Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.111673
Revised: August 5, 2025
Accepted: August 21, 2025
Published online: October 27, 2025
Processing time: 110 Days and 16.6 Hours
Emerging evidence has demonstrated that ANXA13 is closely related to the occurrence and development of malignant tumors. However, the functions and underlying molecular mechanisms of ANXA13 in hepatocellular carcinoma (HCC) have not been defined.
To examine the expression of ANXA13 in HCC, investigate its correlation with clinicopathological features.
Quantitative real-time PCR and western blotting were performed to detect the ANXA13 expression in HCC tissues and cell lines at the mRNA and protein levels, respectively. Transwell and cell counting kit-8 assays were performed to assess the effects of ANXA13 overexpression on the proliferation and migration of Huh7 cells.
ANXA13 mRNA was significantly downregulated in HCC tissues, while protein levels were elevated. ANXA13 expression correlated positively with tumor diameter and tumor node metastasis stage. In HCC cell lines (Hep3B and Huh7), ANXA13 expression was higher. Overexpression of ANXA13 enhanced the proliferation and migration capabilities of Huh7 cells.
ANXA13 was upregulated in HCC. Its overexpression promotes tumor progression and is associated with advanced clinicopathological features, suggesting ANXA13 as a potential biomarker and therapeutic target for HCC.
Core Tip: In this study, we demonstrate that ANXA13 is up-regulated in hepatocellular carcinoma (HCC) and postulate that it contributes to tumor progression via post-transcriptional mechanisms. Correlation analyses indicate that ANXA13 may serve as a potential biomarker for HCC, thereby providing a reference for clinical diagnosis and the development of novel thera
- Citation: Yang SK, Zhao XK, Cui YY, Liu DZ, Zhao ZJ, Zhou L, Wang L, Zhang F. ANXA13 expression patterns in hepatocellular carcinoma and impact on tumor behavior. World J Gastrointest Surg 2025; 17(10): 111673
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/111673.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.111673
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent malignancy and the third leading cause of cancer-related mortality worldwide[1]. In China, HCC remains a major public health concern due to its disproportionately high incidence and mortality rates in Asia, posing a significant threat to population health. The pathogenesis of HCC involves a complex interplay of molecular mechanisms, where dysregulation of cancer-associated genes drives hepatocarcinogenesis, progression, and metastasis[2]. These genetic alterations critically influence tumor initiation and aggressiveness, underscoring the urgent need to identify novel biomarkers for improving early diagnosis, therapeutic strategies, and prognostic evaluation in HCC. Annexins are one class of such Ca2+-regulated proteins that can bind to certain membrane phospholipids in a Ca2+-dependent manner, providing a link between Ca2+ signaling and membrane functions. This property links annexins to many membrane-related events, such as the regulated organization of membrane domains and/or membrane-cytoskeleton linkages, certain exocytic and endocytic transport steps, and the regulation of ion fluxes across membranes[3]. ANXA13 is a member of the annexin family of proteins. In the field of oncology, ANXA13 is emerging as a pan-cancer biomarker with distinct diagnostic and therapeutic implications across multiple malignancies: In hepatobiliary and pancreatic cancers, its marked over-expression in cholangiocarcinoma vs pancreatic ductal adenocarcinoma refines differential diagnosis[4,5], while in gastrointestinal tumors such as colorectal and gastroesophageal junction cancers, ANXA13 up-regulation drives tumor invasion, lymph-node metastasis, and poor prognosis[6-8]. Beyond these sites, elevated ANXA13 similarly promotes malignant phenotypes in lung adenocarcinoma, clear-cell renal-cell carcinoma, and breast cancer, yet its knock-down or targeted inhibition reverses these effects and sensitizes cells to rapamycin[9-11]. collectively positioning ANXA13 as a promising, actionable target for both diagnostic stratification and therapeutic intervention across diverse solid tumors. However, research on the ANXA13 gene in HCC remains limited.
Our previous bioinformatics analysis revealed that the ANXA13 gene is significantly overexpressed in HCC tissues compared to adjacent non-tumor tissues. It also exhibits distinct diagnostic properties that may influence clinical prognosis. In this study, we aim to validate the expression of ANXA13 in HCC and adjacent tissues obtained from surgical resections, using tissue microarrays, normal liver cell lines (WRL), and various HCC cell lines. Additionally, we will explore its functional role and investigate the relationship between ANXA13 expression and clinical features of HCC, to identify potential therapeutic targets and provide evidence to improve patient prognosis.
All HCC tissues and corresponding adjacent non-tumor tissues were obtained through surgical resection at the De
WRL and various HCC cell lines, including HepG2, Hep3B, and Huh7, were purchased from Wuhan Punoase Life Science and Technology Co., Ltd. Among these, the Huh7 cell line exhibits relatively low invasive and metastatic potential[12], while HepG2 and Hep3B cell lines demonstrate the lowest invasive and metastatic potential[13]. WRL, HepG2, and Hep3B cell lines were cultured in high-glucose MEM medium, while the Huh7 cell line was cultured in DMEM. Both mediums were supplemented with 10% fetal bovine serum (FBS). All cell lines were maintained at 37 °C in a 5% CO2 incubator, with routine passaging.
A single-cell suspension was prepared using a culture medium containing 20% FBS, and cells were seeded into the wells of the plates according to the required cell density. After 48 hours of incubation, subsequent experimental procedures were performed. One hour before transfection, the cells were replenished with Opti-MEM (serum-free medium). The plasmid DNA in Opti-MEM was mixed with Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, United States) in Opti-MEM and incubated for 20 minutes. This plasmid-lipofectamine complex was then added to the cells, and after 6 hours, the medium was replaced with a complete culture medium containing 10% FBS.
Total RNA was extracted from HCC tissues and various cell lines using the Trizol reagent (Acri Biotech Co., Ltd.). The RNA was then reverse-transcribed into complementary DNA (cDNA). Quantitative PCR (qPCR) was performed using the SYBR Green ProTaqHS qPCR Kit (Acri Biotech Co., Ltd.), with all procedures conducted in the dark to prevent degradation. The reaction mixture for each sample consisted of 2 μL cDNA, 10 μL 2 × SYBR Green ProTaqHS Premix, 0.8 μL of each forward and reverse primer, 0.4 μL ROX Reference Dye (4 μM), and 6 μL RNase-free water, for a total volume of 20 μL. The primer sequences for ANXA13 were as follows: Forward 5’-CAAGCAGTTACGAGCCACCTT-3’ and reverse 5’-CCTCACAATCCTGGGCACATC-3’. The primer sequences for the housekeeping gene GAPDH were: Forward 5’-GGAAGCTTGTCATCAATGGAAATC-3’ and reverse 5’-TGATGACCCTTTTGGCTCCC-3’. The qPCR conditions were as follows: Initial denaturation at 95 °C for 30 seconds, followed by 40 cycles of denaturation at 95 °C for 5 seconds, and annealing/extension at 60 °C for 30 seconds. The expression level of ANXA13 was analyzed using the 2-ΔΔCt method.
Tissue samples and cells were lysed using RIPA buffer (Shanghai Weiao Biotechnology Co., Ltd., Shanghai, China). Protein concentrations were determined using the BCA protein assay kit (WB0123, Shanghai Weiao Biotechnology Co., Ltd., Shanghai, China). Protein samples (30 μg per lane) were separated by 12.5% SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 10% skim milk at room temperature for 2 hours and then incubated overnight at 4 °C with the following primary antibodies. After primary antibody incubation, the membranes were incubated with anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies (WB0177/WB0176, Shanghai Weiao Biotechnology Co., Ltd., Shanghai, China) at room temperature for 1 hour. Protein bands were visualized using the ECL Ultra-Sensitive Chemiluminescence Kit (WB0164, Shanghai Weiao Biotechnology Co., Ltd., Shanghai, China) and scanned using an automated chemiluminescence imaging system (Shanghai Tanneng Life Science Co., Ltd., Shanghai, China). Protein band densities were quantified using ImageJ software (version 1.8.0). The experiment was performed using the ANXA13a isoform (NM_004306.4/NP_004297.2).
Cell proliferation was assessed using the cell counting kit-8 (CCK-8) (Shanghai Donghuan Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer's protocol. Briefly, cells were seeded on 96-well plates at a density of 2 × 103 cells/well and incubated for 24 hours. At the indicated time points (0, 24, 48, and 72 hours), 10 μL of CCK-8 solution was added to each well, followed by incubation at 37 °C in the dark for 2 hours.
The harvested tissue was immediately immersed in a 10% formalin fixative solution for 72 hours. After fixation, appropriate tissue samples were subjected to dehydration, clearing, wax infiltration, and embedding processes, followed by sectioning. The paraffin sections were baked at 45 °C for 120 minutes, followed by xylene deparaffinization. Antigen retrieval was performed by placing the sections in antigen retrieval solution and microwaving at medium-high temperature for 15 minutes, followed by natural cooling to room temperature. The sections were then washed three times with phosphate-buffered saline (PBS) to complete the antigen retrieval process. After blocking endogenous peroxidase activity, a circle was drawn around the tissue using a hydrophobic barrier pen, and 100 µL of serum was applied to block non-specific binding for 30 minutes. The primary antibody was incubated overnight at 4 °C, and subsequently rewarmed at room temperature for 1 hour the next day, with three washes in PBS. The secondary antibody was incubated at room temperature for 1 hour, followed by three PBS washes. The sections were stained with DAB, counterstained with hematoxylin, dehydrated through ethanol, and mounted with neutral gum. Images were captured under a microscope.
For each stained section, ten non-overlapping high-power fields (400 × magnification) were randomly selected. Immunoreactivity was quantified using McCarty’s H-score, calculated as Σ (intensity × percentage of positive cells). Staining intensity was scored as absent (0), weak (1+), moderate (2+), or strong (3+). The H-score analysis was carried out independently by two experienced pathologists who were blinded to the final clinical diagnosis of all cases studied. A third pathologist would review the score when there was an inconsistency between the two pathologists.
Cell migration ability was evaluated using 24-well Transwell chambers with 8 μm pore polycarbonate membranes (Corning, United States). Briefly, 2 × 104 cells in 200 μL serum-free medium were seeded into the upper chamber. The lower chamber was filled with 600 μL complete medium containing 10% FBS as a chemoattractant. After incubation for 24 hours at 37 °C in 5% CO2, non-migrated cells on the upper surface of the membrane were removed using cotton swabs. Migrated cells on the lower surface were fixed with 4% paraformaldehyde for 15 minutes and stained with 0.1% crystal violet for 20 minutes at room temperature. Five random fields were counted for each membrane. All experiments were performed in triplicate and repeated three times independently.
Western blot data were analyzed for grayscale values using ImageJ software. Other data were visualized with GraphPad Prism software (version 9.0; GraphPad Software, Inc.). Positive area analysis of immunohistochemistry (IHC) and Transwell cell migration images was conducted using ImageJ and K-viewer software. Statistical analysis and graphical representation were performed with GraphPad Prism software. Statistical analyses were performed using Shapiro-Wilk normality tests, F-tests for homogeneity of variances, t-tests, and analysis of variance (ANOVA). Benjamini-Hochberg false-discovery rate correction was applied to all multiple comparisons. P < 0.05 was considered statistically significant.
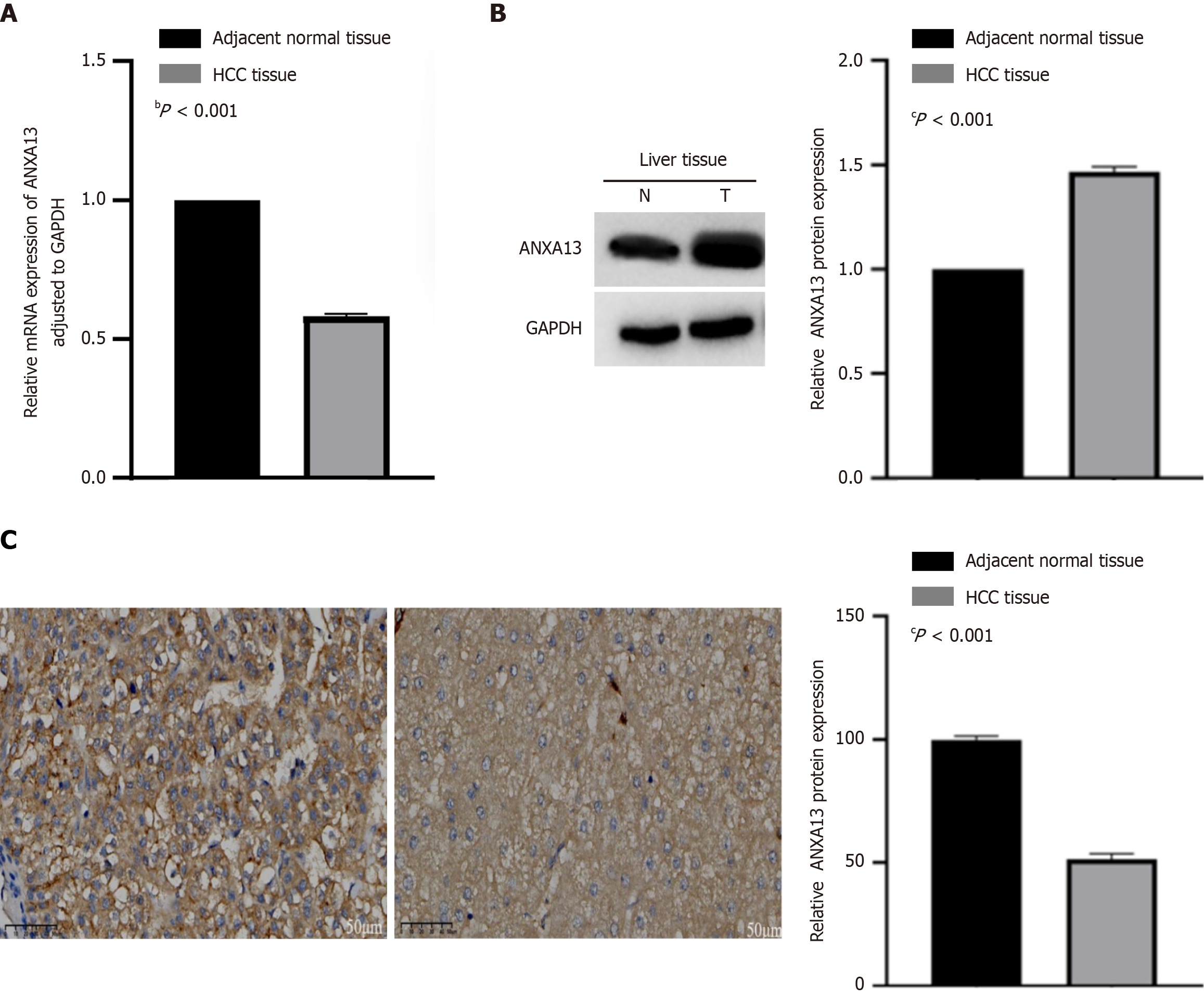
We validated the expression of the ANXA13 gene in clinical HCC tissues and corresponding adjacent non-tumor tissues. Quantitative real-time PCR (qRT-PCR) was used to assess differences in ANXA13 mRNA levels between HCC tissues and adjacent non-tumor tissues. The results indicated that ANXA13 expression was lower in cancer tissues than in adjacent non-tumor tissues, showing a statistically significant difference (P < 0.05) (Figure 1A).
We further analyzed the expression of ANXA13 protein in HCC and adjacent non-tumor tissues using western blotting (Figure 1B). Grayscale value analysis was performed using ImageJ software, with normalization to adjacent non-tumor tissues as the control. The expression levels of ANXA13 protein in HCC tissues were significantly higher than that in adjacent non-tumor tissues (P < 0.05) (Table 1).
| Group | Number of cases | ANXA13/GAPDH | P value |
| HCC tissue | 50 | 1.466 ± 0.1753 | < 0.05 |
| Adjacent normal tissue | 50 | 1.000 ± 0.0000 |
To further confirm the expression of ANXA13 protein, we performed IHC on HCC and adjacent non-tumor tissues. IHC results showed that ANXA13 protein was predominantly localized in the cytoplasm and cell membrane in both HCC and adjacent non-tumor tissues, and ANXA13 protein staining was more intense in HCC tissues (P < 0.05) (Figure 1C; Table 2). This indicates that the expression of ANXA13 is higher in cancer tissues.
| Group | Number of cases | H-score (mean ± SD) |
| HCC tissue | 48 | 99.83 ± 11.00 |
| Adjacent normal tissue | 48 | 51.50 ± 15.13 |
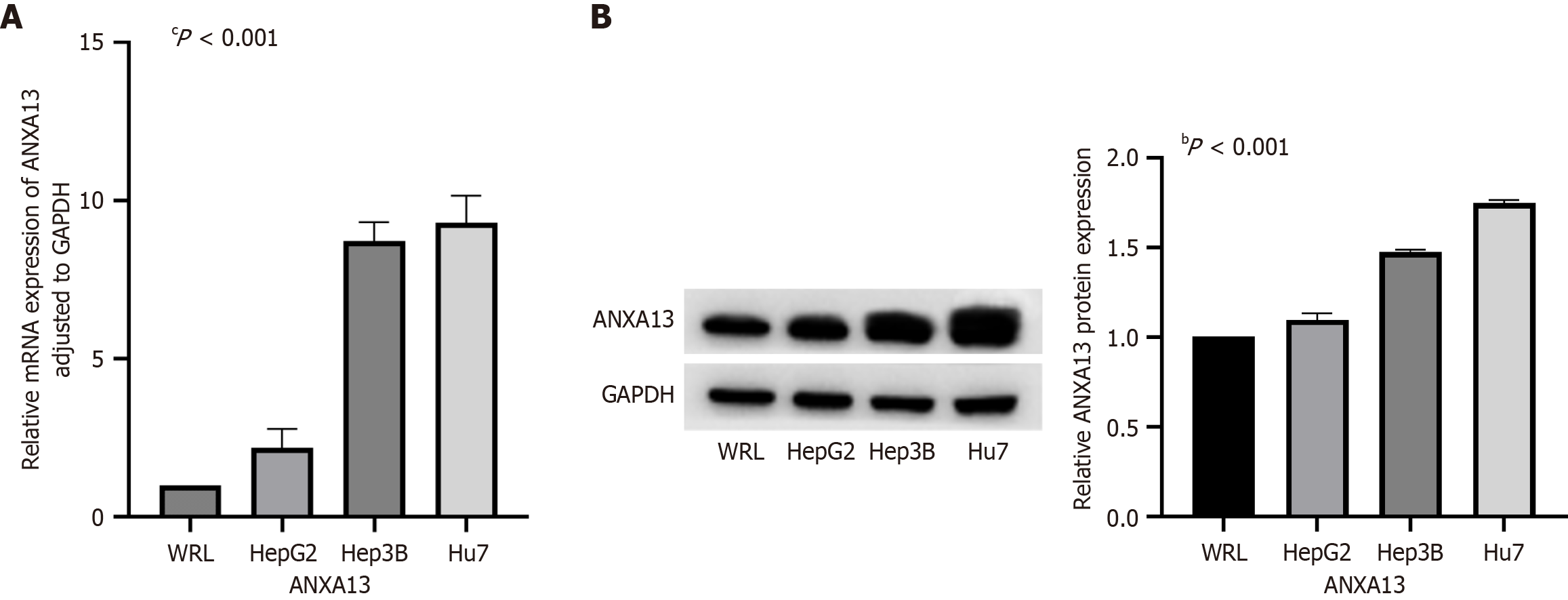
We selected one WRL and three HCC cell lines (Hep3B, Huh7, and HepG2) to investigate the expression of the ANXA13 gene in cells. The expression levels of ANXA13 mRNA were measured in both normal liver cells and HCC cell lines using qRT-PCR. The results revealed that ANXA13 mRNA expression was significantly higher in the Hep3B and Huh7 HCC cell lines compared to the WRL (P < 0.05) (Figure 2A).
Western blot analysis demonstrated that, compared to the WRL, ANXA13 protein expression was mildly elevated in HepG2 cells (P < 0.05). At the same time, it was significantly increased in Hep3B cells and, particularly, in Huh7 cells
We examined the relationship between ANXA13 expression levels (ANXA13 immunohistochemical staining was evaluated using the H-score, with an H-score ≥ 100 defined as high expression and < 100 as low expression) and the clinical pathological data of HCC patients to investigate the correlation between ANXA13 gene expression and HCC staging and progression. The results indicated that ANXA13 expression was significantly associated with tumor diameter and tumor node metastasis (TNM) staging in HCC patients (Table 3; P < 0.05). However, no significant correlation was found between ANXA13 expression and patient age, gender, or pathological grading (P > 0.05).
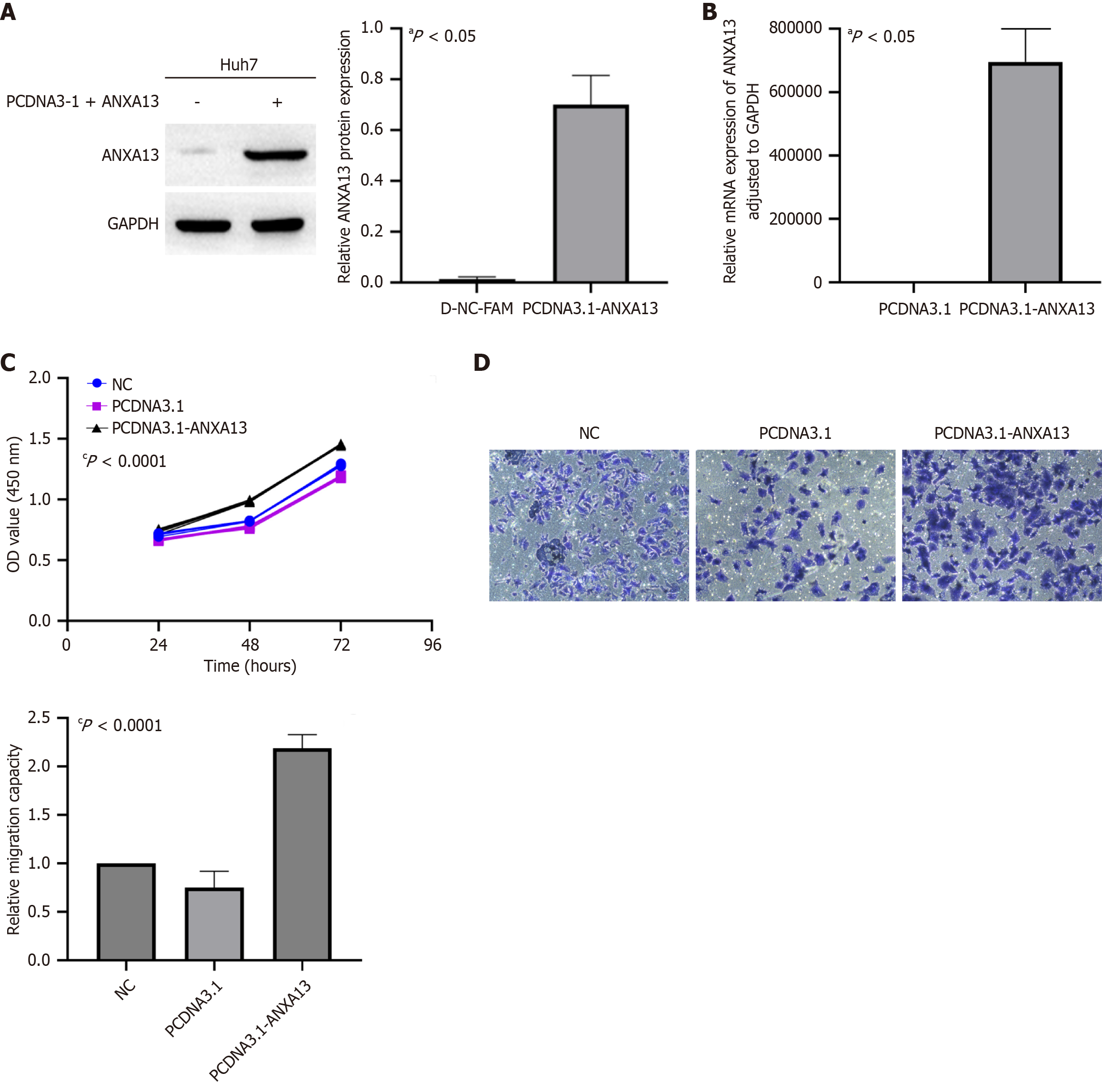
In the following experiments, we selected Huh7 tumor cells as the model for further investigation. The cells were transfected with pcDNA3.1-ANXA13 and the negative control pcDNA3.1 to evaluate the mRNA and protein expression levels of ANXA13. Furthermore, qRT-PCR and Western blot confirmed that the Huh7 cell line was successfully transfected (Figure 3A and B). The impact of ANXA13 expression on cell proliferation was assessed using the CCK-8 assay. The data demonstrated that, compared to control cells, overexpression of ANXA13 significantly increased the proliferation rate of Huh7 cells at 48 and 72 hours post-transfection (Figure 3C). Additionally, the migratory ability of Huh7 cells was evaluated using the Transwell assay. The number of migrated cells was significantly higher in the pcDNA3.1-ANXA13 group compared to the control group (Figure 3D). Given the low endogenous expression of ANXA13 in target tissues, we exclusively employed overexpression approaches to investigate its functional role.
ANXs are a family of intracellular proteins that bind to membrane phospholipids in a calcium ion (Ca2+)-dependent manner[14]. The annexin family consists of five subfamilies: A, B, C, D, and E. The A subfamily is primarily present in human and mammalian cells, the B subfamily is found in invertebrate cells, the C subfamily is present in fungi and some unicellular eukaryotes, the D subfamily is found in plants, and the E subfamily is observed in protozoa[15]. The ANXA subfamily includes twelve members (ANXA1–11 and ANXA13). Among them, the ANXA13 gene is considered the ancestral gene of the twelve annexins in vertebrates. ANXA13 is the only annexin known to undergo N-myristoylation, a post-translational modification. Its amino-terminal region contains a conserved G2 myristoylation site, which enables ANXA13 to bind membrane phospholipids in a calcium-independent manner after N-myristoylation[16]. Studies have shown that ANXA13 expression is elevated in various diseases, including lupus nephritis[17] and glomerulosclerosis[18]. Additionally, ANXA13 is emerging as a pan-cancer biomarker with distinct diagnostic and therapeutic implications across multiple malignancies: Across hepatobiliary and pancreatic malignancies, ANXA13 is markedly overexpressed in cholangiocarcinoma but not in pancreatic ductal adenocarcinoma, thereby refining differential diagnosis[4,5]. In gastrointestinal cancers, including colorectal and gastro-oesophageal junction tumors, elevated ANXA13 drives tumor invasion, lymph-node metastasis, and poor prognosis[6-8]. Beyond these sites, high ANXA13 Levels similarly promote malignant phenotypes in lung adenocarcinoma, clear-cell renal cell carcinoma, and breast cancer, whereas its genetic knockdown or pharmacological inhibition reverses these effects and sensitises tumour cells to rapamycin[9-11]. However, its expression in HCC and its potential impact on clinical prognosis in liver cancer require further investigation.
To further investigate the expression of ANXA13 in HCC, our research team utilized qRT-PCR, western blotting, IHC, and tissue microarray analysis. We also explored its correlation with clinical features associated with liver cancer. Our findings revealed that ANXA13 expression levels were significantly associated with tumor diameter and TNM staging in HCC patients. However, no significant correlation was observed with patient age, gender, or pathological grading. These results suggest that the dysregulated expression of ANXA13 may be indicative of the onset and progression of HCC. The biological characteristics of tumors can often be reflected by the proliferative status of cancer cells, which is a crucial factor in tumor initiation, progression, and prognosis. Collectively, these findings indicate that the ANXA13 gene possesses significant clinical relevance.
The analysis revealed that there was no statistically significant correlation between the expression level of ANXA13 and pathological grading in the tissue microarray. We suggest that this lack of significance may be attributed to the relatively small sample size used in the study. An insufficient sample size may compromise the reproducibility and reliability of our findings by introducing statistical limitations and amplifying biological heterogeneity. Conversely, enlarging the tissue cohort could uncover additional statistically significant correlations with clinical parameters.
The research subjects of this study include liver cancer tissues, adjacent non-cancerous tissues, and three different liver cancer cell lines. qRT-PCR results demonstrated that the expression level of ANXA13 was lower in liver cancer tissues compared to adjacent non-cancerous tissues. However, results from Western blot and IHC showed the opposite trend. The ANXA13 protein expression is significantly higher in liver cancer tissues than in adjacent non-cancerous tissues. This difference was statistically significant, and the result was consistent across multiple independent experimental repeti
In normal hepatocyte cell lines and HCC cell lines, qRT-PCR and western blot results showed upregulation of ANXA13 expression in the HCC cell lines. To further investigate the relationship between the abnormal expression of ANXA13 and cancer cell proliferation, we established a Huh7 HCC cell line with ANXA13 overexpression through plasmid trans
Based on the aforementioned results, we conclude that ANXA13 is upregulated in HCC and holds diagnostic potential. Additionally, it can promote the proliferation and migration of HCC cells. Analysis of clinical pathological data suggests that ANXA13 may influence tumor progression in HCC patients, providing a reference for clinical diagnosis and the development of novel therapeutic strategies, with evident translational relevance. Across lung adenocarcinoma, clear-cell renal cell carcinoma, and breast cancer, overexpression of ANXA13 has been shown to drive malignant phenotypes, whereas its genetic knockdown or pharmacological inhibition not only reverses these effects but also markedly sensitizes tumor cells to rapamycin[9-11]. Leveraging these cross-cancer findings, we posit that a comprehensive elucidation of ANXA13 function in HCC will provide a critical mechanistic foundation for refining prognostic stratification and therapeutic strategies in affected patients. Although this study examined the expression of ANXA13 in HCC tissues and cell lines, the underlying mechanisms of its overexpression and post-transcriptional regulation in HCC remain unexplored. Moreover, the study is limited by a small sample size and insufficient postoperative follow-up data, preventing a comprehensive analysis of the correlation between ANXA13 and patient prognosis. Due to time constraints, animal experiments were not conducted. Future research should aim to expand the sample size, include detailed postoperative survival follow-up, and investigate the impact of ANXA13 on patient survival prognosis. Furthermore, establishing animal models and exploring related functional mechanisms will be essential to further elucidate the molecular pathways through which ANXA13 influences HCC.
In summary, this study demonstrates for the first time that ANXA13 is significantly overexpressed in HCC cell lines and tissues, and its expression pattern correlates with advanced clinicopathological features of HCC patients. More importantly, the expression pattern of ANXA13 could be used as a biomarker for poor survival. In addition, ANXA13 overexpression promotes tumor progression. Taken together, this study presents ANXA13 as a novel molecular target for HCC therapy.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12729] [Article Influence: 6364.5] [Reference Citation Analysis (8)] |
| 2. | Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 3. | Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1144] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 4. | Padden J, Ahrens M, Kälsch J, Bertram S, Megger DA, Bracht T, Eisenacher M, Kocabayoglu P, Meyer HE, Sipos B, Baba HA, Sitek B. Immunohistochemical Markers Distinguishing Cholangiocellular Carcinoma (CCC) from Pancreatic Ductal Adenocarcinoma (PDAC) Discovered by Proteomic Analysis of Microdissected Cells. Mol Cell Proteomics. 2016;15:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Geramizadeh B, Sehat M, Mehrmozayan A, Ali Reza AR. Annexin Expression in Cholangiocarcinoma, and Metastatic Pancreatic Ductal Adenocarcinoma "Is it be Helpful for Differential Diagnosis of These Tumors in the Liver?". Iran J Pathol. 2021;16:433-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Fernández-Lizarbe S, Lecona E, Santiago-Gómez A, Olmo N, Lizarbe MA, Turnay J. Structural and lipid-binding characterization of human annexin A13a reveals strong differences with its long A13b isoform. Biol Chem. 2017;398:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Jiang G, Wang P, Wang W, Li W, Dai L, Chen K. Annexin A13 promotes tumor cell invasion in vitro and is associated with metastasis in human colorectal cancer. Oncotarget. 2017;8:21663-21673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | van Duin M, van Marion R, Vissers KJ, Hop WC, Dinjens WN, Tilanus HW, Siersema PD, van Dekken H. High-resolution array comparative genomic hybridization of chromosome 8q: evaluation of putative progression markers for gastroesophageal junction adenocarcinomas. Cytogenet Genome Res. 2007;118:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Xue GL, Zhang C, Zheng GL, Zhang LJ, Bi JW. Annexin A13 predicts poor prognosis for lung adenocarcinoma patients and accelerates the proliferation and migration of lung adenocarcinoma cells by modulating epithelial-mesenchymal transition. Fundam Clin Pharmacol. 2020;34:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Niu X, Zhao K, Zheng Y, Wang Y, Liu R, Zhang Y, Wang L, Wu Y, Bai X, Qiao B. ANXA13 promotes cell proliferation and invasion and attenuates apoptosis in renal cell carcinoma. Heliyon. 2023;9:e18009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Kuhn AR, Schlauch K, Lao R, Halayko AJ, Gerthoffer WT, Singer CA. MicroRNA expression in human airway smooth muscle cells: role of miR-25 in regulation of airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2010;42:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Ma Y, Bian J, Zhang F. Inhibition of perillyl alcohol on cell invasion and migration depends on the Notch signaling pathway in hepatoma cells. Mol Cell Biochem. 2016;411:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu X, Sun J, Yang S, Poon RT, Fan ST. Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular carcinoma. Int J Cancer. 2012;131:E163-E172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Li YZ, Wang YY, Huang L, Zhao YY, Chen LH, Zhang C. Annexin A protein family in atherosclerosis. Clin Chim Acta. 2022;531:406-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (11)] |
| 15. | Wang J, Guo C, Liu S, Qi H, Yin Y, Liang R, Sun MZ, Greenaway FT. Annexin A11 in disease. Clin Chim Acta. 2014;431:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Turnay J, Lecona E, Fernández-Lizarbe S, Guzmán-Aránguez A, Fernández MP, Olmo N, Lizarbe MA. Structure-function relationship in annexin A13, the founder member of the vertebrate family of annexins. Biochem J. 2005;389:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Benjachat T, Tongyoo P, Tantivitayakul P, Somparn P, Hirankarn N, Prom-On S, Pisitkun P, Leelahavanichkul A, Avihingsanon Y, Townamchai N. Biomarkers for Refractory Lupus Nephritis: A Microarray Study of Kidney Tissue. Int J Mol Sci. 2015;16:14276-14290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Bruschi M, La Porta E, Panfoli I, Candiano G, Petretto A, Vidal E, Kajana X, Bartolucci M, Granata S, Ghiggeri GM, Zaza G, Verrina E. Proteomic profile of mesothelial exosomes isolated from peritoneal dialysis effluent of children with focal segmental glomerulosclerosis. Sci Rep. 2021;11:20807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Prieto-Fernández L, Menéndez ST, Otero-Rosales M, Montoro-Jiménez I, Hermida-Prado F, García-Pedrero JM, Álvarez-Teijeiro S. Pathobiological functions and clinical implications of annexin dysregulation in human cancers. Front Cell Dev Biol. 2022;10:1009908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Patil SS, Panchal V, Røstbø T, Romanyuk S, Hollås H, Brenk R, Grindheim AK, Vedeler A. RNA-binding is an ancient trait of the Annexin family. Front Cell Dev Biol. 2023;11:1161588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 906] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Beyer A, Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 2016;165:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 2286] [Article Influence: 228.6] [Reference Citation Analysis (0)] |
| 23. | Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R, Rodriguez EH, Fields AP, Schwartz S, Raychowdhury R, Mumbach MR, Eisenhaure T, Rabani M, Gennert D, Lu D, Delorey T, Weissman JS, Carr SA, Hacohen N, Regev A. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science. 2015;347:1259038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 379] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 24. | Buccitelli C, Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet. 2020;21:630-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 782] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 25. | Cheng Z, Teo G, Krueger S, Rock TM, Koh HW, Choi H, Vogel C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol Syst Biol. 2016;12:855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 26. | Wang H, Zhu Y, Hu L, Li Y, Liu G, Xia T, Xiong D, Luo Y, Liu B, An Y, Li M, Huang Y, Zhong Q, Zeng M. Internal Ribosome Entry Sites Mediate Cap-Independent Translation of Bmi1 in Nasopharyngeal Carcinoma. Front Oncol. 2020;10:1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Xu L, Wang P, Feng X, Tang J, Li L, Zheng X, Zhang J, Hu Y, Lan T, Yuan K, Zhang Y, Ren S, Hao X, Zhang M, Xu M. SETD3 is regulated by a couple of microRNAs and plays opposing roles in proliferation and metastasis of hepatocellular carcinoma. Clin Sci (Lond). 2019;133:2085-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 3073] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/