Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.111672
Revised: July 20, 2025
Accepted: September 2, 2025
Published online: October 27, 2025
Processing time: 110 Days and 18.9 Hours
Intestinal transplantation (ITx) has emerged as a pivotal life-saving intervention for patients with irreversible intestinal failure unresponsive to conventional medical and nutritional therapies. Despite its growing clinical acceptance, ITx remains among the most immunologically complex and technically demanding procedures in the field of solid organ transplantation. This review comprehen
Core Tip: This review systematically elucidates the current status and challenges of intestinal transplantation (ITx) as a life-saving therapy for patients with irreversible intestinal failure. Despite therapeutic hurdles such as the high immunogenicity of the intestine, complex microbiota microenvironment, and susceptibility to rejection, surgical innovations-including robot-assisted vascular anastomosis and multivisceral transplantation-along with breakthroughs in ex vivo perfusion technology have significantly improved clinical outcomes. This review highlights that bidirectional immune reactions (host-vs-graft response and graft-vs-host disease) and chronic rejection remain the primary clinical obstacles. Analysis herein reveals marked differences in immunological profiles and clinical manifestations between pediatric and adult recipients, necessitating individualized treatment strategies. Based on current research advances, future directions include precision immunotherapy, gut microbiota modulation, and bioengineering innovations such as 3D bioprinted grafts. The review emphasizes that only through multidisciplinary collaboration can ITx evolve into a safer and more effective treatment, ultimately achieving comprehensive improvements in long-term survival rates and quality of life for patients.
- Citation: Rong Y, Nie CY, Zhou JD, Wang ZC, Wu DL, Wu SW, Xie ZY. Intestinal reengineering: Scientific advances in intestinal transplantation. World J Gastrointest Surg 2025; 17(10): 111672
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/111672.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.111672
The small intestine is one of the largest immune organs in the human body and plays a critical role in maintaining health, sustaining life, and improving patients’ quality of life[1]. Over the past few decades, organ transplantation has achieved remarkable success in the cardiovascular, gastrointestinal, and urologic systems, with increasingly refined techniques saving the lives of countless patients. Despite persistent challenges-including donor shortages, immune rejection, and complexities in long-term postoperative management-these procedures have significantly advanced human health. Compared with established procedures such as liver, kidney, and heart transplantation, intestinal transplantation (ITx) remains a relatively recent advancement. Although improvements in surgical techniques and immunosuppressive therapies have facilitated progress, ITx continues to face considerable barriers, particularly high rates of graft rejection and postoperative complications, which limit its broader clinical application[2]. The small intestine is densely populated with lymphoid tissue within the mucosal layer, and hosts a vast array of microorganisms in the lumen, making it a highly immunogenic organ and a critical site of mucosal immune activity[3]. Immune interactions between host tissues and microbial communities can trigger infections and provoke intense immune responses post-transplantation, including host-vs-graft reaction (HVGR) and graft-vs-host reaction (GVHR)[4]. Moreover, many ITx candidates are immunocompromised due to long-term dependence on parenteral nutrition (PN), creating a dual challenge of immune deficiency and heightened rejection risk, both of which contribute to reduced surgical success rates[5,6].
In this article, we provide a comprehensive review of the historical evolution and recent breakthroughs in ITx surgical techniques, examine its unique immunological features and underlying mechanisms, and explore current clinical challenges and future directions. Our goal is to offer a theoretical foundation and practical strategies to support the successful clinical translation and broader application of ITx.
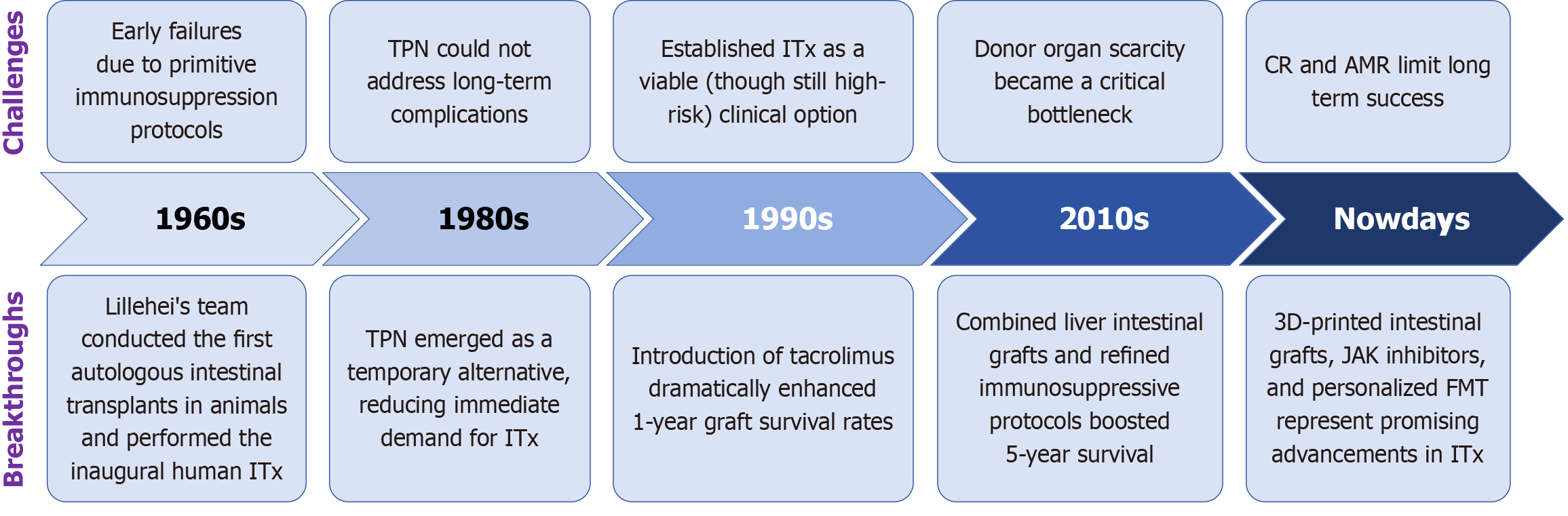
The development of ITx has progressed over six decades, marked by key innovations in immunosuppression, surgical technique, and organ preservation. Initial attempts in the 1960s, including those by Lillehei and colleagues, failed due to inadequate immunologic control[7-9]. The introduction of total PN (TPN) in the 1980s temporarily reduced the urgency for ITx[10].
A major breakthrough occurred in the early 1990s with the clinical application of tacrolimus, which significantly improved one-year graft survival and established ITx as a viable treatment option for irreversible intestinal failure[11]. Since then, the field has entered a period of steady advancement, including the development of multivisceral transplan
Intestinal failure is defined as the inability of the gut to absorb sufficient nutrients, fluids, and electrolytes to sustain growth, development, or homeostasis without parenteral support. It can result from either anatomical loss or functional impairment of the intestine. Anatomical causes include short bowel syndrome (SBS) due to massive small bowel resection, mesenteric ischemia, trauma, congenital intestinal atresia, or enterocutaneous fistulas. Functional causes include disorders such as chronic intestinal pseudo-obstruction, severe motility disorders, mucosal diseases (e.g., microvillus inclusion disease), and radiation enteritis[19,20]. These conditions may be congenital or acquired and can affect both children and adults.
Although PN remains the cornerstone of treatment for patients with intestinal failure, long-term PN dependence is associated with significant morbidity, including catheter-related bloodstream infections, metabolic bone disease, loss of venous access, and intestinal failure-associated liver disease (IFALD). ITx is not considered a first-line or standard therapy for intestinal failure but is instead reserved for carefully selected patients who either develop life-threatening complications from PN or in whom intestinal rehabilitation has failed to achieve nutritional autonomy[21]. The internationally accepted clinical indications for ITx include: (1) Irreversible IFALD with evidence of progressive liver dysfunc
Importantly, with advances in multidisciplinary rehabilitation, the threshold for proceeding to ITx has risen, and many patients who would previously have been considered transplant candidates can now be successfully managed with integrated medical-nutritional approaches. As such, ITx remains a vital but highly specialized therapy, reserved for a minority of patients with refractory or complicated intestinal failure.
Since its clinical introduction in the 1980s, ITx has substantially improved both survival rates and quality of life for patients dependent on PN. While allogeneic transplantation shares fundamental immunological and surgical principles across age groups, pediatric and adult recipients differ significantly in terms of etiology, anatomical and physiological characteristics, perioperative management, and long-term outcomes. These differences necessitate tailored transplanta
Currently, most studies focus on either pediatric or adult ITx cohorts in isolation, with limited systematic comparisons between the two groups. Addressing this gap through well-designed comparative studies represents an important future direction for advancing individualized care and optimizing outcomes across the lifespan.
The differences between pediatric and adult ITx primarily arise from distinct etiologies and clinical indications. In pediatric patients, intestinal failure is most commonly caused by congenital or perinatal conditions, such as intestinal atresia or necrotizing enterocolitis (NEC), which may result in intestinal dysfunction or SBS following surgical resection. Additionally, prolonged dependence on PN in pediatric SBS frequently leads to IFALD-a term now preferred over the outdated “PN-associated liver disease”-to better reflect its multifactorial pathophysiology involving PN, recurrent sepsis, and underlying intestinal compromise. The development of IFALD in pediatric patients is a major trigger for early trans
Another critical factor contributing to the divergence in pediatric vs adult ITx lies in anatomical and physiological differences, which directly impact surgical complexity, postoperative recovery, and long-term outcomes. Pediatric intestines are approximately six times the length of the body-often exceeding adult intestinal length-highlighting key anatomic distinctions[29,30]. Furthermore, the pediatric mesenteric vasculature and intestinal lumen are markedly narrower, increasing technical demands during transplantation. This necessitates highly precise vascular anastomosis of major structures such as the portal vein and mesenteric arteries. Nevertheless, the relatively longer intestinal length in pediatric patients may also confer superior regenerative capacity compared to adults.
From an immunological standpoint, pediatric ITx recipients exhibit a higher risk of AR compared to adults, primarily due to their immature immune systems and limited immunocompetence. This necessitates the use of more precise and per
Collectively, these factors establish a unique “high-responsiveness/high-controllability” immunological paradigm in pediatric recipients, ultimately contributing to their superior long-term graft survival. In contrast, adult recipients are more prone to antibody-mediated rejection (AMR) and chronic graft dysfunction, likely due to a greater burden of preformed antibodies and memory T cells[36], underscoring the fundamental immunological differences between the two groups. The development of combined liver-ITx and refinement of immunosuppressive strategies have helped mitigate rejection risks in adults, a protective effect thought to arise from the liver’s intrinsic immunomodulatory capacity[37]. This protective effect is believed to play a role in attenuating intestinal graft rejection. Both adult and pediatric recipients are vulnerable to complications arising from prolonged immunosuppressive therapy, although their risk profiles vary significantly. Adults are more likely to develop metabolic and cardiovascular side effects-such as post-transplant diabetes mellitus, hypertension, and osteoporosis-often worsened by preexisting comorbidities and age-related organ decline[38]. In contrast, pediatric patients, owing to their longer life expectancy and continued growth, face cumulative toxicities, including growth retardation, nephrotoxicity, neurocognitive effects, and increased infection risk[39]. Therefore, the severity and manifestation of immunosuppression-related complications are not necessarily greater in one population, but rather reflect distinct physiological and temporal vulnerabilities, necessitating tailored long-term management strategies[40].
Currently, immune induction regimens differ slightly between pediatric and adult ITx patients. Pediatric protocols typically include rATG, alemtuzumab (used increasingly in recent practice), basiliximab, and rituximab (used less frequently over time). The reported rates of no rejection, moderate rejection, and severe rejection in the perioperative period are approximately 65%, 22%, and 13%, respectively. In adults, commonly used induction agents include rATG (gradually tapered in clinical use), basiliximab (increasingly utilized), alemtuzumab (gradually reduced), and rituximab (used more frequently in recent protocols). The corresponding rates of no rejection, moderate rejection, and severe rejection in adults are 79%, 15%, and 6%, respectively. Maintenance immunosuppressive regimens are generally similar across both populations and include tacrolimus (91%), corticosteroids (55%), sirolimus (16%), mycophenolate mofetil (12%), azathioprine (6%), cyclosporine (3%), and other agents (4%).
Data from the IITR and recent clinical studies indicate that pediatric ITx recipients generally achieve higher 5-year survival and graft survival rates compared to adult recipients[41]. This favorable outcome is attributed to immunological adaptability, better regenerative capacity, and improved perioperative management in pediatric patients. Although children are more prone to early infectious complications, they exhibit higher rates of rejection reversal and demonstrate more durable long-term graft function[42].
In pediatric ITx recipients, the rank order of graft survival duration is as follows: Total abdominal organ cluster transplantation > combined liver-ITx > isolated small bowel transplantation > modified abdominal cluster transplanta
The incidence of long-term complications also varies between pediatric and adult populations. Post-transplant insulin resistance occurs in approximately 1.8% of pediatric patients compared to 7% in adults; hypertension in 9% of children vs 16% in adults; continuous renal replacement therapy is required in 3% of pediatric vs 6% of adult recipients; and the incidence of post-transplant lymphoproliferative disorder (PTLD) is 8% in children compared to 4% in adults. These findings highlight age-related disparities in complication profiles, likely reflecting differences in baseline comorbidities, immune status, and immunosuppressant pharmacokinetics.
In conclusion, the fundamental differences in ITx outcomes between pediatric and adult patients arise from a complex interplay of etiological, anatomical, immunological, and psychosocial factors. Future efforts should focus on optimizing donor-recipient matching in pediatric ITx, designing immunosuppressive regimens with reduced toxicity, and strengthening multidisciplinary post-transplant care to improve long-term prognosis.
ITx represents the most technically demanding procedure among abdominal organ transplants, requiring precise surgical decision-making to balance the extent of bowel resection during the initial operation. The goal is to avoid excessive resection that may precipitate intestinal failure, while also minimizing complications associated with retaining dysfunctional or marginal bowel segments[43]. In pediatric patients, assessment of residual bowel length must be made in relation to age-specific norms. Early stoma closure is often considered, and in some cases, this is complemented by proximal effluent refeeding to promote intestinal adaptation and enteral autonomy[44]. When significant bowel dilation is present, reconstructive techniques such as serial transverse enteroplasty (STEP) may be employed to improve functional outcomes. In contrast, adult patients often respond adequately to simpler bowel tapering procedures, which are effective in managing dilated segments and enhancing nutrient transit[31,45]. In refractory cases, comprehensive surgical and functional evaluation is essential to determine whether reconstructive approaches alone are sufficient or whether ITx is warranted.
Recent advances in multivisceral transplantation techniques and refined vascular anastomosis methods have led to notable improvements in 5-year survival rates among adult ITx recipients. However, critical challenges persist, including donor organ shortages, size mismatches, and the ongoing risk of chronic rejection (CR). Ultimately, successful ITx outcomes depend on individualized surgical planning that incorporates multiple patient-specific factors, including age, anatomical variability, underlying pathology, and residual intestinal function-fully aligning with the principles of precision medicine.
Vascular anastomosis forms the cornerstone of many modern surgical procedures and has undergone continuous refinement in the context of ITx. In early ITx animal experiments, even when vascular continuity was successfully re-established through precise vessel suturing, graft loss frequently occurred due to uncontrolled immune rejection. This highlighted that vascular anastomosis alone could not ensure graft survival, and only after the introduction of effective immunosuppressive agents did ITx gain momentum, enabling meaningful advances in vascular reconstruction techniques[7]. Traditional ITx involves direct anastomosis of the donor mesenteric artery to the recipient’s abdominal aorta and inferior vena cava. However, this approach is technically challenging due to the depth and short length of the native vessels, which increases the risk of thrombosis and prolongs operative time. To overcome these challenges, the "vascular bridge" technique was developed, allowing the use of donor iliac vessels or synthetic grafts to convert complex end-to-side anastomoses into more manageable end-to-end configurations, thereby reducing operative time and vascular complications[46].
In complex surgical scenarios-such as patients with traumatic abdominal wall defects, multiple cesarean deliveries, or extensive intestinal fistulas leading to loss of abdominal domain and severe scarring-reconstruction of the abdominal wall may require abdominal wall vascularized composite allotransplantation. This approach is often performed in conjunction with ITx or multivisceral transplantation, and in these cases, vascular anastomosis and revascularization are pivotal to procedural success[47]. Therefore, techniques for vascular anastomosis and revascularization in this combined transplan
In a related innovation, small bowel autotransplantation has been introduced as a potential option for the resection of tumors involving the mesenteric vasculature. This technique allows radical tumor removal while maintaining bowel viability and intestinal continuity[49]. Cipriani et al[50] proposed a microvascular end-to-end anastomosis technique between the donor and recipient infra-abdominal vessels, while preserving the donor’s iliofemoral vasculature. This strategy enhanced vascular control and reduced donor morbidity. Later, Giele et al[51] subsequently introduced a two-stage approach, initially anastomosing the graft’s infra-abdominal vessels to the recipient’s forearm vessels to allow early graft perfusion and stabilization. The graft was then transferred to the abdominal cavity after recipient stabilization. More recently, Erdmann et al[52] described a technique enabling simultaneous hemodialysis by constructing a vascular ring between the saphenous vein and the common femoral artery. However, this method is unsuitable in cases of prior saphenous vein injury or thrombosis.
Robotic-assisted surgery has demonstrated considerable advantages in urological operations, including partial and radical nephrectomy, due to its enhanced three-dimensional visualization, refined instrument articulation, and significant reduction in intraoperative blood loss[53]. Importantly, growing evidence supports robotic-assisted kidney transplan
In the domain of ITx, vascular anastomosis-especially involving the superior mesenteric artery (SMA)-presents substantial technical challenges. Adequate blood perfusion must be ensured while minimizing ischemia-induced graft injury[56]. Although conventional open surgery remains the clinical standard for SMA reconstruction, robotic-assisted platforms offer promising solutions through magnified visualization, tremor suppression, and high-precision instrument control. The integration of intraoperative fluorescence imaging, such as indocyanine green, enables real-time assessment of mesenteric perfusion, thereby reducing the likelihood of anastomotic failure[57]. Furthermore, robotic systems provide enhanced dexterity for complex vascular procedures-including end-to-end microanastomosis, venous patching, and customized bypass grafting-potentially reducing operative time and improving surgical outcomes in ITx recipients[58]. These capabilities may be particularly valuable in reoperations, pediatric cases, or recipients with prior abdominal interventions.
While clinical reports on robotic-assisted ITx remain limited, the theoretical and technological potential is substantial. Future directions in this field should prioritize: (1) Establishing standardized robotic-assisted protocols tailored to the anatomic and hemodynamic features of intestinal graft vasculature, such as mesenteric artery and vein variants; (2) Conducting multicenter prospective trials to evaluate long-term graft survival, immunologic outcomes, and complication rates in robotic-assisted ITx; (3) Fostering interdisciplinary surgical integration, uniting transplant surgeons, vascular specialists, and robotic experts to optimize perioperative workflows and minimize conversion rates; and (4) Developing next-generation materials and robotic-compatible vascular scaffolds, including anti-adhesive bioabsorbable stents and microvascular sealants specifically designed for intestinal and mesenteric applications. Collectively, these strategies hold promise to transform robotic-assisted ITx from an emerging concept into a reliable and innovative surgical paradigm, enabling safer, more precise, and personalized management of complex intestinal failure.
Allogeneic ITx remains the most widely used modality for treating isolated small bowel failure. For patients with preserved function of other digestive organs, isolated jejunal or ileal ITx is sufficient to restore intestinal continuity and absorption. However, in cases of multi-organ dysfunction-such as coexisting liver failure, pancreatic tumors, or complex hepatobiliary disease-isolated ITx often proves inadequate, prompting the clinical adoption of combined organ transplantation, including liver-small bowel or multivisceral (e.g., small intestine, pancreas, stomach) transplantation[59,60]. Among multivisceral transplant options, liver-small bowel transplantation is most commonly performed, with the liver playing a central immunologic and metabolic role. However, organ selection must be individualized based on the patient's pathology. For example, patients with mild hepatic dysfunction (e.g., early-stage cholestasis) without significant cirrhosis or portal hypertension may be better served by isolated ITx, preserving native liver function and its immuno
In complex digestive reconstructions, extended graft combinations-encompassing stomach, duodenum, pancreas, bile duct, colon, and spleen-are utilized by select transplant centers. These components can be harvested en bloc or as modular grafts depending on donor anatomy, allowing preservation of vascular and neural continuity[64-66]. This method is often referred to as abdominal organ cluster transplantation or modified cluster transplantation, and organ selection should be tailored to the patient's anatomical limitations and risk profile. Despite growing use, regional and institutional variability persists, and further empirical studies are needed to evaluate the survival benefit of various combinations.
A key area of innovation lies in colon inclusion within the ITx graft. Historically, concerns about increased infection risk and graft failure led to reluctance in including the colon. However, recent data demonstrate that colon co-transplantation does not increase infection or mortality rates, and may confer significant advantages. Specifically, colon recipients show improved fluid retention, less dehydration, and better renal outcomes due to increased fecal water reabsorption. Currently, colon inclusion is reported in nearly 20% of ITx cases[67,68]. The inclusion of the spleen remains controversial. On one hand, preserving the recipient spleen may reduce the risk of PTLD[69]. On the other hand, co-transplantation of the donor spleen in multivisceral ITx has been shown to promote immune tolerance and reduce AR, without increasing the incidence of graft-vs-host disease (GVHD)[65,70]. Strategies for gastric graft inclusion also vary widely across transplant centers. Some incorporate the stomach to preserve vagal nerve integrity and enhance neuromodulatory function of the graft, while others avoid gastric inclusion due to uncertain benefits and potential risks. Current evidence is insufficient to establish standardized recommendations, and future research should focus on evidence-based risk-benefit assessment of gastric co-transplantation[66].
Ischemia-reperfusion injury (IRI) remains a major limiting factor in ITx, not only contributing directly to graft dys
Recent developments in machine perfusion have shown that NMP significantly outperforms hypothermic machine perfusion in preserving intestinal grafts. NMP maintains physiological conditions, preserves mucosal architecture and microcirculatory integrity, and reduces epithelial disruption, thereby prolonging viable preservation times[12]. Experimental porcine models of EVNP have been successfully established, allowing for real-time evaluation of intestinal viability and perfusion parameters, bridging the gap between bench and bedside[75,76]. Concurrently, the emergence of 3D-bioprinting technologies has enabled the creation of bioengineered intestinal scaffolds using light-polymerized hydrogels that mimic villus-crypt architecture and incorporate prevascularized networks. These constructs show promise in addressing post-transplant no-reflow phenomena by facilitating rapid graft revascularization[14,15].
On the pharmacological front, several novel interventions are being integrated into EVNP protocols to enhance graft resilience. Tranilast, for instance, upregulates heme oxygenase-1 (HO-1), whose metabolic product-carbon monoxide-inhibits mitochondrial permeability transition pore opening, thereby reducing mucosal apoptosis and increasing ischemia tolerance in preclinical settings[72,77]. Combinatorial approaches are also gaining traction. For example, mitochondrial-targeted antioxidants such as MitoQ scavenge ROS to preserve ATP synthesis and mitochondrial membrane potential[78], while probiotic strains like Clostridium butyricum help maintain microbial homeostasis through competitive exclusion of pathogens and secretion of short-chain fatty acids (SCFAs), collectively reducing IRI severity and extending ex vivo preservation limits[79].
The convergence of machine perfusion, tissue engineering, and molecular pharmacology marks a transformative phase for ITx. Future research should focus on refining EVNP protocols compatible with multi-organ preservation platforms to extend graft viability beyond 24 hours, validating 3D-bioprinted vascularized scaffolds in large-animal transplantation models, and designing synergistic therapeutic regimens that combine HO-1 inducers with microbiota-targeting strategies to promote immune quiescence and graft adaptability. These integrative approaches are expected to expand the donor organ pool, alleviate post-transplant immunological stress, and accelerate the development of next-generation ITx grafts characterized by enhanced immunotolerance and functional resilience.
ITx remains a vital intervention for irreversible intestinal failure; however, its distinct immunological complexity renders it the most rejection-prone abdominal organ transplant. This high immunogenicity stems from the small intestine’s unique structural and immune properties, including its vast mucosal surface, rich lymphoid architecture, and dense commensal microbiota-factors that collectively heighten the risks of graft rejection and infectious complications[80].
The immune microenvironment of the small intestine comprises specialized immune-active compartments such as lamina propria lymphocytes, Peyer’s patches, and mesenteric lymph nodes[81]. Following ITx, the recipient’s immune system detects donor-derived alloantigens-primarily mismatched HLA molecules-activating both adaptive and innate immune pathways. This immunological cascade culminates in graft-directed immune injury, presenting clinically as AR or CR[82]. HVGR is driven by the activation of recipient T and B lymphocytes upon recognition of foreign HLA, initiating cytotoxic and antibody-mediated mechanisms of graft injury. The incidence of AR in ITx is reported at 30%-50%, which is significantly higher than that observed in other solid organ transplants such as the liver or kidney, and untreated CR often leads to progressive fibrosis and irreversible graft dysfunction[83,84].
In contrast to other abdominal organ transplants, ITx is uniquely susceptible to GVHD, in which immunocompetent donor-derived T cells recognize host tissues as foreign and mount a systemic immune attack. GVHD in ITx primarily targets recipient skin, liver, and gastrointestinal tract, often manifesting as severe inflammatory injury[85]. Although its pathogenesis remains incompletely understood, recent evidence implicates the activation of innate immunity via patho
Following ITx, the transplanted intestine serves both as a frontline defense against external antigens and a persistent source of donor-derived alloantigens, rendering it highly immunogenic and especially vulnerable to rejection compared to other solid organ transplants[90]. Immune cells play a pivotal role in this delicate immune balance-orchestrating both AR and CR processes, while also mediating immune tolerance through regulatory pathways. Table 1 outlines the primary immunological cell types involved in ITx and their associated mechanisms. A comprehensive understanding of the dual roles of immune cells-in mediating rejection and promoting tolerance-is essential for optimizing immunosuppressive regimens and developing next-generation immune-modulating therapies.
| Immune cell type | Infiltration characteristics and functions | Related mechanisms/molecular markers | Clinical significance | Ref. |
| T cell (CD8+) | Key effector cells in early AR; may develop exhausted-like phenotype under persistent antigen stimulation | PD-1, TIM-3, IFN-γ | AR biomarker; PD-1 inhibition must consider graft-vs-host disease risk | [209-211] |
| T cell (CD4+) | Th1/Th17 subsets drive rejection; Treg maintains mucosal homeostasis by suppressing inflammation | Th17: IL-17; Treg: FoxP3, IL-10, TGF-β | Th17/Treg ratio reflects rejection risk; modulating this axis improves outcomes | [91,92,212] |
| Regulatory T cells (Tregs) | Inhibit effector T cell activation; key regulatory cells promoting transplant tolerance | FoxP3, CTLA-4, IL-10, TGF-β | Treg expansion strategies may enhance tolerance and reduce rejection | [104,105,213] |
| B cell | Mediate chronic rejection and vascular injury via DSA production | IgG/IgM, complement activation, intimal hyperplasia | DSA levels correlate with chronic rejection; rituximab as therapeutic option | [164,214,215] |
| Natural Killer Cell (NK) | Contribute to rejection via ADCC; low-activity NK may support tolerance | NKG2D, perforin/granzyme, IL-10 inhibition | Regulating NK may reduce antibody-mediated rejection and mucosal injury | [101,216,217] |
| DC | Activate T cells in lymph nodes; tolerogenic DCs induce Treg differentiation | cDC1/cDC2 antigen presentation; pDC: IFN-α; tolerogenic DC: PD-L1 | DC subpopulation imbalance promotes Th1 polarization; targeting PD-L1 can enhance transplant tolerance | [218-220] |
| Macrophage | M1 aggravates ischemia reperfusion injury; M2 promotes tissue repair and immune suppression | M1: TNF-α, IL-6; M2: ARG1 | M1/M2 balance affects prognosis and barrier preservation | [221-224] |
| Mast cell | Disrupt mucosal barrier; associated with anastomotic leakage and infection | Histamine, IL-4, IL-13, eosinophil recruitment | Antihistamines or stabilizers may prevent complications | [225,226] |
| Myeloid inhibitory cells (MDSC) | Suppress T cell responses; excessive accumulation may lead to fibrosis | ARG1, ROS, M-MDSC | Targeted MDSC modulation needed to avoid immune imbalance | [227-229] |
| γδT cell | Regulate epithelial repair and microbial homeostasis; produce IL-17 and IL-22 | γδT17 subset, IL-17, IL-22, villous regeneration | Support mucosal regeneration; crucial in post-ITx repair | [230,231] |
| ILC | ILC3 preserve epithelial integrity via IL-22; ILC2 mediate type 2 responses and may influence fibrosis | ILC3: RORγt, IL-22; ILC2: IL-5, IL-13 | Dysfunction linked to chronic inflammation; IL-22 as repair target | [232,233] |
T cells are central mediators of adaptive immunity and contribute significantly to graft rejection and tolerance following ITx. CD4+ Th1 cells promote the activation of M1 macrophages via IFN-γ and upregulate iNOS, resulting in apoptosis of intestinal epithelial cells[91,92]. Th17 cells impair mucosal barrier integrity by secreting IL-17A, which downregulates tight junction proteins such as occludin, leading to cryptitis and villous atrophy[93]. Kroemer et al[94] identified elevated levels of TNF-α and IL-17 in CD4+ T cells within rejecting grafts unresponsive to ATG, and demonstrated that anti-TNF-α agents (e.g., infliximab) could reverse these rejections, highlighting the Th17/TNF-α axis as a potential therapeutic target.
CD8+ cytotoxic T cells initiate direct killing of donor epithelial cells via the perforin-granzyme pathway, manifesting histologically as crypt cell apoptosis and epithelial desquamation[95]. Tissue-resident memory T cells (Trm) constitute a long-lived subset residing in the intestinal mucosa and can persist in the graft microenvironment for years. Donor-derived Trm have been shown to survive in recipient intestines for up to 5 years and exhibit dual immunologic functions. While they may participate in early rejection episodes, their long-term persistence also supports the induction of graft tolerance. Bartolomé-Casado et al[96] reported high β2-integrin expression in donor Trm by single-cell RNA sequencing, a potential marker for predicting rejection. Fitzpatrick et al[97] further demonstrated that β2-integrin+ CD8+ Trm cells were enriched in the rejection microenvironment and associated with immune cell infiltration and proinflammatory signaling. Weiner et al[98] revealed that Trm cells harboring host-reactive T cell clones could exacerbate GVHD-like responses, particularly when enriched in the graft. As such, therapeutic strategies aimed at modulating Trm may represent a novel frontier in ITx immunotherapy.
In addition to adaptive immune responses, innate immune cells also play a crucial role in graft injury during AR. Neutrophils exacerbate IRI via the release of NETs, which activate plasmacytoid dendritic cells through TLR9 signaling, promoting activation of the IL-23/IL-17 axis and amplifying early inflammatory responses[99,100]. Additionally, donor-derived NK cells may contribute to rejection by targeting graft endothelial cells through MHC-I mismatch recognition via the "missing self" mechanism[101]. Together, these innate and adaptive immune cells form an integrated effector network that drives AR in ITx.
Conversely, regulatory T cells (Tregs) are instrumental in promoting immune tolerance. Tregs (CD45+/CD25+/FOXP3+) suppress effector T cell activation through anti-inflammatory cytokines such as IL-10 and TGF-β, thereby maintaining mucosal homeostasis and reducing rejection risk[102]. In particular, donor-specific Tregs play a critical role in facilitating long-term tolerance. The Leuven protocol, for instance, has demonstrated enhanced graft survival by inducing Treg expansion under reduced immunosuppressive conditions[103]. Elevated circulating Treg levels correlate with FoxP3 expression and suppression of DC co-stimulation via CTLA-4-mediated downregulation of CD80/CD86, thereby attenuating effector T cell proliferation[104,105]. The role of FOXP3 is further supported by IPEX syndrome, in which its mutation results in profound autoimmune enteropathy, highlighting its essential role in intestinal immune regulation[106]. Tregs also exert suppressive effects through the enzymatic conversion of extracellular ATP to adenosine via CD39 and CD73, with elevated adenosine levels positively associated with prolonged graft survival[107]. Tregs also exert suppressive effects through the enzymatic conversion of extracellular ATP to adenosine via CD39 and CD73, with adenosine levels positively associated with graft survival[108,109]. M2 macrophages, in turn, promote Treg expansion via TGF-β and reinforce immunosuppressive feedback by inducing IL-10 secretion, establishing a self-amplifying tolerance loop[110].
Post-transplant, the interplay between donor and recipient T cells creates a dynamic chimeric microenvironment, and the stability of this chimerism profoundly influences rejection risk. Notably, T cell chimerism exceeding 1% is associated with reduced rejection rates, particularly in multi-organ transplants such as liver-ITx[111]. Early rejection is typically characterized by clonal expansion of recipient-derived T cells, whereas stable graft acceptance correlates with donor T cell predominance. Furthermore, recipients with low DSA titers exhibit higher likelihood of tolerance induction, potentially due to the regulatory effects mediated by Tregs[111].
Understanding the dual roles of immune cells in both immunogenicity and tolerance is crucial for developing precise, personalized immunomodulatory strategies. Future research should leverage single-cell multi-omics technologies to elucidate the complex cellular networks the ITx microenvironment and guide the design of personalized immune the
The gut microbiota plays a dynamic, bidirectional role in post-ITx immune homeostasis, making it a critical determinant of transplantation outcomes. On one hand, microbial translocation can trigger systemic inflammation and exacerbate graft rejection. Following ITx, the intestinal barrier is compromised by IRI, surgical disruption of epithelial integrity, and impaired epithelial renewal resulting from immunosuppressive therapy. These factors facilitate bacterial and metabolite translocation-such as lipopolysaccharide (LPS), peptidoglycan, and lipoteichoic acid-into mesenteric lymph nodes and systemic circulation[36,112-114]. These PAMPs are recognized by pattern recognition receptors (PRRs) on intestinal epithelial and innate immune cells, including TLRs and NOD-like receptors (NLRs)[115-117]. For instance, TLR1, TLR2, TLR4, TLR6, and TLR10 specifically detect microbial ligands such as LPS and lipoproteins. These receptors subsequently activate canonical proinflammatory signaling cascades-most notably the TLR4/NF-κB pathway-which culminate in the release of tumor necrosis TNF-α, IL-6, and IL-1β, thereby amplifying both mucosal and systemic inflammation[118-120].
Additionally, specific bacterial taxa-including segmented filamentous bacteria, Salmonella, and Proteus mirabilis-can drive Th1/Th17-polarized adaptive responses via TLR engagement and antigen presentation pathways, amplifying effector T cell-mediated rejection[121,122]. Post-transplant dysbiosis, particularly the overgrowth of Enterobacteriaceae, has been significantly correlated with a high incidence of acute cellular rejection[123]. Moreover, the translocation of opportunistic pathogens such as Enterococcus faecalis can aggravate graft injury and increase the risk of sepsis[124].
On the other hand, accumulating evidence indicates that certain commensal bacteria may promote graft tolerance by modulating host immunity[125]. For example, Clostridium spp. ferment dietary fiber into SCFAs, such as butyrate and propionate, which activate G protein-coupled receptors (e.g., GPR43) and inhibit histone deacetylases, ultimately en
| Bacterial genus | Specific species | Immune mechanism | Receptor/pathway | Association with intestinal transplantation | Potential applications/risks | Ref. |
| Bifidobacterium | B. longum | Promotes mucosal immune maturation, enhances epithelial integrity | TLR2/4 | Loss post-transplant linked to GVHD predisposition | Probiotic supplementation may mitigate GVHD risk | [234,235] |
| B. infantis | Balances Th1/Th2 response, modulates cytokine milieu | GPR43 (SCFA-mediated) | Suppresses systemic inflammation, fosters tolerance | May enhance immunosuppressive efficacy | [236] | |
| Lactobacillus | L. plantarum | Downregulates pro-inflammatory cytokines (IL-6, IL-8) | TLR2 | Limits endotoxemia and systemic inflammation | Helps prevent post-transplant sepsis | [237] |
| L. reuteri | Promotes IL-10 production and Treg induction | AhR | Enhances barrier repair, reduces mucosal inflammation | Therapeutic for immune enteropathies | [238] | |
| Bacteroides | B. fragilis | Induces Treg via PSA antigen | TLR2 | Shown to promote tolerance in transplant models | Potential microbial immunotherapy target | [239] |
| B. thetaiotaomicron | Promotes IgA secretion, modulates dendritic cell response | TLR4 | Supports mucosal immunity, limits pathogen overgrowth | May prevent Enterobacteriaceae dominance | [240,241] | |
| Escherichia coli | Commensal E. coli | Activates basal innate immunity | TLR5/MyD88 | Maintains immune tone, but overgrowth risks rejection | Surveillance for strain virulence needed | [242] |
| Pathogenic E. coli (e.g., EHEC) | Secretes shiga-like toxins, induces strong cytokine storm | GB3 receptor | Triggers mucosal necrosis, worsens ischemia-reperfusion injury | Requires antibiotic prophylaxis | [243] | |
| Clostridium | C. perfringens | Produces α-toxin, damages tight junctions | - | Can cause graft necrosis, sepsis | Early diagnosis critical | [244] |
| C. difficile | Induces pseudomembranous colitis via toxin A/B | Common post-transplant pathogen | Fecal microbiota transplantation emerging as a salvage therapy | [245] | ||
| Fusobacterium | F. nucleatum | Activates NF-κB, enhances IL-6/IL-8 release | TLR2/4 | Linked to chronic inflammation and neoplasia | Considered a pro-inflammatory marker | [246,247] |
| Enterococcus | E. faecalis | Inhibits inflammasome, alters antimicrobial peptide balance | Contributes to barrier disruption and bacteremia | Targeted decolonization recommended | [248] | |
| Prevotella | P. copri | Modulates Th17 response | Implicated in dysregulated immunity post- intestinal transplantation | Microbiome-guided regulation needed | [249] | |
| Akkermansia | A. muciniphila | Enhances mucus production, improves tight junctions | GPR43 | Shown to improve gut permeability and glucose homeostasis | Probiotic candidate under investigation | [250] |
| Roseburia | Roseburia spp. | Butyrate production, anti-inflammatory effect | FFAR3 (GPR41) | Maintains Treg differentiation and epithelial repair | Supports mucosal tolerance post-transplant | [251] |
| Sutterella | Sutterella spp. | Alters IgA response and epithelial interaction | Poorly understood but potentially modulates rejection | Requires further research | [252] |
Broad-spectrum antibiotics such as vancomycin and meropenem, though routinely used to prevent post-ITx infection, profoundly disrupt gut microbial composition and may impair immune homeostasis[132]. Retrospective studies have demonstrated that long-term use of broad-spectrum antibiotics reduces microbial diversity-particularly decreasing Clostridium spp. - and correlates with increased AR incidence[133,134]. Therefore, preoperative microbiome profiling to guide narrow-spectrum antibiotic selection, along with postoperative probiotic supplementation, may help restore microbial balance and improve graft outcomes.
In summary, the bidirectional regulation of immune responses by the intestinal microbiota-via both proinflammatory and tolerogenic pathways-suggests that clinical management of ITx should strike a balance between infection control and microbial homeostasis. A future-oriented therapeutic paradigm could integrate microbiome sequencing, tailored antibiotic regimens, and targeted microbial therapies, ultimately shifting from immunosuppression alone to precision “immune-microbiota co-regulation”.
The immunologic complexity of ITx stems from the small intestine’s unique anatomical structure and highly immunoactive environment. As the largest lymphoid organ in the body, the small intestine harbors a dense network of gut-associated lymphoid tissue and resident immune cells, contributing to a heightened risk of rejection compared to other solid organs[135]. This unique immune milieu endows ITx with three hallmark immunological features: (1) Bidirectional immune reactivity, characterized by both HVGR and GVHD. A high proportion of naïve donor T cells within passenger lymphocyte populations serves as the principal effector population for GVHD, particularly in multivisceral transplants[136]; (2) Microbiota-immune interactions, whereby disruption of intestinal flora post-transplant can influence immune activation. For example, the expansion of Enterococcus is closely associated with increased rejection risk and can exacerbate mucosal damage through innate immune signaling pathways, as previously described[137]; and (3) Microbiota-immune interactions, whereby disruption of intestinal flora post-transplant can influence immune activation. For example, the expansion of Enterococcus is closely associated with increased rejection risk and can exacerbate mucosal damage through innate immune signaling pathways, as previously described[138].
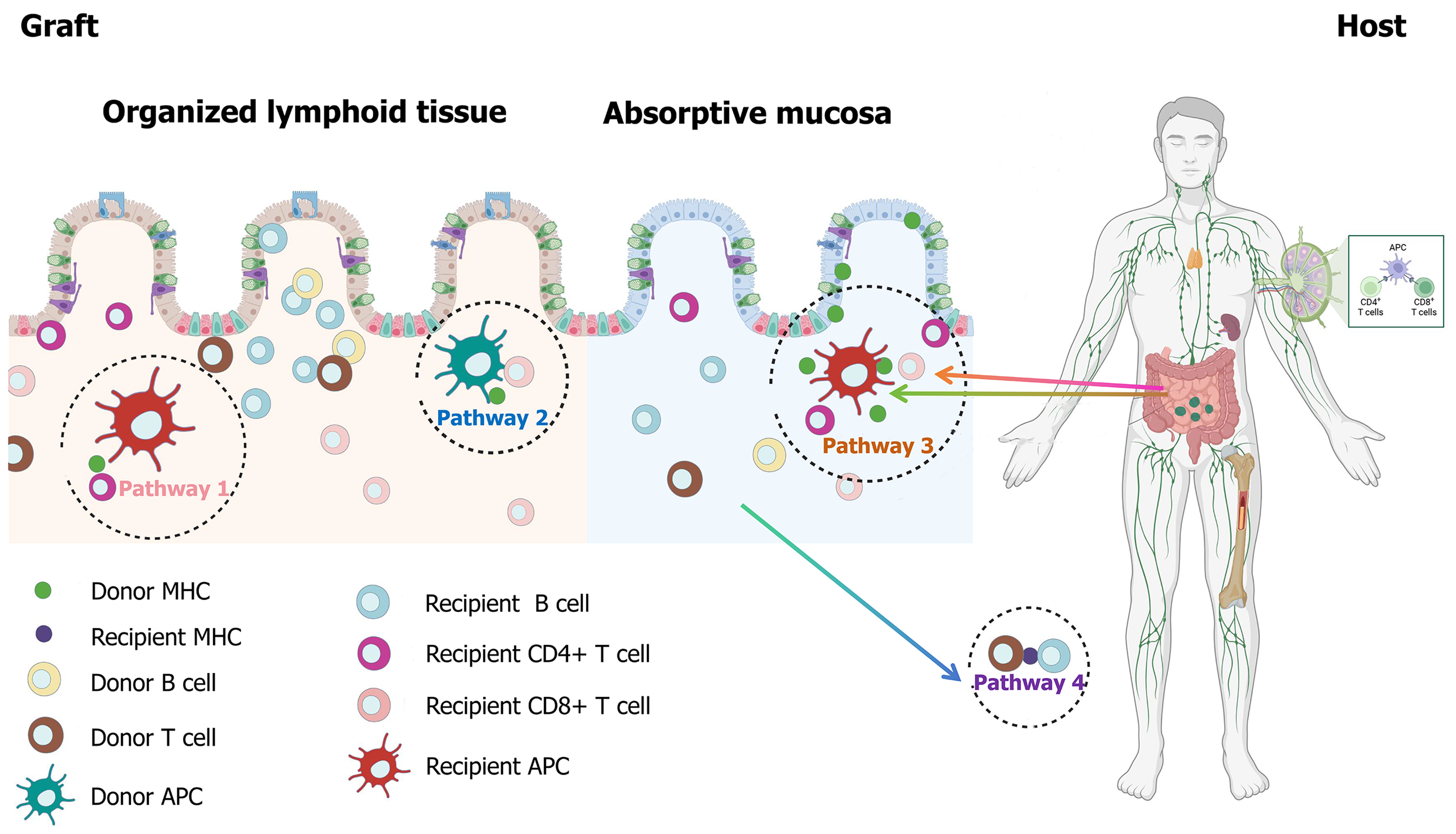
Immunological mechanisms of AR in ITx: AR after ITx is driven by a convergence of multiple allorecognition pathways, forming an intricate and partially redundant immunological network (Figure 2). The direct pathway dominates the early phase of AR, where donor antigen-presenting cells (APCs) directly activate recipient CD8+ and CD4+ T cells via donor MHC molecules. This leads to the generation of cytotoxic T lymphocytes (CTLs) and the release of pro-inflammatory cytokines, exacerbating epithelial injury and crypt apoptosis[138,139]. In parallel, the indirect pathway, where recipient APCs process and present donor antigens, promotes CD4+ T cell activation and facilitates the development of DSAs via B cell differentiation-central to CR and AMR[140]. The semi-direct pathway is mediated by donor-derived vesicles or apoptotic fragments containing intact MHC-peptide complexes, which are transferred to recipient APCs. This dual-mode recognition simultaneously activates T cells and promotes humoral sensitization[141-143]. A fourth route, the inverted direct pathway, involves donor CD4+ T cells directly stimulating recipient B cells, accelerating the formation of DSAs and potentially contributing to early-stage rejection not effectively controlled by T cell-targeted immunosuppression[144]. DSAs are identified in approximately 30% of ITx rejection episodes and mediate injury through complement-dependent cytotoxicity (CDC) and ADCC[74]. In CDC, DSAs initiate the classical complement cascade via C1q binding, forming membrane attack complexes that disrupt graft endothelial integrity. In ADCC, DSAs engage Fcγ receptors on NK cells or macrophages, triggering targeted destruction of the graft epithelium[145,146]. Importantly, preformed high-titer DSAs are predictive of AR within the first year post-transplant[147], and their persistent presence can provoke thrombotic microangiopathy through sustained endothelial activation and microvascular injury[148]. Collectively, these findings support the model where the direct pathway primarily drives early AR, while the indirect and semi-direct pathways underpin chronic and late-stage immune responses[149]. Nevertheless, the overlap and synergy among these pathways challenge the efficacy of conventional immunosuppressive regimens.
In addition to recognition pathways, the spatiotemporal orchestration of effector immune cells critically dictates rejection outcomes. CD4+ and CD8+ T cells are the dominant cellular mediators in AR, with CD4+ T cells differentiating into Th1 and Th17 phenotypes. Th1 cells secrete IFN-γ and TNF-α to activate macrophages and amplify CTL responses, while Th17 cells drive neutrophil recruitment and local tissue inflammation through IL-17A and IL-21[150-153]. An increased frequency of Th17 cells within the lamina propria correlates with histopathologic severity in AR, suggesting a pathogenic role for the IL-23/IL-17 axis in mucosal injury[154].
Concurrently, innate immune responses substantially contribute to the onset of AR. After transplantation, the exposure of intestinal tissue to microbial products rapidly activates PRRs such as TLRs and NLRs on innate immune cells[155]. Variants in the TLR4 gene have been linked to altered susceptibility to rejection, likely due to dysregulated responses to LPS[156]. In clinical observations, a high density of MPO+ neutrophils within graft biopsies is significantly associated with severe AR episodes and poor outcomes[157].
Together, these findings underscore the multifactorial nature of immune rejection in ITx, highlighting both adaptive and innate contributors, as well as complex crosstalk between cellular and humoral effectors. Future strategies must shift toward precision immunotherapy, integrating real-time immune monitoring and pathway-specific interventions to achieve durable graft survival.
Immunological mechanisms of CR in ITx: CR following ITx is driven by a multifaceted immunological cascade, characterized by persistent low-grade inflammation and progressive tissue remodeling. At the cellular level, both CD4+ and CD8+ T lymphocytes recognize donor MHC antigens via direct presentation or donor peptide-MHC complexes processed through the indirect pathway by recipient APCs[158]. The resulting T cell activation leads to elevated secretion of proinflammatory cytokines such as IFN-γ and IL-17, establishing a Th1/Th17-skewed microenvironment that promotes endothelial apoptosis and crypt epithelial injury[159,160]. Effector memory T cells, notable for their long-term persistence post-transplant and reduced susceptibility to immunosuppressive agents, may underlie the insidious progression and treatment resistance observed in CR[161].
On the humoral front, DSAs bind to endothelial antigens via their Fab regions, initiating complement activation (evidenced by C4d deposition) and Fcγ receptor-mediated immune responses through their Fc domains[162,163]. Emerging evidence suggests that the continuous presence of DSAs may stem from long-lived plasma cells that evade immunosuppression, resulting in ongoing B-cell-mediated injury and immune amplification[164].
Innate immunity plays an increasingly recognized and synergistic role in CR. Macrophage polarization exhibits stage-dependent functional divergence: In early rejection, IFN-γ-driven M1 macrophages secrete high levels of TNF-α and IL-6, fueling local inflammation; whereas in late-stage rejection, M2 macrophages promote fibrogenesis and tissue remodeling via secretion of TGF-β[165,166]. NK cells contribute to vascular injury through ADCC, while neutrophils promote micro
The pathological hallmark of CR is chronic inflammation-induced fibrosis, orchestrated by a dense cytokine signaling network. TGF-β activates myofibroblasts and promotes their transdifferentiation via the canonical Smad3 pathway, while platelet-derived growth factor enhances their proliferation through the PI3K/Akt signaling axis[167,168]. In advanced stages, Th2-biased immunity becomes dominant. IL-13, via STAT6 signaling, upregulates fibrogenic gene expression (e.g., COL1A1, TIMP1), exacerbating matrix accumulation and structural distortion of the graft[169,170]. Notably, IL-13 knockout significantly attenuates fibrosis in animal models, supporting its pathogenic role[171]. A key pathological amplifier in CR is vascular injury-induced ischemia. Histopathological studies have shown that chronically rejected intestinal grafts exhibit typical intimal hyperplasia and arterial luminal narrowing resembling atherosclerosis[172-174]. The consequent hypoperfusion activates hypoxia-inducible factor-1α, promoting pro-fibrotic signaling and forming a self-perpetuating cycle of ischemia and fibrosis[175].
Importantly, immunosuppressive escape presents a major clinical barrier in CR. The lymphoid-rich architecture of the small intestine permits persistent donor leukocyte chimerism, predisposing to GVHD and sustained alloimmune activation[176]. Concurrently, microbial dysbiosis-especially loss of commensals and overgrowth of opportunistic pathogens-can activate TLR/NLR pathways and disrupt immune homeostasis, undermining the efficacy of immunosuppression[177]. Beyond immunological mechanisms, several non-immunologic factors also contribute to CR. IRI, a key upstream insult, triggers DAMPs such as high-mobility group box protein 1 (HMGB1), which activate NF-κB via TLR4 and RAGE, promoting cytokine storm and chronic inflammation[178,179]. Surgical factors, including lymphatic disruption and autonomic denervation, may also alter mucosal immune dynamics, aggravating chronic allograft dysfunction[180].
In summary, CR in ITx arises from a complex interplay network of adaptive and innate immune interactions, stromal-vascular remodeling, and extrinsic surgical insults. This underscores the urgency to develop multi-targeted interventions that transcend traditional immunosuppression, incorporating anti-fibrotic, anti-hypoxia, and microbiome-based stra
To mitigate immune rejection after ITx, current clinical strategies adopt a phase-based immunosuppressive approach encompassing induction and maintenance therapy, tailored to the recipient’s immune risk profile. For induction therapy, commonly used agents include ATG, basiliximab (anti-IL-2R), and alemtuzumab (anti-CD52). Among these, ATG remains the mainstay, particularly in high-risk recipients due to its potent T-cell depletion capacity[181]. Basiliximab, with a more favorable safety profile and reduced infection risk, is often preferred in non-sensitized patients and allows for corticosteroid minimization[103,182]. Alemtuzumab may offer protection against GVHD, but its association with PTLD and infections restricts its broader application[183,184].
The cornerstone of maintenance therapy continues to be tacrolimus, often in combination with corticosteroids. Mycophenolate mofetil is frequently incorporated to reduce calcineurin inhibitor-induced nephrotoxicity[185]. Sirolimus, via mTOR inhibition, has shown potential in late-phase immunosuppression and may provide additional protection against CR and GVHD[186]. In cases of AR, high-dose corticosteroids remain first-line; for steroid-refractory rejection, ATG or targeted biologics such as vedolizumab (anti-α4β7 integrin) and infliximab (anti-TNF-α) are employed. Notably, infliximab has demonstrated efficacy in suppressing Th17-mediated inflammation, offering a viable option for steroid-resistant AR[94].
Nevertheless, immunosuppressive toxicity presents a major clinical challenge. Long-term tacrolimus use results in renal impairment in up to 50% of recipients, typically reflected by a > 30% decline in glomerular filtration rate[187]. Treatment resistance is frequently driven by long-lived TEM and plasma cells evading immunosuppression, perpetuating DSA production and AMR[188]. Genetic polymorphisms in CYP3A5 contribute to substantial variability in tacrolimus pharmacokinetics, complicating drug level optimization[189]. Furthermore, persistent activation of TGF-β/Smad signaling promotes irreversible fibrotic remodeling, underlining the elevated risk of late graft loss[190]. A persistent clinical dilemma remains: Intensifying immunosuppression curbs rejection but increases viral reactivation (e.g., cytomegalovirus, Epstein-Barr virus), while de-escalation may trigger recurrent rejection episodes, posing a therapeutic paradox for clinicians[191].
Recent advances in targeted immunotherapies offer new hope. In B-cell-directed strategies, rituximab (anti-CD20) reduces DSA levels and improves graft survival[192]. Bortezomib, a proteasome inhibitor targeting plasma cells, has demonstrated efficacy in AMR, though it carries the caveat of heightened infection risk, necessitating cautious use in the ITx setting[193,194]. JAK inhibitors such as ruxolitinib have shown benefit in steroid-refractory GVHD by dampening JAK-STAT-mediated proinflammatory cytokine signaling[16,17]. For modulation of innate immunity, TAK-242-a TLR4 inhibitor-has been shown to ameliorate IRI by blocking HMGB1-TLR4 signaling[195]. Targeting macrophage polarization is another promising avenue: Colony-stimulating factor-1 receptor inhibitors selectively deplete profibrotic M2 macro
Microbiome-based interventions are rapidly gaining traction. FMT has been shown to restore microbial diversity, elevate IL-25 Levels, and promote mucosal repair, offering intestinal protection[18]. Specific probiotic strains can augment Tregs via SCFA production, reducing AR incidence[198]. Moreover, FMT and probiotics have demonstrated therapeutic potential in GVHD, expanding their role beyond infection prevention.
In humoral rejection, the complement inhibitor eculizumab targets C5 convertase, effectively reducing C4d deposition in graft endothelium, representing a novel therapy for AMR[199]. Concurrently, advances in tissue engineering provide innovative approaches for CR. Decellularized intestinal scaffolds, preserving native extracellular matrix and vascular architecture, when seeded with mesenchymal stem cells, promote mucosal regeneration and attenuate fibrosis in preclinical models[200,201].
Emerging diagnostics enhance early rejection surveillance. Exosomal miR-155-5p outperforms traditional histology in detecting AR and holds promise as a noninvasive biomarker[202]. Luminex-based multiplex assays enable precise DSA quantification, with studies linking mean fluorescence intensity > 10000 to a fourfold increase in CR risk[203]. Artificial intelligence is reshaping transplant monitoring-deep learning models integrating histological and transcriptomic data have achieved 92% predictive accuracy for rejection events, offering robust decision-making support[204].
Future directions will likely emphasize multi-target synergistic immunoregulation. Experimental evidence suggests that donor-derived hematopoietic chimerism combined with targeted cellular therapy enables successful weaning of immunosuppression without rejection, representing a path toward “immune independence”[205,206]. A combinatorial strategy involving JAK inhibitors, anti-fibrotic agents, and microbiome modulation may allow synchronized regulation of adaptive immunity, fibrosis, and the gut-immune axis. Additionally, gene-editing technologies such as CRISPR-Cas9 to delete MHC class I genes in donor organs have prolonged graft survival in models, indicating transformative potential[207]. Finally, neuroimmune modulation is an emerging frontier. A clinical trial investigating vagus nerve stimulation aims to suppress proinflammatory cytokines via α7 nicotinic acetylcholine receptor activation, potentially unlocking a novel anti-rejection paradigm[208]. Collectively, these advances signify a paradigm shift toward precision immunotherapy and immune tolerance in ITx.
ITx has evolved into a critical therapeutic strategy for patients with irreversible intestinal failure, especially those unresponsive to PN or facing life-threatening complications. Over the past decades, significant progress has been made in surgical techniques, immunosuppressive strategies, and perioperative management. Advances such as optimized vascular anastomosis, multi-organ transplantation approaches, and NMP have improved short-term outcomes and extended graft viability.
However, ITx continues to face unique and formidable challenges. The small intestine’s exceptional immunogenicity, complex mucosal immune environment, and dense microbial population create a distinctive setting for both HVGR and GVHR. Moreover, chronic immune rejection, the toxicity of long-term immunosuppressive therapy, and persistent postoperative complications remain major barriers to long-term graft survival and patient quality of life.
To overcome these obstacles, future research must embrace a multidisciplinary and translational approach. Key priorities include the development of precision immunosuppressive regimens tailored to individual immune risk, deeper understanding of host-microbiota-immune interactions, and the clinical translation of novel technologies such as gene editing, 3D-bioprinted grafts, immune monitoring using machine learning, and microbiome-targeted therapies. Further
In conclusion, the trajectory of ITx is shifting from a last-resort intervention toward a personalized, technology-driven therapy. Through collaborative innovation across surgery, immunology, microbiology, and bioengineering, ITx is ex
The authors gratefully acknowledge the contributions of the General Surgery Department and all clinicians and resear
| 1. | Wang RF, Fagelman EJ, Smith NK, Sakai T. Abdominal Organ Transplantation: Noteworthy Literature in 2020. Semin Cardiothorac Vasc Anesth. 2021;25:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Gondolesi GE, Nikoupour H, Matsumoto CS. Intestinal Transplantation in the Developing World. Gastroenterol Clin North Am. 2024;53:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2276] [Article Influence: 189.7] [Reference Citation Analysis (5)] |
| 4. | Assadiasl S, Nicknam MH. Intestinal transplantation: Significance of immune responses. Arab J Gastroenterol. 2024;25:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Harrison E, Allan P, Ramu A, Vaidya A, Travis S, Lal S. Management of intestinal failure in inflammatory bowel disease: small intestinal transplantation or home parenteral nutrition? World J Gastroenterol. 2014;20:3153-3163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 6. | Gondolesi GE. History of clinical intestinal transplantation. Hum Immunol. 2024;85:110788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | LILLEHEI RC, GOOTT B, MILLER FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 212] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Deltz E, Schroeder P, Gundlach M, Hansmann ML, Leimenstoll G. Successful clinical small-bowel transplantation. Transplant Proc. 1990;22:2501. [PubMed] |
| 9. | Starzl TE, Rowe MI, Todo S, Jaffe R, Tzakis A, Hoffman AL, Esquivel C, Porter KA, Venkataramanan R, Makowka L. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449-1457. [PubMed] |
| 10. | Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA. 1968;203:860-864. [PubMed] |
| 11. | Paulo Guzman J, Maklad M, Osman M, Elsherif A, Fujiki M. Updates in induction immunosuppression regimens for intestinal transplantation. Hum Immunol. 2024;85:110800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Weissenbacher A, Vrakas G, Nasralla D, Ceresa CDL. The future of organ perfusion and re-conditioning. Transpl Int. 2019;32:586-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Fard A, Pearson R, Lathan R, Mark PB, Clancy MJ. Perfusate Composition and Duration of Ex-Vivo Normothermic Perfusion in Kidney Transplantation: A Systematic Review. Transpl Int. 2022;35:10236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Karageorgos FF, Alexiou M, Tsoulfas G, Alexopoulos AH. Hydrogel-Based Vascularized Organ Tissue Engineering: A Systematized Review on Abdominal Organs. Gels. 2024;10:653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Xu ZY, Huang JJ, Liu Y, Chen CW, Qu GW, Wang GF, Zhao Y, Wu XW, Ren JA. Extracellular matrix bioink boosts stemness and facilitates transplantation of intestinal organoids as a biosafe Matrigel alternative. Bioeng Transl Med. 2023;8:e10327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Li Q, Zhang Q, Wang C, Tang C, Zhang Y, Li N, Li J. Fish oil enhances recovery of intestinal microbiota and epithelial integrity in chronic rejection of intestinal transplant. PLoS One. 2011;6:e20460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Kaufman SS, Hussan E, Kroemer A, Timofeeva O, Pasieka HB, Guerra JF, Yazigi NA, Khan KM, Ekong UD, Subramanian S, Hawksworth JS, Girlanda R, Ghobrial SS, Fishbein TM, Matsumoto CS. Graft Versus Host Disease After Intestinal Transplantation: A Single-center Experience. Transplant Direct. 2021;7:e731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Jan N, Hays RA, Oakland DN, Kumar P, Ramakrishnan G, Behm BW, Petri WA Jr, Marie C. Fecal Microbiota Transplantation Increases Colonic IL-25 and Dampens Tissue Inflammation in Patients with Recurrent Clostridioides difficile. mSphere. 2021;6:e0066921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, Forbes A, Gabe S, Gillanders L, Holst M, Jeppesen PB, Joly F, Kelly D, Klek S, Irtun Ø, Olde Damink SW, Panisic M, Rasmussen HH, Staun M, Szczepanek K, Van Gossum A, Wanten G, Schneider SM, Shaffer J; Home Artificial Nutrition & Chronic Intestinal Failure; Acute Intestinal Failure Special Interest Groups of ESPEN. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 20. | Bielawska B, Allard JP. Parenteral Nutrition and Intestinal Failure. Nutrients. 2017;9:466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Kaufman SS, Atkinson JB, Bianchi A, Goulet OJ, Grant D, Langnas AN, McDiarmid SV, Mittal N, Reyes J, Tzakis AG; American Society of Transplantation. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Pironi L, Arends J, Bozzetti F, Cuerda C, Gillanders L, Jeppesen PB, Joly F, Kelly D, Lal S, Staun M, Szczepanek K, Van Gossum A, Wanten G, Schneider SM; Home Artificial Nutrition & Chronic Intestinal Failure Special Interest Group of ESPEN. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 516] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 23. | Venick RS, Duggan E, Whatley J. Current status of pediatric intestinal transplantation in the United States. Curr Opin Organ Transplant. 2020;25:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Soltys KA, Bond G, Sindhi R, Rassmussen SK, Ganoza A, Khanna A, Mazariegos G. Pediatric intestinal transplantation. Semin Pediatr Surg. 2017;26:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Matsumoto CS, Subramanian S, Fishbein TM. Adult Intestinal Transplantation. Gastroenterol Clin North Am. 2018;47:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Caporilli C, Giannì G, Grassi F, Esposito S. An Overview of Short-Bowel Syndrome in Pediatric Patients: Focus on Clinical Management and Prevention of Complications. Nutrients. 2023;15:2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 28. | Massironi S, Cavalcoli F, Rausa E, Invernizzi P, Braga M, Vecchi M. Understanding short bowel syndrome: Current status and future perspectives. Dig Liver Dis. 2020;52:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, Rhee S, Sudan D, Mercer D, Martinez JA, Carter BA, Soden J, Horslen S, Rudolph JA, Kocoshis S, Superina R, Lawlor S, Haller T, Kurs-Lasky M, Belle SH; Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723-8.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 30. | Nayyar NS, McGhee W, Martin D, Sindhi R, Soltys K, Bond G, Mazariegos GV. Intestinal transplantation in children: a review of immunotherapy regimens. Paediatr Drugs. 2011;13:149-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003;38:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Horslen SP, Smith JM, Weaver T, Cafarella M, Foutz J. OPTN/SRTR 2020 Annual Data Report: Intestine. Am J Transplant. 2022;22 Suppl 2:310-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | de Ville de Goyet J, Baumann U, Karam V, Adam R, Nadalin S, Heaton N, Reding R, Branchereau S, Mirza D, Klempnauer JL, Fischer L, Kalicinski P, Colledan M, Lopez Santamaria M, de Kleine RH, Chardot C, Yilmaz S, Kilic M, Boillot O, di Francesco F, Polak WG, Verkade HJ; European Liver, Intestine Transplant Association. European Liver Transplant Registry: Donor and transplant surgery aspects of 16,641 liver transplantations in children. Hepatology. 2022;75:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 34. | Sindhi R, AshokKumar C, Mazariegos G, Nayyar N, Ningappa M, Soltys K, Bond G, Sun Q, Humar A, Abu-Elmagd K, Zeevi A. Immune monitoring in small bowel transplantation. Curr Opin Organ Transplant. 2010;15:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Lee EJ, Mazariegos GV, Bond GJ. Pediatric intestinal transplantation. Semin Pediatr Surg. 2022;31:151181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 36. | Sykes M. Tolerance in intestinal transplantation. Hum Immunol. 2024;85:110793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Tian X, Yang Z, Luo F, Zheng S. Gut microbial balance and liver transplantation: alteration, management, and prediction. Front Med. 2018;12:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Tzvetanov IG, Tulla KA, D'Amico G, Benedetti E. Living Donor Intestinal Transplantation. Gastroenterol Clin North Am. 2018;47:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Byeman CJ, Harshman LA, Engen RM. Adult and late adolescent complications of pediatric solid organ transplantation. Pediatr Transplant. 2024;28:e14766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Mehtani R, Saigal S. Long Term Complications of Immunosuppression Post Liver Transplant. J Clin Exp Hepatol. 2023;13:1103-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Grant D, Abu-Elmagd K, Reyes J, Tzakis A, Langnas A, Fishbein T, Goulet O, Farmer D; Intestine Transplant Registry. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 42. | Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T; Intestinal Transplant Association. Intestinal transplant registry report: global activity and trends. Am J Transplant. 2015;15:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (2)] |
| 43. | Struijs MC, Diamond IR, de Silva N, Wales PW. Establishing norms for intestinal length in children. J Pediatr Surg. 2009;44:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Moschino L, Duci M, Fascetti Leon F, Bonadies L, Priante E, Baraldi E, Verlato G. Optimizing Nutritional Strategies to Prevent Necrotizing Enterocolitis and Growth Failure after Bowel Resection. Nutrients. 2021;13:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Boroni G, Parolini F, Stern MV, Moglia C, Alberti D. Autologous Intestinal Reconstruction Surgery in Short Bowel Syndrome: Which, When, and Why. Front Nutr. 2022;9:861093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 46. | Weih S, Kessler M, Fonouni H, Golriz M, Nickkholgh A, Schmidt J, Holland-Cunz S, Mehrabi A. Review of various techniques of small bowel transplantation in pigs. J Surg Res. 2011;171:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Atia A, Hollins A, Shammas R, Phillips BT, Ravindra KV, Sudan DL, Giele H, Mithani SK, Erdmann D. Surgical Techniques for Revascularization in Abdominal Wall Transplantation. J Reconstr Microsurg. 2020;36:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Levi DM, Tzakis AG, Kato T, Madariaga J, Mittal NK, Nery J, Nishida S, Ruiz P. Transplantation of the abdominal wall. Lancet. 2003;361:2173-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | Hommel MJ, van Baren R, Haveman JW. Surgical management and autologous intestinal reconstruction in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2016;30:263-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Cipriani R, Contedini F, Santoli M, Gelati C, Sgarzani R, Cucchetti A, Lauro A, Pinna AD. Abdominal wall transplantation with microsurgical technique. Am J Transplant. 2007;7:1304-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Giele H, Bendon C, Reddy S, Ramcharan R, Sinha S, Friend P, Vaidya A. Remote revascularization of abdominal wall transplants using the forearm. Am J Transplant. 2014;14:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Erdmann D, Atia A, Phillips BT, Mithani SK, Avashia YJ, Hollister BA, Cendales LC, Ravindra KV, Sudan DL. Small bowel and abdominal wall transplantation: A novel technique for synchronous revascularization. Am J Transplant. 2019;19:2122-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Kallidonis P, Gkeka K, Tatanis V, Katsakiori P, Vrettos T, Liatsikos E. Novel Robotic Platforms for Robot-Assisted Laparoscopic Surgery in Urology: A Narrative Review. J Endourol. 2024;38:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 54. | Slagter JS, Outmani L, Tran KTCK, Ijzermans JNM, Minnee RC. Robot-assisted kidney transplantation as a minimally invasive approach for kidney transplant recipients: A systematic review and meta-analyses. Int J Surg. 2022;99:106264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Territo A, Diana P, Gaya JM, Gallioli A, Piana A, Breda A. Robot-assisted kidney transplantation: State of art. Arch Esp Urol. 2021;74:970-978. [PubMed] |
| 56. | Huard G, Schiano T, Moon J, Iyer K. Choice of Allograft in Patients Requiring Intestinal Transplantation: A Critical Review. Can J Gastroenterol Hepatol. 2017;2017:1069726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Veccia A, Antonelli A, Hampton LJ, Greco F, Perdonà S, Lima E, Hemal AK, Derweesh I, Porpiglia F, Autorino R. Near-infrared Fluorescence Imaging with Indocyanine Green in Robot-assisted Partial Nephrectomy: Pooled Analysis of Comparative Studies. Eur Urol Focus. 2020;6:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Kauffmann EF, Napoli N, Ginesini M, Gianfaldoni C, Asta F, Salamone A, Ripolli A, Di Dato A, Vistoli F, Amorese G, Boggi U. Tips and tricks for robotic pancreatoduodenectomy with superior mesenteric/portal vein resection and reconstruction. Surg Endosc. 2023;37:3233-3245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 59. | Sudan DL, Iyer KR, Deroover A, Chinnakotla S, Fox IJ Jr, Shaw BW Jr, Langnas AN. A new technique for combined liver/small intestinal transplantation. Transplantation. 2001;72:1846-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Rees MA, Amesur NB, Cruz RJ, Borhani AA, Abu-Elmagd KM, Costa G, Dasyam AK. Imaging of Intestinal and Multivisceral Transplantation. Radiographics. 2018;38:413-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Meira Filho SP, Guardia BD, Evangelista AS, Matielo CE, Neves DB, Pandullo FL, Felga GE, Alves JA, Curvelo LA, Diaz LG, Rusi MB, Viveiros Mde M, Almeida MD, Epstein MG, Pedroso PT, Salvalaggio P, Meirelles Júnior RF, Rocco RA, Almeida SS, Rezende MB. Intestinal and multivisceral transplantation. Einstein (Sao Paulo). 2015;13:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 63. | Loo L, Vrakas G, Reddy S, Allan P. Intestinal transplantation: a review. Curr Opin Gastroenterol. 2017;33:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Kato T, Selvaggi G, Gaynor JJ, Takahashi H, Nishida S, Moon J, Levi D, Smith L, Hernandez E, Ruiz P, Tzakis A. Inclusion of donor colon and ileocecal valve in intestinal transplantation. Transplantation. 2008;86:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Kato T, Tzakis AG, Selvaggi G, Gaynor JJ, Takahashi H, Mathew J, Garcia-Morales R, Hernandez E, David A, Nishida S, Levi D, Moon J, Island E, Kleiner G, Ruiz P. Transplantation of the spleen: effect of splenic allograft in human multivisceral transplantation. Ann Surg. 2007;246:436-44; discussion 445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Bhamidimarri KR, Beduschi T, Vianna R. Multivisceral transplantation: where do we stand? Clin Liver Dis. 2014;18:661-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Matsumoto CS, Kaufman SS, Fishbein TM. Inclusion of the colon in intestinal transplantation. Curr Opin Organ Transplant. 2011;16:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Ewald C, Swanson BJ, Vargas L, Grant WJ, Mercer DF, Langnas AN, Merani S. Including colon in intestinal transplantation: a focus on post-transplant renal function - a retrospective study. Transpl Int. 2020;33:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Cruz RJ Jr, Costa G, Bond G, Soltys K, Stein WC, Wu G, Martin L, Koritsky D, McMichael J, Sindhi R, Mazariegos G, Abu-Elmagd KM. Modified "liver-sparing" multivisceral transplant with preserved native spleen, pancreas, and duodenum: technique and long-term outcome. J Gastrointest Surg. 2010;14:1709-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Matsumoto R, Kato T. Intestinal Transplantation: Include the Spleen with Intestinal Graft? Gastroenterol Clin North Am. 2024;53:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Su L, Zhang J, Gomez H, Kellum JA, Peng Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy. 2023;19:401-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 497] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 72. | Chen HD, Jiang MZ, Zhao YY, Li X, Lan H, Yang WQ, Lai Y. Effects of breviscapine on cerebral ischemia-reperfusion injury and intestinal flora imbalance by regulating the TLR4/MyD88/NF-κB signaling pathway in rats. J Ethnopharmacol. 2023;300:115691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Sousa MV, Zollner RL, Mazzali M. Renal transplant patients with preformed anti-HLA antibodies: early biopsy findings and clinical outcomes. J Bras Nefrol. 2019;42:201-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | McArdle R, Cope R, Chaudhry A, Sharkey L, Peacock S. Exploring the immunological relevance of pre-transplant donor-specific antibody in intestinal transplantation, with special consideration to the liver. Int J Immunogenet. 2024;51:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Hamed MO, Barlow AD, Dolezalova N, Khosla S, Sagar A, Gribble FM, Davies S, Murphy MP, Hosgood SA, Nicholson ML, Saeb-Parsy K. Ex vivo normothermic perfusion of isolated segmental porcine bowel: a novel functional model of the small intestine. BJS Open. 2021;5:zrab009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Abraham N, Ludwig EK, Schaaf CR, Veerasammy B, Stewart AS, McKinney C, Freund J, Brassil J, Samy KP, Gao Q, Kahan R, Niedzwiecki D, Cardona DM, Garman KS, Barbas AS, Sudan DL, Gonzalez LM. Orthotopic Transplantation of the Full-length Porcine Intestine After Normothermic Machine Perfusion. Transplant Direct. 2022;8:e1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Sun X, Suzuki K, Nagata M, Kawauchi Y, Yano M, Ohkoshi S, Matsuda Y, Kawachi H, Watanabe K, Asakura H, Aoyagi Y. Rectal administration of tranilast ameliorated acute colitis in mice through increased expression of heme oxygenase-1. Pathol Int. 2010;60:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Hamed M, Logan A, Gruszczyk AV, Beach TE, James AM, Dare AJ, Barlow A, Martin J, Georgakopoulos N, Gane AM, Crick K, Fouto D, Fear C, Thiru S, Dolezalova N, Ferdinand JR, Clatworthy MR, Hosgood SA, Nicholson ML, Murphy MP, Saeb-Parsy K. Mitochondria-targeted antioxidant MitoQ ameliorates ischaemia-reperfusion injury in kidney transplantation models. Br J Surg. 2021;108:1072-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. 2024;24:577-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 684] [Article Influence: 342.0] [Reference Citation Analysis (0)] |
| 80. | Koyama M, Hippe DS, Srinivasan S, Proll SC, Miltiadous O, Li N, Zhang P, Ensbey KS, Hoffman NG, Schmidt CR, Yeh AC, Minnie SA, Strenk SM, Fiedler TL, Hattangady N, Kowalsky J, Grady WM, Degli-Esposti MA, Varelias A, Clouston AD, van den Brink MRM, Dey N, Randolph TW, Markey KA, Fredricks DN, Hill GR. Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity. Immunity. 2023;56:1876-1893.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 81. | Torow N, Li R, Hitch TCA, Mingels C, Al Bounny S, van Best N, Stange EL, Simons B, Maié T, Rüttger L, Gubbi NMKP, Abbott DA, Benabid A, Gadermayr M, Runge S, Treichel N, Merhof D, Rosshart SP, Jehmlich N, Hand TW, von Bergen M, Heymann F, Pabst O, Clavel T, Tacke F, Lelouard H, Costa IG, Hornef MW. M cell maturation and cDC activation determine the onset of adaptive immune priming in the neonatal Peyer's patch. Immunity. 2023;56:1220-1238.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 82. | Zhang J, Zhan H, Song Z, Liu S. Immune reactions following intestinal transplantation: Mechanisms and prevention. Asian J Surg. 2024;47:3819-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 83. | Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2196] [Cited by in RCA: 2112] [Article Influence: 117.3] [Reference Citation Analysis (30)] |
| 84. | Abu-Elmagd KM, Kosmach-Park B, Costa G, Zenati M, Martin L, Koritsky DA, Emerling M, Murase N, Bond GJ, Soltys K, Sogawa H, Lunz J, Al Samman M, Shaefer N, Sindhi R, Mazariegos GV. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg. 2012;256:494-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Matsuoka KI. [Graft-versus-host disease: current understanding of immune pathogenesis and clinical treatment]. Rinsho Ketsueki. 2021;62:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 86. | Socié G, Kean LS, Zeiser R, Blazar BR. Insights from integrating clinical and preclinical studies advance understanding of graft-versus-host disease. J Clin Invest. 2021;131:e149296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, Ho VT, Bolaños-Meade J, Ferrara JL, Jones R, Arora M, Blazar BR, Holtan SG, Jacobsohn D, Pasquini M, Socie G, Antin JH, Levine JE, Weisdorf DJ. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 88. | Aversa M. Removing Barriers and Mitigating Risk: The Case for Crossing Donor-specific Antibodies in Lung Transplantation With the Use of Perioperative Desensitization. Transplantation. 2023;107:1017-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 89. | Park HB, Lee JE, Oh YM, Lee SJ, Eom HS, Choi K. CTLA4-CD28 chimera gene modification of T cells enhances the therapeutic efficacy of donor lymphocyte infusion for hematological malignancy. Exp Mol Med. 2017;49:e360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Dubois A, Jin X, Hooft C, Canovai E, Boelhouwer C, Vanuytsel T, Vanaudenaerde B, Pirenne J, Ceulemans LJ. New insights in immunomodulation for intestinal transplantation. Hum Immunol. 2024;85:110827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 91. | Chen L, He Z, Reis BS, Gelles JD, Chipuk JE, Ting AT, Spicer JA, Trapani JA, Furtado GC, Lira SA. IFN-γ(+) cytotoxic CD4(+) T lymphocytes are involved in the pathogenesis of colitis induced by IL-23 and the food colorant Red 40. Cell Mol Immunol. 2022;19:777-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 92. | Sharma N, Fan X, Atolagbe OT, Ge Z, Dao KN, Sharma P, Allison JP. ICOS costimulation in combination with CTLA-4 blockade remodels tumor-associated macrophages toward an antitumor phenotype. J Exp Med. 2024;221:e20231263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 93. | Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, Maloy KJ. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 496] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 94. | Kroemer A, Belyayev L, Khan K, Loh K, Kang J, Duttargi A, Dhani H, Sadat M, Aguirre O, Gusev Y, Bhuvaneshwar K, Kallakury B, Cosentino C, Houlihan B, Diaz J, Moturi S, Yazigi N, Kaufman S, Subramanian S, Hawksworth J, Girlanda R, Robson SC, Matsumoto CS, Zasloff M, Fishbein TM. Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab. Am J Transplant. 2021;21:1238-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 95. | Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 742] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 96. | Bartolomé-Casado R, Landsverk OJB, Chauhan SK, Richter L, Phung D, Greiff V, Risnes LF, Yao Y, Neumann RS, Yaqub S, Øyen O, Horneland R, Aandahl EM, Paulsen V, Sollid LM, Qiao SW, Baekkevold ES, Jahnsen FL. Resident memory CD8 T cells persist for years in human small intestine. J Exp Med. 2019;216:2412-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 97. | FitzPatrick MEB, Provine NM, Garner LC, Powell K, Amini A, Irwin SL, Ferry H, Ambrose T, Friend P, Vrakas G, Reddy S, Soilleux E, Klenerman P, Allan PJ. Human intestinal tissue-resident memory T cells comprise transcriptionally and functionally distinct subsets. Cell Rep. 2021;34:108661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 98. | Weiner J, Svetlicky N, Kang J, Sadat M, Khan K, Duttargi A, Stovroff M, Moturi S, Kara Balla A, Hyang Kwon D, Kallakury B, Hawksworth J, Subramanian S, Yazigi N, Kaufman S, Pasieka HB, Matsumoto CS, Robson SC, Pavletic S, Zasloff M, Fishbein TM, Kroemer A. CD69+ resident memory T cells are associated with graft-versus-host disease in intestinal transplantation. Am J Transplant. 2021;21:1878-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Sun S, Duan Z, Wang X, Chu C, Yang C, Chen F, Wang D, Wang C, Li Q, Ding W. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 2021;12:606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 100. | Zhang J, Feng Y, Shi D. NETosis of psoriasis: a critical step in amplifying the inflammatory response. Front Immunol. 2024;15:1374934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 101. | Železnjak J, Lisnić VJ, Popović B, Lisnić B, Babić M, Halenius A, L'Hernault A, Roviš TL, Hengel H, Erhard F, Redwood AJ, Vidal SM, Dölken L, Krmpotić A, Jonjić S. The complex of MCMV proteins and MHC class I evades NK cell control and drives the evolution of virus-specific activating Ly49 receptors. J Exp Med. 2019;216:1809-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Lin CH, Wu CJ, Cho S, Patkar R, Huth WJ, Lin LL, Chen MC, Israelsson E, Betts J, Niedzielska M, Patel SA, Duong HG, Gerner RR, Hsu CY, Catley M, Maciewicz RA, Chu H, Raffatellu M, Chang JT, Lu LF. Selective IL-27 production by intestinal regulatory T cells permits gut-specific regulation of T(H)17 cell immunity. Nat Immunol. 2023;24:2108-2120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 103. | Ceulemans LJ, Braza F, Monbaliu D, Jochmans I, De Hertogh G, Du Plessis J, Emonds MP, Kitade H, Kawai M, Li Y, Zhao X, Koshiba T, Sprangers B, Brouard S, Waer M, Pirenne J. The Leuven Immunomodulatory Protocol Promotes T-Regulatory Cells and Substantially Prolongs Survival After First Intestinal Transplantation. Am J Transplant. 2016;16:2973-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 104. | Soskic B, Jeffery LE, Kennedy A, Gardner DH, Hou TZ, Halliday N, Williams C, Janman D, Rowshanravan B, Hirschfield GM, Sansom DM. CD80 on Human T Cells Is Associated With FoxP3 Expression and Supports Treg Homeostasis. Front Immunol. 2020;11:577655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 105. | Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118:e2023739118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 106. | Harrison OJ, Powrie FM. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol. 2013;5:a018341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 107. | Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis C, Liu JR, Kryczek I, Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 596] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 108. | Izadi D, Layton TB, Williams L, McCann F, Cabrita M, Espirito Santo AI, Xie W, Fritzsche M, Colin-York H, Feldmann M, Midwood KS, Nanchahal J. Identification of TNFR2 and IL-33 as therapeutic targets in localized fibrosis. Sci Adv. 2019;5:eaay0370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 109. | Yuan H, Li Y, Kong Z, Peng L, Song J, Hou X, Zhang W, Liu R, Feng T, Zhu C. IL-33-Pretreated Mesenchymal Stem Cells Attenuate Acute Liver Failure by Improving Homing and Polarizing M2 Macrophages. Stem Cells Int. 2024;2024:1273099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 110. | Salminen A. Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. J Mol Med (Berl). 2021;99:1553-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 111. | Zuber J, Rosen S, Shonts B, Sprangers B, Savage TM, Richman S, Yang S, Lau SP, DeWolf S, Farber D, Vlad G, Zorn E, Wong W, Emond J, Levin B, Martinez M, Kato T, Sykes M. Macrochimerism in Intestinal Transplantation: Association With Lower Rejection Rates and Multivisceral Transplants, Without GVHD. Am J Transplant. 2015;15:2691-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 112. | Ou L, Ye B, Sun M, Qi N, Li J, Lv M, Lin X, Cai H, Hu J, Song Y, Chen X, Zhu Y, Yin L, Zhang J, Liao S, Zhang H. Mechanisms of intestinal epithelial cell damage by Clostridiumperfringens. Anaerobe. 2024;87:102856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 113. | Kip AM, Ceulemans LJ, Hundscheid IHR, Canovai E, Hartog H, Brown RM, Corcos O, Joly F, De Hertogh G, Gupte G, Dejong CHC, Olde Damink SWM, Pirenne J, Mirza D, Lenaerts K. Paneth Cell Alterations During Ischemia-reperfusion, Follow-up, and Graft Rejection After Intestinal Transplantation. Transplantation. 2020;104:1952-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 114. | Kang J, Loh K, Belyayev L, Cha P, Sadat M, Khan K, Gusev Y, Bhuvaneshwar K, Ressom H, Moturi S, Kaiser J, Hawksworth J, Robson SC, Matsumoto CS, Zasloff M, Fishbein TM, Kroemer A. Type 3 innate lymphoid cells are associated with a successful intestinal transplant. Am J Transplant. 2021;21:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 115. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1296] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 116. | Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (2)] |
| 117. | Mu C, Yang Y, Zhu W. Crosstalk Between The Immune Receptors and Gut Microbiota. Curr Protein Pept Sci. 2015;16:622-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 118. | Yang R, Liu Q, Wang D, Zhao Z, Su Z, Fan D, Liu Q. The Toll-like Receptor-2/4 Antagonist, Sparstolonin B, and Inflammatory Diseases: A Literature Mining and Network Analysis. Cardiovasc Drugs Ther. 2025;39:499-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Zhang B, Liang J, Fan H, Lei K, Li H, Liu D, Zheng F, He M, Chen Y. Study on anti-inflammatory effect of Shangkehuangshui in vitro and in vivo based on TLR4/TLR2-NF-κB signaling pathway. J Ethnopharmacol. 2024;323:117709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 120. | de Groot RCA, Zhu H, Hoogenboezem T, de Bruijn ACJM, Eenjes E, 't Jong AEJ, Belo AI, Estevão SC, Bajramovic JJ, Rottier RJ, Kool M, van Rossum AMC, Unger WWJ. Mycoplasma pneumoniae Compared to Streptococcus pneumoniae Avoids Induction of Proinflammatory Epithelial Cell Responses despite Robustly Inducing TLR2 Signaling. Infect Immun. 2022;90:e0012922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 121. | Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 435] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 122. | Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov II, Sugiyama T, Nuñez G, Camp JG, Hattori M, Umesaki Y, Honda K. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 852] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 123. | Gala I, Baltesova T, Hulik S, Kalanin R, Adandedjan D, Katuchova J, Bena L. A single-centre report of acute pyelonephritis in a patient after kidney transplantation - analyses of risk factors. Bratisl Lek Listy. 2023;124:748-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 124. | Perez-Cruz M, Costa C, Mañez R. Boosted rat natural xenoantibodies cross-react with Enterococcus faecalis by targeting melibiose and L-rhamnose. J Innate Immun. 2014;6:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 125. | Pacaud M, Colas L, Brouard S. Microbiota and immunoregulation: A focus on regulatory B lymphocytes and transplantation. Am J Transplant. 2021;21:2341-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 126. | Wang L, Gong Z, Zhang X, Zhu F, Liu Y, Jin C, Du X, Xu C, Chen Y, Cai W, Tian C, Wu J. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes. 2020;12:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 127. | Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: A Double-Edged Sword for Health? Adv Nutr. 2018;9:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 795] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 128. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 4057] [Article Influence: 312.1] [Reference Citation Analysis (0)] |
| 129. | Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 1865] [Article Influence: 143.5] [Reference Citation Analysis (1)] |
| 130. | Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 736] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 131. | Hagihara M, Kuroki Y, Ariyoshi T, Higashi S, Fukuda K, Yamashita R, Matsumoto A, Mori T, Mimura K, Yamaguchi N, Okada S, Nonogaki T, Ogawa T, Iwasaki K, Tomono S, Asai N, Koizumi Y, Oka K, Yamagishi Y, Takahashi M, Mikamo H. Clostridium butyricum Modulates the Microbiome to Protect Intestinal Barrier Function in Mice with Antibiotic-Induced Dysbiosis. iScience. 2020;23:100772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 132. | Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 391] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 133. | Oh PL, Martínez I, Sun Y, Walter J, Peterson DA, Mercer DF. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. Am J Transplant. 2012;12:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 134. | Taur Y, Coyte K, Schluter J, Robilotti E, Figueroa C, Gjonbalaj M, Littmann ER, Ling L, Miller L, Gyaltshen Y, Fontana E, Morjaria S, Gyurkocza B, Perales MA, Castro-Malaspina H, Tamari R, Ponce D, Koehne G, Barker J, Jakubowski A, Papadopoulos E, Dahi P, Sauter C, Shaffer B, Young JW, Peled J, Meagher RC, Jenq RR, van den Brink MRM, Giralt SA, Pamer EG, Xavier JB. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10:eaap9489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 135. | Filardy AA, Ferreira JRM, Rezende RM, Kelsall BL, Oliveira RP. The intestinal microenvironment shapes macrophage and dendritic cell identity and function. Immunol Lett. 2023;253:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 136. | Bleakley M, Sehgal A, Seropian S, Biernacki MA, Krakow EF, Dahlberg A, Persinger H, Hilzinger B, Martin PJ, Carpenter PA, Flowers ME, Voutsinas J, Gooley TA, Loeb K, Wood BL, Heimfeld S, Riddell SR, Shlomchik WD. Naive T-Cell Depletion to Prevent Chronic Graft-Versus-Host Disease. J Clin Oncol. 2022;40:1174-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 137. | Lee JR, Muthukumar T, Dadhania D, Toussaint NC, Ling L, Pamer E, Suthanthiran M. Gut microbial community structure and complications after kidney transplantation: a pilot study. Transplantation. 2014;98:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 138. | Rivera CA, Randrian V, Richer W, Gerber-Ferder Y, Delgado MG, Chikina AS, Frede A, Sorini C, Maurin M, Kammoun-Chaari H, Parigi SM, Goudot C, Cabeza-Cabrerizo M, Baulande S, Lameiras S, Guermonprez P, Reis e Sousa C, Lecuit M, Moreau HD, Helft J, Vignjevic DM, Villablanca EJ, Lennon-Duménil AM. Epithelial colonization by gut dendritic cells promotes their functional diversification. Immunity. 2022;55:129-144.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 139. | Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1255] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 140. | Siu JHY, Surendrakumar V, Richards JA, Pettigrew GJ. T cell Allorecognition Pathways in Solid Organ Transplantation. Front Immunol. 2018;9:2548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 141. | Conlon TM, Saeb-Parsy K, Cole JL, Motallebzadeh R, Qureshi MS, Rehakova S, Negus MC, Callaghan CJ, Bolton EM, Bradley JA, Pettigrew GJ. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol. 2012;188:2643-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 142. | Harper SJ, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M, Qureshi MS, Mallik M, Bolton E, Bradley JA, Pettigrew GJ. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci U S A. 2015;112:12788-12793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 143. | Morelli AE, Bracamonte-Baran W, Burlingham WJ. Donor-derived exosomes: the trick behind the semidirect pathway of allorecognition. Curr Opin Organ Transplant. 2017;22:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 144. | Charmetant X, Chen CC, Hamada S, Goncalves D, Saison C, Rabeyrin M, Rabant M, Duong van Huyen JP, Koenig A, Mathias V, Barba T, Lacaille F, le Pavec J, Brugière O, Taupin JL, Chalabreysse L, Mornex JF, Couzi L, Graff-Dubois S, Jeger-Madiot R, Tran-Dinh A, Mordant P, Paidassi H, Defrance T, Morelon E, Badet L, Nicoletti A, Dubois V, Thaunat O. Inverted direct allorecognition triggers early donor-specific antibody responses after transplantation. Sci Transl Med. 2022;14:eabg1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 145. | Charreau B. Cellular and Molecular Crosstalk of Graft Endothelial Cells During AMR: Effector Functions and Mechanisms. Transplantation. 2021;105:e156-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Crespo M, Yelamos J, Redondo D, Muntasell A, Perez-Saéz MJ, López-Montañés M, García C, Torio A, Mir M, Hernández JJ, López-Botet M, Pascual J. Circulating NK-cell subsets in renal allograft recipients with anti-HLA donor-specific antibodies. Am J Transplant. 2015;15:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 147. | Chong AS. Mechanisms of organ transplant injury mediated by B cells and antibodies: Implications for antibody-mediated rejection. Am J Transplant. 2020;20 Suppl 4:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 148. | Drachenberg CB, Papadimitriou JC. Endothelial injury in renal antibody-mediated allograft rejection: a schematic view based on pathogenesis. Transplantation. 2013;95:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 149. | Gould DS, Auchincloss H Jr. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol Today. 1999;20:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 243] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 150. | Guerra MR, Rossetti M, Zhang Z, Zhou X, Whang EC, Venick RS, Marcus EA, McDiarmid SV, Farmer DG, Reed EF, Wozniak LJ. Characterization of T cell immunophenotypes in intestinal transplantation: A pilot study. Transpl Immunol. 2018;51:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 152. | Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 153. | Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies. J Clin Invest. 2017;127:2492-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 154. | Huang DL, He YR, Liu YJ, He HY, Gu ZY, Liu YM, Liu WJ, Luo Z, Ju MJ. The immunomodulation role of Th17 and Treg in renal transplantation. Front Immunol. 2023;14:1113560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 155. | Kumar V, Stewart JH 4th. cGLRs Join Their Cousins of Pattern Recognition Receptor Family to Regulate Immune Homeostasis. Int J Mol Sci. 2024;25:1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 156. | Testro AG, Visvanathan K, Skinner N, Markovska V, Crowley P, Angus PW, Gow PJ. Acute allograft rejection in human liver transplant recipients is associated with signaling through toll-like receptor 4. J Gastroenterol Hepatol. 2011;26:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 157. | Zelzer S, Stiegler P, Kapitan M, Schaffellner S, Schweiger M, Stettin M, Stojakovic T, Truschnig-Wilders M, Tscheliessnigg KH, Khoschsorur G. Myeloperoxidase as serum marker for detection of CMV infections and rejections in patients after liver or heart transplantation. Transpl Immunol. 2009;20:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 158. | Illigens BM, Yamada A, Anosova N, Dong VM, Sayegh MH, Benichou G. Dual effects of the alloresponse by Th1 and Th2 cells on acute and chronic rejection of allotransplants. Eur J Immunol. 2009;39:3000-3009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 159. | Lee JY, Hall JA, Kroehling L, Wu L, Najar T, Nguyen HH, Lin WY, Yeung ST, Silva HM, Li D, Hine A, Loke P, Hudesman D, Martin JC, Kenigsberg E, Merad M, Khanna KM, Littman DR. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell. 2020;180:79-91.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 317] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 160. | Walrath T, Malizia RA, Zhu X, Sharp SP, D'Souza SS, Lopez-Soler R, Parr B, Kartchner B, Lee EC, Stain SC, Iwakura Y, O'Connor W Jr. IFN-γ and IL-17A regulate intestinal crypt production of CXCL10 in the healthy and inflamed colon. Am J Physiol Gastrointest Liver Physiol. 2020;318:G479-G489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 161. | Curci C, Sallustio F, Serino G, De Palma G, Trpevski M, Fiorentino M, Rossini M, Quaglia M, Valente M, Furian L, Toscano A, Mazzucco G, Barreca A, Bussolino S, Gesualdo L, Stratta P, Rigotti P, Citterio F, Biancone L, Schena FP. Potential role of effector memory T cells in chronic T cell-mediated kidney graft rejection. Nephrol Dial Transplant. 2016;31:2131-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 162. | Kakuta Y, Maegawa-Higa Y, Matsumura S, Fukae S, Tanaka R, Yonishi H, Nakazawa S, Namba-Hamano T, Isaka Y, Nonomura N. Impact of Immunosuppressive Drug Concentrations on Microvascular Inflammation, Negative Donor-Specific Antibodies, and C4d-Negative Status in Kidney Transplant Recipients. Clin Transplant. 2025;39:e70112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 163. | Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, Zhang X, Eich T, Toyoda M, Eriksson BM, Ge S, Peng A, Järnum S, Wood KJ, Lundgren T, Wennberg L, Bäckman L, Larsson E, Villicana R, Kahwaji J, Louie S, Kang A, Haas M, Nast C, Vo A, Tufveson G. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N Engl J Med. 2017;377:442-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 164. | Heeger PS, Haro MC, Jordan S. Translating B cell immunology to the treatment of antibody-mediated allograft rejection. Nat Rev Nephrol. 2024;20:218-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 165. | Song C, Wang G, Ma X, Mao P, Lu W, Zhang H, Liu L, Zhang Y, Li X. The effect of miR-155-5p on M1 polarization of Kupffer cells and immune response during liver transplantation through regulating the expression of KDM5D. Mol Immunol. 2023;155:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 166. | Goto A, Komura S, Kato K, Maki R, Hirakawa A, Tomita H, Hirata A, Yamada Y, Akiyama H. C-X-C domain ligand 14-mediated stromal cell-macrophage interaction as a therapeutic target for hand dermal fibrosis. Commun Biol. 2023;6:1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 167. | Sittewelle M, Monsoro-Burq AH. AKT signaling displays multifaceted functions in neural crest development. Dev Biol. 2018;444 Suppl 1:S144-S155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 168. | Wang Y, Zhang L, Huang T, Wu GR, Zhou Q, Wang FX, Chen LM, Sun F, Lv Y, Xiong F, Zhang S, Yu Q, Yang P, Gu W, Xu Y, Zhao J, Zhang H, Xiong W, Wang CY. The methyl-CpG-binding domain 2 facilitates pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation. Eur Respir J. 2022;60:2003697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 169. | Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 807] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 170. | O'Reilly S. Role of interleukin-13 in fibrosis, particularly systemic sclerosis. Biofactors. 2013;39:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 171. | Sun J, Wu M, Wang L, Wang P, Xiao T, Wang S, Liu Q. miRNA-21, which disrupts metabolic reprogramming to facilitate CD4(+) T cell polarization toward the Th2 phenotype, accelerates arsenite-induced hepatic fibrosis. Ecotoxicol Environ Saf. 2022;248:114321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 172. | Huang F, Zhang F, Huang L, Zhu X, Huang C, Li N, Da Q, Huang Y, Yang H, Wang H, Zhao L, Lin Q, Chen Z, Xu J, Liu J, Ren M, Wang Y, Han Z, Ouyang K. Inositol 1,4,5-Trisphosphate Receptors Regulate Vascular Smooth Muscle Cell Proliferation and Neointima Formation in Mice. J Am Heart Assoc. 2024;13:e034203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 173. | Tuuminen R, Dashkevich A, Keränen MA, Raissadati A, Krebs R, Jokinen JJ, Arnaudova R, Rouvinen E, Ylä-Herttuala S, Nykänen AI, Lemström KB. Platelet-derived Growth Factor-B Protects Rat Cardiac Allografts From Ischemia-reperfusion Injury. Transplantation. 2016;100:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 174. | Valentijn FA, Knoppert SN, Marquez-Exposito L, Rodrigues-Diez RR, Pissas G, Tang J, Tejedor-Santamaria L, Broekhuizen R, Samarakoon R, Eleftheriadis T, Goldschmeding R, Nguyen TQ, Ruiz-Ortega M, Falke LL. Cellular communication network 2 (connective tissue growth factor) aggravates acute DNA damage and subsequent DNA damage response-senescence-fibrosis following kidney ischemia reperfusion injury. Kidney Int. 2022;102:1305-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 175. | Lu L, Ma Y, Tao Q, Xie J, Liu X, Wu Y, Zhang Y, Xie X, Liu M, Jin Y. Hypoxia-inducible factor-1 alpha (HIF-1α) inhibitor AMSP-30 m attenuates CCl(4)-induced liver fibrosis in mice by inhibiting the sonic hedgehog pathway. Chem Biol Interact. 2025;413:111480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 176. | Murase N, Ye Q, Nalesnik MA, Demetris AJ, Abu-Elmagd K, Reyes J, Ichikawa N, Okuda T, Fung JJ, Starzl TE. Immunomodulation for intestinal transplantation by allograft irradiation, adjunct donor bone marrow infusion, or both. Transplantation. 2000;70:1632-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 177. | Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16:38-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 620] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 178. | Chen X, Zhang L, Yu C, Duan A, Jiao B, Chen Y, Dai Y, Li B. The role of HMGB1 on SiC NPs-induced inflammation response in lung epithelial-macrophage co-culture system. Food Chem Toxicol. 2024;190:114762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 179. | Kumphune S, Seenak P, Paiyabhrom N, Songjang W, Pankhong P, Jumroon N, Thaisakun S, Phaonakrop N, Roytrakul S, Malakul W, Jiraviriyakul A, Nernpermpisooth N. Cardiac endothelial ischemia/reperfusion injury-derived protein damage-associated molecular patterns disrupt the integrity of the endothelial barrier. Heliyon. 2024;10:e24600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 180. | He M, He Q, Cai X, Chen Z, Lao S, Deng H, Liu X, Zheng Y, Liu X, Liu J, Xie Z, Yao M, Liang W, He J. Role of lymphatic endothelial cells in the tumor microenvironment-a narrative review of recent advances. Transl Lung Cancer Res. 2021;10:2252-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 181. | Gondolesi GE. Induction in Intestinal Transplantation. Transplantation. 2020;104:1999-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 182. | Elsabbagh AM, Hawksworth J, Khan KM, Kaufman SS, Yazigi NA, Kroemer A, Smith C, Fishbein TM, Matsumoto CS. Long-term survival in visceral transplant recipients in the new era: A single-center experience. Am J Transplant. 2019;19:2077-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 183. | Devine K, Ranganathan S, Mazariegos G, Bond G, Soltys K, Ganoza A, Sun Q, Sindhi R. Induction regimens and post-transplantation lymphoproliferative disorder after pediatric intestinal transplantation: Single-center experience. Pediatr Transplant. 2020;24:e13723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 184. | Trevizol AP, David AI, Dias ER, Mantovani D, Pécora R, D'Albuquerque LA. Intestinal and multivisceral transplantation immunosuppression protocols--literature review. Transplant Proc. 2012;44:2445-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 185. | Hamilton BK, Rybicki LA, Li H, Lucas T, Corrigan D, Kalaycio M, Sobecks R, Hanna R, Rotz SJ, Dean RM, Gerds AT, Jagadeesh D, Brunstein C, Sauter CS, Copelan EA, Majhail NS. Tacrolimus/methotrexate vs tacrolimus/reduced-dose methotrexate/mycophenolate for graft-versus-host disease prevention. Blood Adv. 2023;7:4505-4513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 186. | Lauro A, Dazzi A, Ercolani G, Zanfi C, Golfieri L, Amaduzzi A, Cucchetti A, La Barba G, Grazi GL, D'Errico A, Vivarelli M, Cescon M, Varotti G, Del Gaudio M, Ravaioli M, Di Simone M, Faenza S, Pironi L, Pinna AD. Rejection episodes and 3-year graft survival under sirolimus and tacrolimus treatment after adult intestinal transplantation. Transplant Proc. 2007;39:1629-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 187. | Fioretto P, Najafian B, Sutherland DE, Mauer M. Tacrolimus and cyclosporine nephrotoxicity in native kidneys of pancreas transplant recipients. Clin J Am Soc Nephrol. 2011;6:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 188. | Yue W, Liu J, Li X, Wang L, Li J. Memory B cells and long-lived plasma cells in AMR. Ren Fail. 2022;44:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 189. | Yaowakulpatana K, Vadcharavivad S, Ingsathit A, Areepium N, Kantachuvesiri S, Phakdeekitcharoen B, Sukasem C, Sra-Ium S, Sumethkul V, Kitiyakara C. Impact of CYP3A5 polymorphism on trough concentrations and outcomes of tacrolimus minimization during the early period after kidney transplantation. Eur J Clin Pharmacol. 2016;72:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 190. | Lee JH, Massagué J. TGF-β in developmental and fibrogenic EMTs. Semin Cancer Biol. 2022;86:136-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 203] [Reference Citation Analysis (0)] |
| 191. | Wang B, Zhou A, Wu Y, Pan Q, Wei X, Gao Y, Xiao W, Jin J, Zhou T, Luo Y, Zhan Z, Liu Y, Gao W, Liu Y, Xia Q. Establishment and validation of a predictive model of immune tolerance after pediatric liver transplantation: a multicenter cohort study. Int J Surg. 2024;110:5615-5626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 192. | Jackson AM, Kraus ES, Orandi BJ, Segev DL, Montgomery RA, Zachary AA. A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int. 2015;87:409-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 193. | Fujiwara S, Wada M, Kudo H, Yamaki S, Fujishima F, Ishida K, Nakamura M, Sasaki H, Kazama T, Tanaka H, Nio M. Effectiveness of Bortezomib in a Patient With Acute Rejection Associated With an Elevation of Donor-Specific HLA Antibodies After Small-Bowel Transplantation: Case Report. Transplant Proc. 2016;48:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 194. | Island ER, Gonzalez-Pinto IM, Tsai HL, Ruiz P, Tryphonopoulos P, Gonzalez ML, Solano JP, Rossique M, Selvaggi G, Tekin A, Smith LJ, Tzakis AG. Successful treatment with bortezomib of a refractory humoral rejection of the intestine after multivisceral transplantation. Clin Transpl. 2009;465-469. [PubMed] |
| 195. | Yokoi T, Yokoyama Y, Kokuryo T, Yamaguchi J, Nagino M. Inhibition of Toll-like receptor 4 ameliorates experimental postischemic injury in the cholestatic liver through inhibition of high-mobility group box protein b1 (HMGB1) signaling. Surgery. 2018;163:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 196. | Deng X, Yang Q, Wang Y, Zhou C, Guo Y, Hu Z, Liao W, Xu G, Zeng R. CSF-1R inhibition attenuates ischemia-induced renal injury and fibrosis by reducing Ly6C(+) M2-like macrophage infiltration. Int Immunopharmacol. 2020;88:106854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 197. | Igarashi Y, Wada H, Muto M, Sone R, Hasegawa Y, Seino KI. Amelioration of liver fibrosis with autologous macrophages induced by IL-34-based condition. Inflamm Regen. 2025;45:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 198. | Hu W, Lu W, Li L, Zhang H, Lee YK, Chen W, Zhao J. Both living and dead Faecalibacterium prausnitzii alleviate house dust mite-induced allergic asthma through the modulation of gut microbiota and short-chain fatty acid production. J Sci Food Agric. 2021;101:5563-5573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 199. | Tajima T, Hata K, Kusakabe J, Miyauchi H, Badshah JS, Kageyama S, Zhao X, Kim SK, Tsuruyama T, Kirchner VA, Watanabe T, Uemoto S, Hatano E. Anti-complement 5 antibody ameliorates antibody-mediated rejection after liver transplantation in rats. Front Immunol. 2023;14:1186653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 200. | Liu Z, Rütten S, Buhl EM, Zhang M, Liu J, Rojas-González DM, Mela P. Development of a Silk Fibroin-Small Intestinal Submucosa Small-Diameter Vascular Graft with Sequential VEGF and TGF-β1 Inhibitor Delivery for In Situ Tissue Engineering. Macromol Biosci. 2023;23:e2300184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (4)] |
| 201. | Tardalkar K, Patil S, Chaudhari L, Kshersagar J, Damle M, Kawale A, Bhamare N, Desai V, Pathak N, Gaikwad V, Joshi MG. Decellularized small intestine scaffolds: a potential xenograft for restoration of intestinal perforation. Tissue Barriers. 2024;12:2290940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 202. | Millán O, Ruiz P, Orts L, Ferré P, Crespo G, Santana M, Fortuna V, Quintairos L, Navasa M, Brunet M. Monitoring of miR-181a-5p and miR-155-5p Plasmatic Expression as Prognostic Biomarkers for Acute and Subclinical Rejection in de novo Adult Liver Transplant Recipients. Front Immunol. 2019;10:873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 203. | Guidicelli G, Guerville F, Lepreux S, Wiebe C, Thaunat O, Dubois V, Visentin J, Bachelet T, Morelon E, Nickerson P, Merville P, Taupin JL, Couzi L. Non-Complement-Binding De Novo Donor-Specific Anti-HLA Antibodies and Kidney Allograft Survival. J Am Soc Nephrol. 2016;27:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 204. | Bai Y, Di L, Liu W, Zhou F, Ma J, Meng G, Li M, Sun G. Elucidating immune cell dynamics in chronic lung allograft dysfunction: A comprehensive single-cell transcriptomic study. Comput Biol Med. 2024;173:108254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 205. | Patwardhan S, Gunes ME, Manell E, Hong J, Jordache P, Chauhan I, Almesallmy A, Mulder H, Ekanayake-Alper D, Hajosi D, Ko HM, Shanmugarajah K, Cetrulo CL, Nowak G, Sachs DH, Sykes M, Weiner J. Durable mixed chimerism may permit subsequent immunosuppression-free intestinal transplantation-A proof-of-principle study. Am J Transplant. 2025;25:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 206. | Crosby K, Long KD, Fu J. Chimerism-Mediated Tolerance in Intestinal Transplantation. Gastroenterol Clin North Am. 2024;53:413-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 207. | Naeimi Kararoudi M, Hejazi SS, Elmas E, Hellström M, Naeimi Kararoudi M, Padma AM, Lee D, Dolatshad H. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Gene Editing Technique in Xenotransplantation. Front Immunol. 2018;9:1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 208. | Caravaca AS, Gallina AL, Tarnawski L, Shavva VS, Colas RA, Dalli J, Malin SG, Hult H, Arnardottir H, Olofsson PS. Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proc Natl Acad Sci U S A. 2022;119:e2023285119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 209. | Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, Tihy I, Tartour E. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 210. | Talvard-Balland N, Braun LM, Dixon KO, Zwick M, Engel H, Hartmann A, Duquesne S, Penter L, Andrieux G, Rindlisbacher L, Acerbis A, Ehmann J, Köllerer C, Ansuinelli M, Rettig A, Moschallski K, Apostolova P, Brummer T, Illert AL, Schramm MA, Cheng Y, Köttgen A, Duyster J, Menssen HD, Ritz J, Blazar BR, Boerries M, Schmitt-Gräff A, Sariipek N, Van Galen P, Buescher JM, Cabezas-Wallscheid N, Pahl HL, Pearce EL, Soiffer RJ, Wu CJ, Vago L, Becher B, Köhler N, Wertheimer T, Kuchroo VK, Zeiser R. Oncogene-induced TIM-3 ligand expression dictates susceptibility to anti-TIM-3 therapy in mice. J Clin Invest. 2024;134:e177460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 211. | Karimi MA, Bryson JL, Richman LP, Fesnak AD, Leichner TM, Satake A, Vonderheide RH, Raulet DH, Reshef R, Kambayashi T. NKG2D expression by CD8+ T cells contributes to GVHD and GVT effects in a murine model of allogeneic HSCT. Blood. 2015;125:3655-3663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 212. | Lee GR. The Balance of Th17 versus Treg Cells in Autoimmunity. Int J Mol Sci. 2018;19:730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 614] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 213. | Alhosseini MN, Ebadi P, Karimi MH, Migliorati G, Cari L, Nocentini G, Heidari M, Soleimanian S. Therapy with regulatory T-cell infusion in autoimmune diseases and organ transplantation: A review of the strengths and limitations. Transpl Immunol. 2024;85:102069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 214. | Xu H, Mehta AK, Gao Q, Lee HJ, Ghali A, Guasch A, Kirk AD. B cell reconstitution following alemtuzumab induction under a belatacept-based maintenance regimen. Am J Transplant. 2020;20:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 215. | Crickx E, Weill JC, Reynaud CA, Mahévas M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. 2020;97:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 216. | Arora J, Ayyappan S, Yin C, Smith BJ, Lemke-Miltner CD, Wang Z, Farooq U, Weiner GJ. T-cell help in the tumor microenvironment enhances rituximab-mediated NK-cell ADCC. Blood. 2024;143:1816-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 217. | Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (3)] |
| 218. | Zhang JM, Huang H, Li XQ, Li SP, Zhou LX, Song SY, Zhu ZJ. FLT3(+) DC inhibits immune rejection via interaction with Treg in liver transplantation. Int Immunopharmacol. 2024;137:112289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 219. | Švajger U, Tešić N, Rožman P. Programmed death ligand 1 (PD-L1) plays a vital part in DC tolerogenicity induced by IFN-γ. Int Immunopharmacol. 2021;99:107978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 220. | Yin X, Chen S, Eisenbarth SC. Dendritic Cell Regulation of T Helper Cells. Annu Rev Immunol. 2021;39:759-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 221. | Zhang K, Guo J, Yan W, Xu L. Macrophage polarization in inflammatory bowel disease. Cell Commun Signal. 2023;21:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 222. | Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, Striz I. M1/M2 macrophages and their overlaps - myth or reality? Clin Sci (Lond). 2023;137:1067-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 368] [Reference Citation Analysis (0)] |
| 223. | Lin S, Zhou Z, Zhao H, Xu C, Guo Y, Gao S, Mei X, Tian H. TNF promotes M1 polarization through mitochondrial metabolism in injured spinal cord. Free Radic Biol Med. 2021;172:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 224. | Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 412] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 225. | McLeod JJ, Baker B, Ryan JJ. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 226. | Grenz A, Eltzschig HK. Mast cells and intestinal injury: a novel link between hypoxia and inflammation. Crit Care Med. 2013;41:2246-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 227. | Ohl K, Tenbrock K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front Immunol. 2018;9:2499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 228. | Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 229. | Okano S, Abu-Elmagd K, Kish DD, Keslar K, Baldwin WM 3rd, Fairchild RL, Fujiki M, Khanna A, Osman M, Costa G, Fung J, Miller C, Kayashima H, Hashimoto K. Myeloid-derived suppressor cells increase and inhibit donor-reactive T cell responses to graft intestinal epithelium in intestinal transplant patients. Am J Transplant. 2018;18:2544-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 230. | Xu L, Feng J, Xu X, Li X, Li X, Qian P, Luo Y, Zhao Y, Zhang M, Lin Y, Huang H. IL-17-producing γδT cells ameliorate intestinal acute graft-versus-host disease by recruitment of Gr-1(+)CD11b(+) myeloid-derived suppressor cells. Bone Marrow Transplant. 2021;56:2389-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 231. | Fischer MA, Golovchenko NB, Edelblum KL. γδ T cell migration: Separating trafficking from surveillance behaviors at barrier surfaces. Immunol Rev. 2020;298:165-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 232. | Crinier A, Viant C, Girard-Madoux M, Vivier É. [Innate lymphoid cells]. Med Sci (Paris). 2017;33:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 233. | Bielecki P, Riesenfeld SJ, Hütter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, Lian M, Amezcua Vesely MC, Kroehling L, Xu H, Slyper M, Muus C, Ludwig LS, Christian E, Tao L, Kedaigle AJ, Steach HR, York AG, Skadow MH, Yaghoubi P, Dionne D, Jarret A, McGee HM, Porter CBM, Licona-Limón P, Bailis W, Jackson R, Gagliani N, Gasteiger G, Locksley RM, Regev A, Flavell RA. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature. 2021;592:128-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 234. | Song J, Dai J, Chen X, Ding F, Ding Y, Ma L, Zhang L. Bifidobacterium mitigates autoimmune hepatitis by regulating IL-33-induced Treg/Th17 imbalance via the TLR2/4 signaling pathway. Histol Histopathol. 2024;39:623-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 235. | Devaux CA, Million M, Raoult D. The Butyrogenic and Lactic Bacteria of the Gut Microbiota Determine the Outcome of Allogenic Hematopoietic Cell Transplant. Front Microbiol. 2020;11:1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 236. | Jena PK, Sheng L, Nagar N, Wu C, Barile D, Mills DA, Wan YY. The effect of synbiotics Bifidobacterium infantis and milk oligosaccharides on shaping gut microbiota community structure and NASH treatment. Data Brief. 2018;19:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 237. | Ren C, Zhang Q, de Haan BJ, Zhang H, Faas MM, de Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci Rep. 2016;6:34561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 238. | Wang M, Feng X, Zhao Y, Lan Y, Xu H. Indole-3-acetamide from gut microbiota activated hepatic AhR and mediated the remission effect of Lactiplantibacillus plantarum P101 on alcoholic liver injury in mice. Food Funct. 2023;14:10535-10548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 239. | Yang YH, Qian W, Hou XH, Dai CB. Bifidobacterium bifidum and Bacteroides fragilis Induced Differential Immune Regulation of Enteric Glial Cells Subjected to Exogenous Inflammatory Stimulation. Inflammation. 2022;45:2388-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 240. | Wei F, Jiang H, Zhu C, Zhong L, Lin Z, Wu Y, Song L. The co-fermentation of whole-grain black barley and quinoa improves murine cognitive impairment induced by a high-fat diet via altering gut microbial ecology and suppressing neuroinflammation. Food Funct. 2024;15:11667-11685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 241. | Nakajima A, Vogelzang A, Maruya M, Miyajima M, Murata M, Son A, Kuwahara T, Tsuruyama T, Yamada S, Matsuura M, Nakase H, Peterson DA, Fagarasan S, Suzuki K. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J Exp Med. 2018;215:2019-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 242. | Bambou JC, Giraud A, Menard S, Begue B, Rakotobe S, Heyman M, Taddei F, Cerf-Bensussan N, Gaboriau-Routhiau V. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J Biol Chem. 2004;279:42984-42992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 243. | Hagel C, Krasemann S, Löffler J, Püschel K, Magnus T, Glatzel M. Upregulation of Shiga toxin receptor CD77/Gb3 and interleukin-1β expression in the brain of EHEC patients with hemolytic uremic syndrome and neurologic symptoms. Brain Pathol. 2015;25:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 244. | Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Uzal FA, Rood JI, McClane BA. Toxin plasmids of Clostridium perfringens. Microbiol Mol Biol Rev. 2013;77:208-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 245. | Wu HY, Kuo CJ, Chou CH, Ho MW, Chen CL, Hsu TS, Chen YC, Chiang-Ni C, Chen YM, Chiu CH, Lai CH. Clostridium innocuum, an emerging pathogen that induces lipid raft-mediated cytotoxicity. Virulence. 2023;14:2265048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 246. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 786] [Article Influence: 87.3] [Reference Citation Analysis (1)] |
| 247. | Park SR, Kim DJ, Han SH, Kang MJ, Lee JY, Jeong YJ, Lee SJ, Kim TH, Ahn SG, Yoon JH, Park JH. Diverse Toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect Immun. 2014;82:1914-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 248. | Park OJ, Ha YE, Sim JR, Lee D, Lee EH, Kim SY, Yun CH, Han SH. Butyrate potentiates Enterococcus faecalis lipoteichoic acid-induced inflammasome activation via histone deacetylase inhibition. Cell Death Discov. 2023;9:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 249. | Nii T, Maeda Y, Motooka D, Naito M, Matsumoto Y, Ogawa T, Oguro-Igashira E, Kishikawa T, Yamashita M, Koizumi S, Kurakawa T, Okumura R, Kayama H, Murakami M, Sakaguchi T, Das B, Nakamura S, Okada Y, Kumanogoh A, Takeda K. Genomic repertoires linked with pathogenic potency of arthritogenic Prevotella copri isolated from the gut of patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82:621-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 250. | de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 1531] [Article Influence: 382.8] [Reference Citation Analysis (0)] |
| 251. | Karim MR, Iqbal S, Mohammad S, Morshed MN, Haque MA, Mathiyalagan R, Yang DC, Kim YJ, Song JH, Yang DU. Butyrate's (a short-chain fatty acid) microbial synthesis, absorption, and preventive roles against colorectal and lung cancer. Arch Microbiol. 2024;206:137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 252. | Mir RA, Schaut RG, Allen HK, Looft T, Loving CL, Kudva IT, Sharma VK. Cattle intestinal microbiota shifts following Escherichia coli O157:H7 vaccination and colonization. PLoS One. 2019;14:e0226099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/