Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.108479
Revised: July 3, 2025
Accepted: August 25, 2025
Published online: October 27, 2025
Processing time: 153 Days and 23.2 Hours
The relationship between hepatitis B surface antigen (HBsAg) concentrations, hepatitis B virus (HBV) DNA levels, and hepatic function in individuals with chronic hepatitis B (CHB) remains incompletely characterized.
To examine the association of serum HBsAg concentrations with HBV DNA levels and hepatic function parameters in patients with CHB.
A total of 110 individuals with CHB admitted to Kunming Third People’s Hos
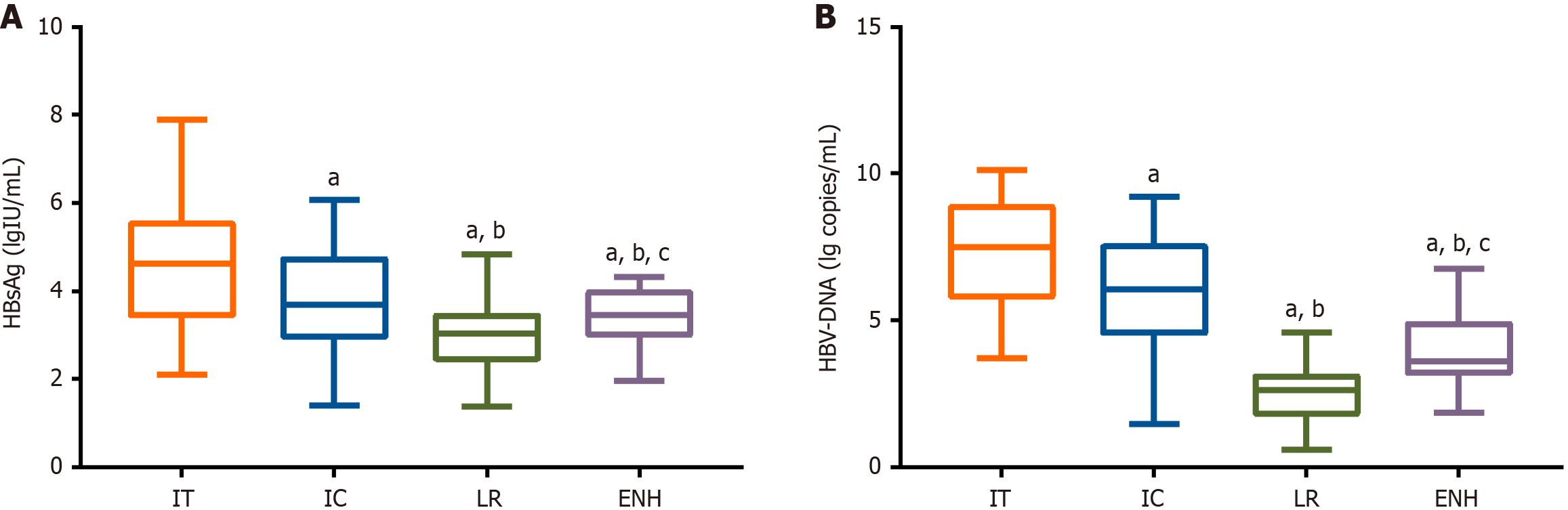
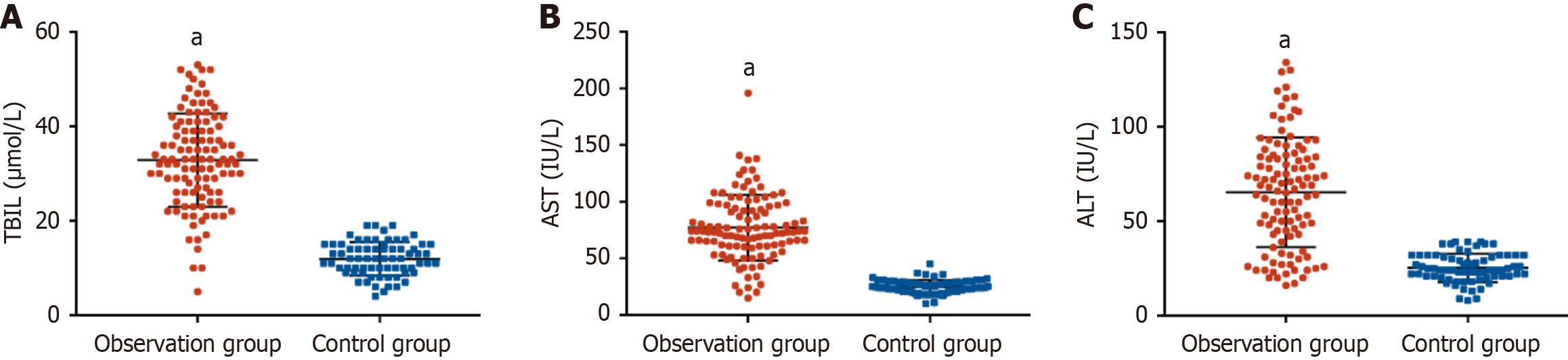
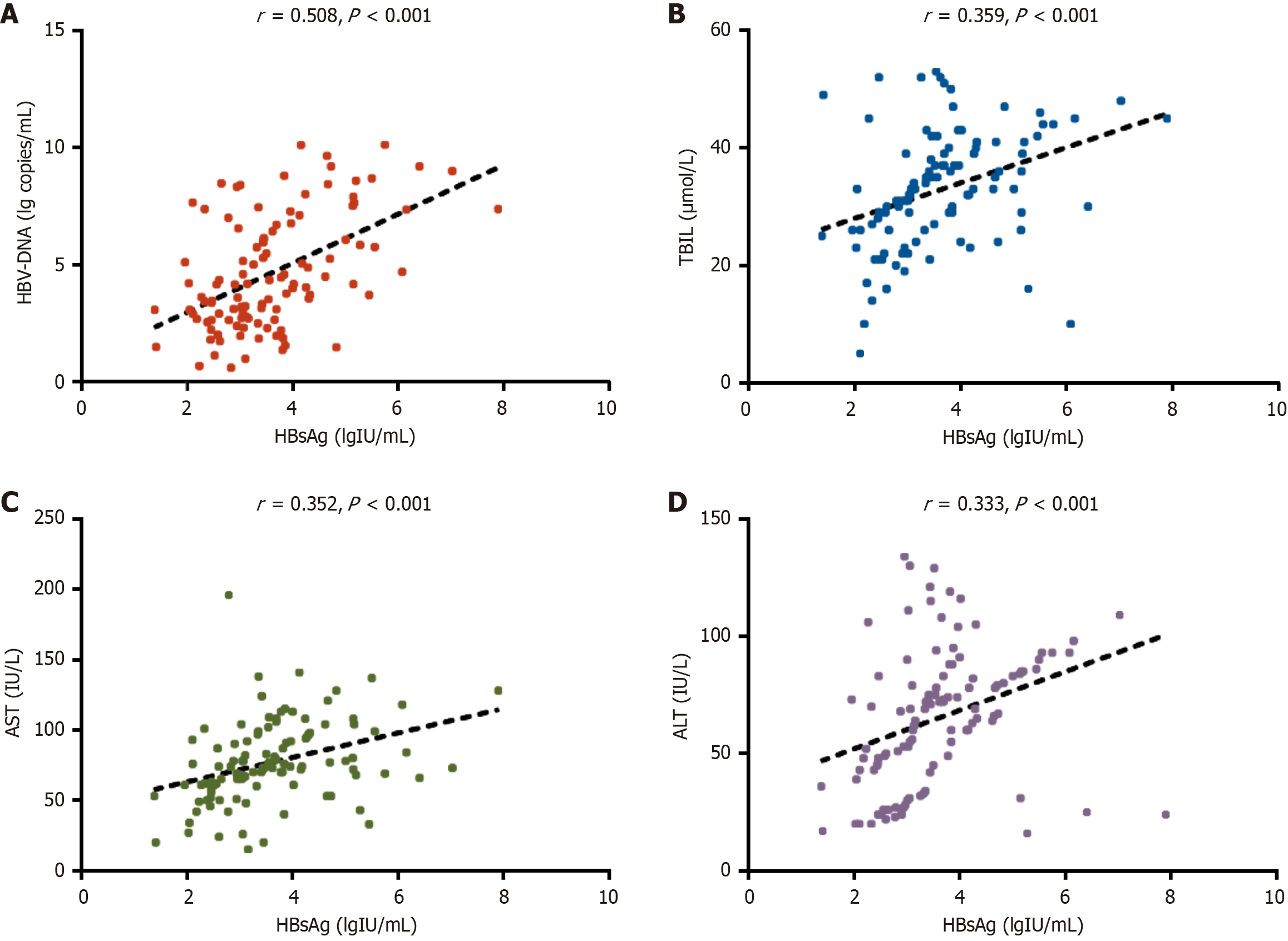
HBsAg levels differed significantly across CHB phases: Immune tolerance (IT) phase (4.62 ± 1.51 lgIU/mL), immune clearance (IC) phase (3.84 ± 1.16 lgIU/mL), low replication (LR) phase (2.99 ± 0.66 lgIU/mL), and HBV e antigen-negative hepatitis (ENH) phase (3.40 ± 0.69 lgIU/mL). Corresponding HBV DNA levels were highest in the IT phase (7.41 ± 1.83 log copies/mL), followed by the IC phase (6.03 ± 1.92 log copies/mL), ENH phase (3.89 ± 1.23 log copies/mL), and LR phase (2.55 ± 1.00 log copies/mL). All hepatic function parameters in patients with CHB were significantly elevated compared to the healthy controls. Pearson correlation analysis showed significant positive associations between serum HBsAg levels and HBV DNA, TBIL, AST, and ALT levels. ROC analysis revealed that an HBsAg cutoff > 4.09 lgIU/mL predicted HBV DNA ≥ 105 IU/mL (high viral load) with 88.57% sensitivity, 78.67% specificity, and an area under the curve (AUC) of 0.868 (P < 0.001), while a cutoff > 4.07 lgIU/mL predicted ALT ≥ 2 × ULN (significant liver injury) with 69.70% sensitivity, 90.91% specificity, and an AUC of 0.821 (P < 0.001).
Serum HBsAg, a noninvasive serological marker, holds significant clinical value in CHB management by aiding in the stratification of viral burden and the prediction of hepatic impairment.
Core Tip: Few studies have comprehensively examined the association between serum hepatitis B surface antigen (HBsAg) levels, hepatitis B virus (HBV) DNA, and liver function in patients with chronic hepatitis B (CHB), particularly regarding its value as a noninvasive predictor of high viral replication and severe liver damage. This study assessed the clinical sig
- Citation: Li J, Li M, Sun H, Yu YT, Zhou YH, Fu F, Yan L. Association of serum hepatitis B surface antigen with hepatitis B virus DNA and hepatic function in patients with chronic hepatitis B. World J Gastrointest Surg 2025; 17(10): 108479
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/108479.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.108479
Hepatitis B virus (HBV) infection, a significant global health burden, afflicts nearly 300 million of the global population and serves as a primary etiology for hepatic decompensation, liver cirrhosis, and hepatic malignancies[1]. The pa
Clinically, both HBV DNA and HBsAg are vital virological biomarkers for monitoring CHB progression[8]: HBV DNA quantification reflects viral replicative activity directly, whereas HBsAg levels indicate cccDNA transcriptional activity and viral protein production capacity—dynamics correlating intimately with HBV infection stages[9]. Notably, HBsAg quantification has demonstrated a close connection with cccDNA transcriptional activity, supporting its utility in iden
While previous studies have provided valuable insights, a comprehensive analysis of the relationship among serum HBsAg levels, HBV DNA load, and liver function parameters in patients with CHB remains limited. Moreover, the potential of HBsAg as a noninvasive predictor of high HBV DNA levels (≥ 105 IU/mL) and significant liver injury—defined as alanine aminotransferase (ALT) levels ≥ 2 times the upper limit of normal (ULN)—remains underexplored. We hypothesize that HBsAg levels are significantly correlated with HBV DNA and liver function indices and may serve as a clinically useful noninvasive biomarker for identifying patients with high viral loads and severe liver damage in chronic HBV infections.
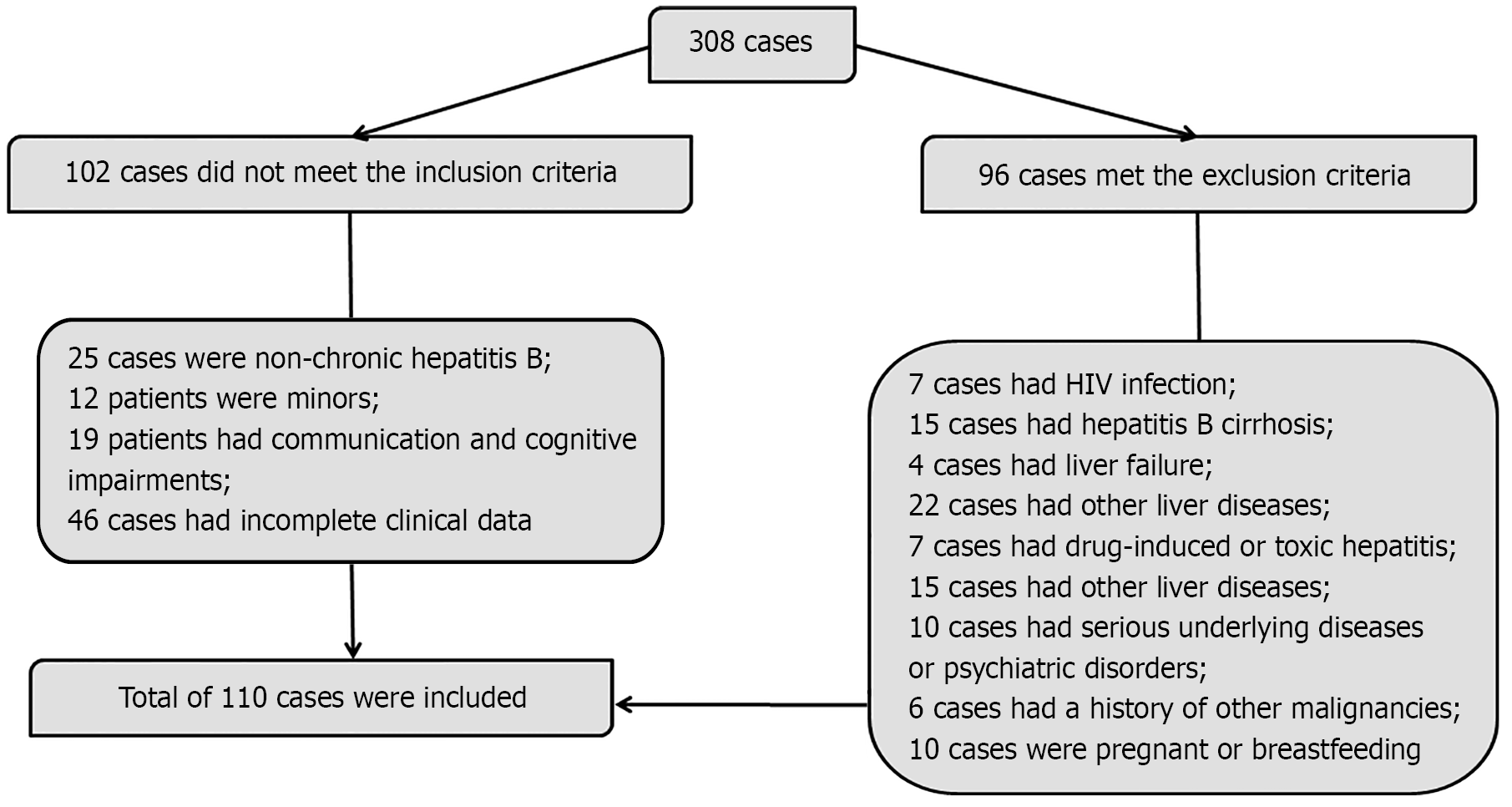
A total of 110 patients with CHB admitted to Kunming Third People’s Hospital (Yunnan, China) between January 2023 and January 2025 were retrospectively enrolled as the observation group. For comparison, a control group of 70 age- and sex-matched healthy individuals from the same period was also included. Both groups were selected strictly in accordance with standardized inclusion and exclusion criteria. Baseline characteristics were comparable between the groups (P > 0.05). Clinical data for patients with CHB were obtained from the electronic health record system of the hospital.
The sample size for this study was determined based on the availability of eligible hospital cases, the need for subgroup analysis, including immune tolerance (IT), IC, low replication (LR), and HBV e antigen (HBeAg)-negative hepatitis (ENH) groups, and reference to comparable studies (e.g., 106 cases in Catanzaro et al[13] and 109 in Wang et al[14]). Following a comprehensive evaluation, a final sample size of 110 was established, providing 84% statistical power (α = 0.05, Cohen’s f = 0.35).
Inclusion criteria: Patients with confirmed diagnosis of CHB based on the Korean Association for the Study of the Liver clinical practice guidelines[15]; age ≥ 18 years; normal cognitive and communication abilities; complete clinical records available.
Exclusion criteria: Coinfection with other hepatitis viruses or human immunodeficiency virus; advanced liver disease, including hepatic cirrhosis or liver failure; other hepatic disorders such as alcoholic liver disease or parasitic liver infections; active infections caused by other pathogens (e.g., influenza virus); drug-induced or toxin-related hepatitis; metabolic liver diseases including hemochromatosis or Wilson’s disease, or the presence of hepatocellular carcinoma; severe cardiovascular, renal, or other systemic diseases; or neuropsychiatric disorders; history of malignancy; current pregnancy or lactation.
HBsAg quantification: Following overnight fasting, 5 mL of early morning peripheral venous blood was collected from each participant, and serum was isolated for analysis. Quantitative measurement of serum HBsAg in the observation group was conducted using a time-resolved fluorescence immunoassay, covering all phases of HBV infection (IT, IC, LR, ENH) with a detection range of 0.05-52000 IU/mL (lower limit: 0.05 IU/mL; upper limit: 52000 IU/mL). All procedures strictly followed the manufacturer’s instructions (EY-elisa1954; Shanghai Yiranzhi Biotech, Shanghai, China). Diagnostic criteria for each CHB phase were defined as follows[16]: (1) IT phase: HBsAg-positive and HBeAg-positive, HBV DNA ≥ 107 copies/mL, normal ALT levels; (2) IC phase: HBsAg- and HBeAg-positive, HBV DNA ≥ 104 copies/mL, sustained or fluctuating ALT elevation; (3) LR phase: HBeAg-negative with anti-HBV e positivity, HBV DNA < 103 copies/mL, normal ALT levels; and (4) ENH phase: HBeAg-negative with anti-HBV e positivity, HBV DNA ≥ 103 copies/mL, persistent or intermittent ALT elevation.
HBV DNA detection: We performed real-time fluorescent polymerase chain reaction to quantify serum HBV DNA concentrations in the observation group. The assay had a linear detection range of 20 IU/mL to 1.0 × 108 IU/mL. All procedures were conducted according to the manufacturer’s instructions for the HBV DNA detection kit (1534248607; Shanghai Jianglai Biotech, Shanghai, China).
Hepatic function assessment: Hepatic function parameters were assessed in both groups using a fully automated biochemical analyzer. Total bilirubin (TBIL; reference range: 5.12-22.24 μmol/L) was measured using the dichlorobenzene diazonium salt method. Aspartate aminotransferase (AST; reference interval: 10-42 IU/L) and ALT (reference interval: 26-40 IU/L) were measured using an enzyme-linked immunosorbent assay.
Quantitative data are expressed as the mean ± SD after confirming the homogeneity of variance and approximate normal distribution using Bartlett’s test and the Kolmogorov-Smirnov test, respectively. Intergroup comparisons of continuous variables were conducted using independent sample t-tests. Categorical variables were presented as frequencies and percentages (n [%]) and compared using the χ2 test. Pearson’s correlation analysis was used to assess the relationships between HBsAg levels and HBV DNA, TBIL, AST, and ALT levels. All statistical analyses were conducted using SPSS software (version 21.0; IBM Statistics, Armonk, NY, United States) and GraphPad Prism (version 7.0; GraphPad Software, Boston, MA, United States). Two-sided P < 0.05 was considered statistically significant.
There were no significant differences between the observation and control groups in demographic and clinical characte
| General data | Observation group (n = 110) | Control group (n = 70) | χ2/t value | P value |
| Sex | 0.420 | 0.517 | ||
| Male | 62 (56.36) | 36 (51.43) | ||
| Female | 48 (43.64) | 34 (48.57) | ||
| Age (years) | 45.71 ± 10.90 | 44.76 ± 9.21 | 0.605 | 0.546 |
| Disease duration (years) | 4.29 ± 2.11 | - | - | - |
| Body mass index (kg/m2) | 22.68 ± 2.19 | 23.24 ± 2.32 | 1.634 | 0.104 |
| Smoking history | 1.645 | 0.200 | ||
| None | 65 (59.09) | 48 (68.57) | ||
| Yes | 45 (40.91) | 22 (31.43) | ||
| Alcoholism history | 3.543 | 0.060 | ||
| None | 72 (65.45) | 55 (78.57) | ||
| Yes | 38 (34.55) | 15 (21.43) | ||
| Disease phase | - | - | ||
| Immune tolerance phase | 22 (20.00) | - | ||
| Immune clearance phase | 27 (24.55) | - | ||
| Low replication phase | 38 (34.55) | - | ||
| HBeAg-negative hepatitis phase | 23 (20.91) | - | ||
| HBsAg (lgIU/mL) | 3.61 ± 1.17 | - | - | - |
| HBV-DNA (lg copies/mL) | 4.66 ± 2.42 | - | - | - |
| ALT (U/L) | 65.26 ± 29.00 | 25.31 ± 7.56 | 11.274 | < 0.001 |
| Platelets (× 109/L) | 164.51 ± 50.42 | 247.43 ± 38.94 | 11.711 | < 0.001 |
Statistical analyses showed markedly reduced HBsAg levels and HBV DNA loads in the IC and LR phases compared to the IT phase (P < 0.05). Although a notable increase in both markers was observed during the ENH phase (P < 0.05), these levels remained significantly lower than those in the IT and IC phases (P < 0.05). Detailed results are presented in Figure 2.
The observation group exhibited significantly higher serum levels of TBIL, AST, and ALT than the healthy controls (P < 0.001), indicating significant hepatic impairment. These findings are presented in Figure 3.
Pearson correlation analysis revealed strong positive associations between serum HBsAg levels and HBV DNA viral load, TBIL, AST, and ALT (P < 0.001). These relationships are presented in Figure 4.
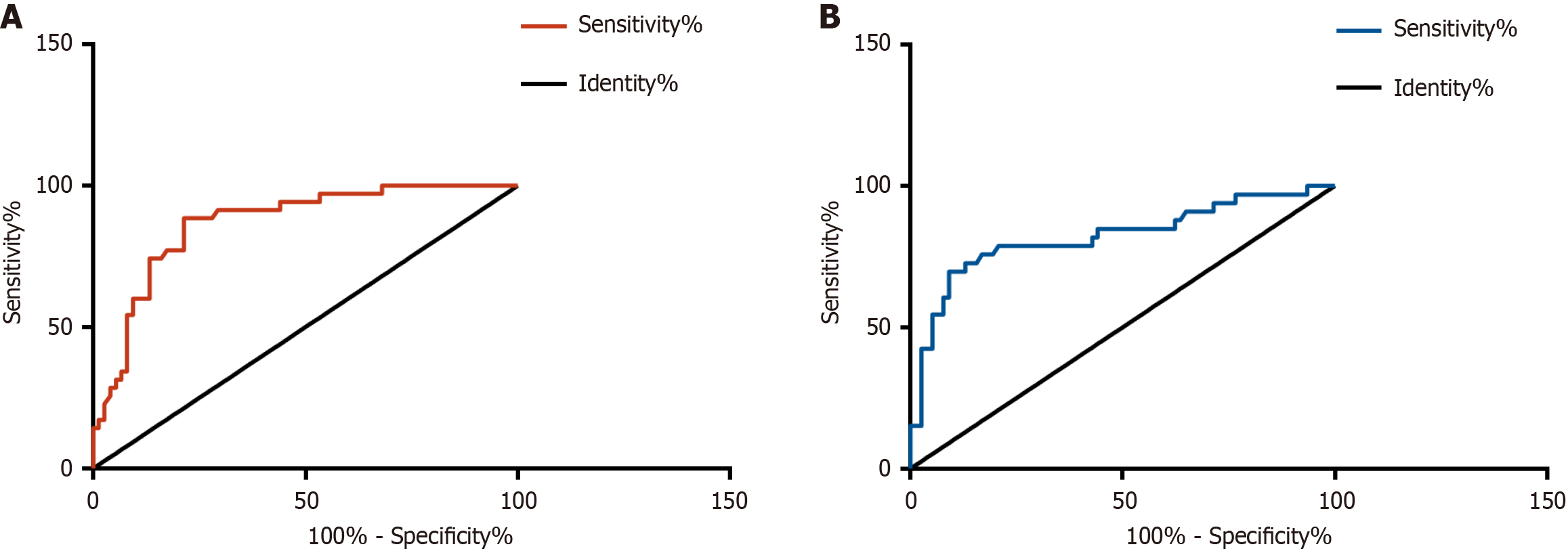
Receiver operating characteristic (ROC) curve analysis identified an HBsAg level > 4.09 LgIU/mL as the optimal threshold for predicting high viral load, with an area under the curve (AUC) of 0.868 (95% confidence interval [CI]: 0.798-0.937), 88.57% sensitivity, and 78.67% specificity. For predicting significant hepatic injury, the optimal cutoff was > 4.07 LgIU/mL, yielding an AUC of 0.821 (95%CI: 0.724-0.917), with 69.70% sensitivity and 90.91% specificity. These predictive values and corresponding diagnostic performance metrics are presented in Figure 5 and Table 2.
| Indicators | Cut-off | AUC | 95%CI | Specificity (%) | Sensitivity (%) | P value |
| High viral load | > 4.09 | 0.868 | 0.798-0.937 | 78.67 | 88.57 | < 0.001 |
| Significant liver injury | > 4.07 | 0.821 | 0.724-0.917 | 90.91 | 69.70 | < 0.001 |
This study included 110 patients with CHB, characterized into the IT, IC, LR, and ENH phases, with 22, 27, 38, and 23 patients in each group, respectively. Longitudinal assessment of serum HBsAg and HBV DNA levels revealed a characteristic dynamic pattern—both markers showed significant downregulation during the IC and LR phases, followed by a partial rebound in the ENH phase, although levels remained below those in the IT and IC phases. The progressive decline in HBsAg titers from the IC to LR phases reflects two critical immunological events: The initial activation of the immune system during the IC phase, followed by effective viral suppression in the LR phase. These findings support the role of quantitative HBsAg as a sensitive biomarker of immune response intensity, facilitating the monitoring of both disease progression and treatment efficacy. Notably, the observed HBsAg rebound during the ENH phase, despite partial preservation of immune control, underscores the clinical importance of concurrent HBV DNA monitoring in detecting potential significant hepatic injury. Wu et al[17] similarly revealed that a sustained post-treatment decline in serum HBsAg levels is associated with continued virological suppression and immune control, representing an ideal therapeutic outcome in CHB management. The phase-associated HBsAg dynamics we documented show strong concordance with Jaroszewicz et al's findings[18], particularly the characteristic elevation during IT and IC phases compared to LR and ENH phases. The phase-specific HBsAg patterns documented by Antaki et al[19] in Middle Eastern populations offer further cross-ethnic validation of our findings.
Furthermore, the observation group exhibited significant increases in serum TBIL, AST, and ALT, reflecting ongoing chronic HBV infection-induced hepatic injury. This liver damage is likely mediated by the direct cytotoxic effects of the virus, immune-mediated inflammatory responses, progressive fibrogenesis, etc.[20-22]. Of note, strong positive associations between HBsAg and both markers of viral replication (HBV DNA) and liver injury (TBIL, AST, ALT) were identified in our research. These relationships may be influenced by HBsAg through direct (e.g., endoplasmic reticulum stress) or indirect mechanisms (e.g., immune system activation) that exacerbate hepatocellular damage[23,24]. These findings suggest the utility of HBsAg as a valuable biomarker for identifying both high HBV DNA viral loads and significant liver injury in CHB. This observation aligns with previous studies. For example, Jaroszewicz et al[18] reported a robust correlation between HBsAg and HBV DNA levels in acute HBV infection, while Zeng et al[25] linked HBsAg to significant liver histopathological changes in chronic HBV. Through further validation, we found that an HBsAg threshold of > 4.09 LgIU/mL effectively predicted HBV DNA levels ≥ 105 IU/mL, with an AUC of 0.868 (95%CI: 0.798-0.937) and high sensitivity. This finding has important clinical implications. In settings with constrained resources, HBsAg measurement may offer an economical first-line screening approach to detect elevated viral loads, thereby decreasing dependence on higher-cost HBV DNA assays. Additionally, this cutoff value may help clinicians prioritize patients for the urgent initiation of antiviral therapy. Additionally, our findings indicate that an HBsAg level > 4.07 LgIU/mL showed strong predictive accuracy for significant liver injury, with an AUC of 0.821 (95%CI: 0.724-0.917) and high specificity. This cutoff may serve as a valuable noninvasive alternative for assessing liver injury in patients for whom liver biopsy is contraindicated. We further recommend enhanced monitoring, for example, FibroScan every 3 months, for patients who exceed this threshold. Previous studies have also explored the utility of serum biomarkers in predicting disease progression in CHB. Wang et al[26] revealed that integrating quantitative hepatitis B core antibody with ALT and HBsAg improves the assessment of liver inflammation severity in patients with HBeAg-positive CHB during the immune-active phase. Similarly, Goyal et al[27] identified HBsAg as a valuable predictor of liver fibrosis in HBeAg-positive chronic HBV infection.
The novelty of this study is reflected in several key aspects. First, by analyzing HBsAg dynamics across different stages of infection, we clarified the changing patterns of HBsAg during HBV progression, which may provide auxiliary evidence for disease stratification. Second, we established a significant correlation between HBsAg levels and high viral loads, as well as liver injury, as indicated by HBV DNA, TBIL, AST, and ALT. Third, HBsAg was validated as a predictor not only of high viral replication but also of hepatic damage. These findings offer clinically valuable guidance for noninvasive monitoring and individualized treatment strategies in patients with CHB.
This study had several limitations that warrant consideration. First, the relatively modest sample size (n = 110) highlights the need for larger multicenter studies to minimize the risk of information collection bias. Second, the exclusion of patients treated with nucleos(t)ide analogs may limit the generalizability of the results to broader CHB populations. Future comparative analyses of HBsAg kinetics in treated vs untreated patients could provide more comprehensive insights and improve applicability. Third, this study did not assess other important indicators, such as fibrosis markers, cccDNA-specific biomarkers, circulating HBV RNA, or hepatitis B core-related antigens. Incorporating these parameters into future studies could enhance the clinical relevance and depth of the findings. Fourth, no correlation analysis was conducted between HBsAg levels and fibrosis assessment tools such as FibroScan. Including this in future studies may help clarify potential associations. Fifth, this study did not evaluate the cost-effectiveness or diagnostic convenience of using HBsAg vs HBV DNA for predicting high viral loads and significant liver injury. Future analysis in this area may help elucidate the clinical merits of HBsAg quantification. Sixth, exact P-values were not reported. Future studies should consider using R software, which can compute extremely small P-values, to provide more precise statistical evidence against the null hypothesis. Seventh, while the ROC-derived threshold (e.g., > 4.09 LgIU/mL) showed robust diagnostic performance, external validation using independent cohorts is essential to confirm clinical utility. Addressing these limitations in future studies will help refine and strengthen the study’s conclusions.
In summary, serum HBsAg levels showed a significant correlation with HBV DNA concentrations and hepatic function parameters in patients with CHB. Moreover, serum HBsAg exhibits substantial predictive value for identifying both high viral loads and the severity of liver injury.
| 1. | Chien RN, Liaw YF. Current Trend in Antiviral Therapy for Chronic Hepatitis B. Viruses. 2022;14:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 2. | Su Z, Chen J, Zhang J, An Y, Liao Y, Wu X, Tao C, Wang L, Cai B. Circulating IL-1β, IL-17, and IP-10 as Potential Predictors of Hepatitis B Virus Infection Prognosis. J Immunol Res. 2022;2022:5202898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Cacoub P, Asselah T. Hepatitis B Virus Infection and Extra-Hepatic Manifestations: A Systemic Disease. Am J Gastroenterol. 2022;117:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. 2023;20:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 365] [Reference Citation Analysis (1)] |
| 5. | Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 6. | Allweiss L, Testoni B, Yu M, Lucifora J, Ko C, Qu B, Lütgehetmann M, Guo H, Urban S, Fletcher SP, Protzer U, Levrero M, Zoulim F, Dandri M. Quantification of the hepatitis B virus cccDNA: evidence-based guidelines for monitoring the key obstacle of HBV cure. Gut. 2023;72:972-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Gong J, Tu W, Liu J, Tian D. Hepatocytes: A key role in liver inflammation. Front Immunol. 2022;13:1083780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 8. | Zeng Z, Liu R, Cao W, Yang L, Lin Y, Bi X, Jiang T, Deng W, Wang S, Lu H, Sun F, Shen G, Chang M, Lu Y, Wu S, Hao H, Xu M, Chen X, Hu L, Zhang L, Wan G, Xie Y, Li M. Study on pathological and clinical characteristics of chronic HBV infected patients with HBsAg positive, HBV DNA negative, HBeAg negative. Front Immunol. 2022;13:1113070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Wang J, Yuan X, Wang Y, Zhang Y, Han M, Lu H, Liu S, Zhang Y, Ge F, Liu Y, Cheng J. PreS1BP mediates inhibition of Hepatitis B virus replication by promoting HBx protein degradation. Virus Res. 2024;341:199326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Li J, Sun X, Fang J, Wang C, Han G, Ren W. Analysis of intrahepatic total HBV DNA, cccDNA and serum HBsAg level in Chronic Hepatitis B patients with undetectable serum HBV DNA during oral antiviral therapy. Clin Res Hepatol Gastroenterol. 2017;41:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Hao X, Chen Y, Bai L, Wei H, Sun R, Tian Z. HBsAg-specific CD8(+) T cells as an indispensable trigger to induce murine hepatocellular carcinoma. Cell Mol Immunol. 2021;18:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Zeng DW, Liu YR, Dong J, Zhu YY, Li YB, Chen J, Zheng Q, Jiang JJ. Serum HBsAg and HBeAg levels are associated with liver pathological stages in the immune clearance phase of hepatitis B virus chronic infection. Mol Med Rep. 2015;11:3465-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Catanzaro R, Aleo A, Sciuto M, Zanoli L, Balakrishnan B, Marotta F. FIB-4 and APRI scores for predicting severe liver fibrosis in chronic hepatitis HCV patients: a monocentric retrospective study. Clin Exp Hepatol. 2021;7:111-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, Cai Y, Wang Y, Zhang X, Chen T, Yao Z, Yang C, Deng R, Zhong Q, Deng X, Chen X, Yang XP, Wei G, Wang Z, Tian J, Chen XP. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16:111-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25:93-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 16. | Vanwolleghem T, Hou J, van Oord G, Andeweg AC, Osterhaus AD, Pas SD, Janssen HL, Boonstra A. Re-evaluation of hepatitis B virus clinical phases by systems biology identifies unappreciated roles for the innate immune response and B cells. Hepatology. 2015;62:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Wu S, Yi W, Gao Y, Deng W, Bi X, Lin Y, Yang L, Lu Y, Liu R, Chang M, Shen G, Hu L, Zhang L, Li M, Xie Y. Immune Mechanisms Underlying Hepatitis B Surface Antigen Seroclearance in Chronic Hepatitis B Patients With Viral Coinfection. Front Immunol. 2022;13:893512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, Cornberg M. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 19. | Antaki N, Zeidane N, Alhaj N, Hadad M, Baroudi O, Antaki F, Abouharb R, Haffar S, Abdelwahab J, Alideeb S, Asaad F, Aljesri A, Doghman D, Aaraj R, Ibrahim N, Ali A, Assil M, Sabah H, Katranji N, Kebbewar K. HBsAg titers in the different phases of hepatitis B infection in Syrian patients. J Clin Virol. 2012;53:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Zai W, Hu K, Ye J, Ding J, Huang C, Li Y, Fang Z, Wu M, Wang C, Chen J, Yuan Z. Long-Term Hepatitis B Virus Infection Induces Cytopathic Effects in Primary Human Hepatocytes, and Can Be Partially Reversed by Antiviral Therapy. Microbiol Spectr. 2022;10:e0132821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Jiang B, Wang L, Liu H, Wang L, Su R, Xu L, Wei G, Li J, Lu F, Chen X. Association of HBV serological markers with host antiviral immune response relevant hepatic inflammatory damage in chronic HBV infection. J Med Virol. 2024;96:e29569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Lin MH, Li HQ, Zhu L, Su HY, Peng LS, Wang CY, He CP, Liang XE, Wang Y. Liver Fibrosis in the Natural Course of Chronic Hepatitis B Viral Infection: A Systematic Review with Meta-Analysis. Dig Dis Sci. 2022;67:2608-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Zhang J, Guo J, Yang N, Huang Y, Hu T, Rao C. Endoplasmic reticulum stress-mediated cell death in liver injury. Cell Death Dis. 2022;13:1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 202] [Reference Citation Analysis (0)] |
| 24. | You H, Qin S, Zhang F, Hu W, Li X, Liu D, Kong F, Pan X, Zheng K, Tang R. Regulation of Pattern-Recognition Receptor Signaling by HBX During Hepatitis B Virus Infection. Front Immunol. 2022;13:829923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Zeng Z, Hao H, Bi X, Lin Y, Yang L, Wang S, Shen G, Chang M, Jiang T, Deng W, Lu H, Sun F, Lu Y, Gao Y, Liu R, Xu M, Chen X, Hu L, Zhang L, Li M, Xie Y. Study on liver histopathology of chronic HBV infected patients with different normal ALT values. Front Immunol. 2022;13:1069752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Wang X, Gao X, Wu R, Chi X, Xu H, Guan Y, Jin Q, Niu J. Serum qAnti-HBc combined with ALT and HBsAg predicts significant hepatic inflammation in HBeAg-positive immune active patients. J Gastroenterol Hepatol. 2022;37:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Goyal SK, Jain AK, Dixit VK, Shukla SK, Kumar M, Ghosh J, Ranjan A, Gupta N, Tripathi M. HBsAg Level as Predictor of Liver Fibrosis in HBeAg Positive Patients With Chronic Hepatitis B Virus Infection. J Clin Exp Hepatol. 2015;5:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/