Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.107741
Revised: May 10, 2025
Accepted: August 12, 2025
Published online: October 27, 2025
Processing time: 210 Days and 6.7 Hours
Esophageal cancer is sixth leading cause of cancer deaths and the eighth most common cancer worldwide. In the recent times, the incidence and mortality rates have increased. To improve the survival in esophageal carcinoma, newer tactics have to be applied to improve outcomes. It is well established that in cases of carcinoma lung and stomach, presence of micrometastasis or spread of tumor cell following surgical manipulation has been shown to predict recurrence and poor prognosis. Similarly, spread of tumor cell during esophagectomy or presence of occult micrometastatic disease in esophageal carcinoma may lead to early tumor recurrence and poor prognosis. The actual incidence of pleural micrometastasis and tumor spillage following thoracoscopic esophagectomy is not clear. The presence of malignant cells in cytologic or immunocytochemical analysis may help in prognostication and further therapeutic decision making.
To assess the incidence of micrometastasis and tumor spillage among the patients undergoing thoracoscopic surgery for esophageal carcinoma.
An observational study was done at Department of Surgical Gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry. Nineteen patients aged 18 to 70 years with slight male preponderance, undergoing elective thoracoscopic esophagectomy for esophageal carcinoma were included in this observational study from June 2021 to June 2023. Pre and post dissection pleural cavity lavage was done with 200 mL saline and the fluid was subjected to cytologic and immunocytological examination. The cytology and immunocytological examination report was interpreted as positive for malignant cells or negative for malignant cells. Immunocytological examination was done and evaluated for presence or absence of tumor markers cytokeratin 7 and p40 signifying presence or absence of tumor spillage.
Rate of pre dissection fluid was negative for the malignant cell by cytological and immunocytological analysis in all cases indicating no micrometastasis in our group of patients. Rate of post dissection fluid was negative for the malignant cell by cytological and immunocytological analysis in all cases indicating no incidence of tumor spillage post-surgery in our patients. No significant association was found between age, gender, body mass index, site of lesion, histological type, neoadjuvant therapy and tumor-nodes-metastasis staging with pre and post dissection pleural fluid cytological and immunohistochemical analysis in our study.
This study assessed the incidence of micrometastasis and tumor spillage following minimal invasive esopha
Core Tip: In carcinoma lung and stomach, presence of micrometastasis or spread of tumor cell after surgery has shown to predict recurrence and poor prognosis. Hence, a routine intraoperative pleural and peritoneal lavage is advocated. Similarly, spread of tumor cell esophagectomy or presence of occult micrometastatic disease in esophageal carcinoma may have detrimental effects leading to recurrence and poor prognosis. The role of routine pleural lavage to detect micrometastasis and tumor spillage is not well established in carcinoma esophagus. It is important to identify such high-risk patients to formulate strategies to prevent tumor spillage and plan management.
- Citation: Shukla A, Kalayarasan R, Gochhait D, Harichandrakumar KT, Pottakkat B. Assessment of the status of micrometastasis and tumor spillage among the patients undergoing thoracoscopic esophagectomy for carcinoma esophagus. World J Gastrointest Surg 2025; 17(10): 107741
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/107741.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.107741
Esophageal cancer is ranked as the sixth leading cause of cancer deaths, and the eighth most common cancer worldwide[1]. In India, it is ranked as the fourth most frequent cause for cancer related deaths[2]. In the recent times, the incidence and mortality rates have increased and it carries poor outcome. Squamous cell carcinoma is the most common histological type followed by adenocarcinoma, other types include sarcomas, small cell carcinomas and rarely lymphomas or melanomas. In “Asian esophageal cancer belt” regions extending from Northeast China to Middle East, intake of hot liquids, poor oral hygiene, high temperature cooking, and low socioeconomic status have been seen to increase squamous cell esophageal carcinoma apart from alcohol and tobacco use in other parts of world[1,3,4]. In esophageal adenocarcinoma, gastroesphageal reflux disease, obesity, alcohol and metabolic syndrome play a major role in etiopathogenesis. Esophageal carcinoma begins from the inner layers and spreads outwards and lacks clinical symptoms in early stages of disease, leading to late detection of disease in advanced stage[5]. To improve the survival in esophageal carcinoma, newer tactics have to be applied for identifying where to use aggressive adjuvant chemotherapy or radiotherapy following surgery.
Esophagectomy is considered as the standard treatment of esophageal carcinoma. Various prospective trials have emphasized that combining surgery with neoadjuvant chemoradiotherapy or chemotherapy has positive impact and improves the outcome in esophageal carcinoma[6,7]. The 5-year survival rates for endoscopic resection, concurrent chemoradiotherapy, radiotherapy alone or esophagectomy according to the Japan Esophageal Society were 88.3%, 32.4%, 24.4% and 59.3% respectively[8]. The actual incidence of micrometastasis and tumor spillage in carcinoma esophagus following surgery is not known. Pre- and post-operative lavage cytology and immunocytochemical analysis are the techniques used to find micrometastasis and tumor cell spillage. Immunocytology is more sensitive and can find as less as one or two cells and the sensitivity can further be enhanced by enrichment methods by at least one order in magnitude.
Only a few retrospective studies have been done in esophageal carcinoma, showing the positive pre and post dis
Patients undergoing surgery for esophageal carcinoma were included in this observational study. All consenting participants with esophageal carcinoma aged 18-70 years planned for thoracoscopic esophagectomy during the study period from June 2021 to June 2023 in the Department of Surgical Gastroenterology of Jawaharlal Institute of Post
Inclusion criteria were all patients with middle and lower third esophageal carcinoma planned for thoracoscopic esophagectomy, aged between 18 and 70 years and Eastern Cooperative Oncology Group (ECOG) performance status I and II. All consecutive patients undergoing planned elective thoracoscopic esophagectomy during the study period in the Department of Surgical Gastroenterology, who met the inclusion criteria were included. Exclusion criteria were patient not undergoing thoracoscopic esophagectomy, obvious pleural metastasis on thoracoscopy and distant metastasis. Primary objective was to assess the incidence of micrometastasis and tumor spillage among the patients undergoing thoracoscopic surgery for esophageal carcinoma and secondary objective was to identify the association of cytological and immunocytological profile with clinical characteristics of the patients undergoing thoracoscopic surgery for esopha
Predissection pleural cavity lavage wash with 200 mL saline was done before starting the dissection after inserting ports. The lavage fluid was suctioned after one minute and anticoagulant was added for fixation and the fluid was centrifuged. A minimum of 100 mL of the lavage fluid was collected for cytological analysis. The sediments were placed on a glass slide and the fluid was subjected to cytologic and immunocytological examination. The cytology examination report was interpreted as positive for malignant cells or negative for malignant cells. The positive report signified micrometastatic disease, whereas a negative report signified absence of micrometastasis. Immunocytological examination was done and evaluated for presence or absence of tumor markers cytokeratin 7 and p40 signifying micrometastasis and absence of micrometastasis.
After the completion of the thoracoscopic esophagectomy pleural cavity lavage was again done with 200 mL saline as before and the fluid was subjected to cytologic and immunocytological examination. The positive cytology report signified tumor spillage, whereas a negative report signified absence of tumor spillage. Immunocytological examination was done and evaluated for presence or absence of tumor markers cytokeratin 7 and p40 signifying presence or absence of tumor spillage (Biogenex laboratories, M944-5M and ANA43-5M, with ready to use dilution). All patients who would be detected to have either micrometastasis or tumor spillage were planned to receive adjuvant chemotherapy as per routine institutional protocol.
At the time of recruitment for thoracoscopic esophagectomy, preoperative clinical data was collected from each patient. Similarly, postoperative results were obtained and the data was entered in a prospectively maintained excel sheet database. Independent variables included age, gender, body mass index, preoperative and post-operative histopathological examination (HPE), ECOG performance status, site of lesion, tumor-nodes-metastasis stage, blood loss, number of blood transfusions, preoperative neoadjuvant therapy, and number of lymph nodes positive. Dependent variables included cytological and immunocytological profiles. The distribution of categorical data such as age, gender, pre
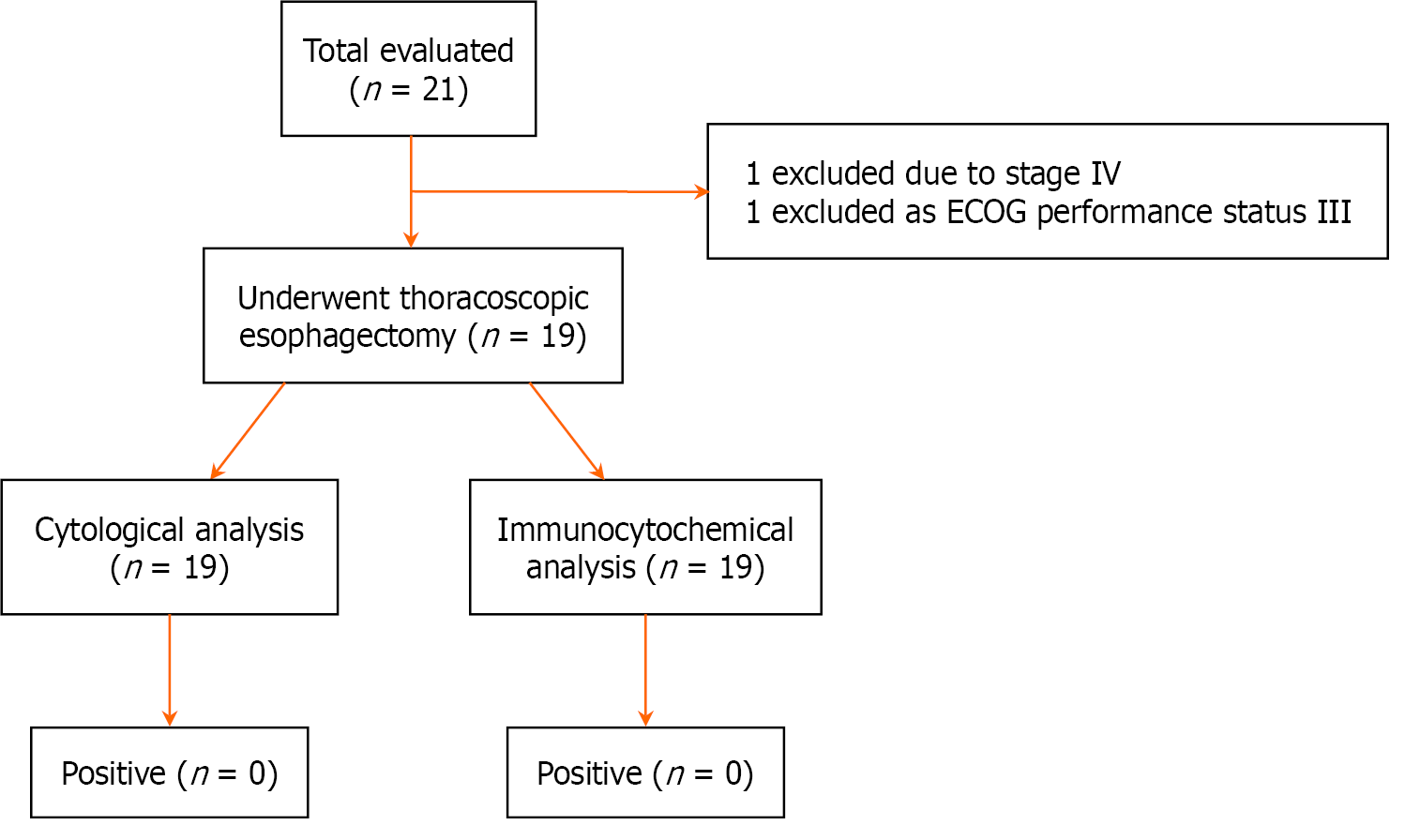
During the study period from June 2021 to June 2023, 21 consecutive patients who were planned for thoracoscopic esophagectomy at the Department of Surgical Gastroenterology of Jawaharlal Institute of Postgraduate Medical Education and Research were evaluated for eligibility. Out of these two patients were excluded as one patient had metastatic disease and one patient had ECOG performance status III. After implementation of the inclusion and the exclusion criteria total of 19 patients were included for thoracoscopic esophagectomy followed by pleural cavity lavage with normal saline and further cytological evaluation. All the patients underwent thoracoscopic esophagectomy followed by preoperative pleural cavity lavage and followed by postoperative pleural cavity lavage after completing of dissection. The study’s outline is depicted in the flowchart (Figure 1).
The median age of the participants in the study was 52 years (range: 26-70 years). Among these 19 patients approximately one third of the patients (7/19) were elderly in their 7th decade of life. The study population had 10 male patients (52.6%) and 9 females (47.4%). The mean body mass index was 22.03 kg/m2 with 1 (5.3%) patient being underweight and 3 (15.8%) patients being overweight. Most frequent site of the lesion was lower thoracic esophagus with gastroesophageal junction (GEJ) involvement in 9 (47.4%) patients and mid thoracic esophagus involvement in 6 (31.6%) patients and only lower thoracic esophagus in 3 (15.8%) patients and one patient (5.3%) had involvement of both mid and lower thoracic esophagus. Out of the 19 patients, squamous cell carcinoma was more common and found in 15 (78.9%) patients and adenocarcinoma in 4 (21.1%) patients. In 9 patients with carcinoma of lower esophagus with GEJ involvement, 5 (55.6%) had squamous cell carcinoma and 4 (44.4%) patients had adenocarcinoma on histology respectively.
Fifteen out of 19 patients (78.9%) received neoadjuvant therapy, 11 (57.8%) received chemoradiotherapy, 4 (21.1%) patients received chemotherapy; however, 4 (21.1%) patients did not receive any form of neoadjuvant therapy and underwent upfront surgery. All 19 patients underwent minimal invasive surgery robot assisted esophagectomy in 11 (57.8%) patients and thoracolaparoscopic esophagectomy in 8 (42.2%) patients with lymphadenectomy. Postoperatively 19 patients who underwent surgery 12 (63.2%) patients were diagnosed as squamous cell carcinoma on final histopa
According to the 8th edition of the American Joint Committee on Cancer staging system most of the patients had pathological stage I disease in 9 (47.4%) patients followed by stage IIIB in 5 (26.3%) patients, stage II disease was seen in 4 (21.1%) patients and stage IVB in 1 (5.3%) patient. Overall T2 tumors were noted in 9 (47.4%) specimens, T3 tumors in 6 (31.6%) specimens, T0 tumors in 3 (5.3%) specimens and T1 tumor in 1 (5.3%) patient. Most of the patients in our cohort had N0 disease in 12 (63.2%) participants, N1 disease in 2 (10.5%) participants, N2 disease in 4 (21.1%) participants and N3 disease in 1 (5.3%) participant. Median total lymph node yield in our patients was 14 (range: 3-27). Out of the 19 patients operated 7 (36.8%) patients had positive nodes. The demographic and clinicopathological profile of patients with esophageal carcinoma is depicted in (Table 1).
| Total number of patients, n = 19 | Percentage (%) | |
| Age, years | 52 (range: 26-70) | |
| Sex | ||
| Male | 10 | 52.6 |
| Female | 9 | 47.4 |
| ECOG PS I | 19 | |
| Body mass index, kg/m2, mean ± SD | 22.03 ± 3.24 | |
| Underweight | 1 | 5.3 |
| Normal | 15 | 8.9 |
| Overweight | 3 | 15.8 |
| Obese | 0 | 0 |
| Site of lesion esophagus | ||
| Mid thoracic | 6 | 31.6 |
| Lower thoracic | 3 | 15.8 |
| Lower thoracic with GEJ | 9 | 47.4 |
| Mid and lower thoracic | 1 | 5.3 |
| Histologic type (pre-surgery) | ||
| Squamous cell carcinoma | 15 | 78.9 |
| Adenocarcinoma | 4 | 21.1 |
| Neoadjuvant therapy | ||
| Chemoradiotherapy | 11 | 57.8 |
| Chemotherapy | 4 | 21.1 |
| Upfront surgery | 4 | 21.1 |
| Histologic type (post-surgery) | ||
| Squamous cell carcinoma | 12 | 63.2 |
| Adenocarcinoma | 4 | 21.1 |
| No residual tumor | 3 | 15.8 |
| TNM stage | ||
| Stage I | 9 | 47.4 |
| Stage II | 4 | 21.1 |
| Stage III | 5 | 26.3 |
| Stage IV | 1 | 5.3 |
Postoperative analysis of the 19 patients with preoperative and post dissection cytological examination did not show positive cytology results with respect to all stages of the carcinoma, depth of tumor, positive or negative lymph nodes, squamous cell carcinoma or adenocarcinoma, with or without neoadjuvant chemotherapy and site of the primary lesion. Similarly, the postoperative analysis of the 19 patients with preoperative and post dissection immunocytochemical examination did not show positive results in relation all the stages of the carcinoma, depth of tumor positive or negative lymph nodes, squamous cell carcinoma or adenocarcinoma, with or without neoadjuvant chemotherapy and site of the primary lesion.
For improving the outcomes in esophageal carcinoma novel tactics have to be applied for identifying where to use aggressive adjuvant chemotherapy or radiotherapy following surgery. Lavage cytology is one of the techniques already in use for various malignancies of gastrointestinal tract and lungs[9]. First reported use of pleural cavity lavage washing was done by Spjut et al[10], in the year 1958 for 49 patients undergoing thoracotomy for lung cancer, in which 16 patients (32.7%) had positive cytology for malignant cells. Similarly Eagan et al[11], in 1984 published their results of pleural cavity lavage following pulmonary resection for carcinoma lungs showing 12 out of 135 positive cytology (8.9%), both the studies conducted the cytologic examination following resection not taking in to consideration the prognostic value of cytologic examination. Kondo et al[12] in 1989, published data on positive pleural cytology following thoracotomy in carcinoma lung patients predicting its prognostic value. Subsequently, Buhr et al[13], conducted their pre dissection and post dissection pleural cavity lavage study, on carcinoma lung patients to discriminate between exfoliation of tumor cells due to surgical manipulation or preexistence of tumor cells before dissection and emphasized the prognostic significance of positive cytological findings. In the current literature, lung cancer pleural lavage cytology is considered an inde
Since 1970s, there has been focus on the presence of malignant cells in the peritoneal cavity in gastric cancer[15]. Malignant cell found in peritoneal washes in gastric cancer have been documented to have significant prognostic impact on recurrence and overall survival. Nakajima et al[16] found more peritoneal cytology positive results in patients having serosal invasion. Kaibara et al[17], in their study showed that the depth of invasion in gastric cancer strongly correlates with peritoneal cytology results. Bando et al[18], in a large study of 1297 patients reported slightly higher peritoneal lavage cytology rate of 27%, although most of the case in this study had advanced gastric carcinoma. Similarly, Ribeiro et al[19], in their report of 49 patients documented high incidence positive lavage cytology of 41%, which also included metastatic disease. Studies have shown that half of patients with serosal involvement harbor peritoneal recurrence following curative resection[20,21]. In the Dutch gastric cancer trial, positive lavage cytology was reported as 7.1% in all cases and 12% in cases with serosal involvement[22]. Various studies have found that even T1 and T2 gastric carcinoma can have positive peritoneal cytology without peritoneal disease on diagnostic laparoscopy, contributing it to be due to perinodal tissue involvement rather than serosal involvement[23,24].
The Japanese Gastric Cancer Association in 1998 suggested that the detection of free cancer cell in the peritoneal washing on cytology be considered as independent prognostic marker[25]. Due to the significant impact of positive peritoneal cytology as a prognostic marker Union International Control Cancer has classified patients with positive peritoneal cytology as stage IV gastric carcinoma and the National Comprehensive Cancer Network recommends that patients having T1b or higher stage of gastric carcinoma should undergo diagnostic laparoscopy with peritoneal washing[26]. Nakagawa et al[27], in their study of 100 patients documented upstaging by 44% with staging laparoscopy and peritoneal lavage cytology, furthermore they had patients (11/18) who still had positive cytology even after neoadjuvant therapy. Pecqueux et al[28], in their meta-analysis of 12338 patients from 64 studies, showed that positive peritoneal cytology is associated with poor overall survival and poor peritoneal recurrence free survival.
In carcinoma esophagus, the index case of positive pleural lavage cytology was an incidental finding after iatrogenic insertion of subclavian catheter for parentral nutrition in a case of dysphagia with accumulation of fluid in pleural cavity, which turned out to be positive on cytology for malignant cells and published in 1974 as letter to editor[29]. Our study was conducted with the primary objective to assess the rate of incidence of micrometastasis and tumor spillage among the patients undergoing thoracoscopic surgery and to identify the association of cytological and immunocytological profile with clinical characteristics of the patients undergoing thoracoscopic surgery for esophageal carcinoma. Out of the 19 patients, who underwent thoracoscopic esophagectomy median age of presentation was 52 years with range of 26-70 years and approximately one third of the patients (7/19) were elderly in their 7th decade of life with 52.6% males in the whole group. The mean body mass index was 22.03 kg/m2 in our population with most patients 78.9% were in the normal range and one patient was underweight, also all 19 patients had good performance status.
In a similar study conducted by Jiao et al[30], in their series of 48 patients had a median age of 54.5 years with a range of 42 to 77 which is similar to our study. In the previous studies there is male preponderance, however in our study the ratio of male patients is slightly more than the female patients, which can be attributed to the small sample size of the study[30,31]. In our study, most common site of involvement with esophageal carcinoma was lower thoracic esophagus with involvement of GEJ and three patients had only lower thoracic esophagus involvement and one had lower thoracic esophagus malignancy with mid thoracic involvement making a total of 13 out of a total of 19 patients where lower thoracic esophagus was involved, whereas mid thoracic esophagus alone was involved only in 6 patients. This is in contrary to the earlier studies where the involvement of mid thoracic carcinoma is found to be more. Jiao et al[30] found more positive cytology results in upper third and Natsugoe et al[31] had more positive cytology in the middle thoracic esophageal malignancies.
Most common histological type in our cohort was squamous cell carcinoma. Most of the patients received neoadjuvant therapy (78.9%) and on post-surgery HPE three patients had no residual disease. Maximum patients were in the early stage in our study (13/19). Overall T2 tumor was the most frequently seen and N0 nodes were noted in 12 patients each. We had a lymph node yield of 14 nodes in all patients and seven patients had positive lymph nodes on pathological examination with maximum number of lymph nodes positive was six nodes. All these factors might have an impact on the cytological and immunohcytochemical analysis in our study.
Natsugoe et al[31] had no positive cytology in the pre resection group of patients which is in concordance to our study results. They had four positive cytology out of 78 patients (5.2%) in post resection group of patients. All these patients had advanced disease (pT3/T4) with all patients having lymph node metastases. Anatomically as the esophagus is in retropleural location there is less chance of pre resection positive cytology, surgical manipulation in an advanced malignancy can lead to dissemination of cells in the pleural space. They concluded that pleural cavity lavage cytology is seldom positive to be used as a prognostic indicator[31]. In a study by Jiao et al[30] on 48 patients, 18.8% (9 patients) had positive pleural cytology after open esophagectomy, there was no analysis done for pre resection cytology. They found no significant correlation to age, sex, clinical symptoms, histology, T stage and N stage to positive lavage cytology. They suggested that the positive cytology results may be a hint towards an aggressive tumor biology and proposed the need of further studies.
In one of the largest study by Doki et al[32] on 240 patients, only 1.3% (3 patients) had positive lavage cytology pre resection all three had advanced disease however, cytology was positive in 9.3% (22 patients) post resection cases. In the post resection group also significant parameters for positive cytology results were advanced disease and positive lymph node metastases to 8 or more lymph nodes. They concluded that tumor cell exfoliates to pleural cavity after surgical manipulation, especially when lymph nodal spread is present and proposed the need of adjuvant therapy in this subgroup of patients as positive cytology might be an indicator of systemic spread.
In our study pre dissection rate of cytological and immunocytological results were negative in all patients, which are similar to previous studies[30,31]. As esophagus lies behind the pleural space and only chance of spread to pleural cavity is an advanced stage of disease. Our study had very few cases of advanced disease. Another major difference is that most of the patients in our study group received neoadjuvant therapy (78.9%) which might have had an influence in down staging of the advanced disease. Also in our study the post dissection rate of cytology and immunocytological analysis results were negative in all patients. This can be attributed to the early stage of disease in most of our patients. Maximum number of positive metastatic lymph nodes were six in our patients, as already stated above by Doki et al[32], they found that 8 or more metastatic lymph nodes have significant impact on positive cytology. In our study all the esophagectomy procedures were done by minimally invasive technique either robot assisted or thoracolaparoscopically, whereas in all the previous studies esophagectomy was accomplished by open technique. This might have an impact due to more manipulation during the surgical procedure leading to spread of tumor cells in post dissection lavage fluid, moreover minimal invasive technique has better magnification rendering to precise dissection of tissue planes and less tissue or tumor handling.
The strength of our study is that only a very few retrospective studies have been conducted in esophageal carcinoma for impact of pleural fluid cytology; most of the studies are done in lung carcinoma and other gastrointestinal tumors. These studies have selectively evaluated for the presence of malignant cells either pre dissection or post dissection. In the present study, a combination of both pre and post dissection thoracoscopic lavage using cytology and immunocytochemistry was attempted to detect micrometastasis and also to detect whether tumor spillage occurs secondary to surgery. Our study is the first study using minimally invasive technique and assessing the impact of tumor spillage after dissection.
Our study was at a high-volume tertiary care hospital which followed a prospective design, systematically collecting data and conducting analyses, allowing for a rigorous evaluation of the outcomes and ensuring robust conclusions. The simplicity of our study design facilitated easy data collection without the need for extensive effort. The utilization of routine cytology and immunocytochemistry protocols in the Department of Pathology also ensured that no additional training or specialized skills were required for the research, enhancing the reproducibility of our findings for future investigations.
A major limitation of our study is that it is a single center study, potentially limiting the diversity of patient populations and clinical practices, which should be considered when interpreting the results. The sample size of our study was relatively small impacting the statistical significance of our study. In spite of the study’s novelty, the sample size was a major limiting factor to draw significant conclusion from our study. To establish more significant conclusions, future research should be conducted with a considerably larger sample size. Moreover, conducting follow-up of patients to analyze long-term outcomes, such as relapse free survival, disease-free survival, and overall survival, would provide important insights into the effectiveness and results of such studies.
Esophageal carcinoma can have metastasis from an early stage and has been considered as a devastating carcinoma. Unlike stomach and lung carcinoma, the exact incidence and repercussions of micrometastasis and tumor spillage is not known in esophageal carcinoma. It is important to identify such high-risk patients to formulate strategies to prevent tumor spillage during surgery and plan for accurate stage-based adjuvant therapy. This study on esophageal carcinoma to assess the incidence of micrometastasis and tumor spillage following minimal invasive esophagectomy could not find any tumor cells in both pre and post dissection samples. The results of the present study suggest that tumor spillage does not occur during thoracic esophageal mobilization and mediastinal lymphadenectomy during minimally invasive esophagectomy. However, future researches with larger sample size are required to conclude the findings of the present study.
| 1. | GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:582-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 2. | Samarasam I. Esophageal cancer in India: Current status and future perspectives. Int J Adv Med Health Res. 2017;4:5-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 531] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Dar NA, Islami F, Bhat GA, Shah IA, Makhdoomi MA, Iqbal B, Rafiq R, Lone MM, Abnet CC, Boffetta P. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer. 2013;109:1367-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1930] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Li Y, Xue L, Qu D, Jiang Z, Wang Z, Yang Z, Zhou A. Encouraging Pathological Complete Response Rate from Neoadjuvant Chemotherapy with Albumin-Bound Paclitaxel Plus Cisplatin and Capecitabine for Locally Advanced Esophageal Squamous Carcinoma: Preliminary Outcome of a Retrospective Study. Cancer Manag Res. 2021;13:2163-2170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Watanabe M, Tachimori Y, Oyama T, Toh Y, Matsubara H, Ueno M, Kono K, Uno T, Ishihara R, Muro K, Numasaki H, Tanaka K, Ozawa S, Murakami K, Usune S, Takahashi A, Miyata H; Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Shoji F, Yamazaki K, Kouso H, Mori R, Takeo S. The Impact of Pleural Lavage Cytology Both Before and After Lung Resection on Recurrence of Non-Small Cell Lung Cancer. Ann Thorac Surg. 2016;101:2141-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Spjut HJ, Hendrix VJ, Ramirez GA, Roper CL. Carcinoma cells in pleural cavity washings. Cancer. 1958;11:1222-1225. [PubMed] [DOI] [Full Text] |
| 11. | Eagan RT, Bernatz PE, Payne WS, Pairolero PC, Williams DE, Goellner JR, Piehler JM. Pleural lavage after pulmonary resection for bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1984;88:1000-1003. [PubMed] |
| 12. | Kondo H, Naruke T, Tsuchiya R, Goya T, Suemasu K, Yamagishi K, Kishi K, Noguchi M. Pleural lavage cytology immediately after thoracotomy as a prognostic factor for patients with lung cancer. Jpn J Cancer Res. 1989;80:233-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Buhr J, Berghäuser KH, Gonner S, Kelm C, Burkhardt EA, Padberg WM. The prognostic significance of tumor cell detection in intraoperative pleural lavage and lung tissue cultures for patients with lung cancer. J Thorac Cardiovasc Surg. 1997;113:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Wang CM, Ling ZG, Wu YB, Cai SQ, Tang ZM, Wu C, Chen YQ. Prognostic Value of Pleural Lavage Cytology in Patients with Lung Cancer Resection: An Updated Meta-Analysis. PLoS One. 2016;11:e0157518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Iitsuka Y, Kaneshima S, Tanida O, Takeuchi T, Koga S. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer. 1979;44:1476-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Nakajima T, Harashima S, Hirata M, Kajitani T. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol. 1978;22:225-229. [PubMed] |
| 17. | Kaibara N, Iitsuka Y, Kimura A, Kobayashi Y, Hirooka Y, Nishidoi H, Koga S. Relationship between area of serosal invasion and prognosis in patients with gastric carcinoma. Cancer. 1987;60:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 18. | Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 243] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Ribeiro U Jr, Gama-Rodrigues JJ, Bitelman B, Ibrahim RE, Safatle-Ribeiro AV, Laudanna AA, Pinotti HW. Value of peritoneal lavage cytology during laparoscopic staging of patients with gastric carcinoma. Surg Laparosc Endosc. 1998;8:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, Yamamoto M. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol. 1992;1:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996;83:672-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Benevolo M, Mottolese M, Cosimelli M, Tedesco M, Giannarelli D, Vasselli S, Carlini M, Garofalo A, Natali PG. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol. 1998;16:3406-3411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 26. | Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803-1804. [PubMed] [DOI] [Full Text] |
| 27. | Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. 2007;10:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reißfelder C, Weitz J, Rahbari NN. Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget. 2015;6:35564-35578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Berson D. Letter: Pleural lavage in carcinoma of the esophagus. N Engl J Med. 1974;291:530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 30. | Jiao X, Zhang M, Wen Z, Krasna MJ. Pleural lavage cytology in esophageal cancer without pleural effusions: clinicopathologic analysis. Eur J Cardiothorac Surg. 2000;17:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Natsugoe S, Shimada M, Nakashima S, Tokuda K, Matsumoto M, Kijima F, Baba M, Shimizu K, Tanaka S, Aikou T. Intraoperative pleural lavage in esophageal carcinoma. Ann Surg Oncol. 1999;6:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Doki Y, Kabuto T, Ishikawa O, Ohigashi H, Sasaki Y, Yamada T, Hiratsuka M, Miyashiro I, Kameyama M, Murata K, Imaoka S, Yasuda T, Nakaizumi A, Takenaka A. Does pleural lavage cytology before thoracic closure predict both patient's prognosis and site of cancer recurrence after resection of esophageal cancer? Surgery. 2001;130:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/