Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.107760
Revised: June 22, 2025
Accepted: August 6, 2025
Published online: October 27, 2025
Processing time: 156 Days and 22.8 Hours
Although thoracotomy has been the conventional treatment for patients with early esophageal cancer (EEC), its drawbacks underscore the demand for more effective therapeutic strategies to improve surgical outcomes.
To comprehensively analyze the effect of totally thoracoscopic esophagectomy (TTE) on postoperative complications and serum inflammatory factors in patients diagnosed with EEC.
A total of 113 patients with EEC, who were admitted to our hospital between September 2022 and December 2024, were recruited for this study. Specifically, 55 patients were assigned to the control group and underwent conventional surgical procedures, whereas 58 patients formed the research group and underwent TTE. Subsequently, a series of comparisons and analyses were conducted between the two groups. These comparisons included surgery-related parameters, such as incision length, operation duration, and the number and extent of lymph node dissection; postoperative complications, namely, empyema, pulmonary infection, incision infection, anastomotic fistula, and delayed gastric emptying; postope
Statistical data demonstrated that compared with the control group, the research group exhibited substantially shorter incision length and postoperative hospitalization duration. The two groups had comparable number and extent of lymph node dissection. Notably, both the overall incidence of postoperative complications and the Numerical Rating Scale score on postoperative day 3 were remarkably lower in the research group. Although the levels of IL-6, IL-8, tumor necrosis factor-α, C-reactive protein, cortisol, and adrenaline in the research group increased statistically postoperatively, they were still considerably lower than those in the control group.
In patients with EEC, TTE not only reduces the risk of postoperative complications but also effectively alleviates the body’s inflammatory and stress responses associated with surgery.
Core Tip: Currently, evidence on totally thoracoscopic esophagectomy for early esophageal cancer is limited. This study confirms that compared to conventional surgery, this approach minimizes incision size, decreases hospitalization, reduces complications, lessens pain on postoperative day 3, and attenuates inflammatory stress. The finding supports its role as a clinically superior approach and a valuable reference for optimizing treatment strategies while furnishing practitioners with clinically relevant reference data.
- Citation: Zhao HC, Zhang S, Zhou L, Lou XL, Chen D, Shi CW, Ren Z. Effect of totally thoracoscopic esophagectomy on postoperative complications and serum inflammatory factors in patients with early esophageal cancer. World J Gastrointest Surg 2025; 17(10): 107760
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/107760.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.107760
Esophageal cancer (EC), a highly lethal digestive tract malignancy, ranks as the sixth leading cause of cancer-related mortality and the seventh most prevalent cancer worldwide[1]. According to statistics from the American Cancer Society, there were 19260 new cancer diagnoses and 15530 associated fatalities in the United States in the year 2021 alone[2]. This type of cancer is notable for its high invasiveness and pronounced tendency for lymph node metastasis. Clinically, patients typically experience a choking sensation, a sense of a foreign body while swallowing food, or may suffer from retrosternal pain[3]. Regrettably, in the early disease stage, the symptoms and physical manifestations are often subtle and not specific; as a result, the majority of the patients have middle or advanced disease stages upon diagnosis. This not only leads to a dismal prognosis, with a 5-year survival rate of < 20%, but also incurs exorbitant treatment expenses[4,5]. Timely treatment for patients with early EC (EEC) is crucial in averting the infiltration and metastasis of cancer cells, thus safeguarding patients’ physical well-being and avoiding the life-threatening effect of tumor invasion[6,7].
Currently, surgery remains the principal treatment modality for patients diagnosed with EEC, and thoracotomy is the conventional surgical approach[8]. Thoracotomy, performed under direct visual inspection, does yield certain therapeutic outcomes; however, it is encumbered with several limitations. The extensive surgical incision not only augments the susceptibility to infection but also protracts the recovery period, thereby presenting formidable impediments to the patient’s postoperative convalescence[9,10]. In contrast, totally thoracoscopic esophagectomy (TTE), a type of minimally invasive surgery, improves the capability of a thoracoscope to effectively widen the surgical field of vision. This not only substantially enhances the precision and efficiency of the surgical procedure but also mitigates the body’s exposure to unnecessary trauma, thereby minimizing the overall physiological influence[11]. Li et al[12] reported that thoracoscopic and laparoscopic minimally invasive esophagectomy constituted 73.4%, open surgery accounted for 17.5%, and hybrid surgery made up 9.1% among all surgical procedures in China. These statistical data unequivocally evince the wi
Currently, clinical evidence of its use in EEC remains scarce. To gain a more in-depth understanding of the clinical advantages associated with TTE in patients with EEC and bridge this research gap, a clinical comparative analysis is imperative to confirm the purported benefits and furnish evidence-based insights for the optimization of treatment strategies in this patient cohort.
This study adopted a retrospective research design. All patients who were definitively diagnosed with EC via gastroscopy in combination with pathological examination were included[14]. They strictly met the established indications for surgical intervention. According to the tumor node metastasis staging system, their disease was classified as stage 0-I[15]. Notably, they had no evidence of overt tumor invasion (T4b) and distant metastatic foci. Additionally, these patients had no history of thoracic surgical procedures. Their clinical data records were comprehensive, and they demonstrated high levels of compliance throughout the study process. Conversely, patients who had undergone radiotherapy or che
Through meticulous screening based on the aforementioned inclusion and exclusion criteria, a total of 113 patients diagnosed with EEC, who were admitted to our hospital between September 2022 and December 2024, comprised the study population. Based on the different surgical approaches, participants were allocated into two treatment groups: The control group (n = 55) received conventional surgical treatment, whereas the research group (n = 58) underwent TTE.
With α = 0.05 and β = 0.2, the sample size calculation yielded 48 participants per group. Considering a 15% dropout rate, 55 participants were recruited per group (total of 113), ensuring methodological rigor. Post-hoc analysis revealed that the final sample size exceeded the standard 80% power threshold significantly.
The control group underwent conventional surgery. Specifically, the patient was placed in the left lateral decubitus position, and combined anesthesia was administered. Single-lumen tracheal intubation for ventilation of both lungs was then performed. Subsequently, an incision measuring 15-20 cm in length was made at the fourth intercostal space on the right anterolateral chest wall. The intrathoracic cavity was meticulously examined to precisely ascertain the location, dimensions, and morphological characteristics of the tumor. Subsequently, the thoracic segment of the esophagus was dissociated, and thoracic lymph nodes were dissected thoroughly. The patient was then placed in the supine position. To facilitate the dissection of the abdominal esophagus and lymph node dissection, a midline epigastric longitudinal incision (15 cm) was made. Additionally, an approximately 5-cm-long arcuate incision was created at the cervical sternum. The esophagus was transected at the cardia, and an end-to-end anastomosis was performed between the cervical esophageal stump and the fundus of the gastric esophageal end.
The research group underwent TTE (McKeown’s three-incision approach). Briefly, the patient was initially placed in the supine position, combined anesthesia was then administered, and single-lumen tracheal intubation was performed for bilateral lung ventilation. Subsequently, the right side of the patient’s body was elevated to approximately 30°, and the upper arm was repositioned toward the cephalic region. An incision of approximately 1 cm in length was made at the seventh intercostal space along the right midaxillary line, and a thoracoscope was inserted therein as the observation port. To establish an artificial pneumothorax, carbon dioxide was then insufflated, with the pressure precisely maintained at 6 mmHg. Subsequently, to establish operative ports for thoracoscopic access, additional incisions, each approximately 1 cm in length, were created at the fifth and eighth intercostal spaces and at the fourth intercostal space along the right anterior axillary line. The target lesions were identified, and the mediastinal pleura were carefully incised using an electrocautery hook. The azygos vein arch was meticulously dissected and subsequently transected, and a comprehensive mediastinal lymphadenectomy covering the upper, middle, and lower mediastinal regions was performed to ensure complete nodal clearance. The esophagus was then mobilized up to the cervical level for subsequent anastomosis. Thereafter, to facilitate gastric mobilization, an appropriately sized abdominal incision was made. A tubular gastric conduit was constructed while preserving the right gastroepiploic vascular arcade and greater curvature. For thorough nodal removal, systematic abdominal lymphadenectomy was performed, including dissection of the para-aortic and perigastric lymph nodes. The prepared gastric conduit was then carefully transposed to the cervical region for anastomosis. A transverse cervical incision exposed the cervical esophagus. Following the resection of the involved esophageal segment, the tubular gastric conduit was delivered to the neck. An esophagogastric anastomosis was performed using either a stapled or hand-sewn technique, providing meticulous attention to ensure tension-free anastomosis and reduce postoperative leakage risk. Thereafter, the anastomotic site was carefully inspected for vascular integrity and airtightness, with a cervical drain placed as needed. After the surgery for both groups of patients, the surgical cavities were closed with drainage tubes in place. The tubes were removed within 1-2 days after surgery if the output was < 100 mL per day without evidence of chylous effusion. Antibacterial agents were routinely administered to prevent potential infections. Given the technical complexity of TTE, all the surgeons involved in this study were at least at the attending level or higher, with each having completed a minimum of 50 thoracoscopic procedures. Postoperative pain management consisted of patient-controlled intravenous analgesia with sufentanil, with dosages adjusted according to real-time pain assessment scores and tolerance of each patient.
Surgery-related indicators: The intraoperative incision length, operation duration, number of lymph nodes, and extent (superior mediastinal/celiac lymph node stations) of lymph node dissection of both groups were observed and recorded.
Postoperative complications: Adverse events, including empyema, pulmonary infection, incision infection, anastomotic fistula, and delayed gastric emptying, were closely monitored, precisely documented, and graded based on the Clavien-Dindo grading standards[16]. The incidence rates were then calculated to quantitatively evaluate the safety and potential risks associated with each surgical approach.
Postoperative pain and postoperative hospitalization duration: The Numerical Rating Scale (NRS) was employed to assess the pain intensity in both groups preoperatively and postoperative day 3[17]. The NRS, with scores ranging from 0 point to 10 points, serves as a reliable tool for pain quantification: Higher scores indicate more severe pain perception. Additionally, the postoperative hospitalization duration of the two groups was carefully observed and recorded.
Serum inflammatory factors: In both groups, 5 mL of venous blood samples were collected from patients in the fasting state during the early morning, both before surgery, and on postoperative day 3. These samples were then centrifuged and subsequently dispatched to the laboratory for analysis. The levels of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α were measured using the enzyme-linked immunosorbent assay technique.
Stress response-related markers: The enzyme-linked immunosorbent assay technique was employed to measure the levels of C-reactive protein (CRP), cortisol, and adrenaline before and 3 days after surgery.
For standardized measurement protocols, both serum inflammatory markers and stress response indicators were analyzed using identical methodologies. The data reflected measurements at specific time points, not peak concentrations. Sampling was performed at strictly synchronized time points in the research and control groups. Furthermore, all patients underwent concurrent NRS assessments during these predetermined time intervals.
Measurement data are presented as mean ± SD. For the between-group comparison of measurement data, an in
The control group included 25 male and 30 female patients, while the research group included 22 male and 36 female patients. The mean ages of the control and research groups were 45.53 ± 6.98 years and 46.31 ± 7.44 years, respectively. The control and research groups had mean body mass index values of 22.04 ± 1.45 kg/m² and 21.64 ± 1.72 kg/m², respectively. The average lesion diameter of the control group was 2.20 ± 0.59 cm compared with 2.12 ± 0.88 cm in the research group. After a comprehensive statistical comparison, no significant differences in sex distribution, age, body mass index, lesion diameter, and place of residence were detected between the two groups (P > 0.05). Detailed data are presented in Table 1.
| Indicators | Control group (n = 55) | Research group (n = 58) | χ2/t | P value |
| Sex | - | - | 0.658 | 0.417 |
| Male | 30 (54.55) | 36 (62.07) | - | - |
| Female | 25 (45.45) | 22 (37.93) | - | - |
| Age (year), mean ± SD | 45.53 ± 6.98 | 46.31 ± 7.44 | 0.574 | 0.567 |
| BMI (kg/m2), mean ± SD | 22.04 ± 1.45 | 21.64 ± 1.72 | 1.333 | 0.185 |
| Lesion diameter (cm), mean ± SD | 2.20 ± 0.59 | 2.12 ± 0.88 | 0.564 | 0.574 |
| Place of residence | - | - | 0.404 | 0.525 |
| Urban | 40 (72.73) | 39 (67.24) | - | - |
| Rural | 15 (27.27) | 19 (32.76) | - | - |
The research group demonstrated a significantly shorter incision length than the control group (P < 0.001). However, the operative duration was notably longer in the research group (P < 0.001). No statistical intergroup differences were noted in the number of dissected lymph nodes or the dissection range (P > 0.05). Detailed data are presented in Table 2.
| Indicators | Control group (n = 55) | Research group (n = 58) | χ2/t | P value |
| Incision length (cm) | 19.02 ± 6.62 | 12.83 ± 2.12 | 6.766 | < 0.001 |
| Operation duration (minute) | 300.95 ± 28.01 | 322.41 ± 30 | 3.925 | < 0.001 |
| Number of dissected lymph nodes (n) | 28.62 ± 4.32 | 27.36 ± 5.71 | 1.317 | 0.190 |
| Extent of lymph node dissection, n (%) | ||||
| Superior mediastinal lymph node stations | 55 (100) | 58 (100) | - | - |
| Celiac lymph node stations | 49 (89.09) | 51 (87.93) | 0.037 | 0.847 |
A comprehensive statistical analysis and comparison clearly demonstrated that the overall incidence rate of postoperative complications was notably lower in the research group than in the control group. Specific details are presented in Table 3.
| Indicators | Control group (n = 55) | Research group (n = 58) | χ2 | P value |
| Empyema (IIIb) | 6 (10.91) | 2 (3.45) | - | - |
| Pulmonary infection (II) | 3 (5.45) | 2 (3.45) | - | - |
| Incision infection (II) | 5 (9.09) | 1 (1.72) | - | - |
| Anastomotic fistula (IIIa) | 6 (10.91) | 3 (5.17) | - | - |
| Delayed gastric emptying (IIIb) | 3 (5.45) | 1 (1.72) | - | - |
| Total | 23 (41.82) | 9 (15.52) | 9.620 | 0.002 |
Before surgery, the NRS scores of the two groups were not significantly different (P > 0.05). On postoperative Day 3, the NRS scores of both groups demonstrated a marked decline (P < 0.05). Notably, the NRS score of the research group was markedly lower than that of the control group (P < 0.05). In addition, the postoperative hospitalization duration of the research group was also significantly shorter than that of the control group (P < 0.05). Detailed data are presented in Table 4.
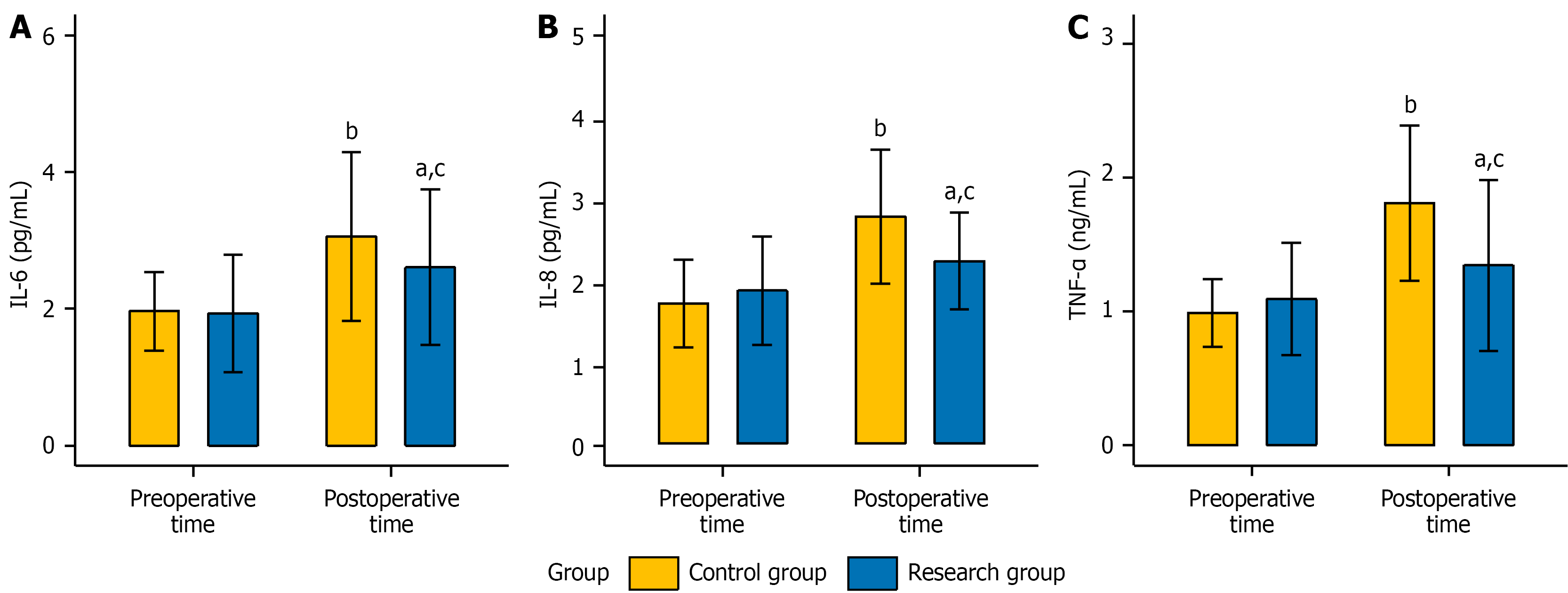
Preoperatively, the levels of serum inflammatory factors, including IL-6, IL-8, and TNF-α, were not significantly different between the two groups (P > 0.05). Postoperatively, the levels of all these factors increased significantly in both groups (P < 0.05). Nevertheless, the levels of all these factors were lower in the research group than in the control group (P < 0.05). Detailed data are presented in Figure 1.
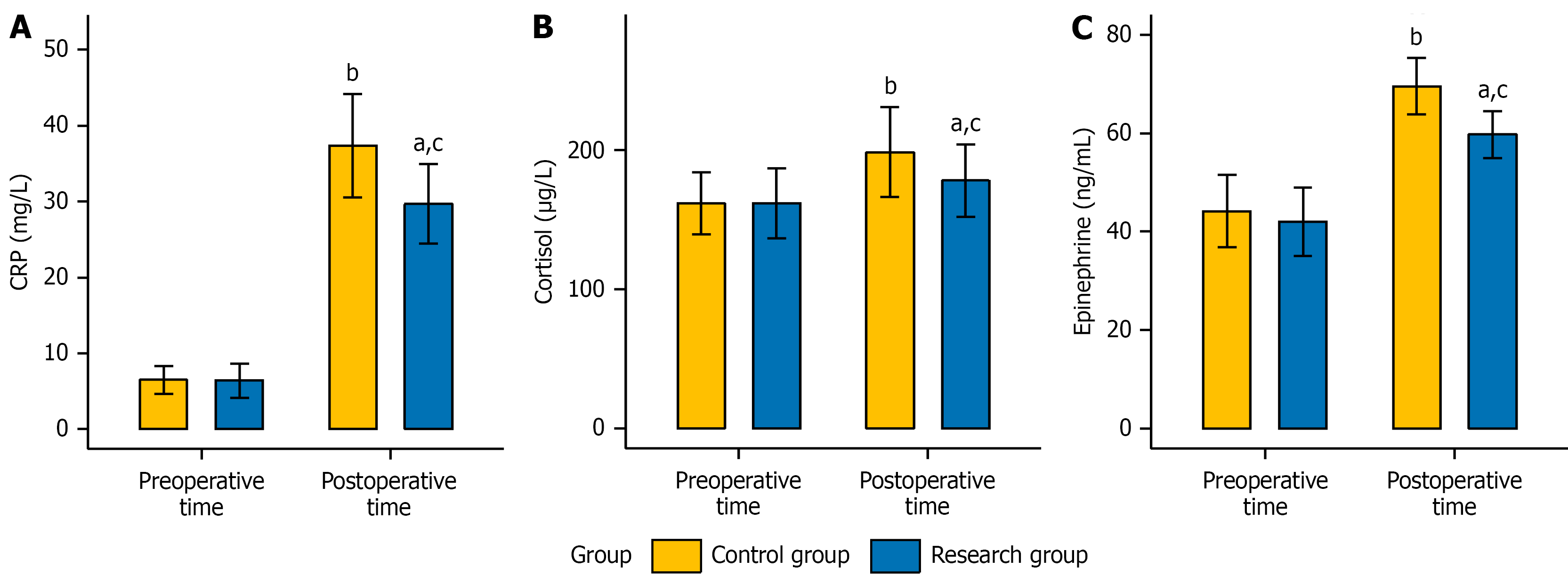
Before surgery, no significant differences were observed in the levels of stress response-related markers, namely, CRP, cortisol, and adrenaline, between the two groups (P > 0.05). Postoperatively, the levels of the aforementioned indicators significantly increased in both groups (P < 0.05). Nevertheless, the levels of these indicators in the research group were notably lower than those in the control group (P < 0.05). Detailed data are presented in Figure 2.
EC is closely linked to multiple factors, including obesity, unhealthy diet, tobacco smoking, and alcohol intake. Epidemiological evidence indicates the predilection of this malignancy for the male population, and the risk of developing EC escalates concomitantly with age advancement[18,19]. EEC refers to cancerous tissue that is confined to the mucosa and submucosa and has not yet metastasized to the lymph nodes. For patients diagnosed with EEC, early radical surgery can remarkably boost the survival rate to approximately 90%[20,21]. This study is dedicated to analyzing the clinical advantages of TTE in EEC management to not only present more efficacious surgical treatment strategies for these patients but also to furnish surgeons with more dependable clinical insights and guidelines.
In the treatment of patients with EEC, TTE manifests distinct advantages over conventional surgical approaches, including substantially shortened incision length and comparable lymph node yield, albeit with longer operative duration. Regarding safety, TTE demonstrated a remarkable advantage with a relatively lower overall incidence of postoperative complications. The complication rate decreased substantially from 41.82% to 15.52%, strongly suggesting that TTE is instrumental in mitigating the incidence of postoperative complications. This marked reduction in postoperative morbidity may be attributed to: (1) Minimized tissue trauma through microinvasive ports; (2) Attenuated systemic inflammatory responses; and (3) Enhanced surgical precision reducing iatrogenic injury. Similarly, Hoshino et al[22] further attested to the benefits of TTE. In treating patients with advanced esophageal squamous cell carcinoma, TTE leverages the magnifying effect of the thoracoscope to achieve a relatively high complete resection rate while ensuring a certain degree of safety. In addition, when performed on patients with EEC, TTE considerably alleviated the pain on postoperative day 3 and was conducive to remarkably shortening the duration of postoperative hospitalization. Mo
This study shows room for improvement. First, the retrospective design imposed constraints on data availability, resulting in the inability to collect information on intraoperative blood loss, NRS scores at other critical time points (e.g., postoperative days 1 and 7), and analgesic consumption. Thus, future studies should adopt a prospective design to systematically gather relevant data, which would enable more robust clinical conclusions. Second, although the baseline characteristics were well-balanced between the two groups, the nonrandomized nature of the study means that unmeasured confounders - such as variations in surgical expertise, patient adherence, smoking status, or underlying comorbidities - may have influenced the outcomes. In future research, multivariate regression analysis or propensity score matching could help isolate the true effect of the surgical technique, thereby providing more reliable evidence to guide clinical decision-making. Finally, long-term prognostic evaluation should be supplemented to further elucidate the potential clinical advantages of TTE.
In summary, TTE presents multiple benefits for patients diagnosed with EEC. It can notably decrease the incision length, shorten postoperative hospitalization duration, mitigate the risk of postoperative complications, relieve the pain experienced on postoperative day 3, and alleviate the inflammatory stress response. Given these substantial advantages, this surgical approach is highly deserving of clinical application and popularization.
| 1. | Lei J, Xu F, Deng C, Nie X, Zhong L, Wu Z, Li J, Wu X, He S, Chen Y. Fusobacterium nucleatum promotes the early occurrence of esophageal cancer through upregulation of IL-32/PRTN3 expression. Cancer Sci. 2023;114:2414-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 2. | Hang TP, Spiritos Z, Gamboa AM, Chen Z, Force S, Patel V, Chawla S, Keilin S, Saba NF, El-Rayes B, Cai Q, Willingham FF. Epidemiology of early esophageal adenocarcinoma. Clin Endosc. 2022;55:372-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Zeng Y, Liu Y, Li J, Feng B, Lu J. Value of Computed Tomography Scan for Detecting Lymph Node Metastasis in Early Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2025;32:1635-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Predescu D, Achim F, Constantinoiu S, Moraru AC, Rotariu A, Rosianu CG, Scripcariu DV, Constantin A. From Caustic Stenosis to Esophageal Cancer, a Challenging Evolution - Narrative Review. Chirurgia (Bucur). 2024;119:515-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Lundberg E, Lagergren P, Mattsson F, Lagergren J. Life Expectancy in Survivors of Esophageal Cancer Compared with the Background Population. Ann Surg Oncol. 2022;29:2805-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Qu HT, Li Q, Hao L, Ni YJ, Luan WY, Yang Z, Chen XD, Zhang TT, Miao YD, Zhang F. Esophageal cancer screening, early detection and treatment: Current insights and future directions. World J Gastrointest Oncol. 2024;16:1180-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (2)] |
| 7. | Wang HK, Wei Q, Yang YL, Lu TY, Yan Y, Wang F. Clinical usefulness of the lymphocyte-to-monocyte ratio and aggregate index of systemic inflammation in patients with esophageal cancer: a retrospective cohort study. Cancer Cell Int. 2023;23:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 8. | Lianyong J, Fengqing H, Xiao X, Xuefeng Z, Rui B. Single-Stage Surgical Procedure for Patients with Primary Esophageal and Lung Cancers. Thorac Cardiovasc Surg. 2024;72:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Cheng L, Liu J, Lian L, Duan W, Guan J, Wang K, Liu Z, Wang X, Wang Z, Wu H, Chen Z, Wang J, Jian F. Predicting deep surgical site infection in patients receiving open posterior instrumented thoracolumbar surgery: A-DOUBLE-SSI risk score - a large retrospective multicenter cohort study in China. Int J Surg. 2023;109:2276-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Zhu H, Ma X, Ye T, Wang H, Wang Z, Liu Q, Zhao K. Esophageal cancer in China: Practice and research in the new era. Int J Cancer. 2023;152:1741-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 11. | Wang L, Ge L, Song S, Ren Y. Clinical applications of minimally invasive uniportal video-assisted thoracic surgery. J Cancer Res Clin Oncol. 2023;149:10235-10239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Li Y, Liu WX, Qi L, Li Y, Liu JF, Fu JH, Han YT, Fang WT, Yu ZT, Chen KN, Mao YS. Changes in the recent three decades and survey on the current status of surgical treatment for esophageal cancer in China. Thorac Cancer. 2024;15:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Avasarala SK, Lentz RJ, Maldonado F. Medical Thoracoscopy. Clin Chest Med. 2021;42:751-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A, Smyth EC; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 437] [Article Influence: 109.3] [Reference Citation Analysis (1)] |
| 15. | Marom G. Esophageal Cancer Staging. Thorac Surg Clin. 2022;32:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Abbassi F, Pfister M, Lucas KL, Domenghino A, Puhan MA, Clavien PA; Outcome Reporting Group. Milestones in Surgical Complication Reporting: Clavien-Dindo Classification 20 Years and Comprehensive Complication Index 10 Years. Ann Surg. 2024;280:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Suri P, Heagerty PJ, Timmons A, Jensen MP. Description and initial validation of a novel measure of pain intensity: the Numeric Rating Scale of Underlying Pain without concurrent Analgesic use. Pain. 2024;165:1482-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Liu CQ, Ma YL, Qin Q, Wang PH, Luo Y, Xu PF, Cui Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer. 2023;14:3-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 227] [Reference Citation Analysis (0)] |
| 19. | Kwon MJ, Kang HS, Choi HG, Kim JH, Kim JH, Bang WJ, Hong SK, Kim NY, Hong S, Lee HK. Risk for Esophageal Cancer Based on Lifestyle Factors-Smoking, Alcohol Consumption, and Body Mass Index: Insight from a South Korean Population Study in a Low-Incidence Area. J Clin Med. 2023;12:7086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 20. | Lim H, Kim DH, Jung HY, Gong EJ, Na HK, Ahn JY, Kim MY, Lee JH, Choi KS, Choi KD, Song HJ, Lee GH, Kim JH. Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver. 2015;9:159-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Ma H, Wang L, Chen Y, Tian L. Convolutional neural network-based artificial intelligence for the diagnosis of early esophageal cancer based on endoscopic images: A meta-analysis. Saudi J Gastroenterol. 2022;28:332-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Hoshino I, Gunji H, Kuwayama N, Kurosaki T, Tonooka T, Soda H, Takiguchi N, Nabeya Y, Takayama W. Efficacy of thoracotomy and thoracoscopic-assisted esophageal surgery in conversion and salvage surgeries: a retrospective study. World J Surg Oncol. 2022;20:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 382] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 24. | Song S, Zhang Y, Duan X, Liu C, Du Y, Wang X, Luo Y, Cui Y. HIF-1α/IL-8 axis in hypoxic macrophages promotes esophageal cancer progression by enhancing PD-L1 expression. Cancer Gene Ther. 2023;30:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Forkasiewicz A, Stach W, Wierzbicki J, Stach K, Tabola R, Hryniewicz-Jankowska A, Augoff K. Effect of LDHA Inhibition on TNF-α-Induced Cell Migration in Esophageal Cancers. Int J Mol Sci. 2022;23:16062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 26. | Qi FQ, Sun Y. Efficacy and prognostic analysis of carbon nanotracers combined with the da Vinci robot in the treatment of esophageal cancer. World J Clin Cases. 2024;12:4924-4931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Li Y, Dong H, Tan S, Qian Y, Jin W. Effects of thoracic epidural anesthesia/analgesia on the stress response, pain relief, hospital stay, and treatment costs of patients with esophageal carcinoma undergoing thoracic surgery: A single-center, randomized controlled trial. Medicine (Baltimore). 2019;98:e14362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Liu S, Huang H, Zhang C, Chen L, Feng X, Wu Y, Xia Q, Huang X. Postoperative leukocyte counts as a surrogate for surgical stress response in matched robot- and video-assisted thoracoscopic surgery cohorts of patients: A preliminary report. J Robot Surg. 2024;18:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Sato H, Miyawaki Y, Lee S, Sugita H, Sakuramoto S, Tsubosa Y. Effectiveness and safety of a newly introduced multidisciplinary perioperative enhanced recovery after surgery protocol for thoracic esophageal cancer surgery. Gen Thorac Cardiovasc Surg. 2022;70:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Shi K, Qian R, Zhang X, Jin Z, Lin T, Lang B, Wang G, Cui D, Zhang B, Hua X. Video-assisted mediastinoscopic and laparoscopic transhiatal esophagectomy for esophageal cancer. Surg Endosc. 2022;36:4207-4214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/