of 148 patients, yielding an overall accuracy of 51.35%. This was lower than the 100% accuracy of pathological diagnosis, and the difference was statistically significant (P < 0.05), as shown in Figure 1F.
Among the 82 patients pathologically diagnosed with N0 stage, HR-MRI combined with DCE-MRI correctly identified 45 cases. The sensitivity for N0 staging was 54.88% (45/82), and the specificity was 84.85% (56/66). Among the 46 patients diagnosed with N1 stage, 17 were correctly identified, with a sensitivity of 36.96% (17/46) and specificity of 72.55% (74/102). Among the 20 patients diagnosed with N2 stage, 14 were correctly identified, yielding a sensitivity of 70.00% (14/20) and specificity of 73.44% (94/128), as shown in Table 4.
| N stage | Sensitivity (%) | Specificity (%) |
| N0 | 54.88 | 84.85 |
| N1 | 36.96 | 72.55 |
| N2 | 70.00 | 73.44 |
This study evaluated the utility of HR-MRI combined with DCE-MRI in the preoperative staging of rectal cancer in older patients. The results demonstrated strong agreement between HR-MRI combined with DCE-MRI and pathological diagnosis in T staging (k = 0.8174), with excellent sensitivity for advanced T3 and T4 stages (89.47%-90.48%)[21,22]. These findings are consistent with those of previous studies showing that HR-MRI can accurately delineate tumor infiltration depth by providing high-resolution visualization of rectal wall layers and perirectal fat planes. By clearly displaying the relationship between the tumor and surrounding tissues, MRI facilitates reliable T stage assessment.
Although sensitivity for T1 and T2 stages was lower (62.50%-75.00%), likely due to age-related anatomical changes such as muscular atrophy and fat infiltration that obscure early tumor boundaries, the high specificity (94.35%-100%) highlights the technique’s reliability in ruling out superficial tumors. This supports its clinical utility in guiding decisions regarding sphincter-preserving surgery.
In contrast, the diagnostic efficacy for N staging was significantly limited, with poor agreement (k = 0.259) and substantial variability in sensitivity and specificity across stages—most notably the low sensitivity for N1 (36.96%). These findings align with those of prior studies underscoring MRI’s limitations in detecting lymph node metastases, particularly in small nodes or cases with few metastatic deposits. Three age-related factors further contribute to this limitation: (1) Mesenteric arteriosclerosis in older patients alters DCE-MRI contrast kinetics, reducing the discriminative power of perfusion parameters (e.g., Ktrans) for metastatic nodes; (2) Chronic inflammation, common in this population, promotes reactive lymph node hyperplasia that mimics metastasis on morphology-based MRI; and (3) Over 60% of missed N1 metastases involve nodes smaller than 5 mm, which lie beyond the spatial resolution of conventional MRI. Additionally, age-related comorbidities may obscure imaging features, compounding the challenge of accurate N staging.
Although HR-MRI combined with DCE-MRI performs well in T staging, complementary techniques are needed to improve N staging accuracy. Prior studies suggest that diffusion-weighted imaging (DWI) can enhance diagnostic performance by quantifying restricted diffusion in malignant lymph nodes, as metastatic nodes typically demonstrate lower apparent diffusion coefficient values compared with benign nodes[23-25]. Positron emission tomography-computed tomography (PET-CT), although limited by cost and accessibility, can detect occult metastases through metabolic activity (fludeoxyglucose uptake), thereby overcoming the size-based limitations of MRI. In contrast, CT offers poor soft-tissue resolution, resulting in lower N staging accuracy (61.25%), and is therefore suboptimal for older patients requiring precise staging. A multimodal approach (HR-MRI + DWI ± PET-CT) is thus recommended to optimize N staging, particularly in older patients with vascular comorbidities.
Emerging multi-omics strategies show promise in overcoming current limitations. Cicalini et al[26] reported that integrating MRI radiomics with serum metabolomic profiles improved prediction of nodal metastasis in locally advanced rectal cancer (area under the curve: 0.92 vs 0.78 for MRI alone). Similarly, combining serum microRNA-378 expression with DW-MRI increased N staging accuracy to 96.67% by distinguishing inflammatory from metastatic lymph nodes. Such approaches may help address age-related diagnostic challenges by capturing molecular and functional heterogeneity that extends beyond conventional anatomical imaging.
This study has several limitations. First, the retrospective design may have introduced selection bias. Second, the sample size (n = 148) limited the ability to perform subgroup analyses by tumor location (upper, middle, or lower rectum) and by pathological subtype (e.g., mucinous vs conventional adenocarcinoma, with only six mucinous cases), restricting assessment of imaging performance heterogeneity. Third, technical parameters for image acquisition and analysis were not fully standardized, which may affect reproducibility. Fourth, the study cohort consisted exclusively of female patients, reflecting higher recruitment rates from our gynecologic oncology unit, which limits generalizability to male patients—among whom rectal cancer incidence is 1.5-2 times higher. Future large-scale prospective trials should validate these findings in sex-balanced older cohorts and include stratified analyses by tumor location and histology.
HR-MRI combined with DCE-MRI exhibits good diagnostic performance for preoperative T staging of rectal cancer, with high accuracy and consistency, making it a valuable tool for preoperative assessment in older patients. However, its diagnostic efficacy for N staging remains limited, underscoring the need for cautious interpretation in clinical practice and the integration of complementary imaging modalities to improve accuracy. A comprehensive, multimodal approach can better support individualized treatment planning. Future studies should focus on advancing MRI techniques and combining them with other imaging modalities to enhance the accuracy and reliability of rectal cancer staging, ultimately improving treatment strategies and patient outcomes.
| 1. | Wang R, Li L, Xu J, Ding ZT, Qiao J, Redding SR, Xianyu YY, Ouyang YQ. Effects of Structured Expressive Writing on Quality of Life and Perceived Self-Care Self-Efficacy of Breast Cancer Patients Undergoing Chemotherapy in Central China: A Randomized Controlled Trial. Healthcare (Basel). 2022;10:1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Londoño-Berrio M, Castro C, Cañas A, Ortiz I, Osorio M. Advances in Tumor Organoids for the Evaluation of Drugs: A Bibliographic Review. Pharmaceutics. 2022;14:2709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Shen Y, Zhao X, Zhao H, Chen N, Wang J, Zhuang H, Zhang X. Clinical Application of Enteral Nutrition Combined with Microbial Preparation for Intestinal Preparation in Elderly Patients with Colorectal Cancer. Med Sci Monit. 2022;28:e935366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Zou Y, Yang Q, Guan B, Fu X, Wang J, Li Y. Survey on Mental Health Status and Quality of Life and Correlation among Patients with Permanent Stoma of Colorectal Tumor. Comput Math Methods Med. 2022;2022:5792312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Liu S, Qu D, Li W, He C, Li S, Wu G, Zhao Q, Shen L, Zhang J, Zheng J. miR‑647 and miR‑1914 promote cancer progression equivalently by downregulating nuclear factor IX in colorectal cancer. Mol Med Rep. 2017;16:8189-8199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Chakradhar S. Colorectal cancer: 5 big questions. Nature. 2015;521:S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Brody H. Colorectal cancer. Nature. 2015;521:S1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 8. | Syed Soffian SS, Mohammed Nawi A, Hod R, Abdul Manaf MR, Chan HK, Abu Hassan MR. Disparities in Recommendations for Colorectal Cancer Screening Among Average-Risk Individuals: An Ecobiosocial Approach. Risk Manag Healthc Policy. 2022;15:1025-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Väyrynen JP, Haruki K, Lau MC, Väyrynen SA, Ugai T, Akimoto N, Zhong R, Zhao M, Dias Costa A, Borowsky J, Bell P, Takashima Y, Fujiyoshi K, Arima K, Kishikawa J, Shi SS, Twombly TS, Song M, Wu K, Chan AT, Zhang X, Fuchs CS, Meyerhardt JA, Giannakis M, Ogino S, Nowak JA. Spatial Organization and Prognostic Significance of NK and NKT-like Cells via Multimarker Analysis of the Colorectal Cancer Microenvironment. Cancer Immunol Res. 2022;10:215-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 698] [Article Influence: 99.7] [Reference Citation Analysis (1)] |
| 11. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 12. | Chuanji Z, Zheng W, Shaolv L, Linghou M, Yixin L, Xinhui L, Ling L, Yunjing T, Shilai Z, Shaozhou M, Boyang Z. Comparative study of radiomics, tumor morphology, and clinicopathological factors in predicting overall survival of patients with rectal cancer before surgery. Transl Oncol. 2022;18:101352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Ivan D, Ohlerth S, Richter H, Verdino D, Rampazzo A, Pot S. 3T high-resolution magnetic resonance imaging, conventional ultrasonography and ultrasound biomicroscopy of the normal canine eye. BMC Vet Res. 2022;18:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Jarnagin WR, Schwartz LH, Gultekin DH, Gönen M, Haviland D, Shia J, D'Angelica M, Fong Y, DeMatteo R, Tse A, Blumgart LH, Kemeny N. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 15. | Zhao M, Guo LL, Huang N, Wu Q, Zhou L, Zhao H, Zhang J, Fu K. Quantitative analysis of permeability for glioma grading using dynamic contrast-enhanced magnetic resonance imaging. Oncol Lett. 2017;14:5418-5426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Kim HR, Kim SH, Nam KH. Association between Dynamic Contrast-Enhanced MRI Parameters and Prognostic Factors in Patients with Primary Rectal Cancer. Curr Oncol. 2023;30:2543-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Tong T, Sun Y, Gollub MJ, Peng W, Cai S, Zhang Z, Gu Y. Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging. 2015;42:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Intven M, Reerink O, Philippens ME. Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging. 2015;41:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Intven M, Monninkhof EM, Reerink O, Philippens ME. Combined T2w volumetry, DW-MRI and DCE-MRI for response assessment after neo-adjuvant chemoradiation in locally advanced rectal cancer. Acta Oncol. 2015;54:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Deng J, Zhou S, Wang Z, Huang G, Zeng J, Li X. Comparison of Prognosis and Lymph Node Metastasis in T1-Stage Colonic and Rectal Carcinoma: A Retrospective Study. Int J Gen Med. 2022;15:3651-3662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol. 2022;43:101739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 22. | Bates DDB, Homsi ME, Chang KJ, Lalwani N, Horvat N, Sheedy SP. MRI for Rectal Cancer: Staging, mrCRM, EMVI, Lymph Node Staging and Post-Treatment Response. Clin Colorectal Cancer. 2022;21:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Lv B, Cheng X, Cheng Y, Kong X, Jin E. Predictive value of MRI-detected tumor deposits in locally advanced rectal cancer. Front Oncol. 2023;13:1153566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | McMahon CJ, Smith MP. Magnetic resonance imaging in locoregional staging of rectal adenocarcinoma. Semin Ultrasound CT MR. 2008;29:433-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Catalano OA, Lee SI, Parente C, Cauley C, Furtado FS, Striar R, Soricelli A, Salvatore M, Li Y, Umutlu L, Cañamaque LG, Groshar D, Mahmood U, Blaszkowsky LS, Ryan DP, Clark JW, Wo J, Hong TS, Kunitake H, Bordeianou L, Berger D, Ricciardi R, Rosen B. Improving staging of rectal cancer in the pelvis: the role of PET/MRI. Eur J Nucl Med Mol Imaging. 2021;48:1235-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Cicalini I, Chiarelli AM, Chiacchiaretta P, Perpetuini D, Rosa C, Mastrodicasa D, d'Annibale M, Trebeschi S, Serafini FL, Cocco G, Narciso M, Corvino A, Cinalli S, Genovesi D, Lanuti P, Valentinuzzi S, Pieragostino D, Brocco D, Beets-Tan RGH, Tinari N, Sensi SL, Stuppia L, Del Boccio P, Caulo M, Delli Pizzi A. Multi-omics staging of locally advanced rectal cancer predicts treatment response: a pilot study. Radiol Med. 2024;129:712-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |