Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.106689
Revised: July 21, 2025
Accepted: September 8, 2025
Published online: October 27, 2025
Processing time: 141 Days and 0 Hours
Congenital short bowel syndrome (CSBS) is a rare disorder characterized by a congenital shortage in the length of the small intestines, resulting in compromised intestinal functionality, frequently accompanied by congenital intestinal mal
Case 1 underwent surgical intervention 23 days after birth, but experienced pos
CSBS is rarely encountered in clinical practice and is often accompanied by con
Core Tip: Congenital short bowel syndrome (CSBS) is a rare disorder distinguished by the congenital shortage in the length of the small intestines, resulting in compromised intestinal functionality, frequently accompanied by congenital intestinal malrotation. Prompt identification and surgical management of CSBS are crucial in enhancing the overall prognosis of patients affected by this condition. What is new: This paper summarizes the experience of enteral and parenteral nutrition schemes for two cases of CSBS accompanied by intestinal malrotation to provide a reference for this condition.
- Citation: Ma LL, Li WN, Lei X, Lin XD, Wu ZW, Xu B, Huang GX. Experience in enteral and parenteral nutrition schemes for familial congenital short bowel syndrome: Two case reports. World J Gastrointest Surg 2025; 17(10): 106689
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/106689.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.106689
Congenital short bowel syndrome (CSBS) is a rare disorder characterized by a congenital shortage in the length of the small intestine, compromising intestinal functionality, frequently accompanied by congenital intestinal malrotation. The primary clinical presentations include bilious vomiting, abdominal pain, abdominal distension, high intestinal obstruc
When confronted with patients with SBS exhibiting significant individual differences, medical practitioners often struggle to formulate appropriate and effective individualized treatment strategies. Herein, we report two siblings diagnosed with CSBS accompanied by intestinal malrotation. They were admitted to the Department of Pediatric Surgery of the Women and Children’s Hospital Affiliated with Xiamen University. They have homozygous deletion mutations in exons 3-5 of the Coxsackie and adenovirus receptor-like membrane protein (CLMP) gene. Clinical data were collected, and they were evaluated for disparities in postoperative growth between them, with a focus on nutrition schemes as a potential contributing factor. We summarize the experience of treatment strategy, particularly the nutrition schemes, to provide a reference for this condition.
Case 1: A female neonate (age, 22 days; weight, 2.30 kg) was admitted to our hospital due to a history of 21 days of jaundice and 15 days of intermittent vomiting.
Case 2: The patient was a male infant (aged 1 hour; birth weight, 2.932 kg). Following his birth, imaging of the upper digestive tract and barium enema were performed, confirming the presence of congenital intestinal malrotation.
Case 1: A history of 21 days of jaundice and 15 days of intermittent vomiting.
Case 2: An amniocentesis confirmed the presence of a CLMP gene mutation in fetal DNA, which was identical to the mutation found in his sister.
Cases 1 and 2 were newborns, no history of past illness were found.
Case 1: Gene testing revealed a homozygous mutation in the CLMP gene (variant, NM 024769, nucleic acid deletion, exons 3-5).
Case 2: An amniocentesis confirmed the presence of a CLMP gene mutation in fetal DNA, which was identical to the mutation found in his sister.
Case 1: On day 23 after birth, the patient underwent emergency surgical intervention, during which the small intestine was explored, with a total length of approximately 65 cm and absence of additional structural anomalies. Breastfeeding was resumed 48 hours after surgery. However, the patient experienced symptoms including vomiting and bloody stools.
Case 2: Confirming the presence of congenital intestinal malrotation.
No significant abnormalities in routine blood tests of two newborns.
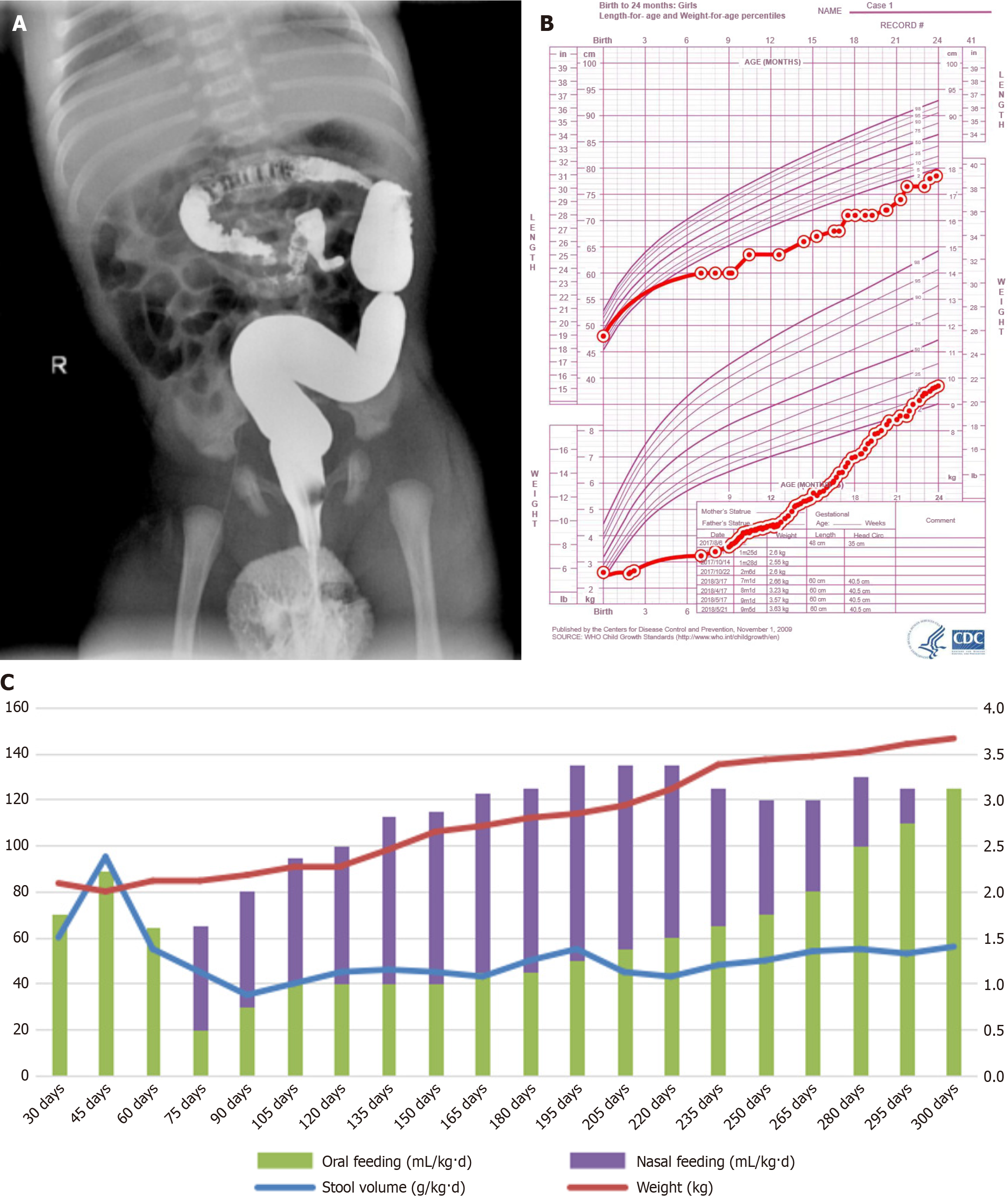
Case 1: Upper gastrointestinal and barium enema imaging revealed intestinal malrotation (Figure 1A).
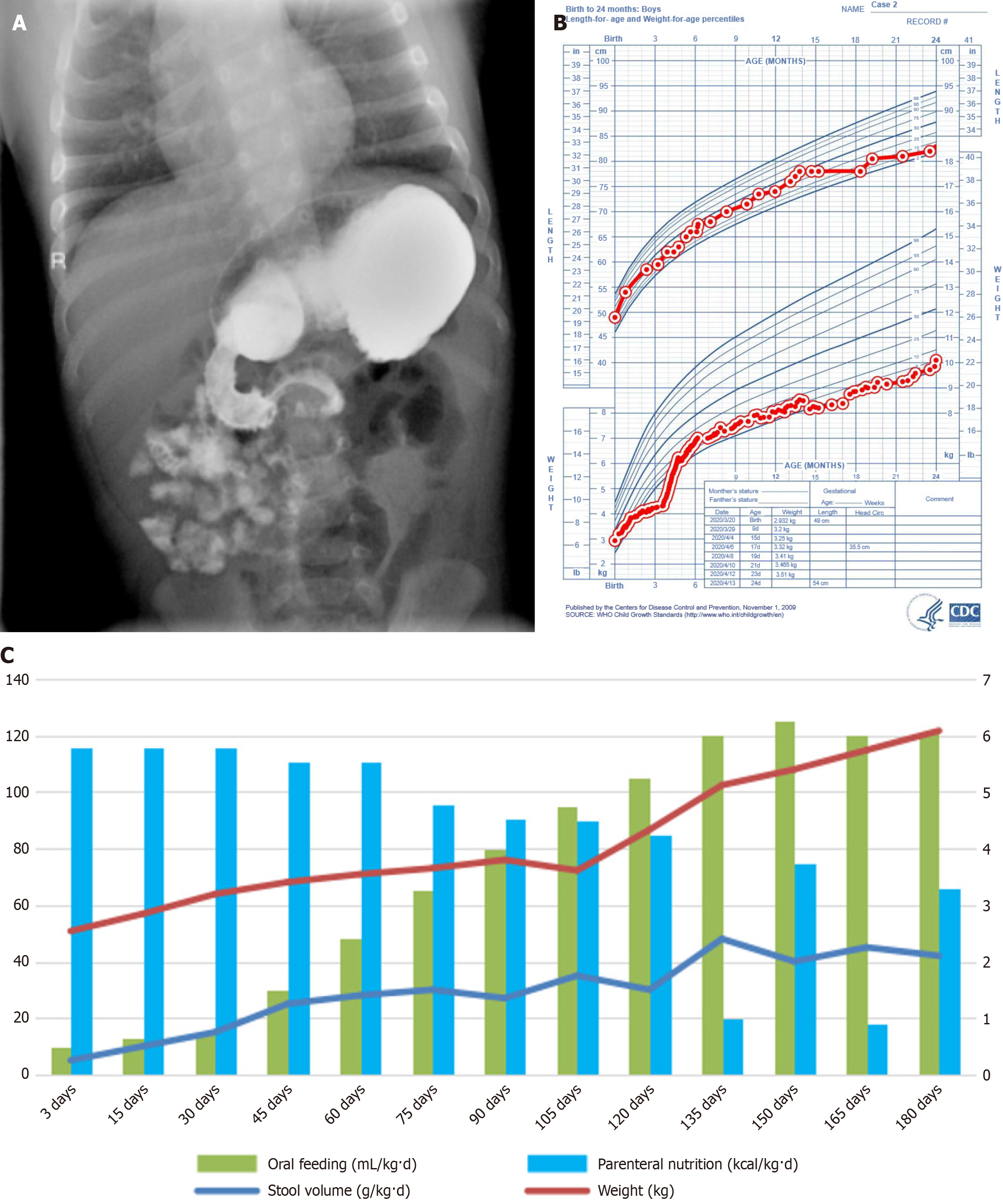
Case 2: Following his birth, imaging of the upper digestive tract and barium enema were performed, confirming the presence of congenital intestinal malrotation (Figure 2A).
The final diagnosis of cases 1 and 2 were familial CSBS.
Allergic enteritis was considered based on the clinical manifestations, so feeding was subsequently substituted with a free amino acid formula. However, oral feeding still led to feeding intolerance, with daily stool volume ranging from 50 to 100 g/kg/day, resulting in a change to EN (continuous nasal feeding) combined with PN treatment. The nasal feeding amount gradually reduced, and transition to oral feeding was planned at the age of 4 months. During the PN period associated with cholestatic liver injury, the intravenous nutrient solution’s fat emulsion was replaced with a fish oil fat emulsion. Concurrently, liver and gallbladder-protecting drugs were simultaneously administered for 1 month, resulting in a notable improvement of liver function. By the age of 6 months, the child successfully transitioned to total EN, discontinuing PN.
Emergency surgical intervention was performed 2 days after birth. The total length of the small intestine was found 51 cm, and the ileocecum and colon were structurally normal. A deeply hydrolyzed formula was administered orally 24 hours postoperatively, along with adequate PN. The intravenous nutrition solution consisted of a fish oil fat emulsion, and the stool volume was monitored daily to maintain it < 30 g/kg/day. At the age of 3 months, oral feeding has reached 80%-90% of physiological requirements. PN has been intermittently discontinued for 10 to 14 days, and the concentration of the deep hydrolysis formula has been increased.
The patient underwent a 36-month follow-up (Figure 1B). The patient exhibited favorable clinical manifestations, such as the absence of abdominal distension, jaundice, ascites, edema, rash, or oral lesions after discharge. Also, the correlation between oral and nasal feeding, fecal volume, and body weight was observed in case 1 during the first 300 days after birth were shown in Figure 1C.
No adverse reactions, such as abdominal distension, jaundice, or bloody stools, were observed during hospitalization. This child was monitored for 24 months in accordance with the child growth standards established by the World Health Organization (Figure 2B)[3]. The correlation between oral feeding, PN, fecal volume, and body weight within the first 180 days after birth in case 2 were shown in Figure 2C. The baseline information of the two children is presented in Table 1, along with supporting data from Figure 2B and C. Furthermore, their social cognition, emotional functioning, and intelligence were within the expected normal range.
| Case 1 | Case 2 | |
| Gender | Female | Male |
| Birth gestational age (weeks) | 40 | 39 weeks + 5 days |
| Weight (kg) | 2.55 | 2.932 |
| Bowel length (cm) | 65 | 51 |
| Surgical age (days) | 23 | 2 |
| Complication | Electrolyte disorder; allergic enteritis; cholestasis induced liver function damage | None |
CSBS is an uncommon medical condition initially documented by Hamilton et al[4] in 1969. It affects approximately < 1 in 1000000 individuals[4]. Regardless of the gene mutation type that causes CSBS, standardized diagnosis and treatment procedures are challenging. With advancements in prenatal examinations, the identification of congenital malformation cases has notably increased. In case 2, genetic examination of the prenatal amniotic fluid was conducted to ascertain the specific gene mutation, which confirmed the diagnosis of CSBS before surgery. Hence, the intestinal adaptation was expedited, and complete enteral feeding was attained effectively.
The primary goal of CSBS management is to ensure that patients achieve optimal nutritional status and growth. Second, we aim to maintain the stability of body fluids and electrolytes and reduce the loss of body fluids, electrolytes, and nutrients in feces. Third, we aim to promote intestinal adaptation. Based on the growth and development patterns of these two children, their diagnosis and treatment processes were summarized as follows.
With the advancement of prenatal diagnostic techniques, an increasing number of newborns are diagnosed with congenital gastrointestinal abnormalities before birth[5]. Specifically, congenital intestinal malrotation can now be detected by ultrasound during the prenatal period[6]. Thus, to ascertain the presence of genetic mutations, this group is recommended to undergo amniocentesis. The prognosis of CSBS can be improved with early diagnosis, particularly when active surgical intervention is pursued in the absence of significant postnatal problems.
The composition, amount, and duration of EN can affect oral feeding, and the demand and tolerance of nutrients vary depending on age, nutritional status, remaining intestinal length and function, and presence of ileocecal valves[7,8]. We speculated that early breastfeeding in case 1 might lead to feeding intolerance, such as vomiting, abdominal distension, diarrhea, and bloody stools, ultimately resulting in slow growth and delayed development. When EN is implemented in the early stage, a study suggested that children with early symptoms of intestinal obstruction have a poorer prognosis than those with late symptoms, whereas children with delayed symptoms have a higher survival rate[9].
With regard to nutrition, no consensus on guidelines for the nutritional treatment of infants with CSBS has been established. In addition, small bowel insufficiency leads to malabsorption, prolonged diarrhea with decreased peristalsis, and recurrent bloating, making medical resuscitation and nutritional therapy challenging. Prompt initiation of total PN (TPN) is a highly effective measure in promoting the early growth and development of individuals with SBS. In case 1, the patient had a notable increase in enteral feeding and a corresponding increase in stool amounts while the caloric intake from PN was inadequate. Thus, adjustments in the feeding schedule were made only when the child’s weight gain was insufficient. In case 2, in whom EN failed to fulfill his physiological requirements, adequate PN led to a consistent and positive trend in weight gain. Moreover, case 2 exhibited more optimal development than case 1. Moreover, literature reviews have indicated that early and prolonged TPN can have a substantial positive effect on the survival rate of CSBS. The primary determinant of survival rate in medical care is adequate nutritional support. Thus, every child is reco
SBS requires long-term intravenous nutrition to sustain health and growth. Regarding infusion techniques, two methods can be distinguished: Continuous and intermittent infusion. Continuous infusion offers several advantages, such as a consistent infusion rate, adequate nutrition supply, and suitability for early TPN. Conversely, intermittent infusion involves administering the necessary nutrient solution over a few hours, typically 12 hours. This approach allows for a departure from the limitations imposed by continuous 24-hour nutrient solution infusion, thereby ensuring the uninterrupted daily activities of patients and their family members. According to a previous study, intermittent infusion effectively mitigated visceral fat accumulation resulting from nutritional support, thereby aligning it closer to human physiology[9]. This approach is consistent with the notion that children already possess a certain degree of EN protection. In children who require a longer intestinal adaptation period and gradual EN supplementation, intermittent PN was employed to adequately address their nutritional requirements.
In case 2, intermittent PN commenced after the child reached 3 months of age. This approach not only effectively met the child’s caloric requirements but also mitigated the risk of cholestasis, thereby preventing potential complications such as liver failure and catheter-related infections. The high likelihood of cholestasis in children with SBS, necessitating long-term intravenous nutrition, underscores the importance of the early administration of fish oil fat emulsion to prevent such occurrence. Ongoing research indicates that the early administration of fish oil may effectively mitigate cholestasis and associated liver function impairment[11]. Currently, clinical comprehension of PN-related complications is inadequate, which has emerged as a prominent subject of academic investigation in recent times. These complications encompass severe cholestasis, PN-related liver disease, and intestinal failure-related liver disease. Compared with adults, infants and young children exhibit a greater susceptibility to these complications[12-14]. The intravenous fish oil administration is an efficacious approach in mitigating the incidence of intestinal failure-associated liver disease, particularly among children with SBS. This intervention effectively ameliorated the cholestasis induced by prolonged infusion of soybean oil fat emulsion, thereby preventing the development of advanced liver disease and reducing mortality rates[15-17].
To date, CSBS has been confirmed to have CLMP gene mutations by genetic testing. All CLMP gene mutations reported in the literature lead to loss of protein function, and the clinical manifestations of the reported cases were limited to the intestine[18]. In summary, no specific mutation type was found to be associated with a specific clinical phenotype. The potential correlation between gene type and growth and development is a topic for investigation. Despite sharing the same genetic mutation, variations in sex, birth weight, and small intestine length may contribute to distinct patterns of intestinal adaptation, growth, and development. Currently, no studies have investigated the potential relationship between certain gene mutation types and intestinal adaptation, as well as the subsequent growth and development in pediatric patients diagnosed with CSBS. Thus, investigations of this matter will be an area of interest.
In recent years, the survival rate of children with CSBS has improved a lot. The enhancement of clinical outcomes encompasses various factors, such as the implementation of professional multidisciplinary management, prompt timing of perinatal diagnosis, advancements in surgical techniques, improvements in enteral and PN schemes, and mitigation of catheter-related bloodstream infections. Continuous monitoring of malnutrition risk and nutritional status in patients with CSBS is crucial, even following the discontinuation of PN. Currently, empirical support for a nutrition regime for children with CSBS is lacking, as very few studies have explored the effectiveness of various feeding strategies, and published data suffer from methodological deficiencies. Nevertheless, enhancing nutritional approaches for intestinal adaptation in children with CSBS, shortening PN duration, and mitigating the associated complications remain im
CSBS management should be approached in a multidisciplinary and comprehensive manner[19]. Individualized plans, particularly nutrition schemes, must be developed to achieve the ultimate goal of enhancing the survival rate and promoting optimal growth and development of patients with CSBS.
| 1. | Chandra R, Kesavan A. Current treatment paradigms in pediatric short bowel syndrome. Clin J Gastroenterol. 2018;11:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Merritt RJ, Cohran V, Raphael BP, Sentongo T, Volpert D, Warner BW, Goday PS; Nutrition Committee of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Intestinal Rehabilitation Programs in the Management of Pediatric Intestinal Failure and Short Bowel Syndrome. J Pediatr Gastroenterol Nutr. 2017;65:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 1693] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 4. | Hamilton JR, Reilly BJ, Morecki R. Short small intestine associated with malrotation: a newly described congenital cause of intestinal malabsorption. Gastroenterology. 1969;56:124-136. [PubMed] |
| 5. | Liang B, Yang F, Huang H, Liu Z, Ji Q, Wang Y, Wu X, Lin Y, Xie L, Zhao W, Cao H, Xu L, Lin N. Prenatal diagnosis of fetal digestive system malformations and pregnancy outcomes at a tertiary referral center in Fujian, China: A retrospective study. Heliyon. 2023;9:e21546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Zulli A, Tocchioni F, Oreglio C, Biagiotti R, Di Maurizio M, Morini F. Prenatal diagnosis of isolated bowel malrotation and its impact on post-natal management. A case report and review of the literature. J Pediatr Surg Case Rep. 2023;92:102627. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Jaksic T. Current short bowel syndrome management: An era of improved outcomes and continued challenges. J Pediatr Surg. 2023;58:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Roy CC, Groleau V, Bouthillier L, Pineault M, Thibault M, Marchand V. Short bowel syndrome in infants: the critical role of luminal nutrients in a management program. Appl Physiol Nutr Metab. 2014;39:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sabharwal G, Strouse PJ, Islam S, Zoubi N. Congenital short-gut syndrome. Pediatr Radiol. 2004;34:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Negri E, Coletta R, Morabito A. Congenital short bowel syndrome: systematic review of a rare condition. J Pediatr Surg. 2020;55:1809-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, Puder M. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197-e201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Sant'Anna AM, Altamimi E, Clause RF, Saab J, Mileski H, Cameron B, Fitzgerald P, Sant'Anna GM. Implementation of a multidisciplinary team approach and fish oil emulsion administration in the management of infants with short bowel syndrome and parenteral nutrition-associated liver disease. Can J Gastroenterol. 2012;26:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Diamond IR, Sterescu A, Pencharz PB, Wales PW. The rationale for the use of parenteral omega-3 lipids in children with short bowel syndrome and liver disease. Pediatr Surg Int. 2008;24:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RA, Lopes S, Duggan C, Puder M. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678-e686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 15. | Hasosah M, Lemberg DA, Skarsgard E, Schreiber R. Congenital short bowel syndrome: a case report and review of the literature. Can J Gastroenterol. 2008;22:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Buchman A. Total parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr. 2002;26:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Ou FF, Li MJ, Mei LB, Lin XZ, Wu YA. Congenital Short-Bowel Syndrome Is Associated With a Novel Deletion Mutation in the CLMP Gene: Mutations in CLMP Caused CSBS. Front Pediatr. 2021;9:778859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Nandivada P, Fell GL, Gura KM, Puder M. Lipid emulsions in the treatment and prevention of parenteral nutrition-associated liver disease in infants and children. Am J Clin Nutr. 2016;103:629S-634S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/