Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.110543
Revised: June 27, 2025
Accepted: August 6, 2025
Published online: October 27, 2025
Processing time: 137 Days and 19.1 Hours
Postoperative nausea-vomiting (PONV) occurs often after surgery performed under general anesthesia. Liberal fluid treatments are a low-cost and a low side-effect alternative to pharmacological treatment in the prevention of PONV.
To compare the effects of perioperative liberal and restrictive fluid therapy on PONV and recovery after laparoscopic cholecystectomy.
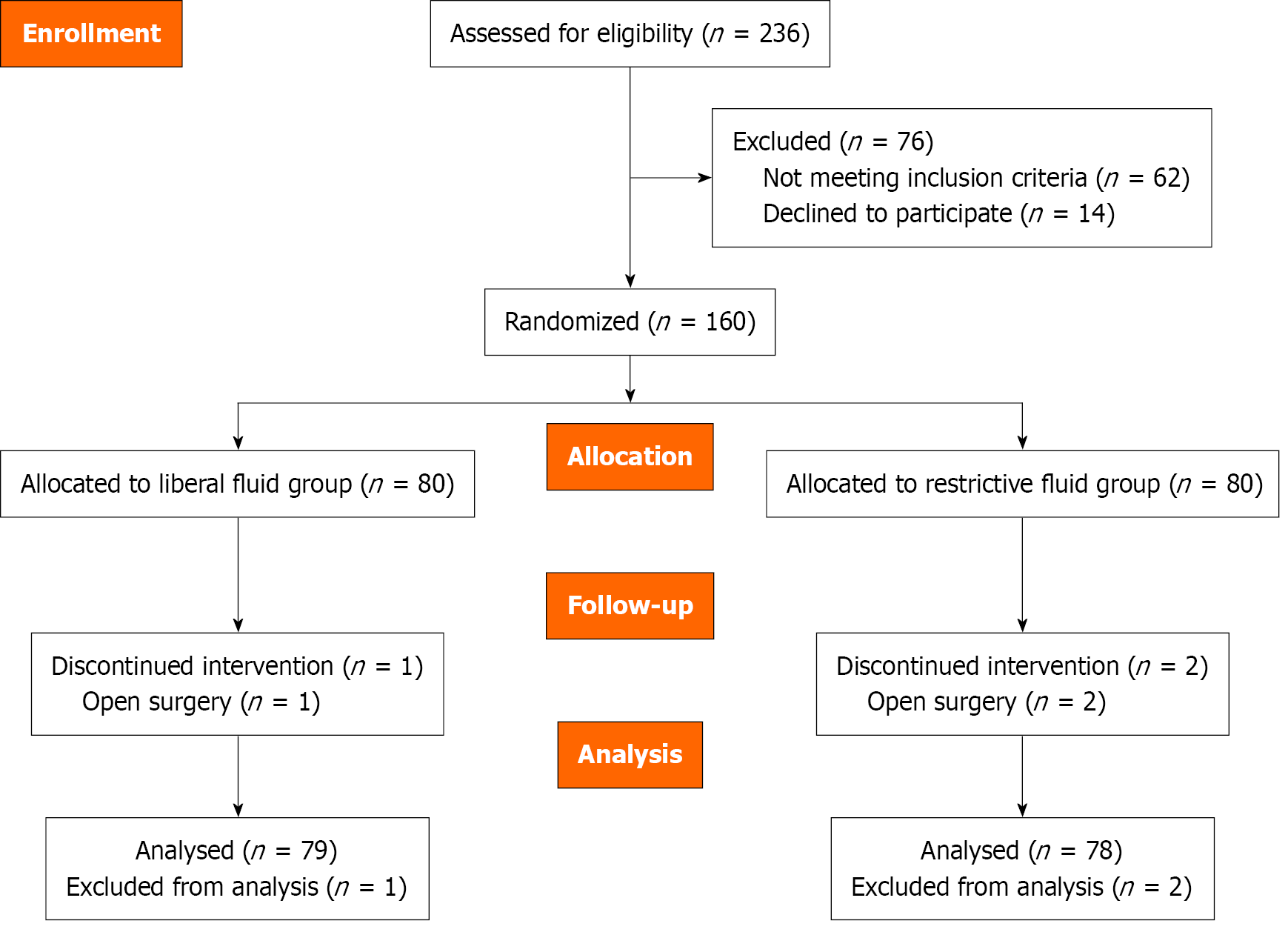
A total of 160 patients were randomly allocated to two groups: Liberal fluid treatment group (group L), and restrictive fluid treatment group (group R). Three patients were excluded. Ringer’s lactate infusion was administered intravenously as follows: 20 mL/kg/hour to group L, and 4 mL/kg/hour to group R. The pri
The incidence of PONV was significantly lower in group L (38.0%) compared with group R (70.5%) (relative risk: 0.54, 95% confidence interval: 0.39-0.74, P < 0.001). The quality of recovery-15 scale scores for overall satisfaction were significantly higher in group L compared with group R [137 (135-141) vs 135 (130-139), P = 0.006].
Perioperative liberal fluid therapy reduced the incidence of PONV and improved the quality of postoperative recovery in patients undergoing laparoscopic cho
Core Tip: Liberal fluid therapy may be an alternative to pharmacological therapy in the prevention of postoperative nausea-vomiting (PONV) because of its low cost and few side effects. This study investigated the effect of liberal fluid therapy and restrictive fluid therapy in the prevention of PONV after laparoscopic cholecystectomy. Perioperative liberal fluid therapy reduced the incidence of PONV and improved the quality of postoperative recovery in patients undergoing laparoscopic cholecystectomy.
- Citation: Korkusuz M, Et T. Effect of perioperative restrictive and liberal fluid regimens on postoperative nausea-vomiting and quality of recovery in laparoscopic cholecystectomy. World J Gastrointest Surg 2025; 17(10): 110543
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/110543.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.110543
Postoperative nausea-vomiting (PONV) frequently occurs after surgeries performed under general anesthesia. PONV can lead to a longer stay in the postoperative care unit (PACU), delayed discharge from the hospital, and an increase in readmission to the hospital. It can also cause serious complications, including suture opening, aspiration of stomach contents, dehydration, and electrolyte disorders[1]. PONV prophylaxis is more cost-effective than placebo in patients who are high risk due to the incremental costs associated with PONV[2]. Anti-emetics as pharmacological prophylaxis of PONV have limited effects on preventing nausea and vomiting along with side effects and increased hospital costs[2]. Liberal fluid treatments are inexpensive and have few side effects. They are potential alternatives to pharmacological treatment in the prevention of PONV[3-5].
Laparoscopic cholecystectomy (LC) has a high risk of PONV. If anti-emetic prophylaxis is not administered to patients undergoing LC, the incidence of PONV is 53%-75%[6,7]. Currently, there are no studies comparing the effects of liberal and restrictive fluid treatments on PONV and the quality of recovery in LC. Therefore, we aimed to compare the effects of perioperative liberal and restrictive fluid treatments on PONV and recovery quality in patients undergoing LC. Postope
The Karamanoglu Mehmetbey University Faculty of Medicine Ethics Committee, No. 10-2023/14 approved this study. This study was registered at clinicaltrials.gov. The registration identification number is NCT06197659. All study pro
The study exclusion criteria were defined as the presence of chronic liver or kidney disease, chronic gastrointestinal system disease, a history of abdominal surgery, chronic opioid use, active smoking, a history of PONV, a history of motion sickness, the presence of diabetes, epilepsy, heart valve disease, congestive heart disease, pregnancy, body mass index (BMI) ≥ 35, the use of anti-emetic drugs within 24 hours before surgery, the development of intraoperative hyper
All patients started fasting at midnight on the day of the surgery. Preoperative fluid status was standardized by providing 200 mL of drinking water at 6:00 am. The sample option in the Minitab® software (version 21; Minitab, Inc, State College, PA, United States) randomly assigned patients into two groups as follows: Liberal fluid treatment group (group L), and restrictive fluid treatment group (group R). Group L received 20 mL/kg/hour of Ringer’s lactate solution (RL). Group R received 4 mL/kg/hour of RL. The consulting anesthesia specialist was informed of the results of the randomization process via the sealed envelope method. Patients were not apprised of their group assignments. Fluid infusion bags and infusion pumps were covered with opaque sheets to blind the surgeon. Additionally, the researchers who collected the data were blind to the group assignments.
The allocated RL amount was infused at a fixed rate for 1 hour immediately before anesthesia induction. At the end of 1 hour, maintenance fluid of 2 mL/kg/hour was administered to all patients until discharge. During the surgery, standard monitoring, including electrocardiography, oxygen saturation, capnography, and noninvasive blood pressure mea
The operation was performed using the standard 4-port surgical technique. Pneumoperitoneum was created at 12 mmHg intraabdominal pressure with non-humidified carbon dioxide.
All patients received 1 g paracetamol every 6 hours and 50 mg dexketoprofen every 12 hours for postoperative analgesia. The first dose of the drugs was administered after anesthesia induction. PONV and Numerical Rating Scale (NRS) were evaluated by a PACU nurse in the PACU and by a ward nurse in the general ward. Both nurses were blinded to the patient groups. The NRS values were recorded at 15 minutes, 30 minutes, and 60 minutes after surgery in the PACU and at 2 hours, 6 hours, 12 hours, and 24 hours after surgery in the general ward. When the Aldrete score was > 9 and the NRS questioning was completed, the patients were discharged from the PACU to the ward.
Patients were informed of the NRS characteristics during the preoperative period and of the necessity to request analgesia when the NRS for pain at rest was ≥ 4. When NRS was ≥ 4, intravenous tramadol (50 mg) was administered. When pain at rest was still ≥ 4 after 30 minutes, additional analgesia (50 mg tramadol) was administered. If the com
Postoperative nausea was defined as an unpleasant feeling associated with the wish to vomit, and postoperative vomiting was defined as the attempt to expel the stomach contents (vomiting or retching). PONV was defined as any nausea, vomiting, or both. Patients were informed before surgery that they should request anti-emetics when they had characteristics of PONV and developed PONV. Patients who requested anti-emetics were intravenously administered 10 mg metoclopramide. If the patient experienced PONV within 4 hours of metoclopramide administration, 8 mg ondansetron was administered. A full response was defined as no nausea or vomiting without any administration and the use of no anti-emetic drugs within 24 hours after surgery, which was the endpoint of the primary outcome. PONV was only assessed during the 24-hour postoperative hospitalization since patients without complications were discharged 24 hours after surgery.
One of the primary outcomes of this study was the postoperative quality of recovery-15 score (QoR-15) that was administered 24 hours after surgery. The QoR-15 evaluates 15 parameters and is accepted as the optimum scale for patient-centered measurement of postoperative recovery[8]. The total points of the QoR-15 range from 0-150 with 150 points indicating excellent recovery. The personnel collecting the QoR-15 data were trained before the start of the study. The QoR-15 data were collected in the native language of the patient at 24 hours postoperatively.
Preoperative demographic characteristics, the time to standing unassisted, and the time to the first oral intake of solid food were recorded for each patient. The time to first mobilization was defined as the time of first standing unassisted in the general ward. Early postoperative mobilization was encouraged, and first oral intake of solid food was permitted 6 hours after surgery. To evaluate the effect on thirst, the patients were permitted to drink water on request after fully awakening postoperatively. The time when the patient first demanded oral fluid was described as the thirst time. If there were no clinical complaints, the decision for discharge was made by the surgeon. Otherwise discharge procedures were applied after 24 hours of observation in the surgical clinic.
The primary aim of the study was to determine the significance of restrictive and liberal fluid therapies on the rates of PONV following LC in adult patients. A power analysis was performed using the two-sample z-test for independent proportions. Using literature data obtained from the studies of Erhan et al[7] and Ashok et al[9] and expert opinions with the aim of obtaining 80% power and a significance level of α = 0.05, the power analysis for a one-tailed hypothesis revealed that a total of at least 154 patients (a minimum of 77 patients in each group) would be required to detect a statistically significant difference of a 19% reduction in the rates between the two groups. The data from 157 patients were analyzed. The determined sample size calculated through priori power analysis was achieved in each group in our study.
Statistical analyses were performed using SPSS software (Version 22; IBM Corp., Armonk, NY, United States). Descriptive statistics for categorical data were reported using frequencies (n) and percentages (%). Numerical data showing normal distribution were reported as mean ± SD values, and non-normal data were reported as median (quartile 1, quartile 3) values. The χ2 test was used to compare proportions between categorical variables. Relative risks were reported with 95% confidence intervals (CIs). The conformity of quantitative data to normal distribution was assessed using the Kolmogorov-Smirnov test, histograms, and Q-Q plots, and the Levene’s test was used to assess the homogeneity of variances. When parametric test assumptions were met, the Student’s t-test was used to compare continuous data between two independent groups. When assumptions were not met, the Mann-Whitney U test was used.
Univariate and multivariate binary logistic regression analyses were conducted to assess the effect of certain risk factors on the incidence of PONV, and odds ratios were reported with 95%CIs. Factors found to be significant with a P < 0.10 in the univariate analysis were included in the multivariate analysis model. In the logistic regression analyses, the enter method was used for variable entry, and the results of the final multivariate model were reported. In all other analyses, a value of P < 0.05 was accepted as statistically significant. Although the sample size calculation was based on a one-tailed hypothesis due to a directional assumption derived from prior evidence suggesting that liberal fluid therapy reduces PONV[7,9], all subsequent statistical analyses were conducted using two-tailed tests to account for potential differences in both directions and ensure methodological neutrality.
A total of 157 patients were recruited, with 78 patients (49.7%) in group R and 79 patients (50.3%) in group L. The total patient group comprised 42 males (26.8%) and 115 females (73.2%) with a mean age of 50.95 ± 11.10 years (minimum-maximum: 24-65) and mean BMI of 28.57 ± 3.76 (21.8-36.4). The mean anesthesia duration was 54.98 ± 7.61 minutes (40-75 minutes), and the mean surgical duration was 40.97 ± 7.00 minutes (25-60 minutes). PONV was observed in 85 patients (54.1%).
The comparisons of the demographic characteristics of the two groups are presented in Table 1. There were no significant differences between the groups for mean age, weight, height, and BMI (P = 0.690, P = 0.592, P = 0.885, P = 0.255, respectively). The sex ratios and American Society of Anesthesiologists scores were similar in both groups (P = 0.755, P = 0.799, respectively). The mean duration of surgery and mean duration of anesthesia were not significantly different between the groups (P = 0.370, P = 0.498, respectively).
| Variable | Group L3, n = 79 | Group R4, n = 78 | P value |
| Age, years | 50.6 ± 9.9 | 51.3 ± 12.2 | 0.6901 |
| Weight, kg | 77.2 ± 13.0 | 78.3 ± 12.4 | 0.5921 |
| Height, cm | 165.3 ± 9.2 | 165.1 ± 8.0 | 0.8851 |
| BMI, kg/m2 | 28.2 ± 3.8 | 28.9 ± 3.8 | 0.2551 |
| Sex, male/female | 22/57 | 20/58 | 0.7552 |
| ASA, I/II/III | 28/45/6 | 25/45/8 | 0.7992 |
| Duration of surgery, minutes | 40.5 ± 8.4 | 41.5 ± 5.2 | 0.3701 |
| Duration of anesthesia in minutes | 54.6 ± 8.9 | 55.4 ± 6.1 | 0.4981 |
The comparisons of postoperative data between the groups are presented in Table 2. The incidence of PONV was statistically significantly lower in group L than group R (relative risk: 0.54, 95%CI: 0.39-0.74, P < 0.001). PONV was not observed in 23 patients (29.5%) in group R and in 49 patients (62.0%) in group L. The time to first rescue anti-emetic was significantly longer in group R than in group L (P = 0.037). The time to oral fluid intake was significantly longer in group L than in group R (P < 0.001). No statistically significant difference was observed between the two groups for the requirement for rescue analgesia, time to first rescue analgesia, total tramadol consumption, first mobilization time, and time of first oral solid food intake (P = 0.413, P = 0.927, P = 0.069, P = 0.123, P = 0.566, respectively).
| Variable | Group L4, n = 79 | Group R5, n = 78 | Relative risk or mean difference (95%CI) | P value |
| Incidence of PONV, n (%) | 30 (38.0) | 55 (70.5) | 0.54 (0.39-0.74) | < 0.0011 |
| Time to first rescue anti-emetic, hours | 3.93 ± 2.44 | 5.49 ± 4.32 | 1.55 (-0.14 to 3.26) | 0.0372 |
| Time to demand oral fluids, minutes | 225.1 ± 94.4 | 164.4 ± 82.5 | -60.7 (-88.6 to 32.7) | < 0.0012 |
| Requirement of rescue analgesia, n (%) | 53 (67.1%) | 57 (73.1%) | 0.91 (0.74-1.12) | 0.4131 |
| Time to first rescue analgesia, hours | 2.94 ± 1.78 | 2.98 ± 2.59 | 0.039 (-0.81 to 0.88) | 0.9272 |
| Total tramadol consumption, mg | 50 (50-100) (68.8 ± 28.1) | 50 (50-50) (59.6 ± 19.9) | -9.21 (-18.3 to -0.06) | 0.0693 |
| First mobilization time, hours | 2.91 ± 1.42 | 3.27 ± 1.46 | 0.35 (-0.09 to 0.81) | 0.1232 |
| Time to first oral solid food intake, hours | 6.68 ± 0.91 | 6.78 ± 1.21 | 0.099 (-0.24 to 0.43) | 0.5662 |
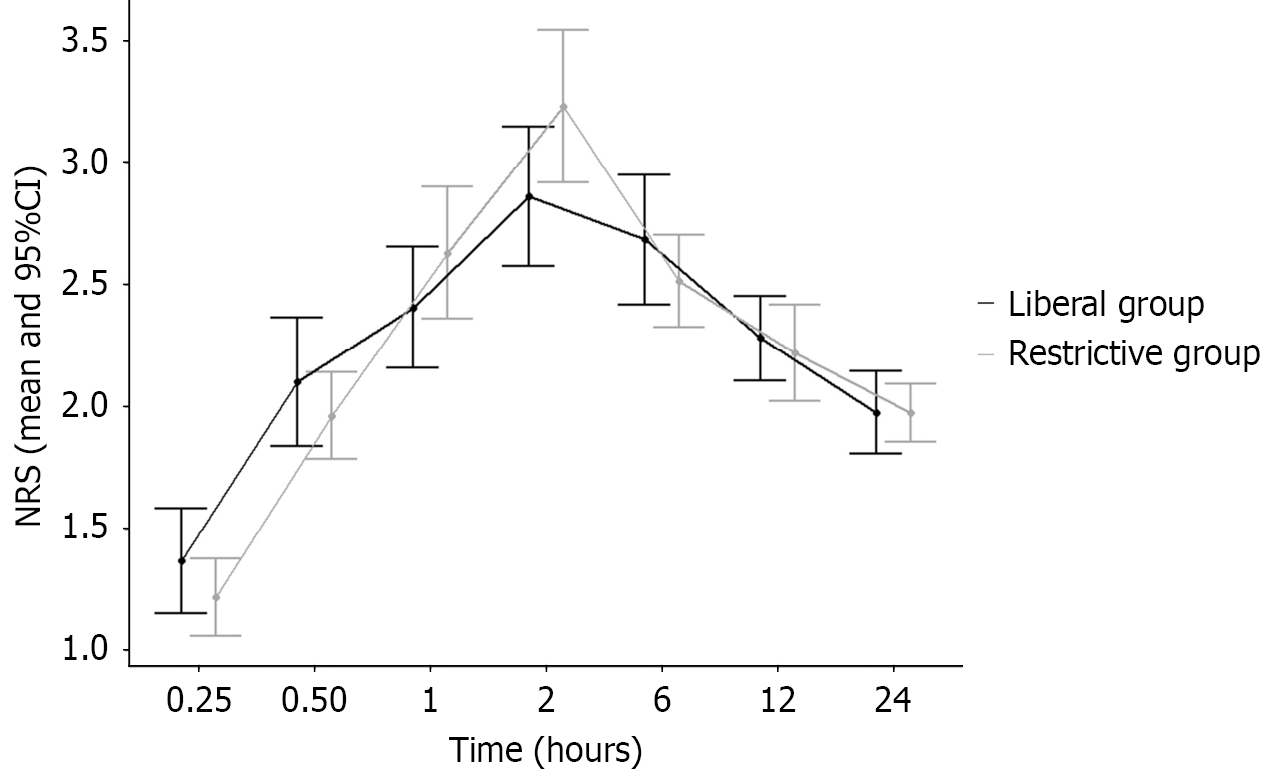
The comparisons of the NRS scores between the groups are presented in Table 3. There were no significant differences in the NRS scores at all timepoints for both groups (P > 0.05 for all). The time-dependent changes in mean values and 95%CIs are shown in Figure 2.
| Variables1 | Group L2, n = 79 | Group R3, n = 78 | P value |
| 15 minutes | 1 (1.0-2.0) (1.4 ± 1) | 1 (1.0-2.0) (1.2 ± 0.7) | 0.342 |
| 30 minutes | 2 (1.0-3.0) (2.1 ± 1.2) | 2 (1.0-3.0) (2.0 ± 0.8) | 0.844 |
| 60 minutes | 2 (2.0-3.0) (2.4 ± 1.1) | 2 (2.0-3.0) (2.6 ± 1.2) | 0.247 |
| 2 hours | 2 (2.0-4.0) (2.9 ± 1.3) | 3 (2.0-4.0) (3.2 ± 1.3) | 0.061 |
| 6 hours | 2 (2.0-3.0) (2.7 ± 1.2) | 2 (2.0-3.0) (2.5 ± 0.8) | 0.676 |
| 12 hours | 2 (2.0-3.0) (2.3 ± 0.8) | 2 (2.0-2.3) (2.2 ± 0.9) | 0.448 |
| 24 hours | 2 (2.0-2.0) (2.0 ± 0.8) | 2 (2.0-2.0) (2.0 ± 0.5) | 0.592 |
The comparisons of the postoperative QoR-15 scale scores of the two groups are presented in Table 4. Patients in group L reported significantly higher scores for the items related to feeling rested, able to return to work or usual home activities, feeling comfortable and in control, and nausea or vomiting (P = 0.022, P = 0.023, P = 0.047, P < 0.001, respectively). No statistically significant difference was found between the groups for the other items (P > 0.05 for all; Table 4). The QoR-15 scale overall satisfaction scores were significantly higher in group L than in group R (P = 0.006).
| Factor1 | Group L3, n = 79, median (Q1, Q3) | Group R4, n = 78, median (Q1, Q3) | Median difference | P value |
| Able to breathe easy | 10 (9.0-10.0) | 10 (9.0-10.0) | 0 | 0.143 |
| Able to enjoy food | 10 (10.0-10.0) | 10 (9.0-10.0) | 0 | 0.097 |
| Feeling rested | 9 (9.0-10.0) (9.09 ± 0.91) | 9 (8.0-10.0) (8.58 ± 1.31) | 0 | 0.0222 |
| Had good sleep | 9 (8.0-10.0) | 9 (8.0-10.0) | 0 | 0.053 |
| Able to look after personal toilet and hygiene unaided | 10 (9.0-10.0) | 10 (9.0-10.0) | 0 | 0.735 |
| Able to communicate with family or friends | 10 (10.0-10.0) | 10 (10.0-10.0) | 0 | 0.747 |
| Getting support from hospital doctors and nurses | 10 (10.0-10.0) | 10 (10.0-10.0) | 0 | 0.990 |
| Able to return to work or usual home activities | 6 (6.0-7.0) (6.32 ± 1.23) | 6 (5.0-7.0) (5.77 ± 1.63) | 0 | 0.0232 |
| Feeling comfortable and in control | 9 (9.0-10.0) (9.22 ± 0.73) | 9 (9.0-9.3) (8.81 ± 1.31) | 0 | 0.0472 |
| Having a feeling of general well-being | 10 (9.0-10.0) | 9 (9.0-10.0) | 1 | 0.115 |
| Moderate pain | 7 (7.0-8.0) | 7 (7.0-8.0) | 0 | 0.760 |
| Severe pain | 9 (9.0-10.0) | 9 (9.0-10.0) | 0 | 0.843 |
| Nausea or vomiting | 10 (8.0-10.0) (9.15 ± 1.00) | 8 (7.0-9.0) (8.12 ± 1.15) | 2 | < 0.0012 |
| Feeling worried or anxious | 10 (10.0-10.0) | 10 (10.0-10.0) | 0 | 0.655 |
| Feeling sad or depressed | 10 (10.0-10.0) | 10 (10.0-10.0) | 0 | 0.758 |
| QoR-15 total score | 137 (135.0-141.0) (137.20 ± 4.88) | 135 (130.0-139.3) (134.00 ± 6.89) | 2 | 0.0062 |
The results of the univariate and multivariate binary logistic regression analyses to determine the effects of demographic and clinical risk factors on PONV incidence are presented in Table 5. The univariate model showed that age, time to request oral fluids, first mobilization time, total tramadol consumption, duration of surgery, and duration of anesthesia had no effect on PONV incidence (P > 0.05 for all; Table 5). The effect of BMI was significant (P = 0.092) in the univariate model but was not statistically significant in the multivariate analysis (P = 0.106).
| Independent variable | Univariate | Multivariate | ||
| P value | OR (95%CI) | P value | OR (95%CI) | |
| Age, years | 0.607 | 0.993 (0.965-1.021) | NA | NA |
| BMI, kg/m2 | 0.092 | 0.929 (0.854-1.012) | NA | NA |
| Female1 | < 0.001 | 4.345 (2.013-9.381) | 0.001 | 4.421 (1.862-10.490) |
| Restrictive group2 | < 0.001 | 3.906 (2.007-7.601) | < 0.001 | 4.874 (2.294-10.350) |
| Time to demand oral fluids, minutes | 0.983 | 1.000 (0.997-1.003) | NA | NA |
| First mobilization time in, hours | 0.412 | 1.096 (0.880-1.365) | NA | NA |
| Total tramadol consumption, mg | 0.320 | 1.009 (0.991-1.027) | NA | NA |
| Time to first oral solid food intake, hours | 0.001 | 1.876 (1.283-2.744) | 0.004 | 1.894 (1.230-2.919) |
| Duration of surgery, minutes | 0.229 | 1.029 (0.982-1.077) | NA | NA |
| Duration of anesthesia, minutes | 0.327 | 1.021 (0.979-1.065) | NA | NA |
The univariate analysis showed that sex, intraoperative fluids, and time to first oral solid food intake had a significant effect on PONV incidence (P < 0.001, P < 0.001, P = 0.001, respectively). After multivariate analysis, the effect of these factors on PONV incidence remained statistically significant (P = 0.001, P < 0.001, P = 0.004, respectively). According to the multivariate model results, the odds ratio for the occurrence of PONV in females compared with males was 4.42 (95%CI: 1.86-10.49, P = 0.001) for group L and 4.87 (95%CI: 2.29-10.35, P < 0.001) for group R. The time to first oral solid food intake was 1.89 (95%CI: 1.23-2.92, P = 0.004).
The incidence of PONV in patients undergoing LC is 53%-75% if anti-emetic prophylaxis is not administered[6,7]. In the current study, 70.5% of the patients in group R (restrictive fluid treatment: 4 mL/kg/hour) experienced PONV within 24 hour after surgery, confirming the rates reported in the literature. In the patients in group L (liberal fluid treatment: 20 mL/kg/hour), only 38% experienced PONV, indicating a full response rate of 62%, a decrease in the relative risk of 46%, and a 54% relative risk of PONV with liberal fluid treatment (95%CI: 0.39-0.74).
Sharma et al[10] administered 10 mL/kg, 20 mL/kg, and 30 mL/kg of RL to patients undergoing LC. They reported that vomiting and the need for rescue anti-emetics occurred in 45% of the group that received 20 mL/kg of RL, consistent with our patients in group L. In the group receiving 10 mL/kg of RL, 80% of their patients reported vomiting and the need for rescue anti-emetics. This result was similar to our patients in group R[10].
The historically reported effects of liberal fluid treatment for preventing PONV are conflicting. Some studies have shown that it improves PONV or that it has no effect[3-5,11,12]. In the current study, liberal fluid treatment resulted in a decrease in PONV incidence. Our results are also supported by a meta-analysis and systematic examination of 41 studies in the Cochrane database that showed that perioperative intravenous crystalloid supplementation could reduce PONV[13]. Although the mechanism between liberal fluid treatment and the anti-emetic effect has not been fully clarified, various mechanisms have been suggested.
A fluid deficit caused by mandatory preoperative fasting creates splanchnic vasoconstriction and mesenteric ischemia, resulting in serotonin production[14]. Serotonin is a hormone that affects nausea and vomiting, and 80% of the total serotonin in the body is found in the intestines[15]. Serotonin is a key neurotransmitter in the gastrointestinal tract and binds to visceral 5-hydroxytryptamine type 3 receptors in the gastrointestinal tract, activating vagal signals and causing chemoreceptor trigger zone activation, nausea, and vomiting. It is hypothesized that liberal intraoperative fluid administration reduces splanchnic hypoperfusion associated with hypovolemia, thereby reducing ischemia-induced serotonin release from enterochromaffin cells, which are known to activate vagal afferents and contribute to vomiting[16]. Another potential mechanism causing PONV is mediated by the anti-diuretic hormone. Anesthetic agents cause relative hy
Operation type, anesthesia type, and patient characteristics all affect PONV incidence. We standardized these variables in our study by performing the same surgery by the same surgeon, applying the same anesthetic technique, and confirming similar demographic characteristics between the two groups. The exclusion of smokers, patients who had previously experienced PONV, and patients with a history of motion sickness also contributed to standardizing the analysis. Therefore, the difference between the groups in the incidence of PONV can be attributed to the differences in the amounts of fluid administered perioperatively. To independently reveal the incidence of PONV, no prophylactic anti-emetic drugs were used because of the low efficacy compared with the cost of anti-emetic prophylaxis[18]. Additionally, fentanyl, which has anti-emetic properties, was used only during anesthesia induction, and then no opioids were used for anesthesia maintenance.
The quality of recovery after surgery, which was an important endpoint in this study, is commonly self-reported by the patient. The QoR-15 is a patient-centered questionnaire with international validity. It is widely used and generally evaluates the quality of recovery after many types of operations[19]. According to the QoR-15 scores, better quality of recovery scores, particularly for the parameter measuring nausea, were obtained in the group given liberal fluid therapy.
Holte et al[20] concluded that the application of fluid at a high dose could reduce complications, primarily nausea and pain, and could improve the recovery process after LC. Although the results seen in the current study were generally similar this study, the different fluid regimens did not have an effect on the postoperative pain scores. As expected, the restrictive fluid treatment group had an earlier mean time to requesting oral fluid than the liberal fluid treatment group. While earlier studies were opposed to early postoperative fluid intake because it could increase PONV incidence, later studies have shown that mandatory postoperative fasting did not decrease the incidence of PONV[21-23].
Although our patients in the restrictive fluid treatment group received oral fluid earlier postoperatively, they ex
According to the multivariate logistic regression analysis, the probability of experiencing PONV was 4.87-fold greater for a patient in the restrictive fluid treatment group than for a patient in the liberal fluid treatment group. The probability of PONV was 4.42-fold greater in females than in males. This result supported the findings of Apfel et al[25]. They analyzed the independent risk factors for PONV and concluded that “PONV is seen more often in females than in males”[25]. Finally, the incidence of PONV increased 1.89-fold for every hour that oral intake of solid food was delayed, indicating that patients experiencing PONV delay oral intake.
There were some limitations to this study. First, sevoflurane was used for anesthesia maintenance and fentanyl for anesthesia induction. There is currently insufficient evidence that another anesthesia regimen for LC is preferred[26]. However, the fentanyl administered to all our patients for induction was standard according to weight, and no other opioid substances were used throughout the operation. Another limitation were potential ethical issues of not administering preventive anti-emetics for LC. However, patients were required to stay overnight in the hospital and were closely monitored in the general surgical ward. Patients were treated with anti-emetics as soon as PONV symptoms were reported. This treatment strategy was acceptable for patient results and satisfaction scores when compared with prophylactic treatment[19].
One of the strengths of our study was the isolation of the effect of intraoperative fluid management strategies (liberal vs restrictive) on PONV by minimizing the influence of known confounders. To achieve this, we excluded patients with chronic systemic diseases, a personal history of PONV, motion sickness, and well-established risk factors for PONV. However, this methodological decision also introduced a limitation. By narrowing the study population to lower-risk individuals, the generalizability of our findings to the broader surgical population was reduced. These exclusion criteria were applied to enhance the internal validity of the study and allow for a clearer interpretation of the impact of fluid therapy alone. Future randomized controlled trials involving more diverse and representative patient populations are necessary to confirm the effects observed in our study and to determine the effect on patients with a higher baseline risk for PONV.
A fixed fluid administration rate of 20 mL/kg/hour was used for the liberal fluid treatment group and 4 mL/kg/hour for the restrictive fluid treatment group, and both groups received 2 mL/kg/hour as maintenance after the first hour. We acknowledge that this standardized protocol did not account for individual patient differences in fluid requirements related to age, weight, comorbidities, or intraoperative hemodynamic variability. The primary goal of this approach was to ensure consistency in fluid volumes to evaluate the isolated impact of liberal vs restrictive fluid strategies on PONV. However, we recognize that this method may reduce the physiological relevance of the intervention as individualized goal-directed fluid therapy is often based on dynamic hemodynamic parameters, such as stroke volume variation or cardiac output, which may better reflect optimal clinical practice[27].
There is no accepted definition for liberal and restrictive fluid treatment methods. The maintenance fluid rate of 2 mL/kg/hour may be considered low by some standards, particularly during laparoscopic procedures with pneumoperitoneum in which insensible losses may increase. Enhanced recovery after surgery protocols generally advocate for a maintenance rate of 1-2 mL/kg/hour, emphasizing early oral intake and rapid transition from intravenous fluids as soon as tolerated[28]. Future studies should focus on the incorporation of individualized fluid management using dynamic monitoring and flexible maintenance strategies based on intraoperative needs to enhance external validity and clinical applicability of findings.
We demonstrated that perioperative liberal fluid treatment (20 mL/kg/hour) significantly reduced the incidence of PONV after LC compared with restrictive fluid treatment (4 mL/kg/hour). Our results support the use of liberal intravenous fluid treatment as an economical alternative to prophylactic anti-emetic use to decrease the incidence of PONV after LC. In addition, perioperative liberal fluid treatment increased the quality of recovery and reduced thirst in the postoperative period.
We thank the operating room staff and general ward nurses in Karaman Training and Research Hospital for their support.
| 1. | Hsieh CY, Poon YY, Ke TY, Chiang MH, Li YY, Tsai PN, Wu SC. Postoperative Vomiting Following Laparoscopic Cholecystectomy Is Associated with Intraoperative Fluid Administration: A Retrospective Cohort Study. Int J Environ Res Public Health. 2021;18:5305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, Bergese SD, Candiotti KA, Chan MT, Davis PJ, Hooper VD, Lagoo-Deenadayalan S, Myles P, Nezat G, Philip BK, Tramèr MR; Society for Ambulatory Anesthesia. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 964] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 3. | Ali SZ, Taguchi A, Holtmann B, Kurz A. Effect of supplemental pre-operative fluid on postoperative nausea and vomiting. Anaesthesia. 2003;58:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Magner JJ, McCaul C, Carton E, Gardiner J, Buggy D. Effect of intraoperative intravenous crystalloid infusion on postoperative nausea and vomiting after gynaecological laparoscopy: comparison of 30 and 10 ml kg(-1). Br J Anaesth. 2004;93:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Maharaj CH, Kallam SR, Malik A, Hassett P, Grady D, Laffey JG. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesth Analg. 2005;100:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Naguib M, el Bakry AK, Khoshim MH, Channa AB, el Gammal M, el Gammal K, Elhattab YS, Attia M, Jaroudi R, Saddique A. Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron and metoclopramide in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind comparison with placebo. Can J Anaesth. 1996;43:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Erhan Y, Erhan E, Aydede H, Yumus O, Yentur A. Ondansetron, granisetron, and dexamethasone compared for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy : A randomized placebo-controlled study. Surg Endosc. 2008;22:1487-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Kleif J, Waage J, Christensen KB, Gögenur I. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth. 2018;120:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 9. | Ashok V, Bala I, Bharti N, Jain D, Samujh R. Effects of intraoperative liberal fluid therapy on postoperative nausea and vomiting in children-A randomized controlled trial. Paediatr Anaesth. 2017;27:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Sharma CS, Gupta V, Dixi MB, Sadhu S, Joshi N. Effect of perioperative intravenous crystalloid infusion on postoperative nausea and vomiting after laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2010;26:383-386. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | McCaul C, Moran C, O'Cronin D, Naughton F, Geary M, Carton E, Gardiner J. Intravenous fluid loading with or without supplementary dextrose does not prevent nausea, vomiting and pain after laparoscopy. Can J Anaesth. 2003;50:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Yogendran S, Asokumar B, Cheng DC, Chung F. A prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesth Analg. 1995;80:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Jewer JK, Wong MJ, Bird SJ, Habib AS, Parker R, George RB. Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting. Cochrane Database Syst Rev. 2019;3:CD012212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 412] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Smith HS, Cox LR, Smith EJ. 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann Palliat Med. 2012;1:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 16. | Andrews PL, Naylor RJ, Joss RA. Neuropharmacology of emesis and its relevance to anti-emetic therapy. Consensus and controversies. Support Care Cancer. 1998;6:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Kim MS, Chey WD, Owyang C, Hasler WL. Role of plasma vasopressin as a mediator of nausea and gastric slow wave dysrhythmias in motion sickness. Am J Physiol. 1997;272:G853-G862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Scuderi PE, James RL, Harris L, Mims GR 3rd. Antiemetic prophylaxis does not improve outcomes after outpatient surgery when compared to symptomatic treatment. Anesthesiology. 1999;90:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Myles PS, Boney O, Botti M, Cyna AM, Gan TJ, Jensen MP, Kehlet H, Kurz A, De Oliveira GS Jr, Peyton P, Sessler DI, Tramèr MR, Wu CL; StEP–COMPAC Group, Myles P, Grocott M, Biccard B, Blazeby J, Boney O, Chan M, Diouf E, Fleisher L, Kalkman C, Kurz A, Moonesinghe R, Wijeysundera D. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth. 2018;120:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 20. | Holte K, Klarskov B, Christensen DS, Lund C, Nielsen KG, Bie P, Kehlet H. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: a randomized, double-blind study. Ann Surg. 2004;240:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Kearney R, Mack C, Entwistle L. Withholding oral fluids from children undergoing day surgery reduces vomiting. Paediatr Anaesth. 1998;8:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Schreiner MS, Nicolson SC, Martin T, Whitney L. Should children drink before discharge from day surgery? Anesthesiology. 1992;76:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Radke OC, Biedler A, Kolodzie K, Cakmakkaya OS, Silomon M, Apfel CC. The effect of postoperative fasting on vomiting in children and their assessment of pain. Paediatr Anaesth. 2009;19:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017;152:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 2431] [Article Influence: 270.1] [Reference Citation Analysis (0)] |
| 25. | Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, Zhang K, Cakmakkaya OS. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 26. | Vaughan J, Nagendran M, Cooper J, Davidson BR, Gurusamy KS. Anaesthetic regimens for day-procedure laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;2014:CD009784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Miller TE, Myles PS. Perioperative Fluid Therapy for Major Surgery. Anesthesiology. 2019;130:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 28. | Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS, Gan TJ, Kennedy RH, Ljungqvist O, Lobo DN, Miller T, Radtke FF, Ruiz Garces T, Schricker T, Scott MJ, Thacker JK, Ytrebø LM, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/