Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.105360
Revised: April 6, 2025
Accepted: August 8, 2025
Published online: October 27, 2025
Processing time: 247 Days and 23.5 Hours
For patients with unresectable hepatocellular carcinoma (HCC), both stereotactic body radiation therapy (SBRT) and transcatheter arterial chemoembolization (TACE) have demonstrated effectiveness in controlling local tumor growth. We investigated whether combining these treatments could provide better outcomes than TACE monotherapy.
To evaluate whether combining SBRT with TACE provides superior clinical out
We conducted a randomized study involving eighty patients diagnosed with unresectable HCC, classified as Barcelona Clinic Liver Cancer stage B and Child-Pugh class A. Participants were divided into two treatment arms: A control group receiving TACE alone (Group A) and an experimental group receiving sequential TACE and SBRT (Group B). The SBRT regimen consisted of 40 Gy administered in five daily fractions over one week. Primary endpoints included local control, pro
The study enrolled 88 patients from April 2021 to January 2023, with 48 assigned to Group A and 40 to Group B. Over a median follow-up period of 20 months, the combination therapy group demonstrated superior outcomes in both tumor control and disease progression metrics. Complete response rates reached 75% in Group B compared to 54.5% in Group A. The combination therapy extended median PFS to 16 months, significantly longer than the 11 months observed with TACE alone (P = 0.003). Neither group had reached median OS by study conclusion. Importantly, both treatment approaches showed comparable safety profiles.
Our findings suggest that supplementing TACE with SBRT offers a well-tolerated and effective treatment strategy for advanced HCC patients. This combination approach achieved better tumor control and delayed disease progression compared to TACE monotherapy, while maintaining an acceptable safety profile.
Core Tip: Combining transcatheter arterial chemoembolization (TACE) and stereotactic body radiation therapy (SBRT) significantly enhanced local tumor control and prolonged progression-free survival in unresectable hepatocellular carcinoma. Despite a mild increase in hepatic toxicity, it remained manageable and reversible, with most patients recovering by three months. The synergy of TACE’s localized chemotherapy and SBRT’s precise high-dose radiation is a promising approach for Barcelona Clinic Liver Cancer stage B, Child-Pugh A patients. Our findings emphasize careful dosing, robust imaging guidance, and close monitoring of liver function to optimize safety. Integration of immunotherapy and further refinements in treatment planning might yield even better outcomes in the future.
- Citation: Chen L, Wang L, Wang H. Comparison of stereotactic body radiotherapy following transcatheter arterial chemoembolization vs transcatheter arterial chemoembolization alone in hepatocellular carcinoma. World J Gastrointest Surg 2025; 17(10): 105360
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/105360.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.105360
Hepatocellular carcinoma (HCC) and gastrointestinal tumors represent major global health challenges, with treatment strategies continuously evolving[1-3]. Stereotactic body radiation therapy (SBRT), as a precise radiation therapy te
With technological advancements, the application of SBRT in liver cancer and gastrointestinal tumor treatment continues to expand. This study aims to investigate the efficacy and toxicity of combined SBRT and traditional transarterial chemoembolization (TACE) vs TACE alone in patients with unresectable HCC, seeking to provide more effective treatment strategies for clinical practice. Our focus is on optimizing SBRT dose fractionation schemes to improve treatment outcomes and exploring the combined application of SBRT with other therapeutic approaches such as targeted therapy and immunotherapy to enhance treatment efficacy.
Furthermore, advances in SBRT technology, including image-guided radiation therapy and adaptive radiation therapy, provide us with more precise treatment tools. The application of artificial intelligence and machine learning technologies has enabled more optimized SBRT planning and precise dose distribution. Meanwhile, studying predictive factors for SBRT-related toxicity and new strategies for managing side effects is crucial for improving treatment safety and tolerability[7-9].
In the field of gastrointestinal tumors, SBRT has shown tremendous potential. We explore the role of SBRT in local control and quality of life improvement for tumors such as pancreatic and gastric cancer, particularly in patients who are inoperable or refuse surgery[10-12]. Additionally, the position of SBRT in multimodal treatment strategies, including synergistic effects with other local treatments and systemic therapies, is also a focus of our research.
This study will provide scientific evidence for the application of SBRT in HCC and gastrointestinal tumor treatment through retrospective review of medical records, comparing the efficacy and toxicity of combined SBRT and TACE vs TACE alone. We expect these findings to promote further development of SBRT technology and provide patients with more treatment options and better outcomes. Through large-scale randomized controlled clinical trials and guideline development, we will provide high-level evidence supporting the role of SBRT in the treatment of liver cancer and gastrointestinal tumors.
Between April 2021 and January 2023, a retrospective analysis was performed at the Departments of Clinical Oncology and Interventional Radiology. The study population consisted of 88 consecutive patients diagnosed with HCC, classified as Barcelona Clinic Liver Cancer (BCLC) stage B and Child-Pugh class A. Eligible patients presented with up to three HCC nodules, with individual nodule diameters not exceeding 50 mm, and absence of vascular invasion. Surgery was not an option due to various factors including poor performance status, patient refusal, or unsuitable conditions for radiofrequency ablation. While patient selection deviated somewhat from standard BCLC stage B criteria, this modification was intentional due to heightened concerns about hepatic toxicity, particularly relevant given Egypt's high prevalence of cirrhosis and viral hepatitis. Treatment allocation divided patients into two cohorts: 48 patients in Group A received TACE as monotherapy, while 40 patients in Group B underwent sequential TACE followed by SBRT. All participants provided written informed consent before treatment initiation.
The TACE procedure employed the Seldinger technique, utilizing a French catheter (4-5F) inserted via the right femoral artery and advanced to the abdominal aorta. Tumor visualization and assessment of residual hepatic tumor staining were accomplished through selective hepatic arteriography[13,14].
Treatment preparation began with patient immobilization in supine position using a wing board support. Imaging protocol included contrast-enhanced computed tomography (CT) with 2 mm slice thickness, performed during breath-hold at inspiration. These imaging datasets were subsequently imported into the treatment planning system.
Treatment volumes were defined using multi-modality imaging [magnetic resonance imaging, triphasic CT, or positron emission tomography (PET) scan], with TACE-induced lipiodol retention serving as an additional guide for tumor localization. The planning target volume (PTV) was generated by adding a 0.5 cm margin to the gross tumor volume, without an intermediate clinical target volume. Critical structures, including spinal cord, functional liver tissue, kidneys, with dose distributions optimized via dose-volume histograms while maintaining organ-at-risk constraints. The prescribed regimen delivered 40 Gy in 5 daily fractions over one week, achieving a biologically effective dose of 88 Gy, with at least 95% PTV coverage. Daily image guidance employed cone-beam CT for precise patient positioning. All treatments were delivered using a Varian "UNIQUE" linear accelerator[15-17].
The monitoring schedule consisted of weekly evaluations throughout the initial month post-treatment, followed by quarterly assessments thereafter. Each evaluation encompassed multiple parameters: Functional status assessment, medical history review, physical evaluation, comprehensive blood analysis (including complete blood count, liver function panels, coagulation studies, and electrolyte measurements), and Child-Pugh scoring. Imaging surveillance, conducted at one month post-treatment and quarterly thereafter, incorporated triphasic abdominopelvic CT or PET/CT imaging, complemented by liver function testing and alpha-fetoprotein (AFP) monitoring. Treatment response evaluation followed modified RECIST criteria, applied after TACE in Group A and post-SBRT in Group B.
The study monitored for two distinct presentations of radiation-induced liver disease (RILD). Classical RILD manifests as subacute hepatic toxicity typically 4-8 weeks post-radiation, characterized by liver enlargement, non-icteric fluid retention, and elevated alkaline phosphatase. Non-classical RILD was defined by either a five-fold increase in liver enzymes.
Data analysis utilized SPSS software (version 22), employing a comprehensive statistical approach. For categorical data analysis, frequency distributions and percentage calculations were performed, with Fisher's exact test or Pearson χ2 test applied based on cell count distributions (threshold: < 20% cells with counts below 5). Continuous data were characterized by mean values, standard deviations, and ranges. Analysis of normally distributed variables employed Student's t-test, while non-parametric data were evaluated using the Mann-Whitney U test. Statistical significance was established at P < 0.05. Survival analytics included progression-free survival (PFS) assessment across treatment groups, visualized through Kaplan-Meier methodology. Inter-group survival comparisons utilized Log-Rank testing, with additional analysis of PFS variations based on recurrence patterns and anatomical distribution.
A total of 88 patients were included in this study, with 48 patients receiving TACE alone (Group A) and 40 patients receiving TACE followed by SBRT (Group B). The median age for all patients was 50 years, with an age range of 50 to 85 years, and the majority of cases were male (over 70%). The median follow-up period was 20 months. The baseline characteristics and disease features were well balanced between the two groups (Table 1).
| Group A | Group B | P value | ||||
| mean ± SD/n | Range/% | mean ± SD/n | Range/% | |||
| Age in years | 60.1 ± 8.0 | 50-85 | 60.3 ± 7.9 | 48-75 | 0.410 | |
| Gender | Male | 36 | 75 | 32 | 80 | 0.823 |
| Female | 12 | 25 | 8 | 20 | ||
| ECOG performance status | 1 | 28 | 58.30 | 24 | 60 | 0.867 |
| 2 | 20 | 41.70 | 16 | 40 | ||
| Hypertension | No | 36 | 75 | 34 | 85 | 0.335 |
| Yes | 12 | 25 | 6 | 15 | ||
| Diabetes | No | 34 | 70.8 | 30 | 75 | 0.276 |
| Yes | 14 | 29.2 | 10 | 25 | ||
| Hepatitis C virus | No | 8 | 16.7 | 12 | 30 | 0.392 |
| Yes | 40 | 83.30 | 28 | 70 | ||
| CHILD status | A | 48 | 100 | 40 | 40 | 0.956 |
| Extent of disease | Bilobar | 20 | 41.7 | 32 | 80 | 0.712 |
| Unilobular | 28 | 58.3 | 8 | 20 | ||
| Degree of liver cirrhosis | Cirrhosis | 44 | 91.70 | 15 | 75 | 0.128 |
| Not known | 4 | 8.30 | 5 | 25 | ||
| Baseline number of lesions | 1 | 26 | 54.20 | 28 | 70 | 0.503 |
| 2 | 22 | 45.80 | 10 | 25 | ||
| 3 | 0 | 0.00 | 2 | 5 | ||
| Baseline size of lesion (cm) | 4.7 ± 0.9 | 3.0 ± 6.5 | 5.2 ± 1.1 | 3.5 ± 7.0 | ||
In Group A, patients achieved a complete response (CR) rate of 54.5%, a partial response (PR) rate of 36.4%, with the remaining 9.1% showing stable disease (SD). In Group B, 75% of patients achieved CR, 15% achieved PR, and 7% showed SD. Compared to Group A, Group B showed a 25% improvement in CR rate (Table 2). The mean AFP levels one month after SBRT completion showed a significant decrease, reaching 18.4 ng/mL in Group B compared to 176.5 ng/mL in Group A, with a P value of 0.003.
| Indicator | Group A | Group B | P value |
| CR (%) | 54.5 | 75.0 | 0.012 |
| PR (%) | 36.4 | 15.0 | 0.008 |
| SD (%) | 9.1 | 7.0 | 0.045 |
| Mean AFP after SBRT (ng/mL) | 176.5 ± 35.0 | 18.4 ± 4.5 | 0.003 |
| Mean ALP after SBRT (U/L) | 120.0 ± 20.0 | 90.0 ± 15.0 | 0.007 |
| Mean ALT after SBRT (U/L) | 45.0 ± 10.0 | 30.0 ± 5.0 | 0.005 |
| Mean AST after SBRT (U/L) | 50.0 ± 12.0 | 35.0 ± 6.0 | 0.006 |
| Mean GGT after SBRT (U/L) | 60.0 ± 15.0 | 40.0 ± 8.0 | 0.004 |
| Mean bilirubin after SBRT (mg/dL) | 1.2 ± 0.3 | 0.8 ± 0.2 | 0.009 |
| Mean platelets after SBRT (103/μL) | 200 ± 40 | 220 ± 30 | 0.021 |
| Mean WBC after SBRT (103/μL) | 6.0 ± 1.0 | 5.5 ± 0.8 | 0.034 |
| Mean hemoglobin after SBRT (g/dL) | 12.0 ± 1.5 | 13.0 ± 1.0 | 0.015 |
Post-SBRT hepatic function monitoring revealed transient liver function deterioration in seven patients at the one-month mark. The majority demonstrated recovery by three months, with Group B showing a marginally higher but statistically insignificant rate of Child-Pugh class progression from A to B (10% in Group B vs 4.54% in Group A, P = 0.181). Notable was the limited incidence of grade 2 non-classic RILD in Group B, affecting only two patients (10%).
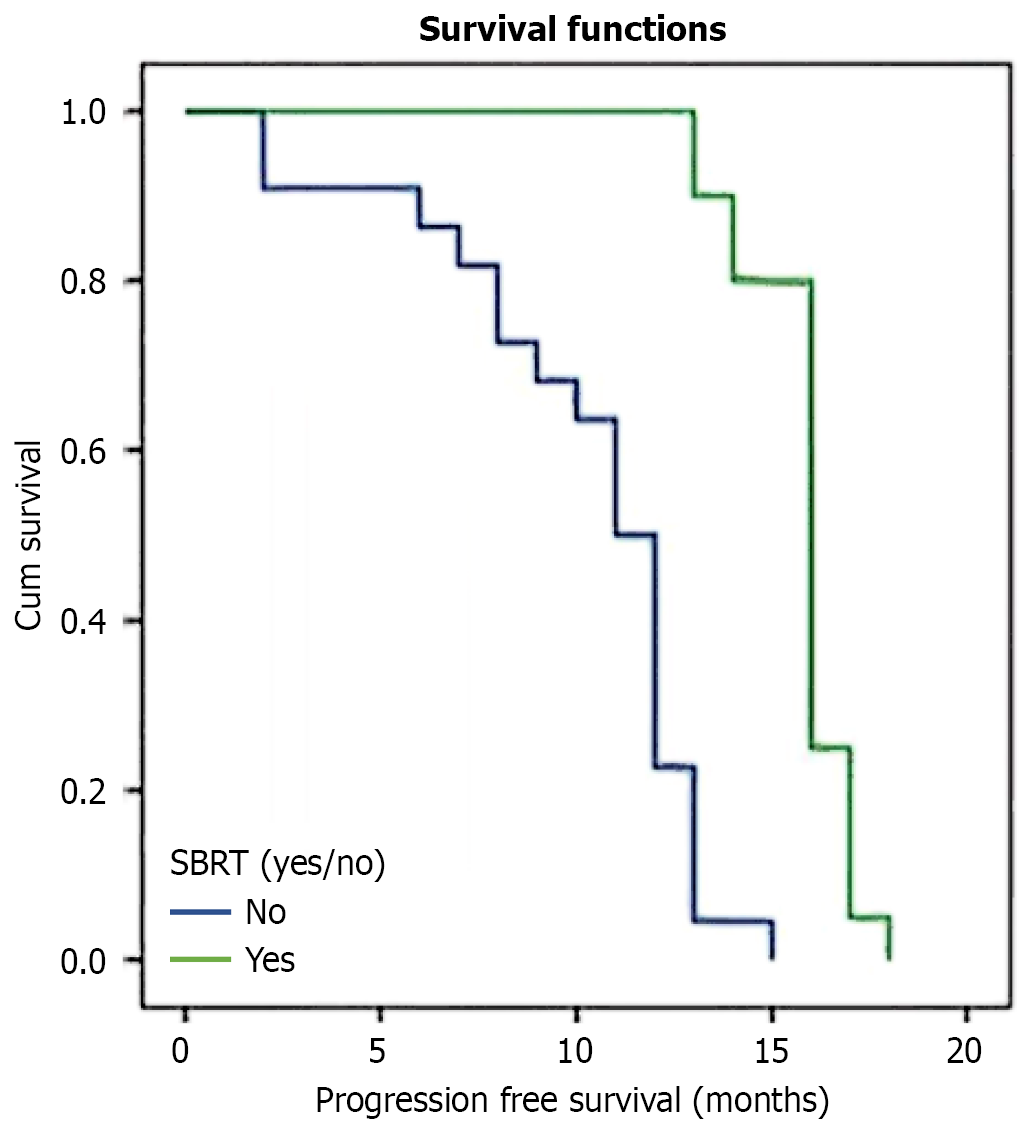
Twenty-month follow-up data demonstrated marked differences in disease progression patterns between treatment groups. The combination therapy cohort (Group B) exhibited significantly extended PFS compared to monotherapy (Group A), with median durations of 16 and 11 months respectively (P = 0.003; Figure 1). Treatment response emerged as a key prognostic indicator, with CR patients achieving substantially longer PFS (16 months) compared to PR cases (8 months, P = 0.002).
Disease control metrics showed consistent advantages for combination therapy across multiple parameters. Local tumor control, measured by median local PFS, favored Group B (16 months in Group B vs 12 months in Group A, P = 0.043). Similarly, distant disease control demonstrated superior outcomes in Group B (median distant PFS 17.5 months vs 13 months, P < 0.001).
Statistical modeling through univariate analysis identified baseline AFP levels and SBRT treatment as significant PFS predictors. Subsequent multivariate analysis, adjusted for age, initial AFP values, and baseline tumor dimensions, confirmed SBRT's protective role against disease recurrence (P = 0.001, HR = 0.07, 95%CI: 0.01-0.32). At study conclusion, median overall survival remained undetermined for both treatment arms (Table 3).
| Univariate | Multivariate | |||||
| P value | HR | 95%CI of HR | P value | HR | 95%CI of HR | |
| Age (years) | 0.095 | 1.02 | 0.98-1.09 | 0.372 | 1.03 | 0.98-1.08 |
| SBRT (yes) | < 0.001 | 0.12 | 0.02-0.28 | 0.002 | 0.08 | 0.02-0.34 |
| Baseline AFP (ng/dL) | 0.005 | 1 | 1-1.02 | 0.068 | 1 | 1-1.02 |
| Baseline size of lesion (cm) | 0.238 | 0.85 | 0.57-1.16 | 0.285 | 0.83 | 0.58-1.19 |
| Baseline number of lesions | 0.975 | 1.02 | 0.50-2.05 | |||
| Number of affected lobes (bilobular) | 0.825 | 0.92 | 0.46-1.90 | |||
| CR after TACE | 0.430 | 0.45 | 0.05-3.65 | |||
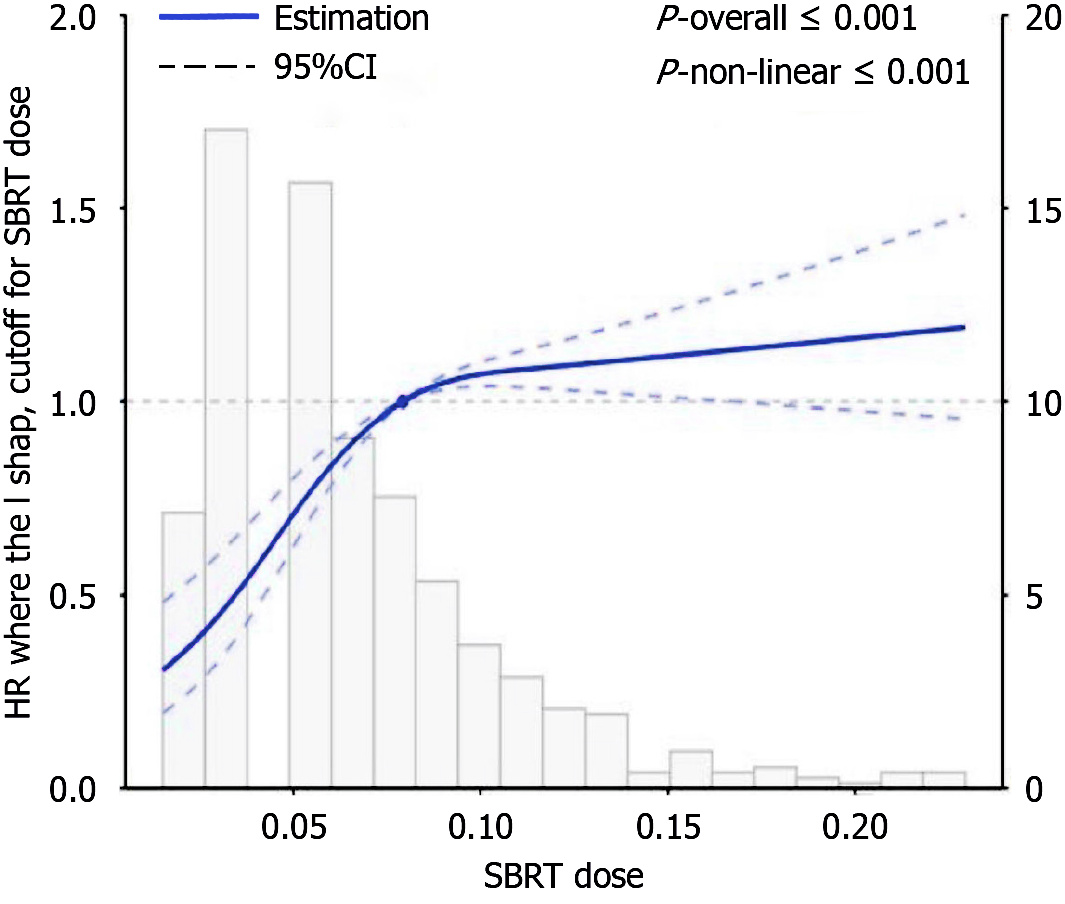
According to the Restricted Cubic Spline (RCS) results shown in the graph, there is a non-linear relationship between SBRT dose and its impact on liver cancer patients. The red solid line indicates that at low doses, the hazard ratio (HR) is slightly below 1, suggesting a possible association with lower risk. As the dose increases, the HR gradually rises and exceeds 1, indicating that higher doses may increase risk. In the statistical analysis, both the overall model and the non-linear relationship showed P-values less than 0.001, demonstrating a significant non-linear relationship between the two factors. The gray histogram shows that most patients received low to medium doses of treatment. In conclusion, the significant non-linear relationship between SBRT dose and survival risk suggests that dose adjustments should be made cautiously to optimize treatment outcomes while minimizing risks (Figure 2).
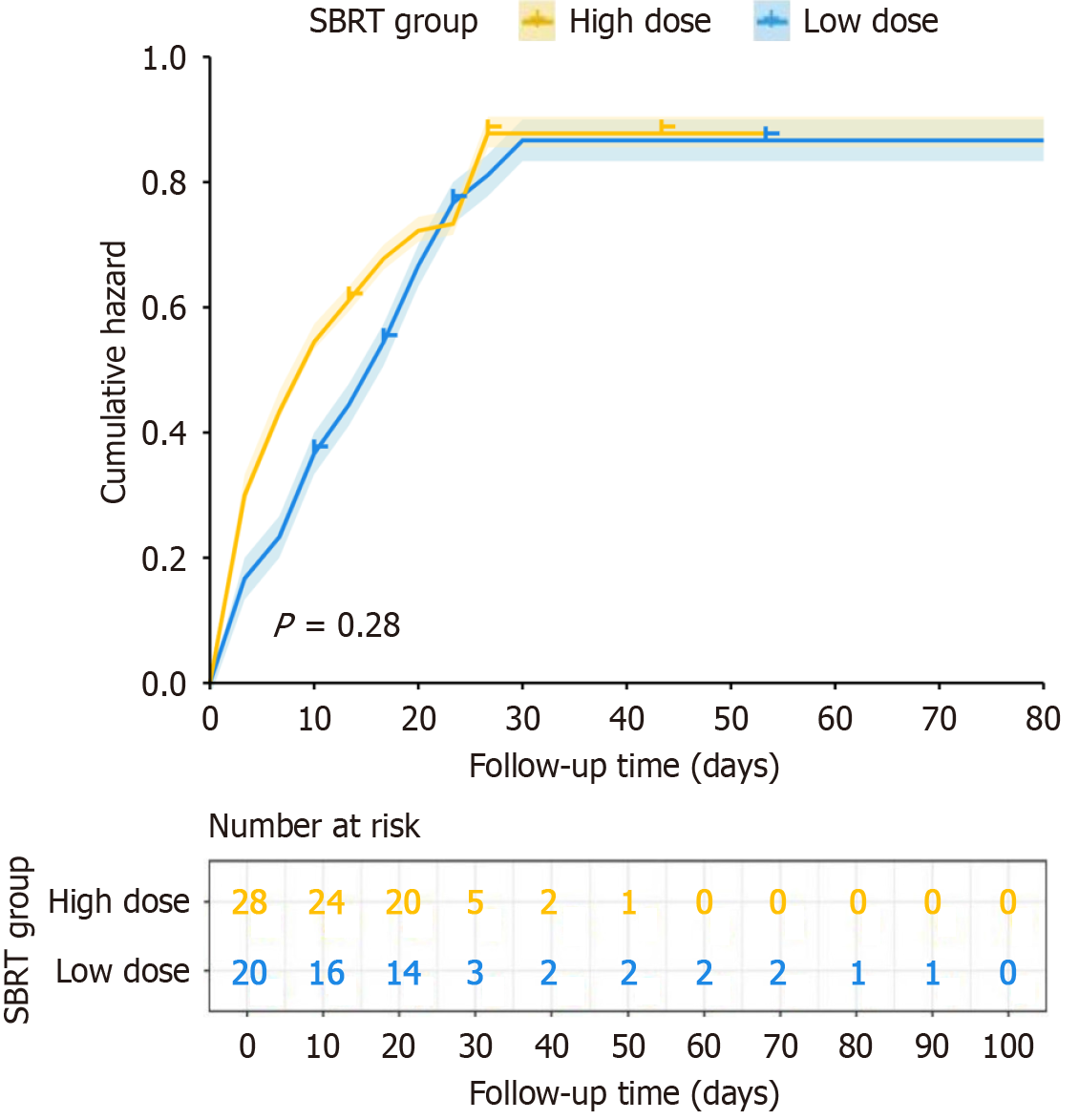
In the survival curves, yellow and blue lines represent the cumulative risk for high-dose and low-dose SBRT groups, respectively. The survival curves between the two groups remain relatively close throughout the follow-up period, indicating no significant difference in survival rates between the groups during the entire follow-up period. Statistical analysis shows a P value of 0.28, indicating no statistically significant difference in survival between the two groups, suggesting that SBRT dose level did not significantly impact survival in this study.
The cumulative risk curves show that both groups experienced a rapid increase in risk during the early follow-up phase but stabilized in the later period. The high-dose group showed slightly higher risk than the low-dose group in the early follow-up period, but the risks between the groups converged over time. The "number at risk" section below the graph shows the number of patients still under observation at each follow-up time point, with sample size gradually decreasing over time. In conclusion, although SBRT is an effective treatment modality, this study found no significant difference in survival outcomes between high and low-dose SBRT for liver cancer patients (Figure 3).
HCC is one of the leading causes of cancer-related deaths globally, presenting particular challenges in advanced stages when surgical options are not feasible[18-20]. Treatment modalities such as TACE and SBRT have emerged as significant interventions for unresectable HCC. TACE involves direct delivery of chemotherapeutic agents to liver tumors while effectively blocking their blood supply, while SBRT provides precise, high-dose radiation targeting tumors with minimal impact on surrounding healthy tissues[21-23]. Recent advances in these therapies show promise for improving patient outcomes, warranting further exploration of their combined application.
In our study, the group receiving TACE alone (Group A) showed a CR rate of 54.5%, PR rate of 36.4%, and SD in 9.1% of patients. In comparison, the group receiving TACE plus SBRT (Group B) achieved a CR rate of 75%, PR rate of 15%, and SD of 7%. This represents a 25% improvement in CR rate for Group B compared to Group A, highlighting the potential synergistic effect of combining SBRT with TACE. Moreover, AFP levels, a biomarker of liver cancer activity, showed significant reduction one month after SBRT, averaging 18.4 ng/mL in Group B compared to 176.5 ng/mL in Group A (P = 0.003). This significant decrease emphasizes the effectiveness of combined therapy in controlling tumor activity.
Regarding liver toxicity following SBRT, seven patients experienced temporary liver function deterioration within one month, with most recovering within three months. The proportion of patients deteriorating from Child-Pugh A to B was 10% in Group B compared to 4.54% in Group A, although this difference was not statistically significant (P = 0.181). Only two patients (10%) in Group B developed grade 2 RILD. These findings suggest that while the addition of SBRT may increase acute toxicity, it remains manageable and reversible, reinforcing its safety in clinical practice.
In our study, the CR rate in the TACE plus SBRT treatment group reached 75%, representing a significant 25% improvement compared to the 54.5% CR rate in the TACE-only group. This remarkable enhancement suggests potential synergistic effects between these two treatment modalities. This synergy may be based on multiple mechanisms: First, TACE-induced tumor hypoxia and decreased blood supply may enhance tumor cell sensitivity to radiation therapy; second, the local inflammatory response triggered by TACE might reshape the tumor microenvironment, promoting post-SBRT immune responses; third, TACE's embolization effect reduces tumor volume, potentially optimizing the target area and radiation dose distribution for subsequent SBRT[24]. Additionally, TACE-induced changes in vascular endothelial growth factor expression may lead to transient vascular normalization, improving the efficacy of subsequent SBRT. Future research should further explore these mechanisms, including analysis of pre- and post-treatment ra
RILD is a potential side effect of radiation therapy. SBRT, while precise in delivering high-dose radiation to tumors while minimizing damage to surrounding normal tissues, may still impact the liver due to its complex structure and function. Acute RILD typically appears within weeks to months after radiation therapy completion, with symptoms potentially including nausea, vomiting, diarrhea, fatigue, and abnormal liver function tests (such as alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin). Chronic RILD appears later after radiation therapy and may lead to persistent liver dysfunction or even cirrhosis. Post-radiation hepatic inflammation can cause liver enlargement, pain, and abnormal liver function tests. Long-term or repeated radiation therapy may lead to liver tissue fibrosis, affecting liver function. Radiation therapy may result in liver dysfunction, impacting metabolic and detoxification capabilities. In some cases, radiation therapy may not completely eliminate tumor cells, leading to tumor recurrence or regrowth[25-27].
This pattern extended to distant disease control, with combination therapy yielding a median distant PFS of 17.5 months compared to 13 months with monotherapy (P < 0.001). Statistical modeling provided deeper insights into outcome determinants. Initial analysis identified both baseline AFP levels and SBRT treatment as significant predictors of PFS. More sophisticated multivariate modeling, accounting for patient age, initial AFP values, and survival parameters, established SBRT as a significant protective factor against disease recurrence (P = 0.001, HR = 0.07, 95%CI: 0.01-0.32). Risk stratification analysis further confirmed substantial survival differences between treatment subgroups. Our RCS analysis revealed a non-linear relationship between SBRT dose and survival risk, a complex relationship that offers new perspectives on liver cancer radiotherapy strategies. The results show that in the lower dose region, the HR is slightly below 1, while as the dose increases, the HR gradually rises and exceeds 1, indicating the existence of a potential "therapeutic window" for dose selection. This relationship likely reflects the balance between tumor control and liver toxicity in radiation therapy - doses that are too low may fail to effectively control the tumor, while doses that are too high may lead to unacceptable liver damage. Radiomics and machine learning technologies offer promising directions for addressing this challenge. By integrating multimodal imaging features, clinical parameters, and biomarkers, these advanced methods have the potential to establish precise prediction models, achieving truly individualized dose optimization. Future research should explore applying artificial intelligence to extract deep features from imaging data to identify tumor heterogeneity and spatial variations in radiation sensitivity, thereby customizing optimal dose regimens for each patient that maximize tumor control while minimizing damage to healthy liver tissue. RetryClaude can make mistakes. Please double-check responses.
For liver function protection and toxicity management, future research should focus on several key areas: First, adaptive radiation therapy techniques can be employed to adjust treatment plans in real-time in response to changes in tumor and normal tissue, thereby reducing radiation dose to functional liver tissue. Second, dose de-escalation strategies should be evaluated in patients with borderline liver function, exploring optimal dose regimens that maintain anti-tumor efficacy while reducing hepatic toxicity. Furthermore, research should focus on identifying radiogenomic markers to predict individual patients' liver radiation sensitivity, enabling truly personalized treatment. Artificial intelligence-assisted treatment planning tools can further optimize dose distribution to maximally protect functional liver tissue.
While our study demonstrates promising results for the combination of TACE and SBRT in unresectable HCC, several limitations should be acknowledged. The findings may not be generalizable to populations with different etiologies of HCC, such as those predominantly caused by hepatitis B virus infection or non-alcoholic steatohepatitis. The patient population in our study may not fully represent the diverse global distribution of HCC risk factors, which vary sig
The combination of SBRT and TACE offers a promising treatment strategy for unresectable HCC, providing improved local control and PFS without increased toxicity. Advances in SBRT technology and predictive models enhance its role in treating liver and gastrointestinal tumors, bringing new hope for improved patient outcomes.
| 1. | Li Y, Li X, Xiao X, Cheng J, Li Q, Liu C, Cai P, Chen W, Zhang H, Li X. A novel hybrid model for predicting tertiary lymphoid structures and targeted immunotherapy outcomes in hepatocellular carcinoma: a multicenter retrospective study. Eur Radiol. 2025;35:3206-3222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Shi CJ, Pang FX, Lei YH, Deng LQ, Pan FZ, Liang ZQ, Xie T, Wu XL, Wang YY, Xian YF, Zeng WQ, Lin HL, Zhang JF. 5-methylcytosine methylation of MALAT1 promotes resistance to sorafenib in hepatocellular carcinoma through ELAVL1/SLC7A11-mediated ferroptosis. Drug Resist Updat. 2025;78:101181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 3. | Sun RQ, Ye YH, Xu Y, Wang B, Pan SY, Li N, Chen L, Pan JY, Hu ZQ, Fan J, Zhou ZJ, Zhou J, Song CL, Zhou SL. Integrated molecular characterization of sarcomatoid hepatocellular carcinoma. Clin Mol Hepatol. 2025;31:426-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Bidarmaghz B, Idrees M, Lee YY, Hodgkinson P. Large hepatocellular carcinoma treated with sequential SBRT and immunotherapy with anti-VEGF (Vascular Endothelial Growth Factor) therapy. BMJ Case Rep. 2023;16:e256931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Chen Y, Hong H, Fang W, Zhang X, Luo H, Chen Z, Yu J, Fan W, Chi X, Peng Y. Toripalimab in combination with Anlotinib for unresectable hepatocellular carcinoma after SBRT: A prospective, single-arm, single-center clinical study. Front Oncol. 2023;13:1113389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Wei L, Aryal MP, Cuneo K, Matuszak M, Lawrence TS, Ten Haken RK, Cao Y, Naqa IE. Deep learning prediction of post-SBRT liver function changes and NTCP modeling in hepatocellular carcinoma based on DGAE-MRI. Med Phys. 2023;50:5597-5608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Brodin NP, Schulte L, Velten C, Martin W, Shen S, Shen J, Basavatia A, Ohri N, Garg MK, Carpenter C, Tomé WA. Organ-at-risk dose prediction using a machine learning algorithm: Clinical validation and treatment planning benefit for lung SBRT. J Appl Clin Med Phys. 2022;23:e13609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Sun W, Mo Z, Li Y, Xiao J, Jia L, Huang S, Liao C, Du J, He S, Chen L, Zhang W, Yang X. Machine learning-based ensemble prediction model for the gamma passing rate of VMAT-SBRT plan. Phys Med. 2024;117:103204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Wimmert L, Nielsen M, Madesta F, Gauer T, Hofmann C, Werner R. Benchmarking machine learning-based real-time respiratory signal predictors in 4D SBRT. Med Phys. 2024;51:3173-3183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Hughson AL, Hannon G, Salama NA, Vrooman TG, Stockwell CA, Mills BN, Garrett-Larsen J, Qiu H, Katerji R, Benoodt L, Johnston CJ, Murphy JD, Kruger E, Ye J, Gavras NW, Keeley DC, Qin SS, Lesch ML, Muhitch JB, Love TMT, Calvi LM, Lord EM, Luheshi N, Elyes J, Linehan DC, Gerber SA. Local Delivery of SBRT and IL12 by mRNA Technology Overcomes Immunosuppressive Barriers to Eliminate Pancreatic Cancer. bioRxiv. 2023;2023.10.30.564833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Liu J, Sidiqi B, McComas K, Gogineni E, Andraos T, Crane CH, Chang DT, Goodman KA, Hall WA, Hoffe S, Mahadevan A, Narang AK, Lee P, Williams TM, Chuong MD. SBRT for Pancreatic Cancer: A Radiosurgery Society Case-Based Practical Guidelines to Challenging Cases. Pract Radiat Oncol. 2024;14:555-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Salas B, Ferrera-Alayón L, Espinosa-López A, Vera-Rosas A, Salcedo E, Kannemann A, Alayon A, Chicas-Sett R, LLoret M, Lara PC. Dose-escalated SBRT for borderline and locally advanced pancreatic cancer. Feasibility, safety and preliminary clinical results of a multicenter study. Clin Transl Radiat Oncol. 2024;45:100753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Zhang H, Wang X, Wang H, Li J, Lei K, Hu R, Liu Z. Development and validation of a model for predicting who can benefit from multiple TACE in HCC patients. Clin Exp Med. 2024;25:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zhou M, Zhang P, Mao Q, Shi Y, Yang L, Zhang X. Multisequence MRI-Based Radiomic Features Combined with Inflammatory Indices for Predicting the Overall Survival of HCC Patients After TACE. J Hepatocell Carcinoma. 2024;11:2049-2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Arumugam S, Young T, Jones C, Pryor D, Sidhom M. Treatment accuracy of standard linear accelerator-based prostate SBRT: the delivered dose assessment of patients treated within two major clinical trials using an in-house position monitoring system. Front Oncol. 2024;14:1372968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Franceschini D, Loi M, Marzo AM, Dominici L, Spoto R, Bertolini A, Lo Faro L, La Fauci F, Marini B, Di Cristina L, Scorsetti M. STRILL: Phase I Trial Evaluating Stereotactic Body Radiotherapy (SBRT) Dose Escalation for Re-Irradiation of Inoperable Peripheral Lung Lesions. Diseases. 2024;12:153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Luca K, Kesarwala AH, Benner K, Tian S, Thomas M, Schreibmann E, Roper J. A lung SBRT treatment planning technique to focus high dose on gross disease. Med Dosim. 2025;50:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Burke L, Hinkson A, Haghnejad V, Jones R, Parker R, Rowe IA. Hepatocellular carcinoma risk scores for non-viral liver disease: A systematic review and meta-analysis. JHEP Rep. 2025;7:101227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Li Y, Chen Y, Zhang Y, Fang Y, Wu L, Zhao Y, Wang D, Qiao X. Integrating multi-omics techniques and in vitro experiments reveals that GLRX3 regulates the immune microenvironment and promotes hepatocellular carcinoma cell proliferation and invasion through iron metabolism pathways. Front Immunol. 2024;15:1496886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Zhuo W, Xia H, Lan B, Chen Y, Wang X, Liu J. Signature of immune-related metabolic genes predicts the prognosis of hepatocellular carcinoma. Front Immunol. 2024;15:1481331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Li X, Tang Z, Pang Q, Wang X, Bai T, Chen J, Wei M, Wei T, Li L, Wu F. The Role of Timing of Progression and Early Salvage Surgery in Unresectable Hepatocellular Carcinoma Treated with TACE Plus TKIs and PD1 Inhibitors. J Hepatocell Carcinoma. 2024;11:1641-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Lu H, Liang B, Zheng C, Xia X. Comparative analysis of efficacy and safety between D-TACE + HAIC + lenvatinib and D-TACE + lenvatinib in the treatment of unresectable massive hepatocellular carcinoma. BMC Cancer. 2024;24:1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Lu SY, Sun HY, Zhou Y, Luo X, Liu S, Zhou WZ, Shi HB, Yang W, Tian W. Prognosis of Patients with Hepatocellular Carcinoma Treated with TACE: A New Score Combining Alpha-Fetoprotein and Des-γ-Carboxy Prothrombin. J Hepatocell Carcinoma. 2024;11:1979-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Deng X, Tan J, Liu H, Zhang H, Li C, Li Q, Zhou J, Xiao Z, Li J. Idarubicin-loaded degradable hydrogel for TACE therapy enhances anti-tumor immunity in hepatocellular carcinoma. Mater Today Bio. 2024;29:101343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | De La Pinta Alonso C. Radiation-induced liver disease in the era of SBRT: a review. Expert Rev Gastroenterol Hepatol. 2020;14:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Gerum S, Heinz C, Belka C, Walter F, Paprottka P, De Toni EN, Roeder F. Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol. 2018;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Robin TP, Raben D, Schefter TE. A Contemporary Update on the Role of Stereotactic Body Radiation Therapy (SBRT) for Liver Metastases in the Evolving Landscape of Oligometastatic Disease Management. Semin Radiat Oncol. 2018;28:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/