Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.110540
Revised: July 12, 2025
Accepted: September 3, 2025
Published online: October 27, 2025
Processing time: 134 Days and 19 Hours
Gastric duplication cysts (GDCs) are rare congenital anomalies, and consensus guidelines for their diagnosis and management are currently lacking. We report a rare case of a GDC in a female child presenting as a submucosal tumor in the gastric antrum. Subtotal resection was achieved using endoscopic submucosal dissection (ESD), resulting in complete symptom relief and pathological confir
A 12-year-old girl presented with abdominal distension and pain for > 1 year. Gastroscopy revealed a protruding lesion approximately 30 mm in diameter in the gastric antrum. Superficial biopsies revealed moderate chronic inflammation and intestinal metaplasia. Contrast-enhanced computed tomography showed a mass protruding into the gastric lumen with homogeneous cyst wall enhancement. Endoscopic ultrasonography identified a hypoechoic mass originating from the muscularis mucosa. The patient underwent ESD for diagnosis and symptom relief. Intraoperatively, due to firm adhesion between the cyst base and the mus
Subtotal resection of GDCs using ESD demonstrates a favorable prognosis.
Core Tip: This study is the first to explore the efficacy of endoscopic submucosal dissection for subtotal resection of intraluminal gastric duplication cyst (GDC). Following near-total resection of an antral submucosal tumor using endoscopic submucosal dissection in a 12-year-old girl, symptoms completely resolved, and histopathology confirmed a GDC. We emphasize the importance of complete resection for definitive diagnosis, while 6-month follow-up confirmed that residual cyst wall preservation had no adverse impact on short-term prognosis. This finding points to a new, safe, and effective minimally invasive therapeutic strategy for GDCs.
- Citation: Zhu N, Chen MY, Li KQ, Zhang YM, Li FL, Li P, Wu J, Zou BC. Subtotal resection of gastric duplication cysts using endoscopic submucosal dissection: A case report. World J Gastrointest Surg 2025; 17(10): 110540
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/110540.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.110540
Gastric duplication cysts (GDCs) are rare congenital malformations, accounting for 2%-8% of all gastrointestinal duplications[1]. Their pathological hallmark is a cyst wall containing a complete muscular layer and a lining of gastrointestinal mucosa, most commonly occurring along the greater curvature of the stomach[2,3]. Although most GDCs are asymptomatic, potential complications include obstruction, bleeding, infection, and malignant transformation (parti
Currently, no standard diagnostic or therapeutic guidelines exist for GDCs. Surgical resection remains the primary approach. With advancements in endoscopic techniques, endoscopic submucosal dissection (ESD) has been attempted for intraluminal GDCs due to its minimally invasive nature. Endoscopic unroofing and submucosal tunneling endoscopic resection have also been reported as successful treatments for GDCs. This article reports a case of pediatric antral GDC achieving complete symptom relief and pathological confirmation after subtotal resection using ESD. It aims to explore the feasibility of endoscopic intervention and emphasizes the critical importance of complete resection for definitive GDC diagnosis.
A 12-year-old female presented to the gastroenterology department with intermittent abdominal distension and pain persisting for > 1 year.
The patient developed intermittent abdominal distension and pain over 1 year ago without an obvious precipitating factor. Symptoms often occurred after meals and were not associated with nausea, vomiting, acid reflux, or heartburn. Symptoms slightly improved with self-administered gastrointestinal prokinetics. One month prior to admission, the frequency of abdominal distension and pain episodes increased, prompting a hospital visit. The patient reported no significant weight change over the past year.
History of past illness was unremarkable.
There was no history of Helicobacter pylori infection, gastrointestinal malignancies, or genetic disorders.
Vital signs were stable, and the abdomen was soft and non-tender. There were no palpable masses or organomegaly.
Routine coagulation parameters and tumor biomarkers, including carcinoembryonic antigen and carbohydrate antigen 19-9, were within normal reference ranges.
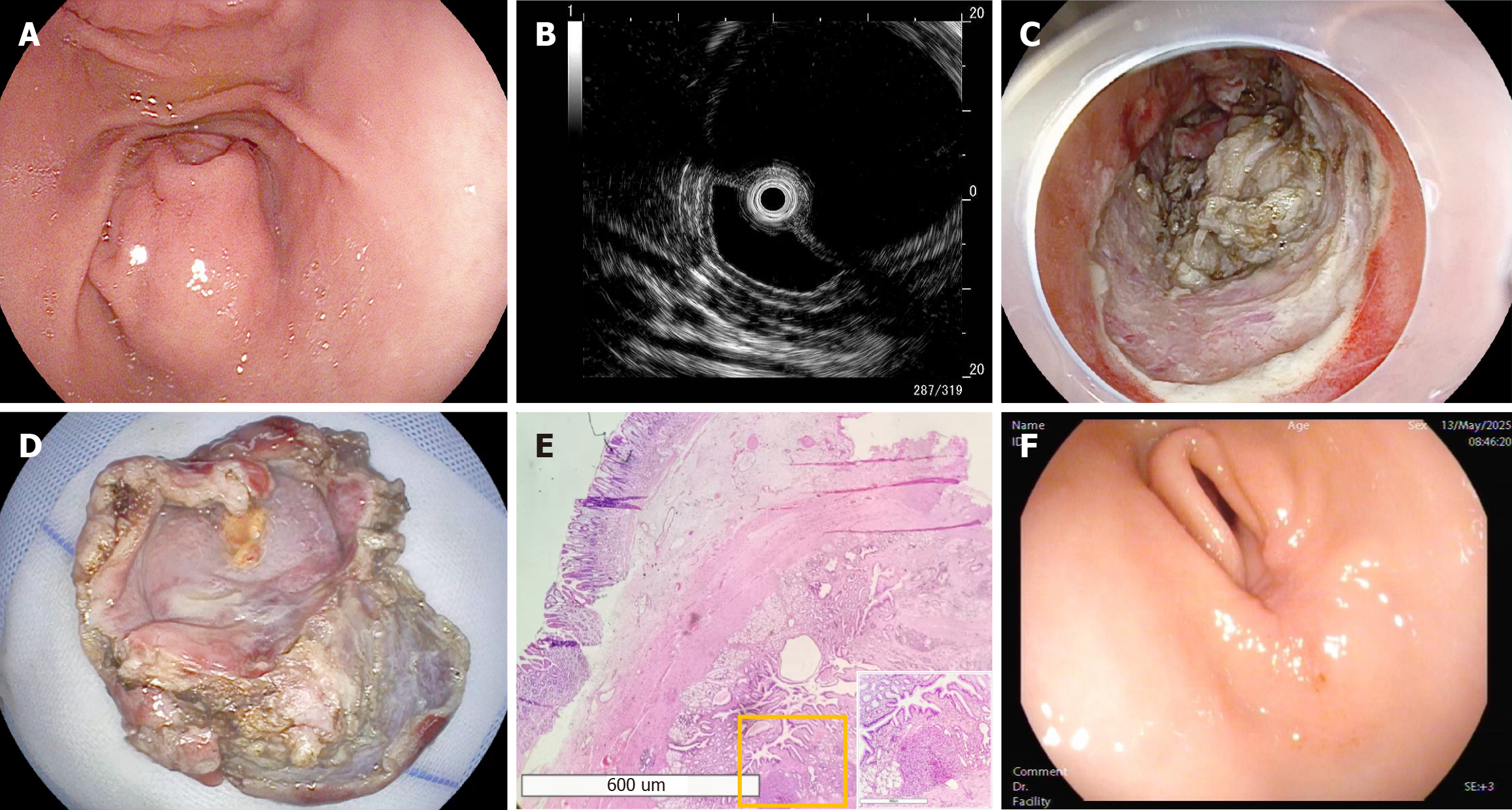
On September 18, 2024, gastroscopy revealed a large, approximately 30 mm × 30 mm submucosal bulge on the greater curvature of the prepyloric region against a non-atrophic background (Figure 1A). The lesion had a smooth surface with a central depression but no ulceration, covered by normal mucosa. Endoscopic ultrasonography (EUS) demonstrated a well-defined, homogeneous hypoechoic cystic structure originating from the muscularis mucosa at the same location, measuring 28.33 mm × 23.28 mm in cross-section (Figure 1B). On September 19, 2024, contrast-enhanced abdominal computed tomography (CT) showed a mass protruding into the gastric lumen with homogeneous enhancement of the cyst wall. On October 12, 2024, ESD was performed (Figure 1C and D; Video). Histopathological examination revealed a cyst lumen lined by gastric mucosal epithelium with traces of pancreatic tissue. Immunohistochemical analysis revealed positive staining for cytokeratin, mucin 5 subtype AC, and mucin 6. The muscular layer exhibited positivity for desmin and smooth muscle actin. Discovered on GIST-1 staining was negative. mast/stem cell growth factor receptor Kit showed scattered positivity in the stromal tissue, confirming the diagnosis of a GDC (Figure 1E).
The final diagnosis was GDC.
Postoperatively, the patient was kept nil by mouth for 3 days, with continuous cardiac monitoring, supplemental oxygen therapy, and a nasogastric tube in place. Treatment included acid suppression therapy, hemostatic agents, nutritional support, and fluid therapy.
The patient’s condition stabilized after 4 days of treatment, with no evidence of active bleeding, and she was discharged. Follow-up gastroscopy at 1 months and 6 months postoperatively showed good healing with no recurrence. The patient remained asymptomatic. Annual follow-up was recommended (Figure 1F).
GDCs are a clinically rare congenital malformation, with an estimated incidence of approximately 17 per million, accounting for 2%-8% of gastrointestinal duplication cysts[1]. They typically occur along the greater curvature of the distal stomach. Most GDCs are located extra-luminally within the abdominal cavity; only a minority protrude into the gastric lumen, presenting as submucosal tumors[2,3]. GDCs can be classified as cystic or tubular. Tubular types communicate with the gastric lumen (< 20%), while cystic types typically do not (> 80%)[4]. Clinically, GDCs are more frequently diagnosed in childhood, with fewer than 25% diagnosed after the age of 12 years[5]. GDCs lack specific clinical manifestations, often presenting with non-specific symptoms such as abdominal pain, nausea, and vomiting. GDCs can lead to severe adverse events, including obstruction, torsion, perforation, hemorrhage, and malignant transformation[4].
Preoperative diagnosis of GDCs is challenging due to their rarity and lack of characteristic features. The imaging characteristics of GDCs exhibits multimodal complementarity: (1) Conventional imaging: Barium meal X-ray may show filling defects in the gastric wall or bulging of the greater curvature; abdominal ultrasound reveals hypoechoic cystic lesions in the upper abdomen; CT demonstrates well-defined, thick-walled cystic masses with homogeneous density and wall enhancement; magnetic resonance imaging shows T1 hypointense and T2 hyperintense cystic lesions with peripheral post-contrast enhancement[3,6]; and (2) Endoscopy and EUS: These offer higher specificity. Gastroscopy may show submucosal bulges or extrinsic compression. EUS definitively reveals anechoic lesions originating from the submucosa or extrinsic to the lumen[7-9]. The clinical utility of EUS-guided fine-needle aspiration remains controversial; proponents advocate its use to exclude malignancy, while opponents’ express concerns about infection risk and question its necessity[10,11]. In summary, multimodal imaging synergistically reveals the morphological features of GDCs. EUS provides the highest diagnostic specificity for determining lesion layer and structure, although the indications and risks of EUS-fine-needle aspiration require validation by higher-level evidence.
Histopathological examination remains the gold standard for diagnosing GDCs. Diagnostic criteria include: (1) A smooth muscle layer within the cyst wall; (2) The inner surface of the cyst wall being lined by gastrointestinal mucosa; and (3) Close attachment to the gastrointestinal tract, sharing a common wall and blood supply[12]. Microscopically, GDCs exhibit normal nuclear morphology in mucosal epithelial cells without neoplastic atypia. The cyst wall may contain ectopic pancreatic tissue. Given the presence of a smooth muscle layer and gastrointestinal mucosal epithelium lining the lumen, immunohistochemistry shows positive expression of smooth muscle actin, mucin 5AC, and/or mucin 6 (gastric markers)[3,4].
Differential diagnoses for GDCs include GIST and leiomyoma: (1) GIST, the most common mesenchymal tumor of the gastrointestinal tract, predominantly occurs in the stomach (54%). On CT imaging, GIST typically presents as a well-circumscribed solid mass with intense enhancement on contrast studies[13,14]. EUS demonstrates a hypoechoic mass originating from the muscularis propria, often containing internal anechoic areas (indicating necrosis)[9]. Histopathological, GIST is characterized by spindle or epithelioid cells exhibiting marked nuclear atypia and frequent mitotic activity (> 5/50 high-power fields), with a propensity for necrosis. Immunohistochemistry reveals diffuse positivity for cluster of differentiation 117[13]; and (2) Leiomyoma, another type of mesenchymal tumor, most frequently arises in the esophagus. CT typically shows a well-defined, spherical mass with homogeneous iso-to-hypodense attenuation, occasionally demonstrating calcification (seen in 10%-15% of cases)[14]. Contrast-enhanced CT reveals mild-to-moderate homogeneous enhancement. EUS features include a uniformly hypoechoic, spherical mass arising from the muscularis propria. Histopathological, leiomyomas consist of intersecting bundles of smooth muscle cells with uniform cigar-shaped nuclei, absence of nuclear atypia, and minimal mitotic activity (< 1/50 high-power fields). Immunohistochemical co-expression of desmin and smooth muscle actin with negative cluster of differentiation 117 confirms the diagnosis[4].
Regarding treatment, no standard guidelines currently exist for GDCs. Most experts recommend surgical resection for symptomatic patients, although the optimal extent of resection is debated, with strategies determined by the surgeon based on experience and patient condition[12]. Observation and follow-up are often recommended for asymptomatic tubular GDCs and small, asymptomatic cystic GDCs. With advancements in endoscopic techniques, endoscopic approaches are increasingly being applied to intraluminal GDCs. Compared to surgical intervention, endoscopic therapy benefits from minimally invasive access via natural orifices, rendering it particularly advantageous for intraluminal GDCs in pediatric, elderly, or comorbid patients. It offers advantages including faster postoperative recovery, shorter hospital stays, and lower costs. However, it also has limitations: Therapeutic efficacy is highly dependent on operator expertise, intraoperative perforation risks are elevated, long-term postoperative follow-up is required, and its efficacy remains limited for extraluminal cysts, large-diameter cysts (> 5 cm), and cysts with complex adhesions[12]. A literature search of the PubMed, EMBASE, and Cochrane Library databases (from their inception until July 6, 2025) revealed that previous reports on ESD for GDCs demonstrated high procedural success rates and favorable patient prognoses[4,12]. Addi
During the ESD procedure in this case, dense adhesions between the cyst base and the muscularis propria necessitated partial cyst wall preservation. To avoid creating a large gastric wall defect following complete resection, only the portion of the cyst wall tightly adherent to the gastric wall was left unresected. Close postoperative follow-up showed structural consistency between the residual cyst wall surface and surrounding gastric mucosa, with no signs of recurrence. The gastric antrum and pylorus exhibited normal anatomy, and the patient reported no clinical discomfort.
GDCs represent rare congenital anomalies with diagnostic and therapeutic challenges due to their nonspecific presentations. In this case, ESD with intentional partial cyst wall preservation was successfully employed for an intraluminal GDC in an adolescent patient, achieving sustained symptom remission and histopathological confirmation. The residual cyst wall exhibited benign integration with surrounding gastric mucosa at the 6-month follow-up, demonstrating no adverse impact on short-term prognosis. This approach provides a viable minimally invasive alternative to surgical resection for select intraluminal GDCs, particularly those with muscular adhesion and in pediatric populations where organ preservation is prioritized. However, definitive diagnosis necessitates complete resection to examine the cyst wall architecture and exclude malignancy. Future multicenter studies with extended follow-up periods are warranted to validate long-term recurrence rates and refine patient selection criteria.
| 1. | Yamasaki A, Onishi H, Yamamoto H, Ienaga J, Nakafusa Y, Terasaka R, Nakamura M. Asymptomatic adenocarcinoma arising from a gastric duplication cyst: A case report. Int J Surg Case Rep. 2016;25:16-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Chen PH, Lee JY, Yang SF, Wang JY, Lin JY, Chang YT. A retroperitoneal gastric duplication cyst mimicking a simple exophytic renal cyst in an adolescent. J Pediatr Surg. 2010;45:e5-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Liu F, Zhao H. Pathological diagnosis, differential diagnosis and origin investigation of easily misdiagnosed adult gastric duplication cysts. Histol Histopathol. 2022;37:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Yuan Z, Wei H, Zhang Y, Cao B, He B, Yuan H. Gastric duplication cysts: literature review and a case report of rare multiple gastric duplication cysts treated by endoscopic submucosal dissection. Postgrad Med. 2023;135:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Passos ID, Chatzoulis G, Milias K, Tzoi E, Christoforakis C, Spyridopoulos P. Gastric duplication cyst (gdc) associated with ectopic pancreas: Case report and review of the literature. Int J Surg Case Rep. 2017;31:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Gupta AK, Guglani B. Imaging of congenital anomalies of the gastrointestinal tract. Indian J Pediatr. 2005;72:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kim GH, Lee MW, Lee BE, Park DY. Endoscopic submucosal dissection for gastric duplication cyst with heterotopic pancreas. Endoscopy. 2021;53:E19-E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Fang Y, Gao T, Yang H, Ma S, Li Q, Zhou PH. Removal of an infant's gastric duplication cyst through endoscopic submucosal dissection: A case report. Medicine (Baltimore). 2019;98:e14820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Varanese M, Spadaccini M, Facciorusso A, Franchellucci G, Colombo M, Andreozzi M, Ramai D, Massimi D, De Sire R, Alfarone L, Capogreco A, Maselli R, Hassan C, Fugazza A, Repici A, Carrara S. Endoscopic Ultrasound and Gastric Sub-Epithelial Lesions: Ultrasonographic Features, Tissue Acquisition Strategies, and Therapeutic Management. Medicina (Kaunas). 2024;60:1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 10. | Fazel A, Moezardalan K, Varadarajulu S, Draganov P, Eloubeidi MA. The utility and the safety of EUS-guided FNA in the evaluation of duplication cysts. Gastrointest Endosc. 2005;62:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Massidda M, Rocchi C, Tomassini G, Vadalà di Prampero SF, Cossu Rocca P, Tondolo V, Manzoni G, Bulajic M. Gastric duplication cyst: a challenging EUS differential diagnosis between subepithelial gastric lesion and exophytic pancreatic cystic neoplasm-a case report and a literature review. Clin J Gastroenterol. 2022;15:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Li Y, Li C, Wu H, Wang Q, Gao ZD, Yang XD, Jiang KW, Ye YJ. Clinical features of gastric duplications: evidence from primary case reports and published data. Orphanet J Rare Dis. 2021;16:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Lai EC, Lau SH, Lau WY. Current management of gastrointestinal stromal tumors--a comprehensive review. Int J Surg. 2012;10:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Wang J, Zhou X, Xu F, Ao W, Hu H. Value of CT Imaging in the Differentiation of Gastric Leiomyoma From Gastric Stromal Tumor. Can Assoc Radiol J. 2021;72:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Chavan R, Nabi Z, Basha J, Sekaran A, Darisetty S, Reddy PM, Reddy DN. Endoscopic resection of a complex gastric duplication cyst using a submucosal tunneling technique. Endoscopy. 2022;54:E168-E169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/