Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.110836
Revised: August 9, 2025
Accepted: August 22, 2025
Published online: October 27, 2025
Processing time: 107 Days and 23.8 Hours
Liver abscess is a serious hepatic infectious disease for which percutaneous drainage has become the preferred treatment method due to its minimally in
To analyze risk factors affecting prognosis after percutaneous drainage in liver abscess patients with fatty liver.
A retrospective analysis of 165 liver abscess patients with fatty liver who under
The poor prognosis group had older age (65.2 ± 11.8 years vs 56.1 ± 11.9 years, P < 0.001), higher diabetes prevalence (70.5% vs 47.2%, P = 0.008), elevated inflammatory markers, and lower serum albumin (26.8 ± 4.2 g/L vs 32.1 ± 5.6 g/L, P < 0.001). Moderate-to-severe fatty liver was more prevalent in the poor prognosis group (68.2% vs 38.0%, P = 0.001). Multivariate analysis identified five indepen
Advanced age, diabetes history, large abscess, hypoalbuminemia, and moderate-to-severe fatty liver are inde
Core Tip: This retrospective study of 165 liver abscess patients with fatty liver undergoing percutaneous drainage identified five independent risk factors for poor prognosis: Advanced age (≥ 65 years), diabetes history, large abscess diameter (≥ 8 cm), hypoalbuminemia (< 30 g/L), and moderate-to-severe fatty liver disease.
- Citation: Zhang L, Yang WF, Wu HD, Zhu HH. Risk factors analysis for poor prognosis after percutaneous drainage in liver abscess patients with fatty liver. World J Gastrointest Surg 2025; 17(10): 110836
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/110836.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.110836
Liver abscess is a serious infectious disease of the liver, classified etiologically into bacterial liver abscess and amoebic liver abscess. Bacterial liver abscess is more common in China, mostly caused by biliary tract infection, portal vein bacteremia, or hematogenous dissemination, with pathogens mainly including Escherichia coli, Klebsiella species, and Streptococcus. In recent years, with the acceleration of population aging, rising incidence of metabolic diseases such as diabetes, and continuous development of medical technology, the characteristics and treatment patterns of liver abscess have been changing. Traditional surgical treatment has gradually been replaced by minimally invasive percutaneous drainage, which has become the preferred treatment method for liver abscess due to its advantages of minimal trauma, rapid recovery, and fewer complications[1,2].
Meanwhile, non-alcoholic fatty liver disease (NAFLD) has become one of the most common chronic liver diseases globally, with its prevalence showing a rapid upward trend worldwide. According to epidemiological surveys, the prevalence of NAFLD in Chinese adults has reached 29.2%, and this proportion continues to grow with lifestyle changes and westernization of dietary patterns. The pathogenesis of fatty liver is complex, mainly related to various factors including insulin resistance, lipid metabolism disorders, oxidative stress, and inflammatory responses. Abnormal fat deposition in hepatocytes not only affects normal liver metabolic function but may also lead to decreased liver immune function and fibrosis progression, thereby increasing the risk of various liver diseases[3-5].
With the continuous rise in fatty liver prevalence, the number of patients with liver abscess combined with fatty liver has also shown a significant increasing trend clinically. This phenomenon is not accidental but results from the combined action of multiple factors. First, fatty liver patients often have comorbidities such as diabetes, obesity, and metabolic syndrome, which are themselves risk factors for liver abscess development. Diabetic patients, due to poor blood glucose control, have impaired immune function and are more susceptible to various infectious diseases, including liver abscess. Second, fatty liver may affect the liver's anti-infection ability through multiple mechanisms: Fat deposition leads to hepatocyte dysfunction, affecting the liver's detoxification and immune functions; chronic inflammatory states associated with fatty liver may weaken local immune responses; fatty liver patients often have intestinal flora imbalance, increasing the risk of bacterial translocation and bloodstream infection[6,7].
More importantly, the presence of fatty liver may significantly affect the treatment outcomes and prognosis of liver abscess patients. The liver tissue structure of fatty liver patients undergoes changes, with hepatocyte swelling and fiber proliferation, which may affect drainage effectiveness; metabolic abnormalities and inflammatory responses associated with fatty liver may delay wound healing and prolong treatment time. Additionally, fatty liver patients often have poor nutritional status and decreased protein synthesis function, with weakened body repair capacity. These factors may all lead to slow recovery after percutaneous drainage and increase the risk of complications[8-10].
Currently, there have been numerous reports on factors affecting prognosis after percutaneous drainage in patients with simple liver abscess, generally considering patient age, underlying diseases, abscess size and number, pathogen type, and laboratory indicators as important prognostic factors. However, prognostic studies specifically targeting the special patient population of liver abscess combined with fatty liver are relatively limited, with existing studies mostly being small-sample, single-center studies lacking systematic risk factor analysis. Particularly, the specific impact of fatty liver severity on liver abscess prognosis and its independent predictive value in multivariate analysis still require more clinical evidence support[11-13].
In clinical practice, physicians often formulate treatment plans based on experience and traditional prognostic assessment indicators, but for liver abscess patients with concurrent fatty liver, targeted prognostic assessment tools and treatment strategies are lacking. In this situation, some patients may receive inappropriate treatment plans due to inaccurate prognostic assessment, leading to poor treatment outcomes or increased complications. Therefore, in-depth research on risk factors affecting prognosis after percutaneous drainage in liver abscess patients with fatty liver, and establishing targeted prognostic assessment models, is of great significance for guiding clinical decision-making, optimizing treatment plans, and improving patient prognosis[14].
In summary, with the continuous rise in fatty liver incidence and changes in population structure, liver abscess combined with fatty liver has become an important clinical issue that cannot be ignored. In-depth understanding of prognostic factors in this special patient population not only helps improve clinicians' understanding of disease comple
This study adopted a retrospective cohort study design, collecting and analyzing clinical data of liver abscess patients with fatty liver who were hospitalized in the Department of Infectious Diseases, Hepatobiliary Surgery, and Interventional Radiology at our hospital from January 2020 to April 2024. The study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (approval number: 2024094-JY). Due to the retrospective design and anonymization of patient data, the ethics committee waived the requirement for informed consent. All patients meeting the criteria and screened through the Hospital Information System and Picture Archiving and Communication System during the study period were consecutively included.
Inclusion criteria included: Age ≥ 18 years, regardless of gender; clinically confirmed liver abscess with typical symptoms (fever, right upper quadrant pain, etc.) and abnormal laboratory tests (elevated white blood cells, abnormal inflammatory markers), imaging examination confirming intrahepatic space-occupying lesions consistent with abscess characteristics; imaging-confirmed fatty liver with liver computed tomography (CT) plain scan density 10 HU lower than spleen density, or magnetic resonance imaging examination indicating liver fat fraction ≥ 5%; all received percutaneous drainage treatment; complete clinical data and follow-up information; hospitalization time ≥ 7 days.
Exclusion criteria included patients with other serious liver diseases (viral hepatitis, cirrhosis, liver cancer, autoim
Patients were grouped according to clinical outcomes within 30 days post-procedure: The good prognosis group was defined as patients whose clinical symptoms completely resolved within 30 days after percutaneous drainage, laboratory indicators returned to normal or significantly improved, imaging examination showed abscess cavity disappearance or significant reduction, no serious complications occurred, successful discharge and completed follow-up; the poor prognosis group was defined as patients who experienced serious complications (major bleeding, bile leak, abdominal infection, septic shock, etc.), treatment failure requiring repeat percutaneous drainage, conservative treatment failure requiring surgical conversion, in-hospital death, or prolonged hospitalization ≥ 30 days without significant improvement within 30 days post-procedure.
Our follow-up protocol included structured outpatient visits at 1 week, 2 weeks, and 1 month post-discharge, primarily assessed through clinical visits, laboratory examinations, and patient interviews. Imaging follow-up was conducted 2-4 weeks post-discharge using abdominal CT or ultrasound to assess abscess cavity changes and treatment response. For high-risk patients or those with concerning clinical findings, follow-up frequency was increased to weekly intervals until complete symptom resolution. Follow-up assessments included clinical symptom evaluation, vital signs monitoring, laboratory tests (complete blood count, inflammatory markers, liver function), and imaging studies as clinically indicated.
The observation indicators collected included patient demographics, laboratory examination indicators at admission (blood routine, biochemical indicators, inflammatory markers, coagulation function), imaging characteristics (abscess characteristics and fatty liver severity assessment), surgery-related indicators, and pathogenic examination results.
Fatty liver severity was classified based on CT liver-spleen density differences according to established criteria: Mild fatty liver was defined as liver CT values 10 HU-20 HU lower than spleen density, moderate fatty liver as 20 HU-30 HU lower, and severe fatty liver as > 30 HU lower than spleen density. This classification system has been validated against histopathological grading and is widely used in clinical practice for non-invasive assessment of hepatic steatosis severity.
The primary endpoint was the incidence of poor prognostic events within 30 days post-procedure, and secondary endpoints included hospitalization time, drainage tube indwelling time, various recovery times, and complication rates.
All clinical data were extracted from electronic medical records by two independent investigators using standardized data collection forms. Discrepancies were resolved through discussion with a third senior investigator. Missing data were handled using complete case analysis, and sensitivity analyses were performed to assess the impact of missing data on study conclusions. Imaging studies were reviewed by experienced radiologists blinded to clinical outcomes to ensure objective assessment of fatty liver severity and abscess characteristics.
Statistical analysis was performed using SPSS 26.0 software. Continuous variables were expressed as mean ± SD or median (interquartile range), and group comparisons were made using t-test or Mann-Whitney U test. Categorical variables were expressed as number (percentage), and group comparisons were made using χ² test or Fisher's exact test. Univariate analysis was used to screen potential risk factors, and variables with P < 0.1 were included in multivariate logistic regression analysis to determine independent risk factors. All statistical tests were two-sided, with P < 0.05 considered statistically significant.
Based on the table data, this study compared baseline characteristics between patients with good prognosis (n = 121) and poor prognosis (n = 44), revealing several significant differences. Demographic and clinical characteristics showed that the poor prognosis group was significantly older (65.2 ± 11.8 years vs 56.1 ± 11.9 years, P = 0.001) and had higher body mass index (28.9 ± 4.6 kg/m² vs 26.1 ± 3.8 kg/m², P = 0.001). These patients also experienced longer duration from onset to admission (12.4 ± 5.2 days vs 8.7 ± 4.1 days, P = 0.001), higher admission temperature (39.4 ± 0.9 °C vs 38.7 ± 0.7 °C, P = 0.001), and more comorbidities (3.2 ± 1.5 vs 1.7 ± 1.1, P = 0.001). The poor prognosis group had lower education levels, with 81.8% having junior high school education or below compared to 74.4% in the good prognosis group (P = 0.031). Lifestyle factors revealed that poor prognosis patients had significantly higher rates of smoking (61.4% vs 42.1%, P = 0.005) and alcohol consumption (68.2% vs 51.2%, P = 0.008). No significant differences were found in gender distribution or residence location. These findings indicate that advanced age, obesity, multiple comorbidities, delayed medical care, lower education, and adverse lifestyle habits are significantly associated with poor patient prognosis (Table 1).
| Characteristics | Good prognosis group (n = 121) | Poor prognosis group (n = 44) | Test statistic | P value |
| Gender | χ² = 0.901 | 0.342 | ||
| Male | 77 (63.6) | 26 (59.1) | ||
| Female | 44 (36.4) | 18 (40.9) | ||
| Age (years) | 56.1 ± 11.9 | 65.2 ± 11.8 | t = 4.852 | 0.001 |
| Body mass index (kg/m²) | 26.1 ± 3.8 | 28.9 ± 4.6 | t = 4.176 | 0.001 |
| Duration from onset to admission (days) | 8.7 ± 4.1 | 12.4 ± 5.2 | t = 5.063 | 0.001 |
| Temperature on admission (°C) | 38.7 ± 0.7 | 39.4 ± 0.9 | t = 5.547 | 0.001 |
| Number of comorbidities | 1.7 ± 1.1 | 3.2 ± 1.5 | t = 7.329 | 0.001 |
| Smoking history | 51 (42.1) | 27 (61.4) | χ² = 7.841 | 0.005 |
| Alcohol consumption history | 62 (51.2) | 30 (68.2) | χ² = 7.026 | 0.008 |
| Residence | χ² = 0.628 | 0.428 | ||
| Urban | 84 (69.4) | 24 (54.5) | ||
| Rural | 58 (47.9) | 20 (45.5) | ||
| Education level | χ² = 4.658 | 0.031 | ||
| Junior high school or below | 90 (74.4) | 36 (81.8) | ||
| High school or above | 52 (43.0) | 8 (18.2) |
In terms of organ dysfunction, chronic kidney disease showed a significantly higher proportion in the poor prognosis group (27.3% vs 11.6%, P = 0.004), and the prevalence of biliary disease was also markedly increased (43.2% vs 28.9%, P = 0.017). These organ dysfunctions may directly impact patients' overall health status, drug metabolism capacity, and disease recovery potential. Regarding cardiopulmonary system diseases, although the poor prognosis group showed numerically higher rates of hypertension (47.7% vs 45.5%), coronary heart disease (22.7% vs 18.2%), and chronic obstructive pulmonary disease (13.6% vs 9.9%), none of these differences reached statistical significance (P values of 0.296, 0.271, and 0.324, respectively). Overall, diabetes mellitus, dyslipidemia, chronic kidney disease, and biliary disease are important comorbidities significantly associated with poor prognosis. These conditions predominantly involve crucial metabolic and excretory functions and may synergistically contribute through multiple mechanisms to decreased bodily reserve function, thereby affecting overall prognosis (Table 2).
| Comorbidity | Total (n = 165) | Good prognosis (n = 121) | Poor prognosis (n = 44) | χ² | P value |
| Diabetes mellitus | 98 (59.4) | 67 (55.4) | 31 (70.5) | 6.983 | 0.008 |
| Hypertension | 76 (46.1) | 55 (45.5) | 21 (47.7) | 1.095 | 0.296 |
| Coronary heart disease | 32 (19.4) | 22 (18.2) | 10 (22.7) | 1.211 | 0.271 |
| Dyslipidemia | 84 (50.9) | 56 (46.3) | 28 (63.6) | 7.542 | 0.006 |
| Chronic kidney disease | 26 (15.8) | 14 (11.6) | 12 (27.3) | 8.294 | 0.004 |
| Chronic obstructive pulmonary disease | 18 (10.9) | 12 (9.9) | 6 (13.6) | 0.974 | 0.324 |
| Biliary disease | 54 (32.7) | 35 (28.9) | 19 (43.2) | 5.673 | 0.017 |
Significant differences were found in systemic constitutional symptoms. The poor prognosis group experienced significantly higher rates of night sweats (65.9% vs 41.3%, P = 0.008) and weight loss (54.5% vs 35.5%, P = 0.045), su
| Variable | Total (n = 165) | Good prognosis (n = 121) | Poor prognosis (n = 44) | χ²/t | P value |
| Right-upper-quadrant pain | 138 (83.6) | 102 (84.3) | 36 (81.8) | 0.168 | 0.682 |
| Nausea & vomiting | 110 (66.7) | 78 (64.5) | 32 (72.7) | 0.872 | 0.350 |
| Fatigue | 122 (73.9) | 86 (71.1) | 36 (81.8) | 1.571 | 0.210 |
| Chills | 126 (76.4) | 89 (73.6) | 37 (84.1) | 1.725 | 0.189 |
| Loss of appetite | 140 (84.8) | 100 (82.6) | 40 (90.9) | 1.476 | 0.226 |
| Abdominal distension | 85 (51.5) | 59 (48.8) | 26 (59.1) | 1.176 | 0.279 |
| Jaundice | 46 (27.9) | 29 (24.0) | 17 (38.6) | 3.060 | 0.080 |
| Cough & sputum | 60 (36.4) | 40 (33.1) | 20 (45.5) | 2.006 | 0.182 |
| Night sweats | 79 (47.3) | 50 (41.3) | 29 (65.9) | 7.086 | 0.008 |
| Weight loss | 67 (40.6) | 43 (35.5) | 24 (54.5) | 4.024 | 0.045 |
| Right-shoulder/back pain | 38 (23.0) | 25 (20.7) | 13 (29.5) | 1.236 | 0.266 |
Inflammatory markers were markedly elevated in the poor prognosis group, with higher white blood cell count (18.2 ± 6.4 × 109/L vs 13.7 ± 4.8 × 109/L), C-reactive protein (145.8 ± 42.3 mg/L vs 98.6 ± 35.7 mg/L), and procalcitonin levels (8.9 ± 3.6 ng/mL vs 4.2 ± 2.1 ng/mL), all P < 0.001, indicating severe systemic inflammation. Liver function and nutritional status showed significant impairment in poor prognosis patients. Serum albumin was markedly lower (26.8 ± 4.2 g/L vs 32.1 ± 5.6 g/L), while total bilirubin (45.7 ± 18.3 μmol/L vs 28.4 ± 12.1 μmol/L) and alanine aminotransferase (89.4 ± 28.7 U/L vs 62.3 ± 21.4 U/L) were significantly elevated, all P < 0.001. Hematologic parameters revealed concerning abnormalities in the poor prognosis group, including lower hemoglobin (106.8 ± 18.2 g/L vs 118.4 ± 16.7 g/L), reduced platelet count (156.7 ± 45.3 × 109/L vs 198.2 ± 52.8 × 109/L), and prolonged prothrombin time (16.8 ± 2.9 seconds vs 14.2 ± 2.1 seconds), all P < 0.001. These findings demonstrate that poor prognosis is associated with severe systemic inflammation, compromised liver function, and hematologic abnormalities (Table 4).
| Parameter (unit) | Good prognosis (n = 121) | Poor prognosis (n = 44) | t/χ² | P value |
| White blood cells (× 109/L) | 13.7 ± 4.8 | 18.2 ± 6.4 | t = 4.62 | < 0.001 |
| C-reactive protein (mg/L) | 98.6 ± 35.7 | 145.8 ± 42.3 | t = 6.31 | < 0.001 |
| Procalcitonin (ng/mL) | 4.2 ± 2.1 | 8.9 ± 3.6 | t = 7.54 | < 0.001 |
| Serum albumin (g/L) | 32.1 ± 5.6 | 26.8 ± 4.2 | t = 5.74 | < 0.001 |
| Total bilirubin (μmol/L) | 28.4 ± 12.1 | 45.7 ± 18.3 | t = 5.93 | < 0.001 |
| Alanine aminotransferase (U/L) | 62.3 ± 21.4 | 89.4 ± 28.7 | t = 5.48 | < 0.001 |
| Hemoglobin (g/L) | 118.4 ± 16.7 | 106.8 ± 18.2 | t = 3.89 | < 0.001 |
| Platelet count (× 109/L) | 198.2 ± 52.8 | 156.7 ± 45.3 | t = 4.27 | < 0.001 |
| Prothrombin time (s) | 14.2 ± 2.1 | 16.8 ± 2.9 | t = 5.12 | < 0.001 |
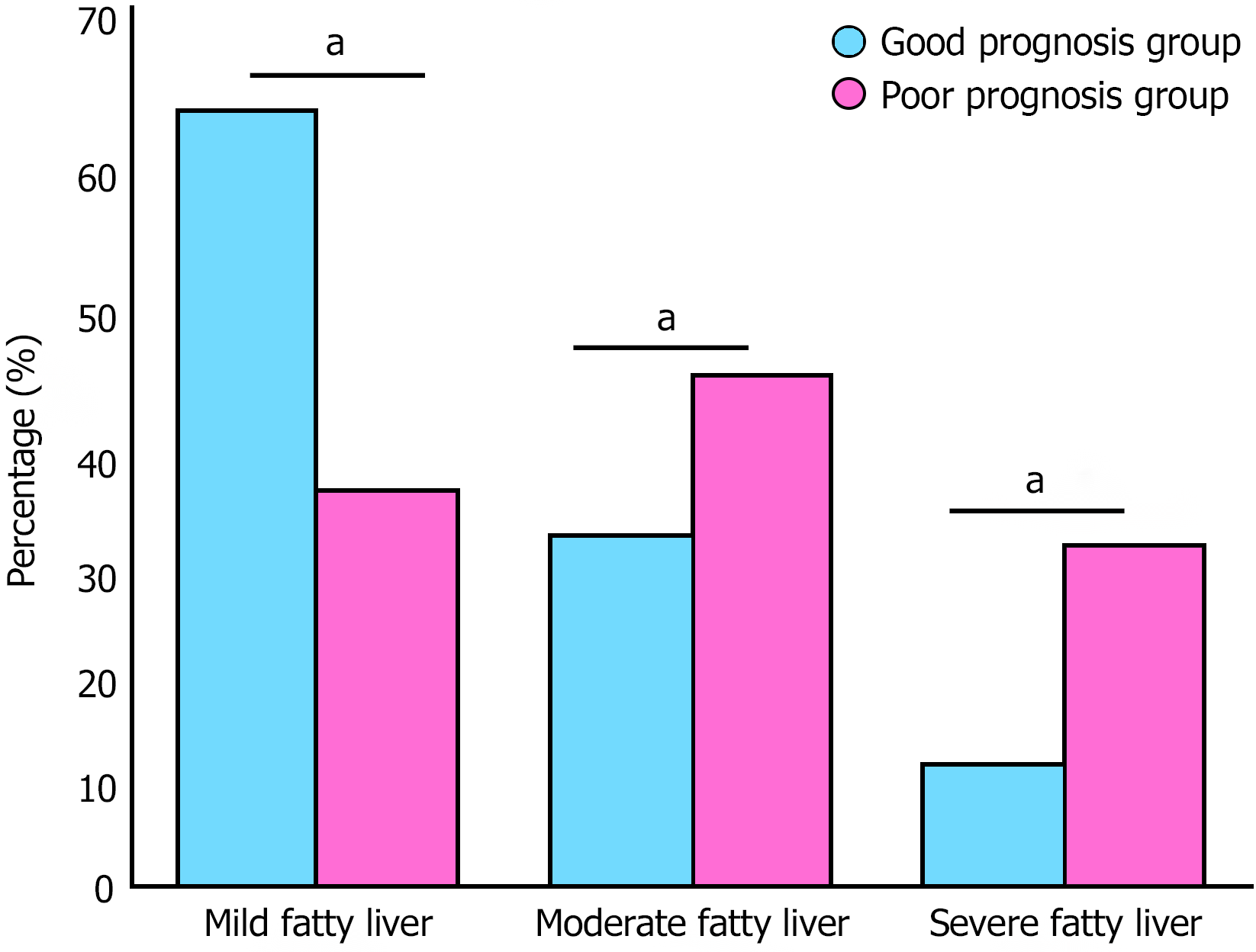
The distribution of fatty liver severity among 165 patients demonstrated a distinct gradient pattern. Based on liver-spleen density difference assessment on non-contrast CT imaging, mild fatty liver was predominant with 90 cases (54.6%), followed by moderate fatty liver with 51 cases (30.9%), and severe fatty liver with 24 cases (14.5%). Further stratified analysis revealed that fatty liver severity was significantly correlated with patient prognosis. The proportion of moderate to severe fatty liver (moderate + severe combined) was markedly higher in the poor prognosis group compared to the good prognosis group (Figure 1).
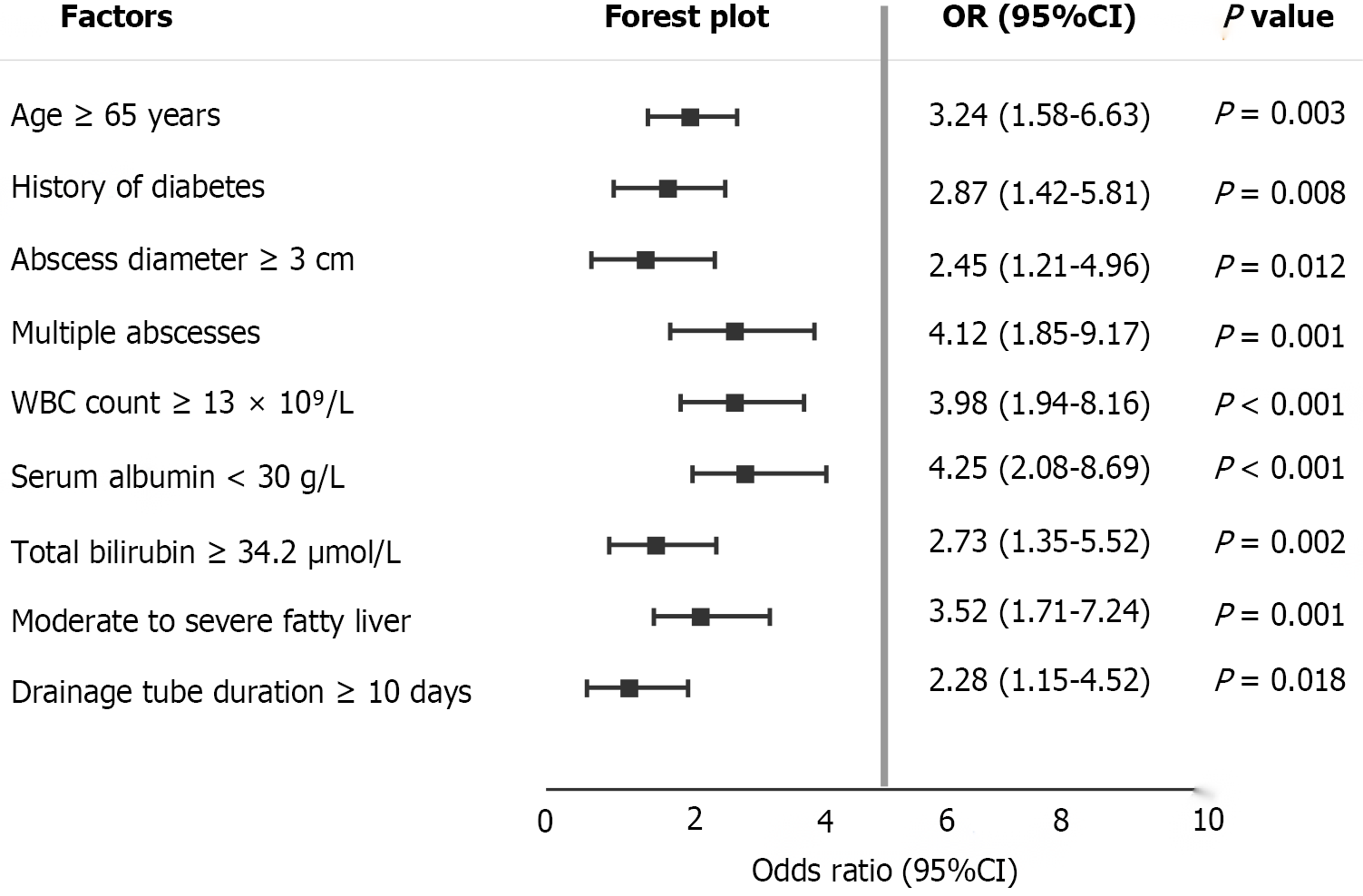
This study systematically evaluated potential risk factors affecting prognosis after percutaneous drainage in liver abscess patients with fatty liver through univariate analysis. The analysis results showed that multiple demographic characteristics, clinical features, laboratory indicators, and imaging parameters were significantly associated with poor prognosis. Regarding demographics and underlying diseases, patients aged ≥ 65 years had significantly increased risk of poor prognosis (OR = 3.24, 95%CI: 1.58-6.63, P = 0.003), and diabetes history was an important prognostic factor (OR = 2.87, 95%CI: 1.42-5.81, P = 0.008), reflecting the adverse effects of decreased body reserve function in elderly patients and diabetes-related immune dysfunction on prognosis. Regarding abscess characteristics, abscess diameter ≥ 8 cm (OR = 2.45, 95%CI: 1.21-4.96, P = 0.012) and multiple abscesses (OR = 4.12, 95%CI: 1.85-9.17, P = 0.001) were both closely related to poor prognosis, suggesting that lesion extent and complexity are key factors determining treatment effectiveness. Laboratory indicator analysis showed that white blood cell count ≥ 15 × 109/L (OR = 3.98, 95%CI: 1.94-8.16, P < 0.001) reflects severe systemic inflammatory response, serum albumin < 30 g/L (OR = 4.25, 95%CI: 2.08-8.69, P < 0.001) indicates malnutrition and impaired liver synthesis function, and total bilirubin ≥ 34.2 μmol/L (OR = 2.73, 95%CI: 1.35-5.52, P = 0.002) indicates the degree of liver function damage. All these indicators were significantly associated with poor prognosis. Imaging assessment showed that moderate-to-severe fatty liver (OR = 3.52, 95%CI: 1.71-7.24, P = 0.001) was an important prognostic predictor, while drainage tube duration ≥ 10 days (OR = 2.28, 95%CI: 1.15-4.52, P = 0.018) among treatment-related factors was also associated with poor prognosis, possibly reflecting poor treatment response and disease complexity. These univariate analysis results laid the foundation for subsequent multivariate analysis and helped identify independent prognostic risk factors (Figure 2).
Liver abscess, as a serious infectious disease of the liver, has undergone significant changes in disease patterns and treatment strategies over the past several decades. Traditionally, liver abscess was mainly seen in malnourished, immunocompromised populations, but with the acceleration of population aging and lifestyle changes, the patient population with liver abscess presents new characteristics. Particularly in recent years, with the rapid rise in incidence of metabolic syndrome, diabetes, and NAFLD, the number of patients with liver abscess combined with fatty liver has shown an obvious increasing trend, attracting widespread attention from clinicians[15-17].
NAFLD has become the most common chronic liver disease globally, with its prevalence showing rapid upward trends in both developed and developing countries. In China, the prevalence of NAFLD in adults has exceeded 25%, reaching over 30% in some regions. The pathogenesis of fatty liver is complex and diverse, mainly involving insulin resistance, lipid metabolism disorders, oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, and gut-liver axis abnormalities. These pathophysiological processes not only lead to abnormal lipid accumulation in hepatocytes but more importantly cause profound changes in the liver microenvironment, including liver sinusoidal endothelial cell dys
The impact of fatty liver on liver immune function is multifaceted. First, the local immune microenvironment of the liver in fatty liver patients undergoes significant changes. Kupffer cells, as the main innate immune cells of the liver, have significantly decreased phagocytic function and antigen presentation ability in fatty liver states, directly affecting the liver's ability to clear pathogenic microorganisms. Second, chronic low-grade inflammatory states associated with fatty liver lead to sustained elevation of pro-inflammatory factors such as tumor necrosis factor-α, interleukin-6, and interleukin-1β. Although this inflammatory environment may appear hyperactivated during acute infection, chronic inflammation actually leads to immune tolerance and decreased immune function. Additionally, fatty liver patients often have intestinal flora imbalance and impaired intestinal barrier function, making bacterial endotoxins and other harmful substances more likely to enter portal circulation, increasing the risk of liver infection[21-24].
The impact of fatty liver on liver structure and function is equally significant. At the histological level, fatty liver presents as massive lipid droplet deposition in hepatocytes, leading to enlarged hepatocyte volume, loose cytoplasm, and loose intercellular connections. These structural changes not only affect normal liver metabolic function but may also influence drug distribution and metabolism within liver tissue. At the vascular level, intrahepatic hemodynamics change in fatty liver patients, with increased liver sinusoidal capillarization and abnormal vascular endothelial function. These changes may affect the effective concentration of therapeutic drugs such as antibiotics at infection sites. At the cellular molecular level, autophagy function in hepatocytes is impaired in fatty liver states, with decreased intracellular waste clearance capacity and weakened antioxidant capacity. All these factors may affect the liver's resistance to infection and tissue repair capacity[25-27].
From a clinical treatment perspective, the presence of fatty liver brings new challenges to liver abscess treatment. Percutaneous drainage, as the current gold standard for liver abscess treatment, depends not only on abscess characteristics but also on the host's overall condition. Changes in liver tissue texture in fatty liver patients may affect puncture tract healing and drainage tube fixation; coagulation dysfunction associated with fatty liver may increase the risk of puncture-related bleeding; common malnutrition in fatty liver patients, particularly protein-energy malnutrition, affects wound healing and tissue repair processes[28,29].
Diabetes, as an important comorbidity of fatty liver, plays a crucial role in the development and progression of liver abscess. Due to chronic hyperglycemic states, diabetic patients have impaired neutrophil function, decreased complement system activity, and abnormal cellular and humoral immune functions. These immune deficiencies make diabetic patients more susceptible to various infectious diseases. Additionally, diabetes-related microvascular and macrovascular diseases affect local blood supply, which is unfavorable for infection control and tissue repair. Hyperglycemic environments also provide favorable nutritional conditions for bacteria, promoting bacterial proliferation and aggravating infection severity[30,31].
Age factors play an important role in liver abscess patients with fatty liver. Elderly patients, due to immunosenescence phenomena, experience varying degrees of decline in the number and function of various immune cells, including T cell subset imbalance, weakened B cell antibody production capacity, and decreased natural killer cell activity. Elderly patients usually have poor nutritional status, with common protein intake deficiency and vitamin and trace element deficiencies. These nutritional factor deficiencies directly affect immune function and tissue repair capacity. Additionally, elderly patients often have multiple chronic diseases such as hypertension, coronary heart disease, and chronic kidney disease. These diseases and their therapeutic drugs may all affect the body's response to infection and recovery process[32].
From a pathophysiological perspective, the development and progression of liver abscess on the basis of fatty liver follow special patterns. Liver microcirculation disorders in fatty liver patients may lead to local ischemia and hypoxia, providing favorable conditions for anaerobic bacterial growth and reproduction. Cholestasis phenomena associated with fatty liver may affect the antibacterial effect of bile, increasing the risk of biliary-origin infections. Portal hypertension and collateral circulation formation common in fatty liver patients may change intrahepatic blood flow distribution, affecting antibiotic distribution and concentration in liver tissue[33].
This study has several important limitations that must be acknowledged. First, the single-center retrospective design may limit the generalizability of our findings, as patient characteristics, treatment protocols, and outcomes may vary across different institutions and healthcare systems. The retrospective nature of data collection introduces potential selection bias and information bias, as certain clinical variables may have been incompletely documented or missing. Second, we were unable to collect several potentially important variables that could influence outcomes, including specific antibiotic regimens, treatment duration, patient adherence to therapy, and detailed glycemic control parameters (such as glycated hemoglobin levels) in diabetic patients.
This study demonstrates that fatty liver disease significantly impacts the prognosis of liver abscess patients undergoing percutaneous drainage. Five independent risk factors were identified: Advanced age (≥ 65 years), diabetes history, large abscess diameter (≥ 8 cm), hypoalbuminemia (< 30 g/L), and moderate-to-severe fatty liver disease.
| 1. | Bhandari A, Koppen J, Wastney T, Hacking C. A systematic review and meta-analysis of spectral CT to differentiate focal liver lesions. Clin Radiol. 2023;78:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Chen P, Yang T, Shi P, Shen J, Feng Q, Su J. Benefits and safety of photodynamic therapy in patients with hilar cholangiocarcinoma: A meta-analysis. Photodiagnosis Photodyn Ther. 2022;37:102712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Gadour E, Awad A, Hassan Z, Shrwani KJ, Miutescu B, Okasha HH. Diagnostic and therapeutic role of endoscopic ultrasound in liver diseases: A systematic review and meta-analysis. World J Gastroenterol. 2024;30:742-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 4. | Gupta P, Singh KK, Balodhi A, Jain K, Deeba F, Salam N. Prevalence of Amoebiasis and Associated Complications in India: A Systematic Review. Acta Parasitol. 2022;67:947-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Li S, Yu S, Peng M, Qin J, Xu C, Qian J, He M, Zhou P. Clinical features and development of Sepsis in Klebsiella pneumoniae infected liver abscess patients: a retrospective analysis of 135 cases. BMC Infect Dis. 2021;21:597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Hussain I, Ishrat S, Ho DCW, Khan SR, Veeraraghavan MA, Palraj BR, Molton JS, Abid MB. Endogenous endophthalmitis in Klebsiella pneumoniae pyogenic liver abscess: Systematic review and meta-analysis. Int J Infect Dis. 2020;101:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Namikawa H, Oinuma KI, Yamada K, Kaneko Y, Kakeya H, Shuto T. Predictors of hypervirulent Klebsiella pneumoniae infections: a systematic review and meta-analysis. J Hosp Infect. 2023;134:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Namikawa H, Oinuma KI, Yamada K, Kaneko Y, Kakeya H, Shuto T. Differences in severity of bacteraemia caused by hypermucoviscous and non-hypermucoviscous Klebsiella pneumoniae. Int J Antimicrob Agents. 2023;61:106767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Al-Sayaghi KM, Alhujaily M, Zaky MK, Alhasan AS, Babikir TB, Alnehmi FS, Abdalrahman HH, Abdelmalik MAA, Ali AM, Fadlalmola HA, Swamy DSV. Percutaneous needle aspiration versus catheter drainage in the management of liver abscess: an updated systematic review and meta-analysis. ANZ J Surg. 2023;93:840-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Gu L, Ai T, Ye Q, Wang Y, Wang H, Xu D. Development and validation of a clinical-radiomics nomogram for the early prediction of Klebsiella pneumoniae liver abscess. Ann Med. 2024;56:2413923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10:2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 13. | Indiran V. Turquoise sign of liver abscess. QJM. 2022;114:891-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Jolobe OMP. Gastrointestinal Origins of Pyogenic Liver Abscess. Am J Med. 2020;133:e210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Kumari D. Hepatic Abscess after Liver-Directed Therapy. J Vasc Interv Radiol. 2023;34:1285-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (1)] |
| 17. | Lin JW, Chen CT, Hsieh MS, Lee IH, Yen DH, Cheng HM, Hsu TF. Percutaneous catheter drainage versus percutaneous needle aspiration for liver abscess: a systematic review, meta-analysis and trial sequential analysis. BMJ Open. 2023;13:e072736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 18. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11:1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 19. | Lobo J, Gallo C, Mejuto P. Liver abscess caused by Arcanobacterium haemolyticum. Rev Esp Enferm Dig. 2020;112:954-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | McNeil T, Daniel S, Gordon DL. Management of pyogenic liver abscess: a South Australian experience. ANZ J Surg. 2020;90:2274-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Meister P, Irmer H, Paul A, Hoyer DP. Therapy of pyogenic liver abscess with a primarily unknown cause. Langenbecks Arch Surg. 2022;407:2415-2422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 23. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 24. | Pandak N, Mahdi AS, Al Majrafi A, Molay M, Deenadayalan SS, Khamis F, Al Balushi Z. Characteristics of Pyogenic Liver Abscess: Experience of a single centre in Oman. Sultan Qaboos Univ Med J. 2022;22:257-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Paramythiotis D, Karakatsanis A, Karlafti E, Bareka S, Psoma E, Hatzidakis AA, Michalopoulos A. Pyogenic Liver Abscess Complicating Acute Cholecystitis: Different Management Options. Medicina (Kaunas). 2022;58:782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Ribeiro Da Costa R, Andres A, Huttner B. [Pyogenic liver abscesses]. Rev Med Suisse. 2020;16:1822-1826. [PubMed] [DOI] [Full Text] |
| 27. | Salehi O, Chen A, Cevik J. Cryptogenic pyogenic liver abscess caused by Fusobacterium necrophorum in an immunocompetent patient. BMJ Case Rep. 2023;16:e255460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Sia T, Yong E. Pasteurella multocida bacteraemia with liver abscess. BMJ Case Rep. 2024;17:e258386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 30. | Suzuki H, Kidder I, Tanaka T, Goto M. Incidence of Colorectal Cancer in Patients Diagnosed With Pyogenic Liver Abscess. JAMA Netw Open. 2023;6:e2348218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 31. | Wakabayashi SI, Kimura T, Tanaka N, Pham J, Tanaka T, Higuchi S, Kobayashi J, Umemura T, Iijima A. Invasive liver abscess syndrome accompanied by spondylodiscitis: a case report and review of the literature. Clin J Gastroenterol. 2020;13:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Wang JL, Hsu CR, Wu CY, Lin HH. Diabetes and obesity and risk of pyogenic liver abscess. Sci Rep. 2023;13:7922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 33. | Wong MF, Ho MP. Endogenous endophthalmitis associated with pyogenic liver abscess. Am J Emerg Med. 2024;76:235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/