Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.109426
Revised: June 24, 2025
Accepted: September 15, 2025
Published online: November 27, 2025
Processing time: 199 Days and 4.9 Hours
One-anastomosis gastric bypass (OAGB) and sleeve gastrectomy (SG) are surgical procedures increasingly performed for weight loss and to achieve remission of diabetes mellitus. Literature comparing the medium-term efficacy of these two procedures is scarce. As such, a meta-analysis comparing OAGB and SG in terms of diabetes remission (DR) and percentage of excess weight loss (EWL) is war
To compare OAGB and SG in terms of DR and EWL% in the medium term.
A comprehensive literature search was conducted in PubMed/MEDLINE, Cochran Library, and Web of Science for relevant articles, from inception through April 2025, using the keywords “one-anastomosis gastric bypass”, “sleeve gas
A total of 1360 articles were identified, and 35 studies were retrieved of which 32 were included in the final analysis. Three full texts were excluded as they did not include data on DR or EWL%. OAGB achieved higher DR than SG at 1 year following surgery [odds ratio (OR) = 1.77, 95% confidence interval (CI): 1.22-2.57, I2 = 76%]. However, DR rates were similar at 3 years and 5 years following surgery (OR = 0.82, 95%CI: 0.61-1.10, I2 = 23% and OR = 0.92, 95%CI: 0.31-2.72, I2 = 75%, respectively). OAGB showed higher EWL% at 1 year (OR = 9.30, 95%CI: 6.45-12.15, I2 = 91%), 3 years (OR = 10.02, 95%CI: 9.40-10.64, I2 = 22%), and 5 years (OR = 11.61, 95%CI: 3.74-19.48, I2 = 97%). OAGB showed higher late complications than adjustable SG. The results were not different in sub-group analysis including only clinical trials, observational studies, and removing studies including super-obese patients and studies contributing most to heterogeneity.
In the medium term, DR rates were similar between OAGB and SG; however, OAGB showed higher EWL% than SG, and late complications were higher in OAGB. Clinical trials investigating the predictors of DR and EWL% are recommended.
Core Tip: Obesity and diabetes are growing at an alarming rate. Bariatric surgery is an effective method for weight management and inducing diabetes remission (DR); therefore, choosing the correct type of bariatric surgery is important. Sleeve gastrectomy is the most frequently performed bariatric surgery, and one-anastomosis gastric bypass has seen an increase in popularity recently. Literature regarding the most effective bariatric surgery for weight reduction and DR is scarce. This review provides broader insights into one-anastomosis gastric bypass and sleeve gastrectomy in the medium term, including their impact on excess weight loss, DR, complications, mortality, and quality of life.
- Citation: Mirghani HO. One-anastomosis gastric bypass vs sleeve gastrectomy for diabetes remission and weight loss: A meta-analysis. World J Gastrointest Surg 2025; 17(11): 109426
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/109426.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.109426
The prevalence of obesity is rising at an alarming rate. According to the global estimates, more than half of the world’s population is expected to be obese or overweight by 2035[1]. The rising rate of obesity is paralleled by an increasing rate of type 2 diabetes (T2D) mellitus, which has a global prevalence of 10.5%[2]. Due to the global burden of diabetes mellitus and obesity, an effective treatment to address both of these serious conditions is a priority[3]. Bariatric/metabolic surgery is an effective and durable treatment for obesity despite the emergence of highly effective anti-obesity medications[4]. Bariatric surgery is recommended for adults with a body mass index (BMI) > 35 kg/m2 regardless of the presence of obesity-related comorbidities. For individuals with T2D and a BMI > 30 kg/m2 or those with a BMI < 35 kg/m2 who have not achieved significant or lasting weight loss, bariatric surgery may also be recommended[5,6]. There are three types of bariatric surgery: Restrictive, malabsorptive, and combined procedures. Restrictive procedures reduce the capacity of the stomach, leading to decreased food intake and promoting a feeling of fullness (satiety) after eating smaller portions. Malabsorptive procedures induce malabsorption by altering gastrointestinal anatomy, resulting in calorie reduction and substantial weight loss. The combined procedures incorporate both mechanisms[7]. The malabsorptive and combined procedures are associated with higher rates of weight loss and better metabolic effects than the restrictive procedures; however, they also carry a higher risk of nutritional deficiencies[8]. Roux-en-Y gastric bypass (RYGB), one-anastomosis gastric bypass (OAGB), and sleeve gastrectomy (SG) are the most frequently performed bariatric surgeries. Ongoing research aims to identify the procedure with the fewest complications[9].
Since its introduction by Rutledge[10] in 1997, OAGB has been increasingly performed. OAGB has been found to effectively mitigate obesity comorbidities and improve patients’ quality of life with manageable side effects[10,11]. OAGB is a safe and effective bariatric surgery for weight reduction in individuals with morbid obesity. Its major benefits are weight reduction, significant remission of obesity-related comorbidities such as dyslipidemia, and a low risk of postoperative leak. OAGB has recently gained increasing popularity among surgeons and patients due to its relatively less invasive approach, easy reversibility and revision, lower morbidity, and promising metabolic outcomes compared to RYGB. However, the occurrence of malnutrition, ulcers, and bile reflux is higher after OAGB compared to SG[12,13].
SG is a restrictive procedure that is achieved by removing the majority of the stomach, specifically the greater curvature, leaving a smaller tubular stomach. This surgery leads to increased levels of glucagon-like peptide 1, a hormone that promotes insulin release, reduces glucagon secretion, and contributes to feelings of fullness (satiety), while simultaneously decreasing ghrelin, the “hunger hormone”. SG is easy to perform and has become the most commonly performed bariatric surgery worldwide since 2016[14,15]. The benefits of SG are weight loss, resolution of comorbidities (including diabetes, hypertension, obstructive sleep apnea, and metabolic-associated fatty liver disease), and improved quality of life[16]. The complications range from 4.4% to 12.8% with bleeding, leaks, and stenosis being the most common; long-term complications include weight regain, malnutrition, and reflux[17].
OAGB (known as a ‘mini gastric bypass’) is a simplified modification of the traditional gastric bypass procedure, which combines both restrictive and malabsorptive components of weight loss surgery and uses a single gastro-jejunal anastomosis. The advantages in the context of the primary metabolic approach are weight loss, resolution of obesity comorbidities, improvement in quality of life, and a low rate of perioperative complications. In addition, it has shown promising results in treating gastroesophageal reflux disease (GERD), particularly as a revisional procedure after other weight loss procedures[18-20]. Importantly, it offers benefits such as reversibility, convertibility, and revisability, and has gained popularity since 2022[14,21]. In this procedure, the stomach is divided between the antrum and body along the lesser curvature, with the division extending upwards (cephalad) towards the angle of His. The pouch is then ana
Meta-analyses comparing OAGB and SG in terms of diabetes remission (DR) and body weight reduction are scare, and have been limited by the small number of included studies, high heterogeneity, and lack of long-term follow-up (Magouliotis et al[4], Quan et al[22], and Wang et al[23]). The previous meta-analyses have also been limited by the inclusion of studies with overlapping data from the same hospital and authors, and published at the same time[24,25]. Therefore, an updated meta-analysis with more recent data on OAGB and SG outcomes is needed. To this end, this meta-analysis was conducted to compare the effects of OAGB and SG on complete DR and percentage of excess weight loss (EWL) in the medium term.
This meta-analysis was conducted to assess the effects of OAGB and SG on complete DR and EWL%. The literature search was conducted in March 2025 and April 2025 with no limitation regarding the study period, strictly adhering to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.
Clinical trials, prospective and retrospective studies, and case-control studies with a minimum period of 1 year and published in the English language were included. The studies compared OAGB and SG regarding complete DR and EWL% in the short and medium term.
Cross-sectional studies, case reports, editorials, opinions, systematic reviews, study protocols without results, and studies with duration < 1 year were excluded. Studies that did not compare OAGB and SG in terms of DR and EWL% were also excluded.
The outcome measures were comparisons between OAGB and SG in terms of EWL% and complete DR.
The definition of T2D remission varies significantly. Therefore, DR was defined as reported by each study: Fasting blood glucose < 126 mg/dL and hemoglobin A1c (HbA1c) < 6.5%[26]; fasting blood glucose < 100 mg/dL and HbA1c < 6%[27]; HbA1c < 6.0% for at least 1 year without anti-diabetes medications[28]; HbA1c < 6.5% without the use of oral hypo
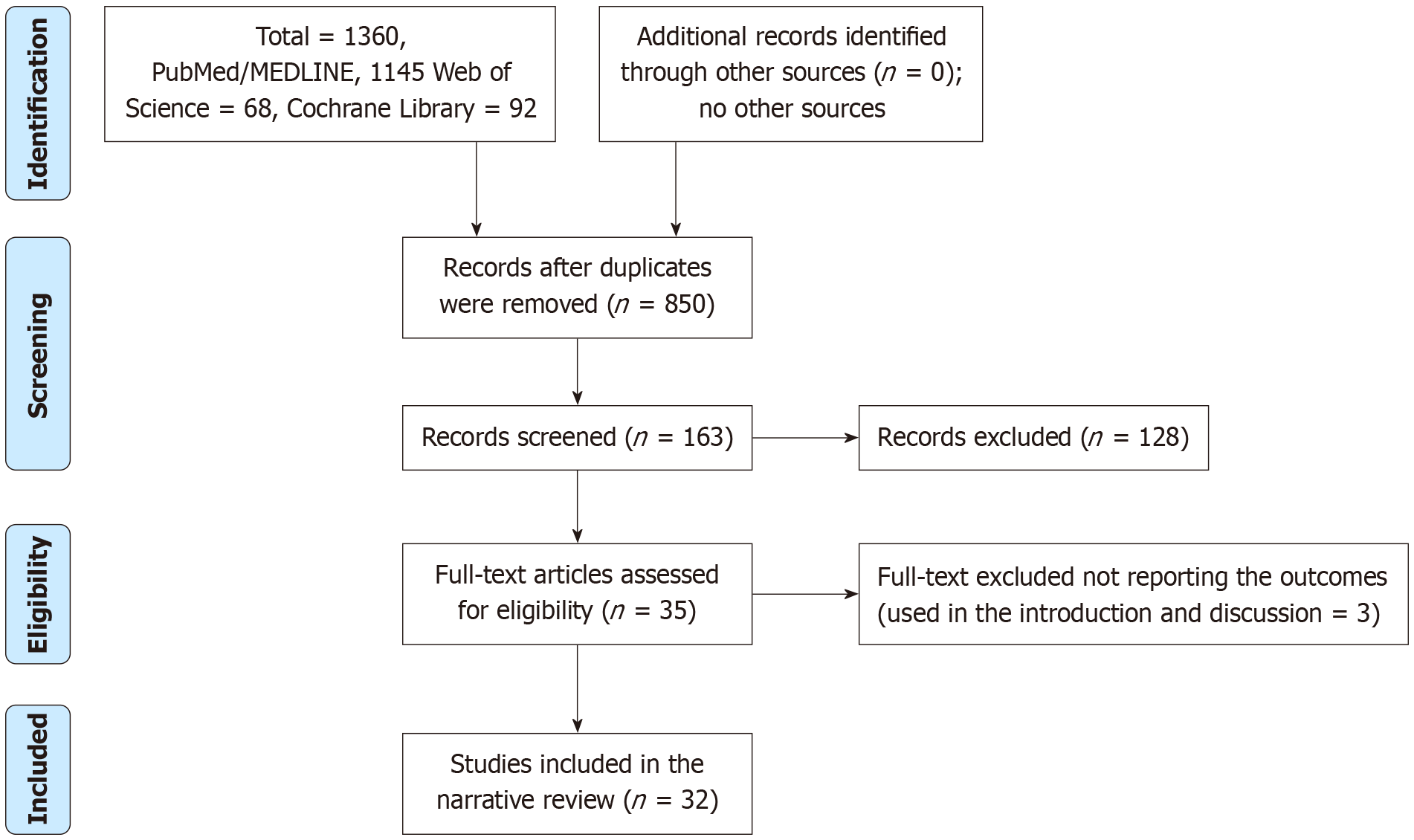
We searched the PubMed/MEDLINE, Cochran Library, and Web of Science databases for articles published in English with no limitation regarding the publication date. The literature search was conducted in March 2025 and April 2025. The keywords used were “one-anastomosis”, “single-anastomosis”, “gastric bypass”, “omega loop”, “sleeve gastrectomy”, “mini-gastric bypass”, “diabetes remission”, and “weight loss”. We identified 1360 articles, and 850 articles remained after the removal of duplicates. The excluded studies did not fulfill the inclusion criteria, which were the number of total DR and EWL%. Some studies measured partial remission/improvement of HbA1c levels. Studies that did not report the EWL% in mean ± SD and those that estimated the BMI pre-intervention and post-intervention were not included. Of the 35 texts retrieved, 32 were included in the final analysis (31 studies assessed complete DR, 21 assessed EWL%, and some studies assessed both outcomes). Three full-texts were excluded because they did not include data on DR or EWL% (Figure 1).
The author used an Excel sheet to extract data from all of the included studies, and a standardized data extraction form was developed. The age of the participants who underwent OAGB and SG, sex distribution, BMI in each group, outcome measures including EWL% and DR, authors, country of publication, study type, and total number of events in OAGB and SG were collected (Tables 1, 2, and 3).
| Ref. | Age (years), OAGB | Age (years), SG | Females, OAGB | Females, SG | BMI (kg/m2), OAGB | BMI (kg/m2), SG | Outcomes | Comments |
| Musella et al[24] | 48.5 ± 8.7 | 49.2 ± 9.1 | 39.6% | 27.3% | 48.3 ± 9.2 | 48.1 ± 7.8 | DR & EWL% | More females in OAGB |
| Abdel-Rahim et al[26] | 42.9 ± 6.17 | 42.95 ± 7.63 | 65% | 85% | 50.88±3.99 | 47.77 ± 6.18 | DR & EWL% | Higher BMI in OAGB |
| Ahmad et al[27] | 47.1 ± 10.8 | 44.6 ± 10.9 | 59.1% | 60.9% | 43.04 ± 7.87 | 42.85 ± 5.02 | DR | No significant differences at the baseline |
| Abdelshafy et al[28] | 44.2 ± 4.99 | 43.6 ± 3.7 | 64.5% | 64.5% | 44.2 ± 4.99 | 43.6 ± 3.7 | DR | No significant differences at the baseline |
| Dayan et al[29] | 67.6 ± 2.8 | 67.6 ± 2.6 | 61.4% | 61.4% | 41.8 ± 7.8 | 43.3 ± 5.9 | DR & EWL% | No significant differences at the baseline |
| Madhok et al[31] | 45.0 ± 25 | 51 ± 30 | 47.4% | 55.4% | 67.0 ± 60 | 65.0 ± 60 | DR & EWL% | OAGB were younger, more males |
| Milone et al[32] | 34.9 ± 6.01 | 33.7 ± 5.61 | 62.2% | 53.5% | 47.3 ± 3.88 | 46.0 ± 4.77 | DR & EWL% | More women in OAGB |
| Toksoy et al[33] | 43.9 ± 12.1 | 40.5 ± 10.8 | 65.3% | 59.3% | 43.1 ± 3.9 | 42.2 ± 4.5 | DR | OAGB were older |
| Alkhalifah et al[36] | 33.8 ± 10.4.1 | 35.2 ± 10 | 70% | 74.9% | 40.4 ± 7.7 | 36.4 ± 7.6 | DR & EWL% | Higher BMI in OAGB |
| Catro et al[37] | 42.4 ± 11 | 43.5 ± 10.2 | 73.2% | 75.9% | 43.8 ± 9.2 | 45.2 ± 9.2 | DR & EWL% | No significant differences at the baseline |
| Das et al[38] | 47.1 | 46.7 | 84.2% | 69.2% | 47.1 ± 9.75 | 52.7 ± 7.17 | DR & EWL% | Less body mass index in OAGB |
| Ding et al[39] | 46 | 30 | 15.4% | 80% | 34.7 | 43.8 | DR | OAGB were older, fewer females, and had lower BMI |
| Gambardella et al[40] | 40.1 ± 5.1 | 38.9 ± 4.6 | 45% | 44.5% | 47.1 ± 2.3 | 46.2 ± 3.6 | DR & EWL% | No significant differences at the baseline |
| Jammu and Sharma[41] | 38 | 23 | 71.2% | 45.4% | 42.5 | 35 | DR | OAGB were older, more females, and high BMI |
| Jain et al[42] | 42.9±14.0 | 39.9±11.7 | 38.6% | 35% | 44.3±7.88 | 44.5±7.16 | DR & EWL% | OAGB were older |
| Kansou et al[43] | 41.2 ± 11.3 | 41.2 ± 12.3 | 93.4% | 91.9% | 42.8 ± 5.0 | 43.4 ± 6.5 | DR & EWL% | No significant differences at the baseline |
| Kular et al[44] | NA | NA | NA | NA | NA | NA | DR & EWL% | NA |
| Moradi et al[45] | 47.3 ± 10.2 | 47.5 ± 10.9 | 77.3% | 80.1% | 44.6 ± 7.0 | 43.8 ± 6.9 | DR | No significant differences at the baseline |
| Plamper et al[46] | 42. ± 11 | 44 ± 11 | 76.2% | 61.8% | 54.1 ± 6.6 | 54.6 ± 10.3 | DR & EWL% | OAGB younger, more females, less BMI |
| Ruiz-Tovar et al[47] | 43.8 ± 11.5 | 43.9 ± 10.9 | 75% | 75% | 45 ± 4.1 | 46.5 ± 3.4 | DR & EWL% | No significant differences at the baseline |
| Schmitz et al[48] | 39.11 ± 0.9 | 41.57 ± 1.07 | 74.5% vs 58.1% | 58.1% | 64.14 ± 0.3 | 66.91 ± 0.6 | DR & EWL% | More females, lower BMI in OAGB |
| Seetharamaiah et al[49] | 42.89 ± 14.02 | 39.89 ± 11.75 | 62% | 65% | 44.32 ± 7.88 | 44.57 ± 7.16 | DR & EWL% | No significant differences at the baseline |
| Shen et al[50] | 42.9 | 42.9 | NA | NA | 39.0 ± 7.2 | 39.0 ± 7.2 | DR | NA |
| Shivakumar et al[51] | 42.9 ± 14.02 | 39.9 ± 11.75 | 61.3% | 65% | 44.3 ± 7.88 | 44.6 ± 7.16 | DR & EWL% | OAGB were older |
| Singla et al[52] | Matched | Matched | Matched | Matched | > 50 | > 50 | DR & EWL% | No significant differences at the baseline |
| Tabesh et al[53] | 41.54 ± 11.20 | 41.42 ± 11.77 | 76% | 84.4% | 46 ± 6.15 | 455.71 | 24 years, 17.2% vs 12.9% diabetes | Higher BMI in OAGB |
| Toh et al[54] | 47 ± 9 | 40 ± 11 | 69.8% | 61.8% | 40.3 ± 9.1 | 43±7.9 | DR & EWL% | OAGB were older with lower BMI |
| Vrakopoulou et al[55] | 46.6 ± 7.8 | 45.9 ± 7.5 | 60% | 57.1% | 52.7 ± 10.8 | 52.2 ± 8.6 | DR & EWL% | No significant differences at the baseline |
| Wazir et al[56] | 48.21 ± 9.77 | 48.21 ± 9.77 | 70.2% females | 70.2% females | 49.80 ± 6.225 | 49.80 ± 6.225 | DR | Not assessed |
| Sari et al[57] | 20-59 | 20-59 | 79.6% females | 79.6% females | ≥ 35 | ≥ 35 | DR & EWL% | Not assessed |
| Yang et al[58] | NA | NA | NA | NA | 32.1 ± 10.3 | 33.9 ± 9.4 | DR & EWL% | No significant differences at the baseline |
| Ref. | Country | Study type | OAGB, 1 year | SG, 1 year | OAGB, 3 years | SG, 3 years | OAGB, 5 years | SG, 5 years |
| Musella et al[24] | Italy | Retrospective | 82/96 | 67/110 | ||||
| Abdel-Rahim et al[26] | Egypt | Prospective | 11/20 | 11/20 | ||||
| Ahmad et al[27] | Syria | Prospective | 110/137 | 57/92 | ||||
| Abdelshafy et al[28] | Egypt | Trial | 18/22 | 14/22 | ||||
| Dayan et al[29] | Israel | Retrospective | 36/41 | 25/83 | ||||
| Lee et al[30] | Taiwan | Trial | 18/30 | 9/30 | ||||
| Madhok et al[31] | United Kingdom | Retrospective | 4/6 | 9/17 | ||||
| Milone et al[32] | Italy | Prospective | 14/16 | 10/15 | ||||
| Toksoy et al[33] | Turkey | Retrospective | 94/108 | 32/39 | ||||
| Alkhalifah et al[36] | Taiwan | Prospective | 496/533 | 186/205 | 488/533 | 205/205 | ||
| Catro et al[37] | Spain | Retrospective | 114/123 | 68/83 | 110/123 | 63/83 | ||
| Das et al[38] | United Kingdom | Retrospective | 3/4 | 3/5 | ||||
| Ding et al[39] | China | Retrospective | 7/10 | 1/3 | ||||
| Gambardella et al[40] | Italy | Prospective | 48/60 | 79/131 | 53/57 | 105/128 | ||
| Jammu and Sharma[41] | India | Retrospective | 59/473 | 13/339 | ||||
| Jain et al[42] | India | Trial | 49/49 | 47/47 | 40/55 | 37/52 | ||
| Kansou et al[43] | France | Retrospective | 25/27 | 19/21 | ||||
| Kular et al[44] | India | Retrospective | 58/63 | 49/61 | ||||
| Moradi et al[45] | Iran | Retrospective | 509/675 | 142/201 | 336/675 | 94/201 | ||
| Plamper et al[46] | Germany | Retrospective | 302/3 19 | 83/98 | NA | NA | NA | NA |
| Ruiz-Tovar et al[47] | Spain | Trial | 66/70 | 53/61 | 61/70 | 50/61 | ||
| Schmitz et al[48] | Germany | Retrospective | 34/51 | 42/45 | ||||
| Seetharamaiah et al[49] | India | Trial | 41/49 | 36/47 | ||||
| Shen et al[50] | Taiwan | Retrospective | 64/81 | 91/130 | ||||
| Shivakumar et al[51] | India | Trial | 41/49 | 36/47 | 46/49 | 44/47 | ||
| Singla et al[52] | India | Retrospective | 58/75 | 64/75 | ||||
| Tabesh et al[53] | Iran | Retrospective | 2/35 | 1/151 | ||||
| Toh et al[54] | Singapore | Retrospective | 23/32 | 60/73 | ||||
| Vrakopoulou et al[55] | Greece | Retrospective | 22/25 | 10/28 | ||||
| Wazir et al[56] | United Kingdom | Retrospective | 1/2 | 10/18 | ||||
| Sari et al[57] | Turkey | Retrospective | 27/28 | 29/31 |
| Ref. | Country | Study type | OAGB, 1 year | SG, 1 year | OAGB, 3 years | SG, 3 years | OAGB, 5 years | SG, 5 years |
| Musella et al[24] | Italy | Retrospective | 64.7 ± 22.9/96 | 52.4 ± 18.3/110 | 22.8 ± 5.9/96 | 20.1 ± 5.3/110 | ||
| Abdel-Rahim et al[26] | Egypt | Prospective | 95.11 ± 7.00/20 | 78.48 ± 19.07/20 | ||||
| Dayan et al[29] | Israel | Retrospective | 67.2 ± 22.3/41 | 45.8 ± 18.0/83 | ||||
| Lee et al[30] | Taiwan | Trial | 78.2 ± 19.7/519 | 68.7 ± 30.3/519 | ||||
| Madhok et al[31] | United Kingdom | Retrospective | 58.0 ± 7.75/19 | 45 ± 21.5/56 | NA | NA | NA | NA |
| Milone et al[32] | Italy | Prospective | 24.19 ± 4.42/16 | 24.33 ± 4.48/15 | ||||
| Alkhalifah et al[36] | Taiwan | Prospective | 84.5 ± 35.2/533 | 64.8 ± 34.3/205 | ||||
| Catro et al[37] | Spain | Retrospective | 72.5 ± 16.4/123 | 68.8 ± 18.6/83 | ||||
| Das et al[38] | United Kingdom | Retrospective | 50.2 ± 28.6/19 | 49.9 ± 19.5/26 | ||||
| Gambardella et al[40] | Italy | Prospective | 83.6 ± 18.1/60 | 74.3 ± 13.8/131 | ||||
| Jain et al[42] | India | Trial | 65.9 ± 10.9/101 | 64.8 ± 14.3/100 | 67.5 ± 16.6/101 | 61 ± 26.4/100 | 65.3 ± 13.9/101 | 55.9 ± 27/100 |
| Kansou et al[43] | France | Retrospective | 79.3 ± 17.8/136 | 71.4 ± 19 /136 | ||||
| Kular et al[44] | India | Retrospective | 63 ± 21.2/104 | 69 ± 22.5/118 | 70 ± 22.6/104 | 61 ± 26.4/118 | 68 ± 24/104 | 51.2 ± 23/118 |
| Plamper et al[46] | Germany | Retrospective | 66.2 ± 13/169 | 57.3 ± 19/118 | NA | NA | NA | NA |
| Ruiz-Tovar et al[47] | Spain | Trial | 97.9 ± 7/70 | 76.3 ± 6/61 | ||||
| Schmitz et al[48] | Germany | Retrospective | 36 ± 0.8/150 | 29 ± 1.2/93 | 42.5 ± 1.9 | 32.4 ± 2.7 | ||
| Seetharamaiah et al[49] | India | Trial | 66.87 ± 10.87/101 | 63.97 ± 13.24/100 | ||||
| Shivakumar et al[51] | India | Trial | 66.2 ± 10.9/101 | 63.9 ± 13.5/100 | 66.5 ± 15.7/93 | 61.2 ± 25.2/92 | ||
| Singla et al[52] | India | Retrospective | 74.57 ± 13.2/75 | 56.20 ± 18.9/75 | ||||
| Toh et al[54] | Singapore | Retrospective | 68 ± 28.5/40 | 61.2 ± 20/195 | 66.2 ± 35.6/31 | 47.9 ± 22.8/53 | 65.2 ± 27.5/8 | 47.3 ± 27.5/15 |
| Vrakopoulou et al[55] | Greece | Retrospective | 98.2 ± 29.0/115 | 79.7 ± 14.5/437 | ||||
| Sari et al[57] | Turkey | Retrospective | 77.66 ± 30.55/62 | 71.11 ± 27.81/129 | ||||
| Yang et al[58] | Taiwan | Prospective | 72 ± 20/89 | 67.2 ± 18.4/32 |
The Cochrane Risk of Bias (RoB) 2 tool[34] and Newcastle Ottawa Scale were used to assess the quality of the included studies[35]. The Cochrane RoB 2 was used to evaluate the studies for potential biases across five key domains, namely selection bias, performance bias, attrition bias, detection bias, and reporting bias. Each domain was evaluated as low RoB, high RoB, and some concerns. The Newcastle Ottawa Scale was used to assess three fundamental aspects of the methodology: Selection of the participants, 0-4 points; confounder adjustment, 0-2; and the determination of outcome indicators, 0-3. A study with a score of 7-9 points was defined as high quality (Tables 4 and 5).
| Ref. | Selection | Compatibility | Exposure | Total score |
| Musella et al[24] | 4 | 2 | 2 | 8 |
| Abdel-Rahim et al[26] | 3 | 2 | 2 | 7 |
| Ahmad et al[27] | 3 | 2 | 3 | 8 |
| Dayan et al[29] | 4 | 2 | 2 | 9 |
| Madhok et al[31] | 3 | 1 | 2 | 7 |
| Milone et al[32] | 4 | 2 | 2 | 8 |
| Toksoy et al[33] | 4 | 2 | 3 | 9 |
| Alkhalifah et al[36] | 3 | 2 | 2 | 7 |
| Catro et al[37] | 4 | 2 | 3 | 9 |
| Das et al[38] | 4 | 2 | 2 | 8 |
| Ding et al[39] | 3 | 2 | 2 | 7 |
| Gambardella et al[40] | 3 | 2 | 3 | 8 |
| Jammu and Sharma[41] | 3 | 2 | 2 | 7 |
| Kansou et al[43] | 4 | 2 | 2 | 8 |
| Kular et al[44] | 4 | 2 | 2 | 8 |
| Moradi et al[45] | 4 | 2 | 3 | 8 |
| Plamper et al[46] | 3 | 2 | 2 | 7 |
| Schmitz et al[48] | 4 | 2 | 2 | 8 |
| Shen et al[50] | 4 | 2 | 2 | 7 |
| Singla et al[52] | 3 | 2 | 2 | 7 |
| Tabesh et al[53] | 3 | 2 | 3 | 8 |
| Toh et al[54] | 4 | 2 | 2 | 8 |
| Vrakopoulou et al[55] | N/A | N/A | N/A | N/A |
| Wasir et al[56] | 4 | 1 | 2 | 7 |
| Sari et al[57] | 3 | 2 | 2 | 7 |
| Yang et al[58] | 3 | 2 | 1 | 6 |
| Ref. | Selection bias1 | Selection bias2 | Performance bias | Attrition bias | Detection bias | Reporting bias | Overall bias |
| Abdelshafy et al[28] | Some concern | Low | Some concern | Low | Low | Some concern | Some concerns |
| Lee et al[30] | Some concern | Some concern | Low | Low | Low | High | Low |
| Jian et al[42] | Low | Low | High | Low | Some concern | Some concern | Some concerns |
| Ruiz-Tovar et al[47] | Low | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Seetharamaiah et al[49] | Low | Some concerns | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Shivakumar et al[51] | Low | Low | Some concerns | Some concerns | Low | Low | Low |
Review Manager version 5.4.1 (Cochrane Collaboration, Oxford, United Kingdom) was used for the data analyses. To analyze data from multiple studies, specifically focusing on the outcomes of complete T2D remission and EWL%, forest plots were used to visualize and summarize the data. Odds ratios (ORs) were used to compare outcomes for dichotomous data, whereas mean differences (MD) were used for continuous data. A random effects meta-analysis was used to pool studies with similar characteristics, particularly when significant heterogeneity existed, and 95% confidence intervals (CIs) were calculated to quantify the precision of the pooled effect estimate. The I2 statistic was used to evaluate the degree of heterogeneity among studies. I2 value < 25% was considered low heterogeneity, whereas a value > 50% indicated substantial heterogeneity, and a random effects model was used. We generated funnel plots to assess potential publication bias in meta-analysis including 10 or more studies. A sub-group analysis was conducted to assess the outcomes in clinical trials and observational studies and after excluding studies with super-obese patients to identify the source of heterogeneity. In addition, the included studies were removed one by one, and their contribution to the heterogeneity was estimated, finally, a sub-group analysis was conducted after removing studies contributing most to the heterogeneity (Tables 6 and 7). A P-value < 0.05 was considered statistically significant.
| Ref. | Effect |
| Musella et al[24] | 1% increase |
| Abdel-Rahim et al[26] | 1% increase |
| Ahmad et al[27] | 1% increase |
| Abdelshafy et al[28] | 1% increase |
| Dayan et al[29] | 9% decrease |
| Lee et al[30] | 1% increase |
| Madhok et al[31] | 1% increase |
| Milone et al[32] | 1% increase |
| Toksoy et al[33] | 1% increase |
| Alkhalifah et al[36] | 1% increase |
| Catro et al[37] | 1% increase |
| Das et al[38] | 1% increase |
| Ding et al[39] | 1% increase |
| Gambardella et al[40] | 1% increase |
| Jammu and Sharma[41] | 1% decrease |
| Jian et al[42] | No effect |
| Kansou et al[43] | 1% increase |
| Kular et al[44] | 1% increase |
| Moradi et al[45] | 2% decrease |
| Plamper et al[46] | No effect |
| Ruiz-Tovar et al[47] | 1% increase |
| Schmitz et al[48] | 7% decrease |
| Seetharamaiah et al[49] | 1% increase |
| Shen et al[50] | 1% increase |
| Shivakumar et al[51] | 1% increase |
| Singla et al[52] | 3% decrease |
| Tabesh et al[53] | No effect |
| Toh et al[54] | 2% decrease |
| Vrakopoulou et al[55] | 2% decrease |
| Wazir et al[56] | 1% increase |
| Sari et al[57] | 1% increase |
| Ref. | Change |
| Musella et al[24] | 1% increase |
| Abdel-Rahim et al[26] | 1% increase |
| Dayan et al[29] | No change |
| Lee et al[30] | 1% increase |
| Madhok et al[31] | 4% decrease |
| Milone et al[32] | No change |
| Alkhalifah et al[36] | No change |
| Catro et al[37] | 1% increase |
| Das et al[38] | 1% increase |
| Gambardella et al[40] | 1% increase |
| Jian et al[42] | No change |
| Kansou et al[43] | 1% increase |
| Kular et al[44] | No change |
| Plamper et al[46] | 1% increase |
| Schmitz et al[48] | 1% increase |
| Seetharamaiah et al[49] | 1% increase |
| Shivakumar et al[51] | 1% increase |
| Singla et al[52] | No effect |
| Toh et al[54] | 1% increase |
| Vrakopoulou et al[55] | No effect |
| Sari et al[57] | No effect |
| Yang et al[58] | 1% increase |
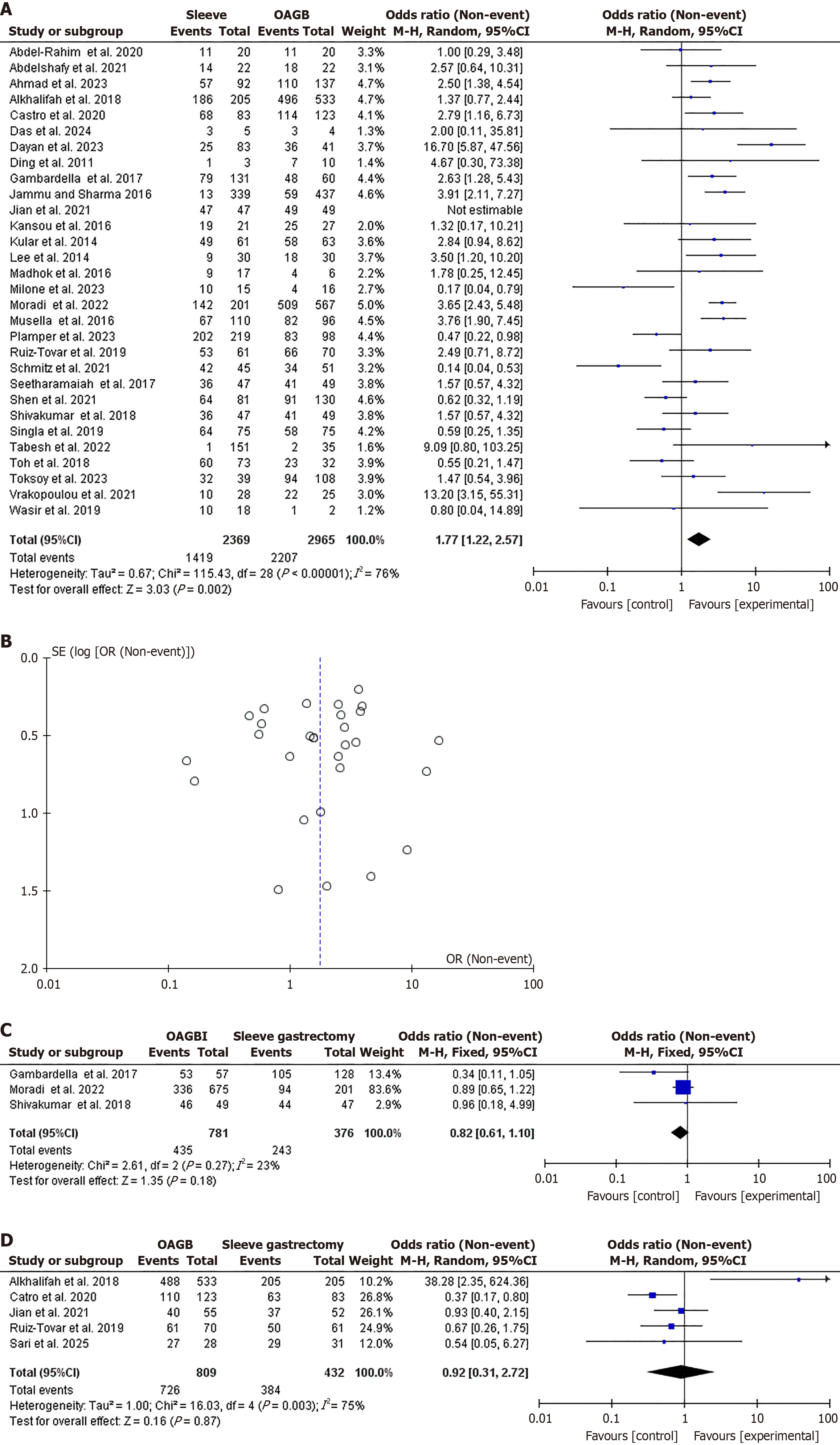
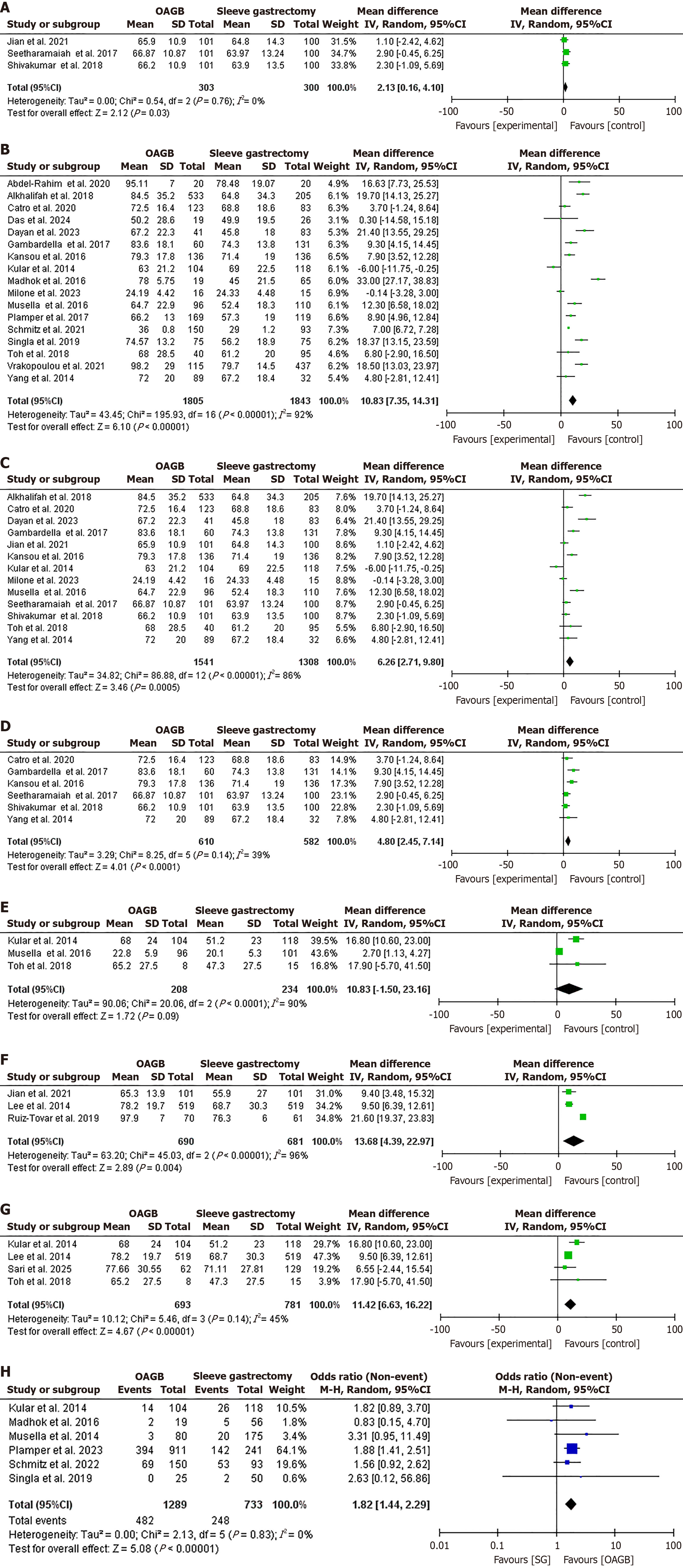
A total of 32 studies[24,26-33,36-56] were included in the meta-analysis, comprising 19 from Asia, 11 from Europe, and 2 from Africa. Twenty-six studies were observational (21 retrospective studies and 4 prospective studies), whereas six were controlled trials. Regarding complete DR, 31 studies were included in the analysis with 5334 patients and 3626 events[24,26-33,36-57]. OAGB achieved higher complete DR at 1 year following surgery compared to SG (OR = 1.77, 95%CI: 1.22-2.57, χ2 = 115.43, P = 0.002, SMD = 28). However, high heterogeneity was found (I2 = 76%, P < 0.001; Figure 2A and B).
No significant difference was found regarding complete DR after 3 years of follow-up in the analysis of three studies that included 1148 patients and 678 events [OR = 0.82, 95%CI: 0.61-1.10, χ2 = 2.61, P = 0.18, standard MD (SMD) = 2][40,45,51]. No significant heterogeneity was found (I2 = 23%, P = 0.27; Figure 2C). No significant difference was observed regarding complete DR after 5 years of follow-up in the analysis of five studies that included 1241 patients and 1110 events[36,37,42,47,57] (OR = 0.92, 95%CI: 0.31-2.72, χ2 = 16.03, P = 0.87, SMD = 4). Significant heterogeneity was found
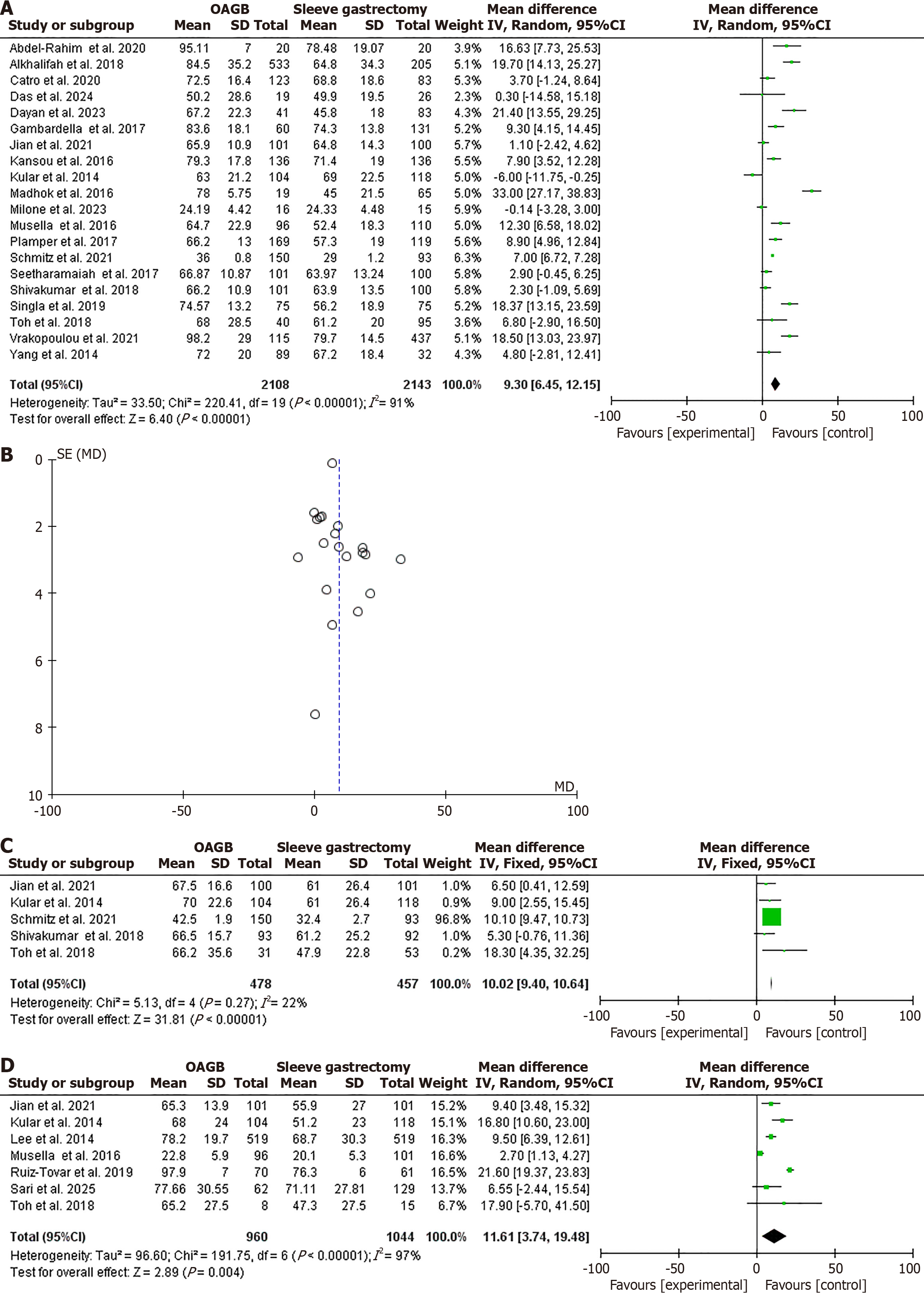
EWL% was higher following OAGB compared to SG after 1 year of follow-up in the analysis of 20 studies with 4251 patients (OR = 9.30, 95%CI: 6.45-12.15, χ2 = 220.41, P < 0.001, SMD = 19)[24,26,29,31,32,36-38,40,42-44,46,48,49,51,52,54,55,58]. However, high heterogeneity was found (I2 = 91%, P < 0.001; Figure 3A and B). EWL% was higher following OAGB after 3 years of follow-up in the analysis of five studies with 935 patients (OR = 10.02, 95%CI: 9.40-10.64, χ2 = 5.13, P < 0.001, SMD = 4)[42,44,48,51,54]. No significant heterogeneity was found (I2 = 22%, P = 0.27; Figure 3C). EWL% was higher following OAGB at the 5-year follow-up in the analysis of seven studies in 2004 (OR = 11.61, 95%CI: 3.74-19.48, χ2 = 191.75, P = 0.004, SMD = 6)[24,30,42,44,47,54,57]. Substantial heterogeneity was found (I2 = 97%, P < 0.001; Figure 3D).
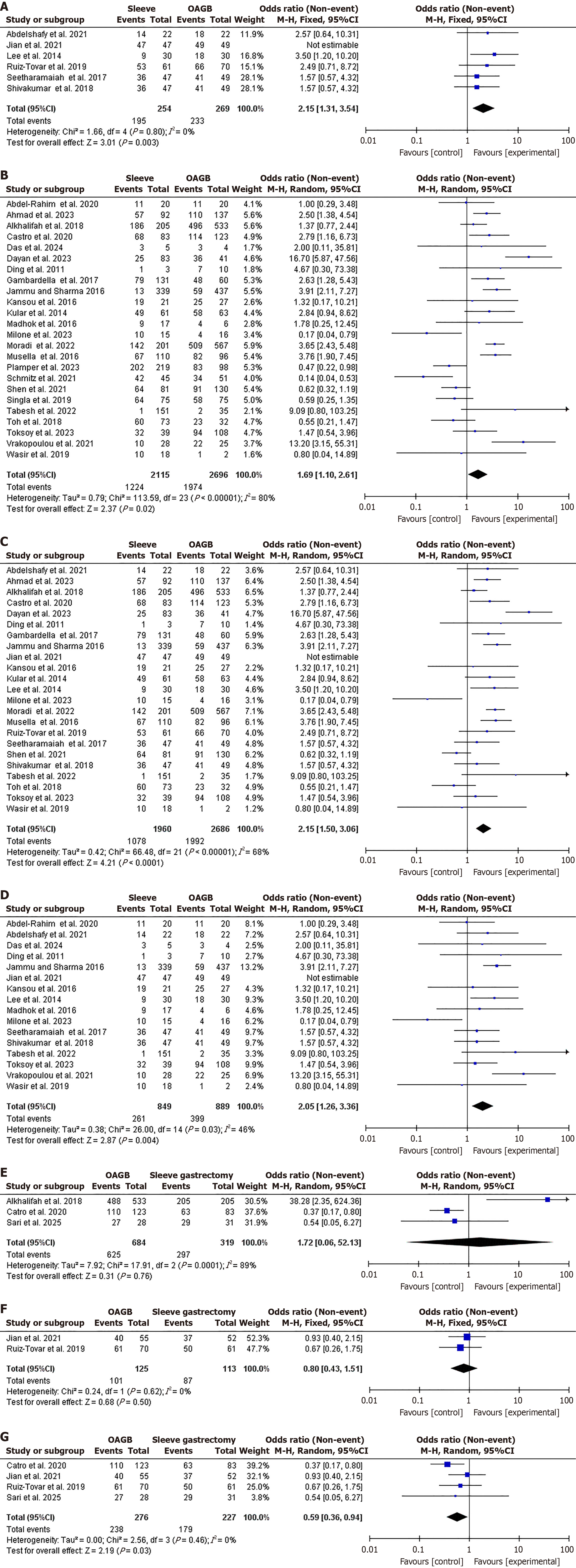
Sub-group analysis for complete DR: A sub-group analysis was conducted including only clinical trials in which complete DR was higher in OAGB (86.6%) compared to SG (76.8%), OR = 2.15, 95%CI: 1.31-3.54, χ2 = 1.66, P = 0.003, SMD = 4. No heterogeneity was found (I2 = 0%, P = 0.80; Figure 4A). Similarly DR was higher in OAGB (86.6%) compared to SG (76.8%) in observational studies, OR = 1.69, 95%CI: 1.10-2.61, χ2 = 113.59, P = 0.02, SMD = 23. Significant heterogeneity was found (I2 = 80%, P < 0.001; Figure 4B). DR was higher in OAGB compared to SG after removing studies with super-obese patients, OR = 2.15, 95%CI: 1.50-3.06, χ2 = 66.48, P < 0.001, SMD = 21. Significant heterogeneity was found (I2 = 68%, P < 0.001; Figure 4C). In a sub-group analysis of studies contributing most to heterogeneity, DR at after 1 year following surgery was not different, OR = 2.05, 95%CI: 1.26-3.36, χ2 = 26.00, P = 0.004, SMD = 14. No significant heterogeneity was found (I2 = 46%, P = 0.03; Figure 4D). DR was not different in OAGB and SG after five years of follow-up when including observational studies and clinical trials, OR = 1.72, 95%CI: 0.06-52.13, χ2 = 17.91, P = 0.76, SMD = 2. Significant heterogeneity was found (I2 = 89%, P < 0.001), and OR = 0.80, 95%CI: 0.43-1.51, χ2 = 0.24, P = 0.50, SMD = 1. No significant heterogeneity was found (I2 = 0%, P = 0.62; Figure 4E and F). In a sub-group analysis of studies contributing most to heterogeneity, DR at after 5 years following surgery was not different, OR = 0.59, 95%CI: 0.36-0.94, χ2 = 2.56, P = 0.03, SMD = 3. No significant heterogeneity was found (I2 = 0%, P = 0.46; Figure 4G).
Sub-group analysis for EWL%: Regarding EWL%, OAGB achieved higher weight loss than SG in clinical trials and observational studies, MD = 2.13, 95%CI: 0.16-4.10, χ2 = 0.54, P = 0.03, SMD = 2, no significant heterogeneity was found
In this meta-analysis, complete DR was higher in patients who underwent OAGB compared to those who underwent SG at 1 year (OR = 1.77, 95%CI: 1.22-2.57, χ2 = 15.43). However, the rates were similar between the two procedures at 3 years and 5 years following surgery (OR = 0.82, 95%CI: 0.61-1.10 and OR = 0.92, 95%CI: 0.31-2.72). Regarding the EWL%, OAGB was superior to SG at 1 year, 3 years, and 5 years following surgery (OR = 9.30, 95%CI: 6.45-12.15; OR = 10.02, 95%CI: 9.40-10.64; and OR = 11.61, 95%CI: 3.74-19.48, respectively). The results were not different in sub-group analysis in clinical trials, observational studies, and in a sub-analysis excluding studies with super-obesity. Barzin et al[59] in their meta-analysis found no differences between OAGB and SG regarding DR in the short term and medium term, with a higher rate of EWL% in OAGB. The findings were similar to our findings regarding EWL%; however, our results differed regarding DR as we found a higher rate of DR following OAGB after 1 year[59]. Plausible explanations could be the small number of included studies in the previous meta-analysis (only nine retrospective studies). In addition, Barzin et al[59] only included patients with a BMI > 50 kg/m2.
Another meta-analysis by Magouliotis et al[4] found a higher EWL% in the OAGB group compared to the SG group in line with current findings. However, the authors only included 10 studies and assessed the outcomes at only 1 year following surgery. Importantly, Magouliotis et al[4] performed incorrect data extraction for EWL% from the article by Kansou et al[43] and unfortunately the number of females from Jammu and Sharma’s study[41] were inaccurately entered, leading to questions about their ultimate results. Furthermore, many recent studies have been published since the meta-analysis by Magouliotis et al[4], and they were included in the current meta-analysis. Our results are not in line with the study by Ali et al[60], who included only 10 studies and found that OAGB had superiority in terms of DR compared to SG. However, the current findings align with Ali et al[60] in terms of EWL%.
Our results were also similar to those by Wu et al[13], who found that OAGB resulted in higher EWL% compared to SG; however, our results differed in terms of DR, as we found a higher rate of DR in patients who underwent OAGB at the 1-year follow-up. The higher rate of DR in OAGB was not sustained at the 3-year and 5-year follow-up. The meta-analysis by Wu et al[13] was limited by its inclusion of studies published by the same authors as well as studies with overlapping patient populations from the same hospitals. In addition, we included more recent studies in the current meta-analysis. The difference in DR rates between OAGB (15.7%) and SG (35%) over time could be influenced by the varying rates of diabetes recurrence after initial remission[61,62]. Other plausible explanations for the discrepancies are the duodenal exclusion effect and biliopancreatic limb length effect[63,64]. More studies including randomized controlled trials but with relatively few patients have recently been published. For example, a literature review by Ding et al[65] showed better DR and EWL% in OAGB compared to SG; however, the authors only included two studies that assessed the outcomes at 5 years. Kermansaravi et al[66] included only eight trials and found similar results to the current findings regarding EWL%; however, the authors found no difference in DR between OAGB and SG at 1 year following surgery, and higher DR after OAGB at 5 years post-surgery in contrast to our findings. We found higher DR after OAGB at 1 year, but this was not sustained at 3 years and 5 years. The discrepancy in results could be explained by the differences in baseline HbA1c, BMI, and duration of diabetes mellitus[67,68].
The mechanisms of DR in bariatric surgery remain unclear; however, weight loss, dietary restriction, effects on gut hormones, bile acid alteration, and microbiota dysregulation are thought to play significant roles[69]. In the present meta-analysis, we found a higher rate of late complications in OAGB compared to SG, our findings were different to Wang et al[23] who included only three studies and found a lower rate of late complications in OAGB, the higher rates of late complications in this study could be explained by higher ulcers and malnutrition in OAGB as reported by Barzin et al[59] and Ali et al[60]. Although OAGB achieved higher weight loss and DR, the procedure is associated with higher nutritional deficiencies, particularly of vitamins and minerals, as well as anemia[70,71]. OAGB is associated with higher rates of ulceration, whereas SG is associated with higher rates of GERD, necessitating conversion surgery[38]. The low rate of GERD after OAGB is explained by the use of a wider gastric tube, leading to low intraluminal pressure[4]. On the other hand, some studies have reported a higher risk of GERD after OAGB, particularly bile reflux, potentially increasing the risk of cancer[72]. Although this risk is minimal according to the International Federation for the Surgery of Obesity and Metabolic Disorders[11], experts recommend against performing OAGB in patients with severe esophagitis (grade C or grade D) or Barrett’s esophagus[73]. One study found that OAGB is associated with less craving for fatty and sweet flavors compared to SG[74]. Another study found that the overall complication rates of OAGB and SG were not significantly different[60]. Regarding mortality, literature is scarce due to the continued reservation of some surgeons regarding OAGB[75]. Singhal et al[76] found no difference in 30-day morbidity and mortality between OAGB and SG. Some studies suggest a better quality of life after gastric bypass surgery compared to SG, potentially due to lower rates of GERD and lower BMI[77,78]. Literature suggests that bariatric surgeries generally lead to a better quality of life than non-surgical approaches, with RYGB offering more substantial and durable long-term benefits than SG. Data on the long-term cost effectiveness of OAGB are lacking[79]; however, some studies have shown that RYBG is more cost-effective than SG[80]. Although SG is more cost-effective in individuals with a BMI between 35 kg/m2 and 39.9 kg/m2[81].
The choice between OAGB and SG could be based on case by case because of the existing controversy regarding the rate of complications (higher GERD in SG and higher ulcer and nutritional deficiency in OAGB). Although, OAGB achieved higher EWL% and short-term DM; however, DM was not maintained in the medium-term. Due to the above, patients could be categorized based on the degree of weight loss needed and the presence of early and late complications.
The strength of this study is the inclusion of recent studies that were not included in previous meta-analyses[29,33,36,40,46,57], and the exclusion of studies published by the same authors to avoid overlap[80,81]. In addition, this meta-analysis has the largest up-to-date number of studies. Finally, we provide valuable information on the long-term impact of the procedures on DR and EWL% by reporting the outcomes at 3 years and 5 years post-procedures.
The current results should be viewed in light of the following limitations. The majority of included studies were observational (26 studies) with only six clinical trials with only a medium-term follow-up period. Significant heterogeneity was observed (> 50%), and studies were pooled using different methodologies. No heterogeneity was found when assessing clinical trials only. However, the heterogeneity persisted in observational studies indicating methodological issues. In this meta-analysis, we could not compare OAGB and SG effects on quality of life and mortality due to insufficient studies. A major limitation of this meta-analysis is that, the study was conducted by a single author, which may have increased the RoB.
OAGB leads to a higher rate of complete DR at 1-year post-operation compared to SG; however, no significant differences were evident at 3 years and 5 years after surgery. OAGB achieves superior EWL% compared to SG at 1 year, 3 years, and 5 years following surgery. The late complications were higher in OAGB. The current findings indicated that the choice between OAGB and SG needs careful patient selection depending on basic characteristics and comorbidities. Patients’ categorization depending on the BMI and comorbidities including GERD, ulcers, and nutritional deficiencies, could help the surgeon to choose between OAGB and SG. Larger controlled trials investigating the predictors of complete DR and EWL% and type of late complications are recommended.
The author gratefully acknowledges the Saudi Digital Library for free access to databases. We would like to acknowledge Ihab Farah from the Faculty of Science, University of Tabuk, for the substantial contribution to data analysis.
| 1. | Mahase E. Global cost of overweight and obesity will hit $4.32tn a year by 2035, report warns. BMJ. 2023;380:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 5730] [Article Influence: 1432.5] [Reference Citation Analysis (37)] |
| 3. | Habib P, Chaconas C, Lyuksemburg V, Sarran M, Quinteros F, Lutfi R. Freedom of Choice in the United States: Patient Autonomy Is Driving Decision-Making in Bariatric Surgery. Obes Surg. 2025;35:2602-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Sioka E, Zacharoulis D. One-Anastomosis Gastric Bypass Versus Sleeve Gastrectomy for Morbid Obesity: a Systematic Review and Meta-analysis. Obes Surg. 2017;27:2479-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Onzi TR, Salgado Júnior W, Bastos ELS, Dantas ACB, Silva LB, Oliveira Neto AA, Tristão LS, Santos CLD, Bernardo WM, Chavez MP. Efficacy and safety of one anastomosis gastric bypass in surgical treatment of obesity: systematic review and meta-analysis of randomized controlled trials. Arq Bras Cir Dig. 2024;37:e1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Brown WA, Liem R, Al-Sabah S, Anvari M, Boza C, Cohen RV, Ghaferi A, Våge V, Himpens J, Kow L, Morton J, Musella M, Pattou F, Sakran N, Clapp B, Prager G, Shikora S; IFSO Global Registry Collaboration. Metabolic Bariatric Surgery Across the IFSO Chapters: Key Insights on the Baseline Patient Demographics, Procedure Types, and Mortality from the Eighth IFSO Global Registry Report. Obes Surg. 2024;34:1764-1777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 7. | Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, De Luca M, Faria SL, Goodpaster KPS, Haddad A, Himpens JM, Kow L, Kurian M, Loi K, Mahawar K, Nimeri A, O'Kane M, Papasavas PK, Ponce J, Pratt JSA, Rogers AM, Steele KE, Suter M, Kothari SN. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2022;18:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 483] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 8. | Arakkakunnel J, Grover K. One Anastomosis Gastric Bypass and Mini Gastric Bypass. 2024 Oct 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 9. | Kamal FA, Fernet LY, Rodriguez M, Kamal F, Da Silva NK, Kamal OA, Ayala Aguilar A, Arruarana VS, Martinez Ramirez M. Nutritional Deficiencies Before and After Bariatric Surgery in Low- and High-Income Countries: Prevention and Treatment. Cureus. 2024;16:e55062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 441] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 11. | De Luca M, Piatto G, Merola G, Himpens J, Chevallier JM, Carbajo MA, Mahawar K, Sartori A, Clemente N, Herrera M, Higa K, Brown WA, Shikora S. IFSO Update Position Statement on One Anastomosis Gastric Bypass (OAGB). Obes Surg. 2021;31:3251-3278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 12. | Parmar CD, Bryant C, Luque-de-Leon E, Peraglie C, Prasad A, Rheinwalt K, Musella M. One Anastomosis Gastric Bypass in Morbidly Obese Patients with BMI ≥ 50 kg/m(2): a Systematic Review Comparing It with Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes Surg. 2019;29:3039-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Wu C, Bai R, Yan W, Yan M, Song M. Clinical Outcomes of One Anastomosis Gastric Bypass Versus Sleeve Gastrectomy for Morbid Obesity. Obes Surg. 2020;30:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1013] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 15. | Keleidari B, Mahmoudie M, Anaraki AG, Shahraki MS, Jamalouee SD, Gharzi M, Mohtashampour F. Six month-follow up of laparoscopic sleeve gastrectomy. Adv Biomed Res. 2016;5:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Felsenreich DM, Bichler C, Langer FB, Gachabayov M, Prager G. Sleeve Gastrectomy: Surgical Technique, Outcomes, and Complications. Surg Technol Int. 2020;36:63-69. [PubMed] |
| 17. | Alshaikhi OA, Salih ME, Awadh AH, Sindi KK, Alkenani AN, Alsaedi RM, Aljidaani MA, Alzubaidi AA, Alshaikhi MA, Himmat M, AlZubaidi HA, Alshaikhi SA. Public Awareness and Knowledge of Sleeve Gastrectomy in the Southwest Region of Saudi Arabia. Cureus. 2024;16:e64344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Maroszczuk T, Lewandowska J, Kapała JM, Lech P, Dowgiałło-Gornowicz N. Is one-anastomosis gastric bypass a good revisional bariatric surgery? A single-center retrospective cohort study. Pol Przegl Chir. 2023;96:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Lessing Y, Nevo N, Pencovich N, Abu-Abeid S, Hazzan D, Nachmany I, Eldar SM. One Anastomosis Gastric Bypass as a Revisional Procedure After Failed Laparoscopic Adjustable Gastric Banding. Obes Surg. 2020;30:3296-3300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Lee WJ, Lin YH. Single-anastomosis gastric bypass (SAGB): appraisal of clinical evidence. Obes Surg. 2014;24:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 562] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 22. | Quan Y, Huang A, Ye M, Xu M, Zhuang B, Zhang P, Yu B, Min Z. Efficacy of Laparoscopic Mini Gastric Bypass for Obesity and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2015;2015:152852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Wang FG, Yu ZP, Yan WM, Yan M, Song MM. Comparison of safety and effectiveness between laparoscopic mini-gastric bypass and laparoscopic sleeve gastrectomy: A meta-analysis and systematic review. Medicine (Baltimore). 2017;96:e8924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Musella M, Apers J, Rheinwalt K, Ribeiro R, Manno E, Greco F, Čierny M, Milone M, Di Stefano C, Guler S, Van Lessen IM, Guerra A, Maglio MN, Bonfanti R, Novotna R, Coretti G, Piazza L. Efficacy of Bariatric Surgery in Type 2 Diabetes Mellitus Remission: the Role of Mini Gastric Bypass/One Anastomosis Gastric Bypass and Sleeve Gastrectomy at 1 Year of Follow-up. A European survey. Obes Surg. 2016;26:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Milone M, Lupoli R, Maietta P, Di Minno A, Bianco P, Ambrosino P, Coretti G, Milone F, Di Minno MN, Musella M. Lipid profile changes in patients undergoing bariatric surgery: a comparative study between sleeve gastrectomy and mini-gastric bypass. Int J Surg. 2015;14:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Abdel-Rahim MM, Magdy MM, Mohamad AA. Comparative study between effect of sleeve gastrectomy and mini-gastric bypass on type 2 diabetes mellitus. Diabetes Metab Syndr. 2018;12:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Ahmad WB, Al Shalabi AG, Kabalan Y. Effect of laparoscopic mini-gastric bypass versus laparoscopic sleeve gastrectomy on hypertension and dyslipidemia in obese type 2 diabetes mellitus patients. Ann Med Surg (Lond). 2023;85:4334-4341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Abdelshafy AM, Elshewy AH, Salah EM, Mohammed OA. Mini-gastric bypass versus sleeve gastrectomy in treatment of type iidiabetes mellitus; a randomized comparative study. Europ J Mol Clin Med. 2021;8:116-129. |
| 29. | Dayan D, Bendayan A, Nizri E, Abu-Abeid S, Lahat G, Abu-Abeid A. One Anastomosis Gastric Bypass Compared with Sleeve Gastrectomy in Elderly Patients: Safety and Long-term Outcomes. Obes Surg. 2023;33:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Lee WJ, Chong K, Lin YH, Wei JH, Chen SC. Laparoscopic sleeve gastrectomy versus single anastomosis (mini-) gastric bypass for the treatment of type 2 diabetes mellitus: 5-year results of a randomized trial and study of incretin effect. Obes Surg. 2014;24:1552-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Madhok B, Mahawar KK, Boyle M, Carr WR, Jennings N, Schroeder N, Balupuri S, Small PK. Management of Super-super Obese Patients: Comparison Between Mini (One Anastomosis) Gastric Bypass and Sleeve Gastrectomy. Obes Surg. 2016;26:1646-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Milone M, Di Minno MN, Leongito M, Maietta P, Bianco P, Taffuri C, Gaudioso D, Lupoli R, Savastano S, Milone F, Musella M. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol. 2013;19:6590-6597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Toksoy M, Akinci O, Ergun S, Tuncay E, Zengin K. Laparoscopic mini-gastric bypass versus laparoscopic sleeve gastrectomy in metabolic surgery A single center experience. Ann Ital Chir. 2023;94:11-18. [PubMed] |
| 34. | Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, Dryden DM. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (2)] |
| 35. | Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 36. | Alkhalifah N, Lee WJ, Hai TC, Ser KH, Chen JC, Wu CC. 15-year experience of laparoscopic single anastomosis (mini-)gastric bypass: comparison with other bariatric procedures. Surg Endosc. 2018;32:3024-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Castro MJ, Jimenez JM, Carbajo MA, Lopez M, Cao MJ, Garcia S, Ruiz-Tovar J. Long-Term Weight Loss Results, Remission of Comorbidities and Nutritional Deficiencies of Sleeve Gastrectomy (SG), Roux-En-Y Gastric Bypass (RYGB) and One-Anastomosis Gastric Bypass (OAGB) on Type 2 Diabetic (T2D) Patients. Int J Environ Res Public Health. 2020;17:7644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Das K, Nadeem F, Kabir SA. Comparison of One-Year Outcomes in Sleeve Gastrectomy vs. One Anastomosis Gastric Bypass in a Single Bariatric Unit. Cureus. 2024;16:e74838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 39. | Ding D, Chen DL, Hu XG, Ke CW, Yin K, Zheng CZ. [Outcomes after laparoscopic surgery for 219 patients with obesity]. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:128-131. [PubMed] [DOI] [Full Text] |
| 40. | Gambardella C, Mongardini FM, Paolicelli M, Lucido FS, Tolone S, Brusciano L, Parisi S, Esposito R, Iovino F, Nazzaro L, Pizza F, Docimo L. One Anastomosis Gastric Bypass vs. Sleeve Gastrectomy in the Remission of Type 2 Diabetes Mellitus: A Retrospective Analysis on 3 Years of Follow-Up. J Clin Med. 2024;13:899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Jammu GS, Sharma R. A 7-Year Clinical Audit of 1107 Cases Comparing Sleeve Gastrectomy, Roux-En-Y Gastric Bypass, and Mini-Gastric Bypass, to Determine an Effective and Safe Bariatric and Metabolic Procedure. Obes Surg. 2016;26:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 42. | Jain M, Tantia O, Goyal G, Chaudhuri T, Khanna S, Poddar A, Majumdar K, Gupta S. LSG vs MGB-OAGB: 5-Year Follow-up Data and Comparative Outcome of the Two Procedures over Long Term-Results of a Randomised Control Trial. Obes Surg. 2021;31:1223-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Kansou G, Lechaux D, Delarue J, Badic B, Le Gall M, Guillerm S, Bail JP, Thereaux J. Laparoscopic sleeve gastrectomy versus laparoscopic mini gastric bypass: One year outcomes. Int J Surg. 2016;33 Pt A:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Kular KS, Manchanda N, Rutledge R. Analysis of the five-year outcomes of sleeve gastrectomy and mini gastric bypass: a report from the Indian sub-continent. Obes Surg. 2014;24:1724-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Moradi M, Kabir A, Khalili D, Lakeh MM, Dodaran MS, Pazouki A, Kermansaravi M, Alibeigi P, Moazenzadeh H, Abdolhosseini MR, Eghbali F, Baradaran HR. Type 2 diabetes remission after Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and one anastomosis gastric bypass (OAGB): results of the longitudinal assessment of bariatric surgery study. BMC Endocr Disord. 2022;22:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Plamper A, Lingohr P, Nadal J, Trebicka J, Brol MJ, Woestemeier A, Schmitz SM, Alizai PH, Neumann UP, Ulmer TF, Rheinwalt KP. A Long-Term Comparative Study Between One Anastomosis Gastric Bypass and Sleeve Gastrectomy. J Gastrointest Surg. 2023;27:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 47. | Ruiz-Tovar J, Carbajo MA, Jimenez JM, Castro MJ, Gonzalez G, Ortiz-de-Solorzano J, Zubiaga L. Retraction Note to: Long-term follow-up after sleeve gastrectomy versus Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc. 2021;35:1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Schmitz SM, Alizai PH, Kroh A, Schipper S, Brozat JF, Plamper A, Neumann UP, Rheinwalt K, Ulmer TF. Clinical outcomes after one anastomosis gastric bypass versus sleeve gastrectomy in super-super-obese patients. Surg Endosc. 2022;36:4401-4407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Seetharamaiah S, Tantia O, Goyal G, Chaudhuri T, Khanna S, Singh JP, Ahuja A. LSG vs OAGB-1 Year Follow-up Data-a Randomized Control Trial. Obes Surg. 2017;27:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Shen SC, Lee WJ, Kasama K, Seki Y, Su YH, Wong SK, Huang YM, Wang W. Efficacy of Different Procedures of Metabolic Surgery for Type 2 Diabetes in Asia: a Multinational and Multicenter Exploratory Study. Obes Surg. 2021;31:2153-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Shivakumar S, Tantia O, Goyal G, Chaudhuri T, Khanna S, Ahuja A, Poddar A, Majumdar K. LSG vs MGB-OAGB-3 Year Follow-up Data: a Randomised Control Trial. Obes Surg. 2018;28:2820-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Singla V, Aggarwal S, Singh B, Tharun G, Katiyar V, Bhambri A. Outcomes in Super Obese Patients Undergoing One Anastomosis Gastric Bypass or Laparoscopic Sleeve Gastrectomy. Obes Surg. 2019;29:1242-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Tabesh MR, Abolhasani M, Zali MR, Bagheri R, Alipour M, Cheraghloo N, Asadzadeh-Aghdaei H, Wong A, Zahedi H, Hobaby S, Shadnoush M, Cheraghpour M. The impact of bariatric surgery procedures on the modulation of cardiometabolic risk factors in patients with severe obesity: a 12-month follow-up. J Int Med Res. 2022;50:3000605221119657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Toh BC, Chan WH, Eng AKH, Lim EKW, Lim CH, Tham KW, Fook-Chong S, Tan JTH. Five-year long-term clinical outcome after bariatric metabolic surgery: A multi-ethnic Asian population in Singapore. Diabetes Obes Metab. 2018;20:1762-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Vrakopoulou GZ, Theodoropoulos C, Kalles V, Zografos G, Almpanopoulos K. Type 2 diabetes mellitus status in obese patients following sleeve gastrectomy or one anastomosis gastric bypass. Sci Rep. 2021;11:4421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Wazir N, Arshad MF, Finney J, Kirk K, Dewan S. Two Years Remission of Type 2 Diabetes Mellitus after Bariatric Surgery. J Coll Physicians Surg Pak. 2019;29:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 57. | Sari AC, Avci MA, Ocak S, Akgun C, Buk OF, Ciftci AB, Daldal E. Which Procedure Yields Better Outcomes: Sleeve Gastrectomy, Roux-en-Y Gastric Bypass or Mini Gastric Bypass? Seven Years Outcome Analysis. Medicina (Kaunas). 2025;61:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Yang PJ, Lee WJ, Tseng PH, Lee PH, Lin MT, Yang WS. Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Relat Dis. 2014;10:1182-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Barzin M, Ebadinejad A, Aminian A, Khalaj A, Ghazy F, Koohi F, Hosseinpanah F, Ramezani Ahmadi A, Valizadeh M, Abiri B. Does one-anastomosis gastric bypass provide better outcomes than sleeve gastrectomy in patients with BMI greater than 50? A systematic review and meta-analysis. Int J Surg. 2023;109:277-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Ali M, Wang Y, Ji J, Wang W, Wang D. One Anastomosis Gastric Bypass Versus Sleeve Gastrectomy for Obesity: a Systemic Review and Meta-analysis. J Gastrointest Surg. 2023;27:2226-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Soong TC, Lee MH, Lee WJ, Chen JC, Wu CC, Chun SC. One Anastomosis Gastric Bypass for the Treatment of Type 2 Diabetes: Long-Term Results and Recurrence. Obes Surg. 2021;31:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Lee MH, Almalki OM, Lee WJ, Chen SC, Chen JC, Wu CC. Laparoscopic Sleeve Gastrectomy for Type 2 Diabetes Mellitus: Long-Term Result and Recurrence of Diabetes. Obes Surg. 2020;30:3669-3674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 339] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 64. | Ke Z, Li F, Gao Y, Zhou X, Sun F, Wang L, Chen J, Tan X, Zhu Z, Tong W. Short versus long biliopancreatic limb in Roux-en-Y gastric bypass surgery for treatment of type 2 diabetes mellitus. Wideochir Inne Tech Maloinwazyjne. 2021;16:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Ding Z, Jin L, Song Y, Feng C, Shen P, Li H. Comparison of single-anastomosis gastric bypass and sleeve gastrectomy on type 2 diabetes mellitus remission for obese patients: A meta-analysis of randomized controlled trials. Asian J Surg. 2023;46:4152-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 66. | Kermansaravi M, Chiappetta S, Kassir R, Bosco A, Giudicelli X, Lainas P, Safieddine M. Efficacy of One Anastomosis Gastric Bypass Versus Sleeve Gastrectomy and Roux-en-Y Gastric Bypass for the Treatment of Type 2 Diabetes Mellitus: a Systematic Review and Meta-Analysis of Randomized Clinical Trials. Obes Surg. 2024;34:4555-4562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 67. | Souteiro P, Belo S, Neves JS, Magalhães D, Silva RB, Oliveira SC, Costa MM, Saavedra A, Oliveira J, Cunha F, Lau E, Esteves C, Freitas P, Varela A, Queirós J, Carvalho D. Preoperative Beta Cell Function Is Predictive of Diabetes Remission After Bariatric Surgery. Obes Surg. 2017;27:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Aminian A, Vidal J, Salminen P, Still CD, Nor Hanipah Z, Sharma G, Tu C, Wood GC, Ibarzabal A, Jimenez A, Brethauer SA, Schauer PR, Mahawar K. Late Relapse of Diabetes After Bariatric Surgery: Not Rare, but Not a Failure. Diabetes Care. 2020;43:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 69. | Yin M, Wang Y, Han M, Liang R, Li S, Wang G, Gang X. Mechanisms of bariatric surgery for weight loss and diabetes remission. J Diabetes. 2023;15:736-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 70. | Futooh Z, ElSafty M, Shinkar A, Orebi HA, Elghany RAEA, Youssef HAA, Sabra HK, Elkaseer M, Agwa M, Mahmoud E, Ebrahim A, Bakr IS, Nafea MA, Shalamesh M, Abosonna K, El-Hady HA. Comparative outcomes of one-anastomosis gastric bypass and sleeve gastrectomy: a retrospective analysis of weight loss and micronutrient deficiencies in super-obese patients in 12 months. J Gastrointest Surg. 2025;29:102112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Romano-Zelekha O, Keren D, Herskovitz Y, Vinograd A, Globus I, Keinan-Boker L. Anemia rates after one-anastomosis gastric bypass versus sleeve gastrectomy: a retrospective cohort study. Surg Today. 2025;55:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 72. | Keleidari B, Dehkordi MM, Shahraki MS, Ahmadi ZS, Heidari M, Hajian A, Nasaj HT. Bile reflux after one anastomosis gastric bypass surgery: A review study. Ann Med Surg (Lond). 2021;64:102248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Kermansaravi M, Parmar C, Chiappetta S, Shahabi S, Abbass A, Abbas SI, Abouzeid M, Antozzi L, Asghar ST, Bashir A, Bhandari M, Billy H, Caina D, Campos FJ, Carbajo MA, Chevallier JM, Jazi AHD, de Gordejuela AGR, Haddad A, ElFawal MH, Himpens J, Inam A, Kassir R, Kasama K, Khan A, Kow L, Kular KS, Lakdawala M, Layani LA, Lee WJ, Luque-de-León E, Loi K, Mahawar K, Mahdy T, Musella M, Nimeri A, González JCO, Pazouki A, Poghosyan T, Prager G, Prasad A, Ramos AC, Rheinwalt K, Ribeiro R, Ruiz-Úcar E, Rutledge R, Shabbir A, Shikora S, Singhal R, Taha O, Talebpour M, Verboonen JS, Wang C, Weiner R, Yang W, Vilallonga R, De Luca M. Patient Selection in One Anastomosis/Mini Gastric Bypass-an Expert Modified Delphi Consensus. Obes Surg. 2022;32:2512-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 74. | Mashkoori N, Ibrahim B, Shahsavan M, Shahmiri SS, Pazouki A, Amr B, Kermansaravi M. Alterations in taste preferences one year following sleeve gastrectomy, Roux-en-Y gastric bypass, and one anastomosis gastric bypass: a cross-sectional study. Sci Rep. 2024;14:25484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 75. | Parikh M, Eisenberg D, Johnson J, El-Chaar M; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. American Society for Metabolic and Bariatric Surgery review of the literature on one-anastomosis gastric bypass. Surg Obes Relat Dis. 2018;14:1088-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Singhal R, Cardoso VR, Wiggins T, Super J, Ludwig C, Gkoutos GV, Mahawar K; GENEVA Collaborators. 30-day morbidity and mortality of sleeve gastrectomy, Roux-en-Y gastric bypass and one anastomosis gastric bypass: a propensity score-matched analysis of the GENEVA data. Int J Obes (Lond). 2022;46:750-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 77. | Varvoglis DN, Lipman JN, Li L, Sanchez-Casalongue M, Zhou R, Duke MC, Farrell TM. Gastric Bypass Versus Sleeve Gastrectomy: Comparison of Patient Outcomes, Satisfaction, and Quality of Life in a Single-Center Experience. J Laparoendosc Adv Surg Tech A. 2023;33:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 78. | Małczak P, Mizera M, Lee Y, Pisarska-Adamczyk M, Wysocki M, Bała MM, Witowski J, Rubinkiewicz M, Dudek A, Stefura T, Torbicz G, Tylec P, Gajewska N, Vongsurbchart T, Su M, Major P, Pędziwiatr M. Quality of Life After Bariatric Surgery-a Systematic Review with Bayesian Network Meta-analysis. Obes Surg. 2021;31:5213-5223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Ruiz-Tovar J, Carbajo MA, Jimenez JM, Castro MJ, Gonzalez G, Ortiz-de-Solorzano J, Zubiaga L. Long-term follow-up after sleeve gastrectomy versus Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: a prospective randomized comparative study of weight loss and remission of comorbidities. Surg Endosc. 2019;33:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 80. | Alsumali A, Eguale T, Bairdain S, Samnaliev M. Cost-Effectiveness Analysis of Bariatric Surgery for Morbid Obesity. Obes Surg. 2018;28:2203-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 81. | Plamper A, Lingohr P, Nadal J, Rheinwalt KP. Comparison of mini-gastric bypass with sleeve gastrectomy in a mainly super-obese patient group: first results. Surg Endosc. 2017;31:1156-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |