Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.108239
Revised: July 22, 2025
Accepted: August 18, 2025
Published online: October 27, 2025
Processing time: 141 Days and 0.1 Hours
Ulcerative colitis (UC) is a chronic relapsing inflammatory bowel disease with rising global incidence. Current therapies for UC often provide incomplete relief and are associated with adverse side effects, highlighting the need for alternatives with increased safety and effectiveness. Compound spleen-tonifying composition (CSTC) contains ingredients, such as Pulsatilla chinensis (Bunge) Regel and Glycyrrhiza uralensis Fisch, that have been shown to be efficacious in the treatment of UC. Its mechanism needs to be investigated further.
To study the therapeutic effect and mechanism of CSTC in dextran sulfate sodium (DSS)-induced UC in rats.
Sprague-Dawley rats were freely given 4% DSS solution for seven days to establish the UC model. After in
DSS administration triggered severe UC symptoms, including weight loss, colon shortening, elevated DAI scores, and histological damage. These symptoms were accompanied with oxidative stress (reduced SOD and GSH-px levels and increased MDA and MPO levels), inflammation (elevated TNF-α, IL-1β, and IL-6 levels), and a reduction in the expression levels of tight junction proteins [zonula occludens-1 (ZO-1) and occluding]. High- and medium-dose CSTC treatment significantly alleviated clinical symptoms, restored colon morphology, normalized oxidative stress markers, suppressed proinflammatory cytokines, and enhanced ZO-1 and occludin levels, demonstrating dose-dependent efficacy. Notably, solvent extraction critically influenced bioactivity: Nonpolar extracts (chlo
The above findings highlight CSTC’s multifaceted anti-UC effects, which are mediated through oxidative stress mitigation and cytokine modulation, while emphasizing the polarity-dependent efficacy of its extracts.
Core Tip: This study reveals that the compound spleen-tonifying composition (CSTC), a traditional Chinese medicine formula, alleviates ulcerative colitis (UC) through dual modulation of oxidative stress, inflammation and tight junction protein expression. Crucially, bioactivity is polarity-dependent: Polar solvent extracts (ethyl acetate/n-butanol) show superior efficacy to non-polar extracts in restoring intestinal barrier integrity, reducing dextran sulfate sodium-induced damage, and normalizing inflammatory/oxidative markers. This is the first report establishing extraction methodology as a critical determinant of CSTC’s therapeutic effects, providing a novel strategy for optimizing herbal formulations against UC.
- Citation: Zhao WC, Zhao QL, Zhang Y, Zhao N, Tian JQ, Wu YY, Zhang W. Compound spleen-tonifying composition alleviates dextran sulfate sodium-induced ulcerative colitis in rats. World J Gastrointest Surg 2025; 17(10): 108239
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/108239.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.108239
Ulcerative colitis (UC) is a chronic inflammatory bowel disease primarily affecting the rectum and colon. It is typically characterized by persistent pain, weakened intestinal peristalsis, and mucus and blood in stools. In recent years, the incidence of UC has increased annually because of changes in people's daily living habits and nutritional intake, posing a serious threat to life and health[1,2]. At present, glucocorticoids, aminosalicylic acid, immunosuppressants, and other common drugs are used to treat UC. Although these drugs can alleviate symptoms, they cannot completely cure the disease and have considerable toxic side effects[2-4]. In recent years, numerous research results have shown that traditional Chinese medicine has remarkable advantages in improving the symptoms of patients with UC, enhancing their quality of life, and reducing treatment-related adverse reactions[5,6].
Traditional Chinese medicine has a long history of treating UC, showing considerable clinical efficacy and minimal adverse reactions. Its function is mainly reflected in its multicomponent and multitarget characteristics. In recent years, numerous studies have demonstrated that single herbs and their active ingredients, as well as traditional Chinese medicine formulas, can enhance the antioxidant capacity of intestinal mucosa through different pathways, reduce inflammation, and thus treat UC. The traditional Chinese medicine formula Pulsatilla decoction added with the herb Rhizoma Atractylodis macrocephalae (A. macrocephalae) has been proven to inhibit inflammation and oxidative damage by regulating the IL-6/signal transducer and activator of transcription 3 pathway, thereby effectively improving dextran sulfate sodium (DSS)-induced UC[7]. In addition, the new Baitouweng decoction containing 13 traditional Chinese medicines has been proven to regulate gut microbiota and alleviate UC[8].
The compound spleen-tonifying composition (CSTC) containing Codonopsis pilosula (C. pilosula), A. macrocephalae, Poria cocos (P. cocos), Pulsatilla chinensis (Bunge) Regel, dandelion, Sanguisorba officinalis (S. officinalis), Bletilla striata (B. striata), and Glycyrrhiza uralensis (G. uralensis) Fisch is a classical traditional Chinese medicine prescription. In clinical practice, we have used CTSC for the treatment of UC with definite efficacy. The traditional Chinese medicine CTSC includes C. pilosula, A. macrocephala, and P. cocos, with the functions of nourishing qi, strengthening the spleen, and promoting dampness[9-11], as the main herbs. P. chinensis (Bunge) Regel and dandelion are medicinal herbs that are effective in regulating the large intestine and liver meridians[12,13]. They have a bitter taste and cold nature. They can separate blood and clear heat, detoxify and cool blood, and stop dysentery. S. officinalis and B. striata are used as adjuncts to restore the liver, stomach, and large intestine meridians to achieve detoxification, blood cooling, convergence, and hemostasis effects[14,15]. G. uralensis Fisch has a mild nature and has the effects of nourishing qi, detoxification, and harmonizing various medicines[16]. CSTC nourishes qi, strengthens the spleen, eliminates dampness, cools and detoxifies blood, and stops bleeding and dysentery. It is suitable for tonifying the spleen and benefits the stomach, spleen deficiency, qi stagnation, diarrhea, defecation, intestinal wind, and blood under the wind. It is especially useful for UC with spleen deficiency and dampness combined with heat toxicity syndrome, such as diarrhea; dysentery; and spleen deficiency and dampness.
In this study, we aim to explore the therapeutic effect of CSTC on DSS-induced UC in rats. CSTC, particularly at high doses, demonstrated robust efficacy against DSS-induced UC by modulating oxidative stress and inflammation. The divergent effects of its solvent extracts emphasize the critical role of extraction methodologies in harnessing phytotherapeutic potential. Our findings position CSTC as a promising candidate for UC treatment.
Eighty Sprague-Dawley rats (equal number of males and females) weighing 200-230 g were purchased from Liaoning Changsheng Biotechnology Co., Ltd. Dried slices of C. pilosula, A. macrocephala, P. cocos, P. chinensis (Bunge) Regel, dandelion, S. officinalis, B. striata, and G. uralensis Fisch were procured from Hebei Chufeng Chinese Herbal Slices Co., Ltd. and identified by the Chief Pharmacist Guo-Cai Sun of the Department of Pharmacy in The 964th Hospital of the Joint Logistic Support Force of the Chinese People's Liberation Army in compliance with the requirements of the Chinese Pharmacopoeia. DSS (MW = 5000) was bought from Shanghai Yuanye Biotechnology Co., Ltd. Superoxide dismutase (SOD), malondialdehyde (MDA), myeloperoxidase (MPO), glutathione peroxidase (GSH-px), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) kits were sourced from Nanjing Jiancheng Bioengineering Institute.
A total of 580 g of medicinal herbs was weighed in accordance with the prescription ratio, soaked in 6000 mL of water for 1 hour, boiled twice for 1 hour each time, and filtered. The filtrate was combined and concentrated. The concentrated solution was extracted sequentially with petroleum ether, chloroform, ethyl acetate, and water-saturated N-butanol in the liquid extract-to-extraction solution ratio of 1:2. Each solution was extracted three times in a row. The final solution of each extraction fraction was obtained, evaporated and concentrated, and dried. Subsequently, the extracted drugs were dissolved in water and brought to a volume of 200 mL. Petroleum ether, chloroform, ethyl acetate, and n-butanol extracts were thus obtained.
A rat model of UC was established by using DSS (4% w/v)[17]. Animals were kept in a controlled setting at 23 °C and reared for one week on a 12 hours dark/Light cycle. After five days of adaptive feeding, 80 SD rats were randomly assigned to 10 groups (n = 8 per group), as follows: Control group: Mice received distilled water ad libitum. DSS group: Mice received 4% (w/v) DSS solution ad libitum for seven days to induce colitis, then gavaged with an equivalent volume of pure water daily for 10 days. Positive group: Mice received 4% DSS solution for seven days, then gavaged with 5-aminosalicylic acid suspension (0.2 g/kg body weight) daily for 10 days. High-, medium-, and low-dose CSTC groups: Mice received 4% DSS for seven days, then gavaged with 4.0, 2.0, and 1.0 g/kg CSTC aqueous solution daily for 10 days. Petroleum ether, chloroform, ethyl acetate, and N-butanol extract groups: Mice received 4% DSS for seven days, then gavaged daily with different extract solutions at the dose of 4.0 g/kg for 10 days. After the final administration, all rats received anesthesia and were sacrificed through cervical dislocation. The colon was subsequently collected for further analysis. Animal experiments were reviewed and approved by the Animal Experiment Committee of the 964th Hospital of the Joint Logistics Support Force.
The behavioral performance, mental state, hair luster, and fecal characteristics of each group were monitored. The presence of bloody and purulent stool was observed. Meanwhile, changes in body weight and food intake were recorded. The disease activity index (DAI) was used to evaluate the condition of the rats, and scoring criteria were based on the literature[8]. This study implemented a multidimensional quantitative system for clinical symptom stratification. Body mass alterations were stratified into five gradients: Grade 0 (no loss), Grade 1 (< 5% reduction), Grade 2 (5%-10% decrease), Grade 3 (10%-20% decline), and Grade 4 (> 20% depletion). Intestinal function was assessed by using a three-tier scale: Baseline status (0 points, formed stools), mild abnormality (2 points, loose consistency), and marked abnormality (4 points, watery discharge). The following three-tier criteria were established for lower gastrointestinal bleeding manifestations to achieve the nuanced differentiation of symptom severity: Absence of bleeding (0 points), minor hemorrhage (2 points), and overt bleeding (4 points).
Rats were anesthetized at 24 hours after the last drug administration, and the colon was quickly dissected. The colon was cut along the longitudinal axis, washed with physiological saline, and fixed. Inflammation and ulceration were observed with the naked eye. A 0.5 cm colon segment was collected 1 cm away from the ileocecal junction and fixed in 4% formaldehyde solution for hematoxylin-eosin staining (HE) and microscopy examination.
Histopathological evaluation employed a dual-axis grading matrix incorporating inflammatory burden and structural integrity parameters. Leukocyte infiltration intensity was quantified on the basis of a quaternary scale (0-3) spanning isolated lamina propria involvement (Grade 0) to circumferential bowel wall penetration (Grade 3). Mucosal pathology assessments utilized parallel stratification (0-3), distinguishing between histologically intact epithelia (baseline) and multilayered bowel wall disintegration (severe), enabling comprehensive histopathological profiling.
The remaining colon tissues were washed with ice-cold saline and homogenized through ultrasonic crushing for SOD, MDA, MPO, GSH-px, TNF-α, IL-1β, and IL-6 detection. Specific operations were performed in accordance with kit instructions.
Total RNA was isolated from colonic tissues by utilizing TRIzol reagent (Invitrogen, the United States), then reverse-transcribed by using a commercial cDNA synthesis kit (Vazyme, China). Quantitative reverse transcription PCR was subsequently performed with iTaq™ Universal SYBR® Green Supermix (Bio-Rad, United States) on an ABI 7500 Real-Time PCR System (Applied Biosystems, United States). The relative expression levels of target genes (ZO-1 and occludin) were normalized against that of the endogenous reference gene GAPDH and quantitatively analyzed through the comparative threshold cycle (2−ΔΔCt) method.
Quantitative data analysis was conducted with SPSS statistical software (version 20.0; SPSS Inc., Chicago, IL, United States). Experimental results were expressed as mean ± SD based on three or more biologically independent replicates. Intergroup comparisons were assessed through unpaired Student's t-test analyses, with statistical significance defined as two-tailed P value < 0.05.
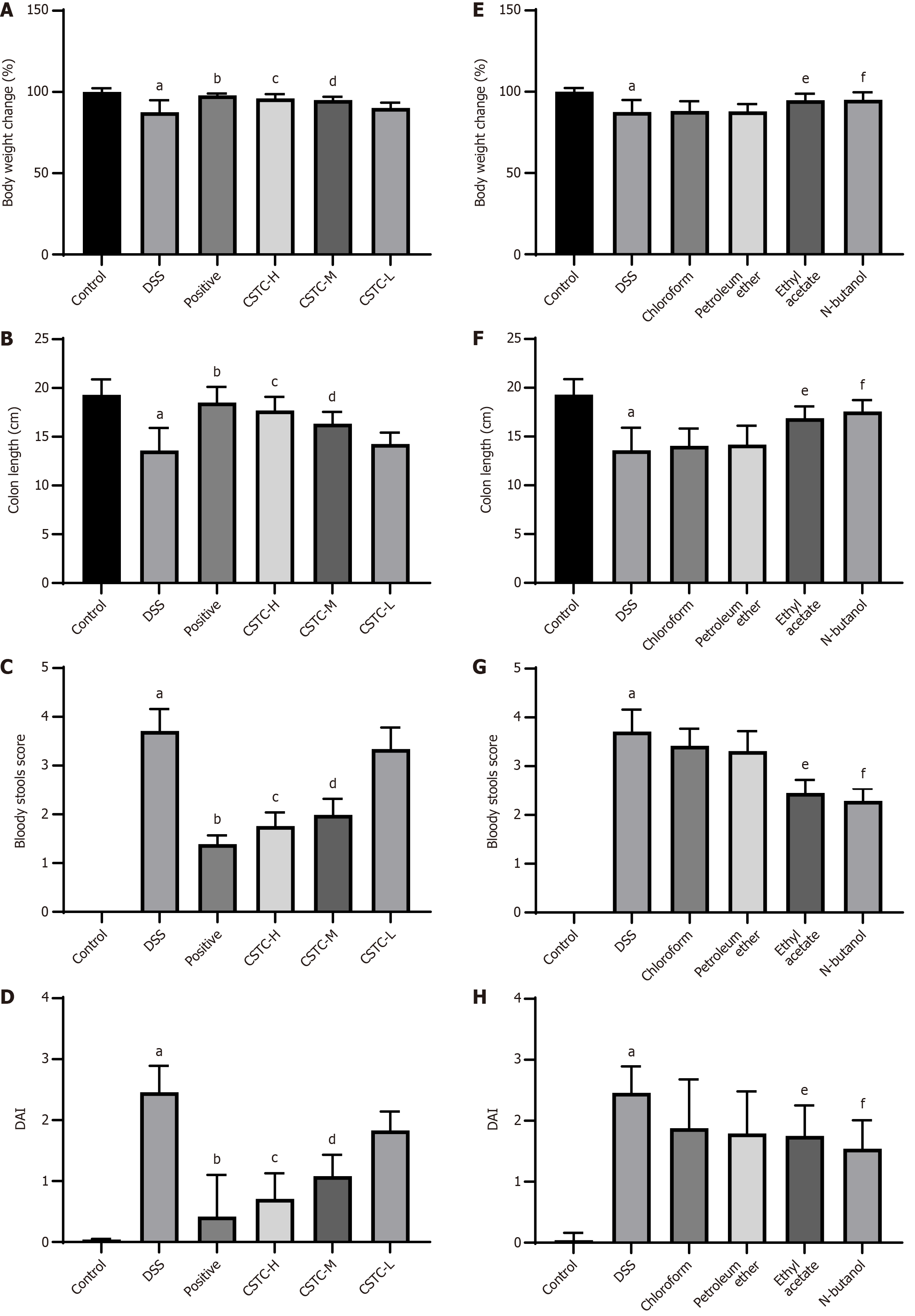
We observed body weights, colon lengths, stool characteristics, and DAI scores in each group to investigate whether CSTC can relieve UC symptoms in rats. The normal group exhibited normal water and food intake; no significant weight loss; and no abnormal changes, such as bloody stools.
In contrast to the control group, the DSS group showed symptoms, such as weight loss, rectal bleeding, decreased appetite, and shortened colon length, as well as significantly increased rectal bleeding and DAI scores (Figure 1A-D). Among the drug-treated experimental groups, the positive and CSTC groups (high- and medium-dose groups) showed significantly reduced DSS-induced body weight and improved colon shortening, bloody stools, and DAI scores (Figure 1A-D). In addition, we extracted CSTC with different extraction solutions (chloroform, petroleum ether, ethyl acetate, and n-butanol) and subsequently used the extracts to treat UC rats. The chloroform and petroleum ether extract groups showed only slight improvements, whereas the groups administered with ethyl acetate and/or n-butanol extracts demonstrated a marked ability to mitigate weight loss induced by DSS exposure. Moreover, these groups showed improvements in colon length, a reduction in the occurrence of bloody stools, and a decrease in DAI scores (Figure 1E-H).
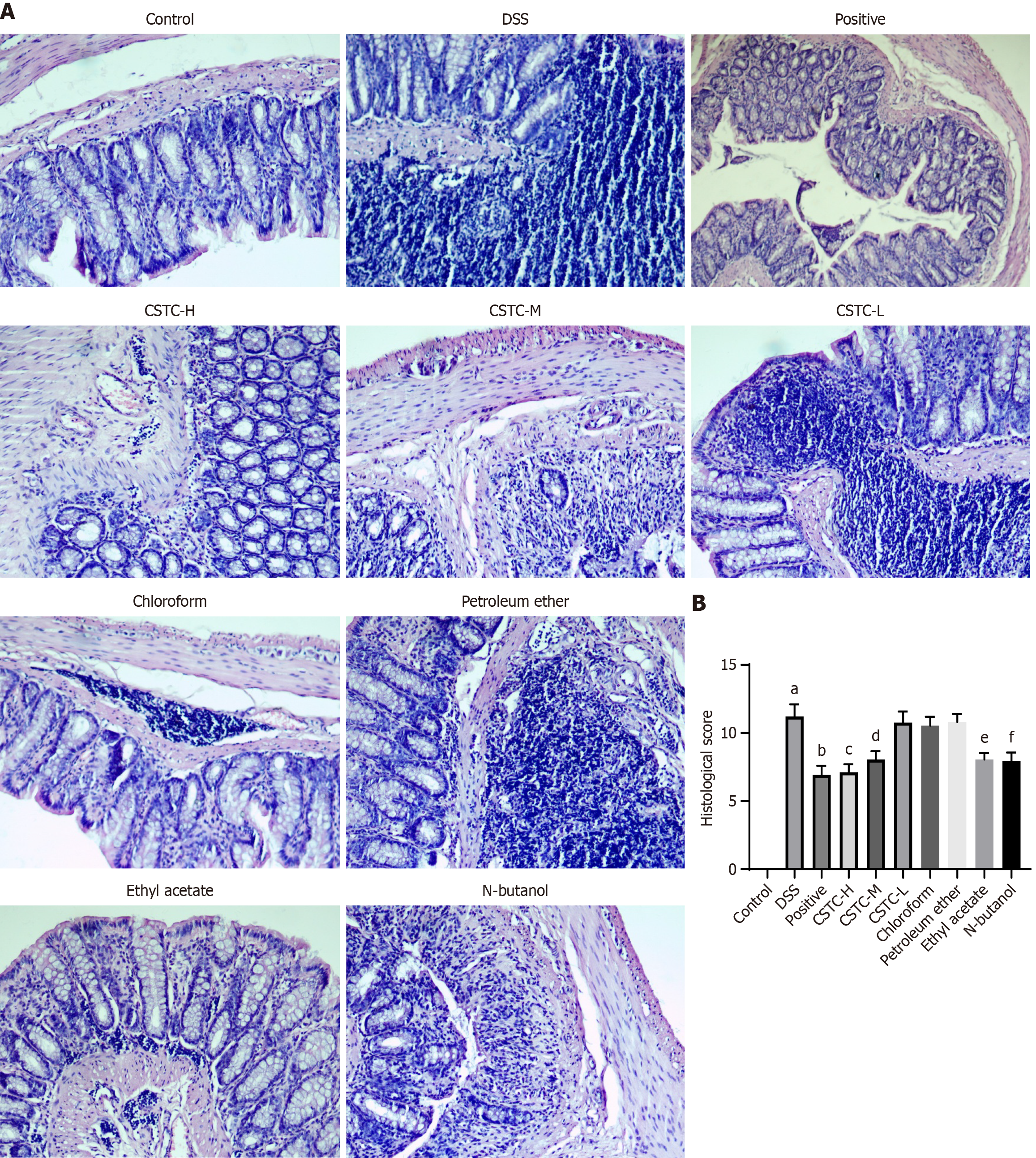
We further visually evaluated the ameliorating effect of CSTC on colon tissue damage through the HE staining of colon tissue. We observed that the colonic mucosa of the control group was intact, with well-arranged glands and no inflammatory cell infiltration or ulcers. We found that the colon structure in DSS group rats was damaged, with mucosal destruction, glandular disorder, extensive mucosal necrosis, and ulceration, leading to a significant increase in pathological scores (Figure 2). The positive group, groups treated with high and medium doses of CSTC, ethyl acetate extract group, and N-butanol extract group effectively showed improved DSS-induced colonic histological damage in the colon mucosa and reduced pathological scores (Figure 2). The changes in intestinal tissue morphology were not significant in the low-dose CSTC, chloroform extract, and petroleum ether extract groups, indicating a weak effect on the repair of colonic mucosa (Figure 2).
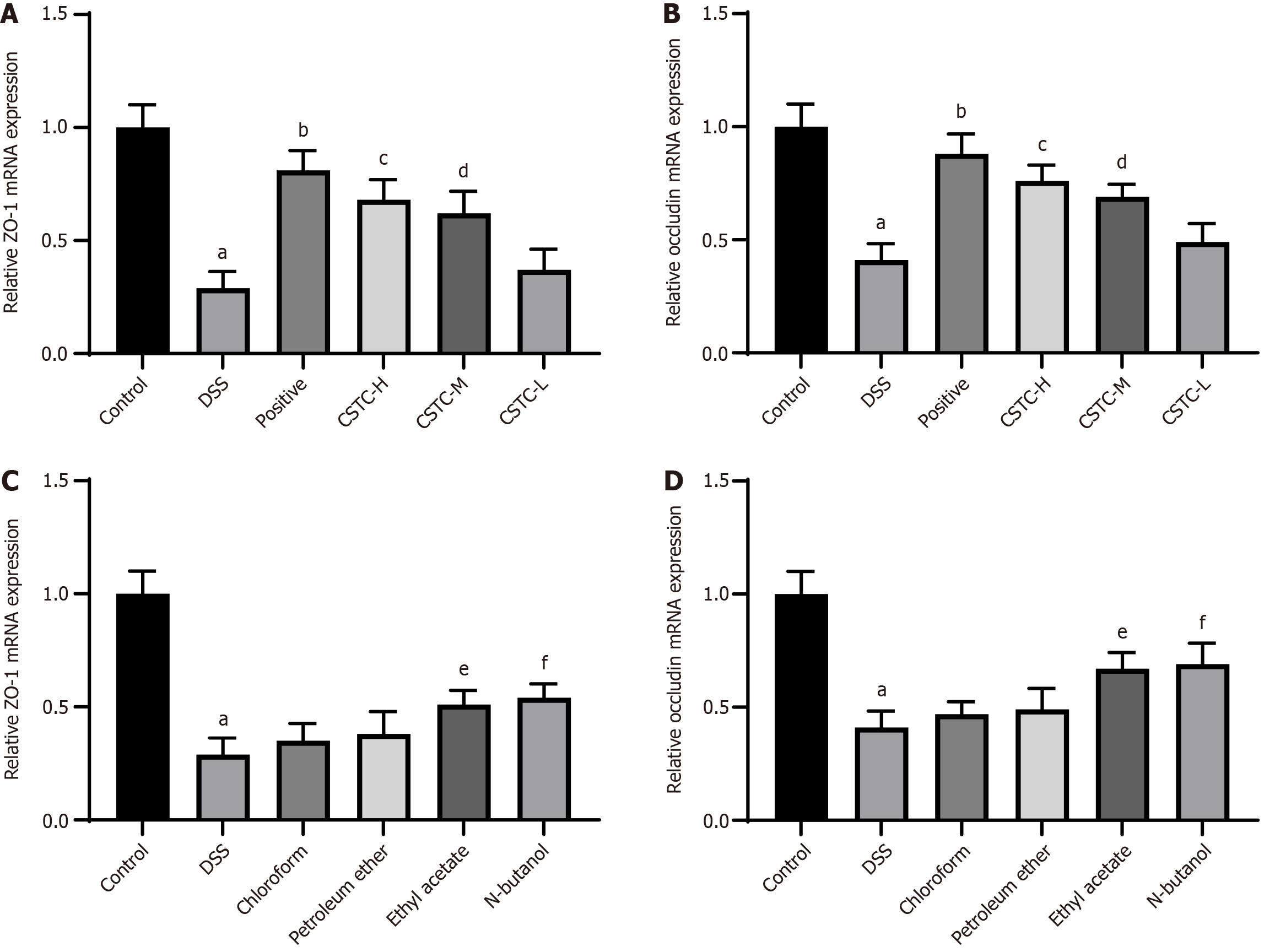
We subsequently measured the expression levels of the representative tight junction proteins ZO-1 and occludin in colon tissue to explore the effect of CSTC on the integrity of the intestinal mucosal barrier. The results showed that the mRNA expression levels of ZO-1 and occludin decreased in the DSS group. However, after treatment with the positive drug and high and medium doses of CSTC, the mRNA expression levels of ZO-1 and occludin in the colon of UC rats significantly increased (Figure 3A and B). In addition, chloroform and petroleum ether extracts were unable to restore the effects of DSS stimulation effectively, resulting in the decreased expression levels of ZO-1 and occludin mRNA. However, ethyl acetate and n-butanol could restore the expression levels of these proteins (Figure 3C and D).
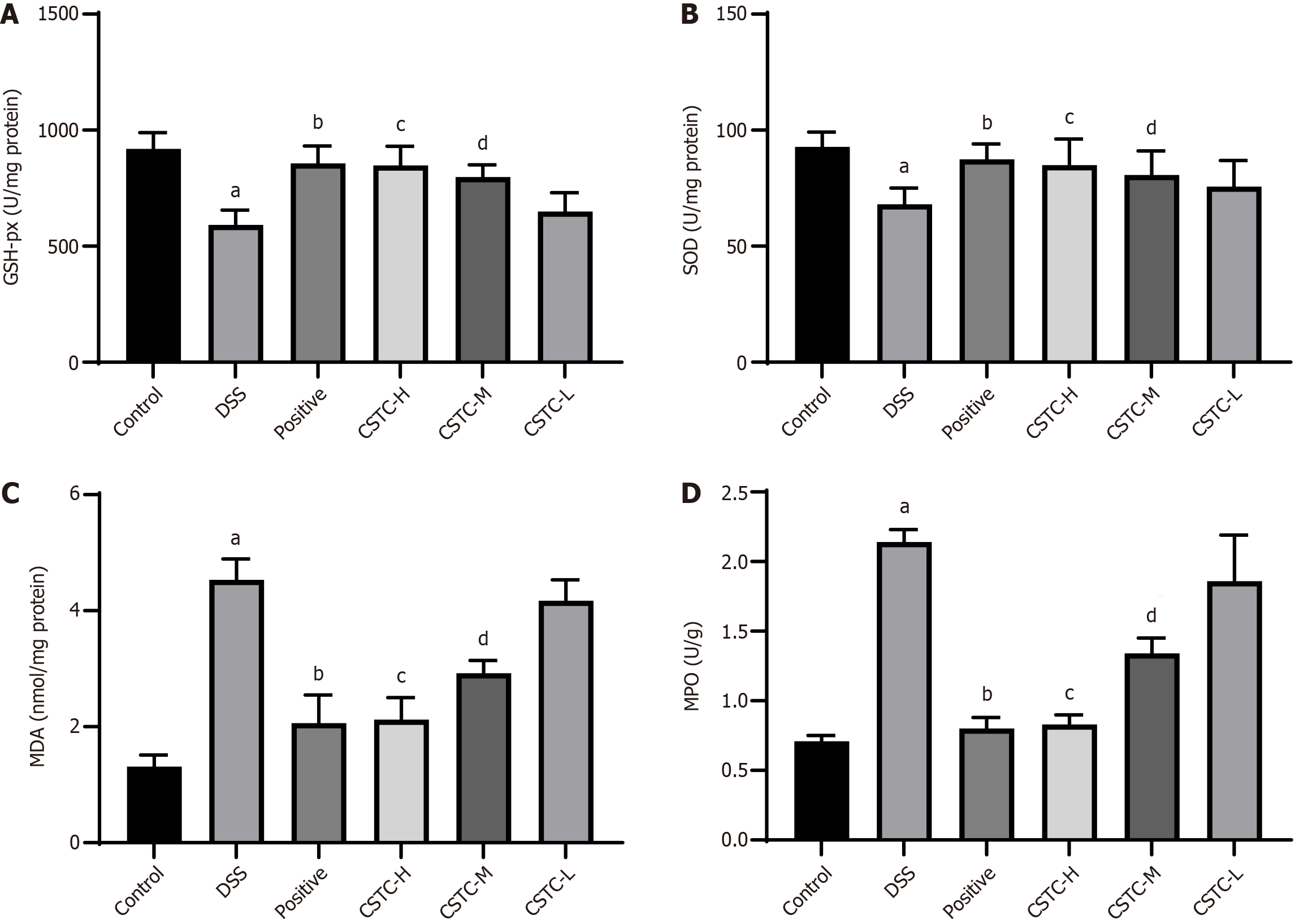
We measured the expression levels of oxidase (MDA and MPO) and antioxidant (SOD and GSH-px) enzymes to understand the antioxidant effects of CSTC in DSS-induced rats. We revealed that DSS stimulation led to a significant reduction in SOD and GSH-px activities, whereas the levels of MPO and MDA in colon tissue significantly increased. After positive drug and CSTC treatment (high and medium doses), the levels of MPO and MDA significantly decreased, whereas the activities of SOD and GSH-px significantly increased (Figure 4).
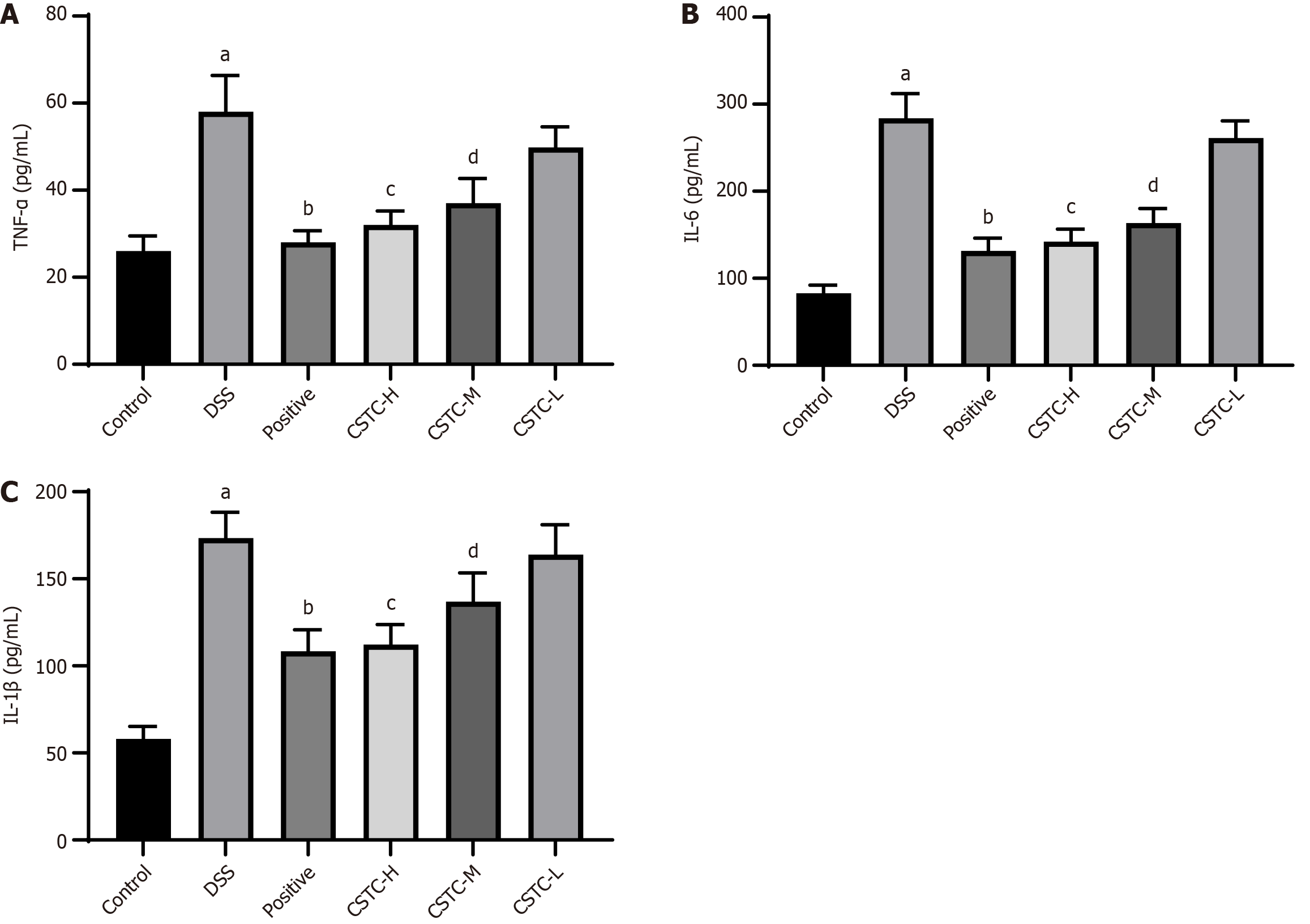
Next, we investigated the anti-inflammatory effect of CSTC in DSS-induced UC. Compared with those after the control treatment, the levels of TNF-α, IL-1β, and IL-6 in rat colon tissue significantly increased after DSS stimulation. The levels of TNF-α, IL-1β, and IL-6 in the colon tissue of rats treated with the positive drug and high and medium doses of CSTC significantly reduced (Figure 5).
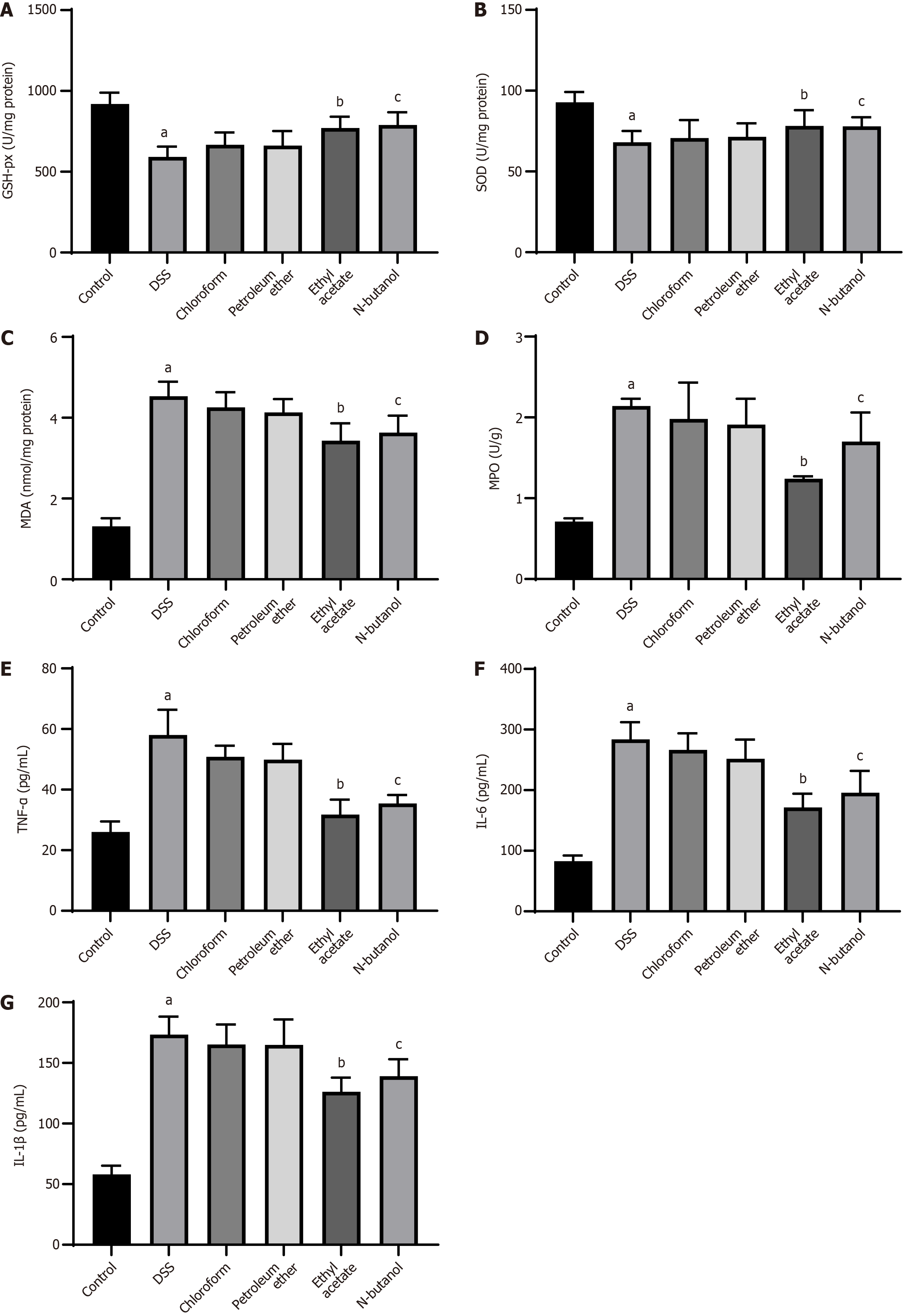
We extracted CSTC by using nonpolar (chloroform and petroleum ether) and polar (ethyl acetate and N-butanol) solvents and explored the effects of the resulting extracts on oxidative stress and inflammation. DSS-induced rats had lower SOD and GSH-px levels and higher MPO and MDA levels than control group rats. These levels were prominently restored by treatment with ethyl acetate or n-butanol extract (Figure 6A-D). In addition, in DSS rats, ethyl acetate and n-butanol extracts showed anti-inflammatory effects similar to those of CSTC, as evidenced by the reduced concentrations of TNF-α, IL-1β, and IL-6 (Figure 6E-G). The use of chloroform and petroleum ether extracts did not affect the secretion of oxidative stress markers and inflammatory factors in DSS rats (Figure 6).
UC, as a chronic inflammatory bowel disease with a prolonged course and recurrent episodes, may increase the risk of cancer. In recent years, the combination of traditional Chinese medicine theory and modern scientific technology and methods has become a research hotspot in the treatment of UC. Establishing the UC model by allowing rats to drink DSS solution with a certain concentration voluntarily has become the most common and reliable UC modeling method. DSS directly damages the colonic epithelium, inducing acute inflammation characterized by neutrophil infiltration, crypt distortion, ulceration, and elevated proinflammatory cytokine (e.g., TNF-α, IL-1β, and IL-6) and oxidative stress marker (e.g., MPO and MDA) levels, features that closely mirror the epithelial barrier dysfunction and inflammatory milieu observed in human UC[18-20]. This fidelity makes this modeling method particularly suitable for evaluating therapeutics targeting mucosal healing, inflammation, and oxidative stress. The expression levels of ZO-1 and occludin significantly reduced in patients with UC and DSS animal models. This effect is closely related to intestinal mucosal barrier damage. Our results indicated that CSTC (high and medium doses) and its ethyl acetate and n-butanol extracts could effectively improve colon injury in UC rats during treatment. Such an improvement is manifested by the inhibition of DSS-induced weight loss, colon shortening, rectal bleeding, diarrhea, and ZO-1 and occludin expression silencing. Notably, the efficacy of CSTC was dose-dependent, with low-dose treatment failing to elicit significant improvements in clinical or histological parameters. This finding aligns with previous results emphasizing the importance of optimal dosing for phytochemical interventions in UC models[12,21].
The etiology and mechanism of UC remain unclear and may involve complex inflammatory reactions. During UC, the expression of proinflammatory cytokines in the intestinal mucosa increases, whereas the secretion of anti-inflammatory cytokines is relatively insufficient, leading to inflammatory reactions in the intestinal mucosa and causing intestinal damage. Therefore, inflammatory factors are usually regarded as important topics in the research on UC[22]. As expected, the anti-inflammatory properties of CSTC and its polar extracts were further underscored by reduced TNF-α, IL-1β, and IL-6 Levels.
Recent studies have found that oxidative stress plays a key role in the pathogenesis of UC. The treatment of UC can be achieved by regulating the level of oxidative stress[23-26]. Oxidative stress can damage intestinal mucosal epithelial cells in various ways, affect the normal function of intestinal epithelial cells, and cause or aggravate the occurrence and development of UC[27]. The levels of MPO, MDA, SOD, GSH-px, and other cytokines can reflect antioxidant capacity in patients with UC[28,29]. Therefore, regulating the activity of the above cytokines and improving antioxidant capacity in patients with UC provide an effective method for the treatment of UC. Here, the observed restoration of colon length and reduction in DAI scores at high CSTC doses may stem from the ability of CSTC to suppress oxidative stress, as evidenced by the normalized SOD, GSH-px, MDA, and MPO levels. These results corroborate the role of antioxidant pathways in countering DSS-induced epithelial damage.
Intriguingly, solvent extraction markedly influenced therapeutic outcomes. While chloroform and petroleum ether extracts exhibited minimal efficacy, ethyl acetate and N-butanol extracts displayed effectiveness. They demonstrated remarkable histological repair, antioxidant activity, and anti-inflammatory effects. The discrepancy between the efficacies of various extracts may arise from the divergent bioactive constituents extracted by polar and nonpolar solvents. Extracts obtained with ethyl acetate and n-butanol, which typically isolate phenolic acids or flavonoids, likely contain anti-inflammatory compounds that mitigate tissue-level damage[30-32]. The inability of chloroform and petroleum ether extracts to modulate cytokines suggests that nonpolar solvents fail to isolate critical anti-inflammatory agents from CSTC. This polarity-dependent bioactivity highlights the importance of solvent selection in optimizing herbal extracts for UC therapy.
While this study provides compelling evidence for the efficacy of CSTC and its polar extracts in the treatment of DSS-induced UC, several limitations warrant acknowledgment. First, the mechanistic exploration in this work focused primarily on downstream effects (oxidative stress, inflammation, and barrier function). Future studies should delve deeply into the upstream signaling pathways (e.g., NF-κB, MAPK, Nrf2, and NLRP3 inflammasome) modulated by CSTC and its active constituents to elucidate its molecular mode of action fully. Second, the gut microbiome plays a crucial role in UC pathogenesis and TCM efficacy. Although our study demonstrated functional improvements, the effect of CSTC on gut microbial composition and function remains unexplored. Investigating these changes could provide valuable insights into additional mechanisms. Addressing these limitations in future research will be crucial for advancing CSTC toward clinical application.
We systematically evaluated the therapeutic potential of CSTC and its solvent-derived extracts in alleviating DSS-induced UC in rats. Our findings demonstrated that the administration of CSTC, particularly at high and medium doses, effectively ameliorated UC-related symptoms, including weight loss, colon shortening, and increased DAI scores. Furthermore, histological and biochemical analyses revealed that CSTC mitigated colonic mucosal damage, oxidative stress, and inflammation and promoted tight junction protein expression, suggesting a multifaceted mechanism of action. These findings position CSTC as a promising candidate for UC treatment, warranting the further exploration of its bioactive constituents and clinical translatability.
| 1. | Nascimento RPD, Machado APDF, Galvez J, Cazarin CBB, Maróstica Junior MR. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020;258:118129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 2. | Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 945] [Reference Citation Analysis (104)] |
| 3. | Ismail Abo El-Fadl HM, Mohamed MFA. Targeting endoplasmic reticulum stress, Nrf-2/HO-1, and NF-κB by myristicin and its role in attenuation of ulcerative colitis in rats. Life Sci. 2022;311:121187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 4. | Christophorou D, Funakoshi N, Duny Y, Valats JC, Bismuth M, Pineton De Chambrun G, Daures JP, Blanc P. Systematic review with meta-analysis: infliximab and immunosuppressant therapy vs. infliximab alone for active ulcerative colitis. Aliment Pharmacol Ther. 2015;41:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Zheng S, Xue T, Wang B, Guo H, Liu Q. Chinese Medicine in the Treatment of Ulcerative Colitis: The Mechanisms of Signaling Pathway Regulations. Am J Chin Med. 2022;50:1781-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Duan ZL, Wang YJ, Lu ZH, Tian L, Xia ZQ, Wang KL, Chen T, Wang R, Feng ZY, Shi GP, Xu XT, Bu F, Ding Y, Jiang F, Zhou JY, Wang Q, Chen YG. Wumei Wan attenuates angiogenesis and inflammation by modulating RAGE signaling pathway in IBD: Network pharmacology analysis and experimental evidence. Phytomedicine. 2023;111:154658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 7. | Huangfu S, Dou R, Zhong S, Guo M, Gu C, Jurczyszyn A, Yang Y, Jiang B. Modified Pulsatillae decoction inhibits DSS-induced ulcerative colitis in vitro and in vivo via IL-6/STAT3 pathway. BMC Complement Med Ther. 2020;20:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Gu X, Miao Z, Wang Y, Yang Y, Yang T, Xu Y. New Baitouweng decoction combined with fecal microbiota transplantation alleviates DSS-induced colitis in rats by regulating gut microbiota metabolic homeostasis and the STAT3/NF-κB signaling pathway. BMC Complement Med Ther. 2022;22:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Guo H, Lou Y, Hou X, Han Q, Guo Y, Li Z, Guan X, Liu H, Zhang C. A systematic review of the mechanism of action and potential medicinal value of codonopsis pilosula in diseases. Front Pharmacol. 2024;15:1415147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Zhu B, Zhang QL, Hua JW, Cheng WL, Qin LP. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J Ethnopharmacol. 2018;226:143-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 11. | Guo ZY, Wu X, Zhang SJ, Yang JH, Miao H, Zhao YY. Poria cocos: traditional uses, triterpenoid components and their renoprotective pharmacology. Acta Pharmacol Sin. 2025;46:836-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Zhou M, Yang M, Jin C, Song Y, Chen J, Gao M, Ai Z, Su D. Pulsatilla chinensis Saponins Ameliorate Inflammation and DSS-Induced Ulcerative Colitis in Rats by Regulating the Composition and Diversity of Intestinal Flora. Front Cell Infect Microbiol. 2021;11:728929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Shi Y, Zou J, Zhang X, Zhai B, Guo D, Sun J, Luan F. Extraction, purification, structural features, biological activities, modifications, and applications from Taraxacum mongolicum polysaccharides: A review. Int J Biol Macromol. 2024;259:129193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 14. | Jang E, Inn KS, Jang YP, Lee KT, Lee JH. Phytotherapeutic Activities of Sanguisorba officinalis and its Chemical Constituents: A Review. Am J Chin Med. 2018;46:299-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | He X, Wang X, Fang J, Zhao Z, Huang L, Guo H, Zheng X. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J Ethnopharmacol. 2017;195:20-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 16. | Ding Y, Brand E, Wang W, Zhao Z. Licorice: Resources, applications in ancient and modern times. J Ethnopharmacol. 2022;298:115594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 17. | Ma J, Zhang J, Wang Y, Huang J, Yang X, Ma J, Liu Z, Wang F, Tang X. Modified Gegen Qinlian decoction ameliorates DSS-induced chronic colitis in mice by restoring the intestinal mucus barrier and inhibiting the activation of γδT17 cells. Phytomedicine. 2023;111:154660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 18. | Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23:6016-6029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 621] [Cited by in RCA: 633] [Article Influence: 70.3] [Reference Citation Analysis (20)] |
| 19. | Zhang W, Zou G, Li B, Du X, Sun Z, Sun Y, Jiang X. Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation. J Microbiol Biotechnol. 2020;30:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1-15.25.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1431] [Article Influence: 119.3] [Reference Citation Analysis (1)] |
| 21. | Li Q, Cui Y, Xu B, Wang Y, Lv F, Li Z, Li H, Chen X, Peng X, Chen Y, Wu E, Qu D, Jian Y, Si H. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol Res. 2021;170:105694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 22. | Li Q, Sun X, Yu K, Lv J, Miao C, Yang J, Wang S, Fu Z, Sun Y, Zhang H, Zhang ZS, Keller ET, Yao Z, Wang Q. Enterobacter ludwigii protects DSS-induced colitis through choline-mediated immune tolerance. Cell Rep. 2022;40:111308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Mao N, Yu Y, He J, Yang Y, Liu Z, Lu Y, Wang D. Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota. Int J Mol Sci. 2024;25:6613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 24. | Li R, Mou J, Zhao L, Hu M, Wang B, Sun Y, Liu J, Qi X, Yang J. Fucoidan from Stichopus chloronotus relieved DSS induced ulcerative colitis through inhibiting intestinal barrier disruption and oxidative stress. Int J Biol Macromol. 2024;283:137811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Xiao HT, Peng J, Wen B, Hu DD, Hu XP, Shen XC, Liu ZG, He ZD, Bian ZX. Indigo Naturalis Suppresses Colonic Oxidative Stress and Th1/Th17 Responses of DSS-Induced Colitis in Mice. Oxid Med Cell Longev. 2019;2019:9480945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Tian T, Wang Z, Zhang J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid Med Cell Longev. 2017;2017:4535194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 480] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 28. | Zhang C, Hu Y, Yuan Y, Guo J, Li H, Li Q, Liu S. Liposome-embedded SOD attenuated DSS-induced ulcerative colitis in mice by ameliorating oxidative stress and intestinal barrier dysfunction. Food Funct. 2023;14:4392-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 29. | Zhao H, Cheng N, Zhou W, Chen S, Wang Q, Gao H, Xue X, Wu L, Cao W. Honey Polyphenols Ameliorate DSS-Induced Ulcerative Colitis via Modulating Gut Microbiota in Rats. Mol Nutr Food Res. 2019;63:e1900638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Li W, Zhang L, Xu Q, Yang W, Zhao J, Ren Y, Yu Z, Ma L. Taxifolin Alleviates DSS-Induced Ulcerative Colitis by Acting on Gut Microbiome to Produce Butyric Acid. Nutrients. 2022;14:1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Wu Z, Huang S, Li T, Li N, Han D, Zhang B, Xu ZZ, Zhang S, Pang J, Wang S, Zhang G, Zhao J, Wang J. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 487] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 32. | Han D, Wu Y, Lu D, Pang J, Hu J, Zhang X, Wang Z, Zhang G, Wang J. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death Dis. 2023;14:656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/