Published online Nov 27, 2024. doi: 10.4240/wjgs.v16.i11.3590
Revised: September 10, 2024
Accepted: September 12, 2024
Published online: November 27, 2024
Processing time: 105 Days and 9.9 Hours
Non-ulcerative necrosis of the stomach and duodenum is rare because of the abundant blood supply in the gastrointestinal tract. Duodenal necrosis is a rare complication of severe acute pancreatitis. Emergency pancreaticoduodenectomy (EPD) is a rare procedure, with extensive duodenal necrosis being one of its indications.
We here report the case of a 57-year-old man who survived EPD for pancreatitis, which resulted in the necrosis of the distal stomach, full-length duodenum, and part of the jejunum.
Despite significant surgical risks, an EPD could be a life-saving procedure in severe cases of pancreatitis.
Core Tip: This case report details a rare instance of successful emergency pancreaticoduodenectomy (EPD) for a patient with severe acute pancreatitis (SAP) which led to extensive necrosis of the distal stomach, full-length duodenum, and part of the jejunum. This is the most extensive case of necrosis reported to date. Despite the high risk of complications, EPD proved to be a life-saving intervention, underscoring its critical role in treating severe gastrointestinal necrosis associated with SAP. Our case study shows that it is crucial to recognize this rare complication in time.
- Citation: Tong KN, Zhang WT, Liu K, Xu R, Guo W. Emergency pancreaticoduodenectomy for pancreatitis-associated necrotic perforation of the distal stomach and full-length duodenum: A case report. World J Gastrointest Surg 2024; 16(11): 3590-3597
- URL: https://www.wjgnet.com/1948-9366/full/v16/i11/3590.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i11.3590
Non-ulcerative necrotic perforation of both the stomach and duodenum is rare because these two organs are characterized by a rich blood supply. Some possible causes of non-ulcerative necrosis of the duodenum include trauma, severe acute pancreatitis (SAP), intestinal obstruction, and ingestion of corrosive toxins[1-4]. SAP can further be complicated by duodenal necrosis, which is extremely rare, with only a few cases reported worldwide. Additionally, most of these cases are limited to the necrosis of the second and third segments of the duodenum[5-8]. SAP complicated by duodenal necrosis has a very poor prognosis, with a mortality rate as high as 40%, requiring emergency surgical intervention[1]. Emergency pancreaticoduodenectomy (EPD) is a rare procedure with both traumatic and non-traumatic indications. Compared to traumatic indications, EPD for non-traumatic diseases is rarer (0.3%-3%)[9]. Herein, we report a case report involving a patient at our center who underwent EPD for SAP resulting in the necrosis of the distal stomach, the full-length of the duodenum, and the proximal part of the jejunum.
A 57-year-old man presented to us with pain in the upper-middle abdomen after alcohol consumption on July 09, 2023. He started vomiting coffee-like gastric contents on July 16, 2023, and experienced neurological symptoms, including impaired consciousness.
The patient initially did not pay much attention to abdominal pain. He was first admitted to the local hospital for vomiting and impaired consciousness. Abdominal computed tomography (CT) suggested pancreatic edema, a small amount of exudate, and abdominopelvic effusion, while cranial CT suggested cerebral infarction on the left side of the circumferential pool. Laboratory examinations suggested a white blood cell count (WBC) count of > 20 × 109 cells/L, blood amylase > 900 U/L, and blood lipase > 600 U/L, as well as metabolic acidosis with elevated creatinine. The local hospital diagnosed him with acute edematous pancreatitis and put him on a water fast. In addition, they provided him with intravenous nutrition, cefoperazone/sulbactam to fight infection, somatostatin to inhibit pancreatic enzymes secretion, and omeprazole to inhibit gastric acid secretion. They continued this conservative treatment for seven days, following which the blood amylase and WBC contents of the patient recovered to normal values. However, the patient continued to vomit blood and have nausea. The patient was then referred to the emergency department of our hospital.
The patient has been suffering from hypertension for the last 5 years, with a maximum blood pressure of 150/90 mmHg. He takes nifedipine (30 mg) once a day regularly to control his blood pressure. The patient denied a history of coronary heart disease, cerebral infarction, hepatitis, tuberculosis and other infectious diseases, other trauma, surgery, or blood transfusion. He also denied a history of food and drug allergies.
The patient denied the history of unclean coitus. He has been smoking for the last more than 30 years, with an average consumption of 10 cigarettes/day. He also has a history of drinking, two bottles of beer/day, for the last more than 30 years. He denied a family history of any hereditary disease.
On admission, the patient was confused, but responding to calls. Bloody gastric fluid was seen in gastrointestinal decompression. He had a flat abdomen, did not show any bowel pattern or peristaltic wave, had weakened bowel sounds (2 beats/min) on auscultation, and did not have any vibratory watery sounds. Predominantly tympanic sounds were noted on percussion. In addition, the hepatic dullness boundary was still present. Abdominal softness was noted on palpation. The mid-upper abdomen was tender. No rebound pain or muscular tension was noted. No pathologic signs were present. No other specific positive physical signs were identified.
At the time of admission to the emergency department, the WBC of the patient was 13.89 × 109/L. His granulocyte rate was 83.5%. Total C-reactive protein was 89.15 mg/L. Total hemoglobin was 81 g/L and total albumin was 25.5 g/L. Blood amylase, calcitoninogen, cardiac enzyme profile, creatinine, and coagulation were all in the normal range.
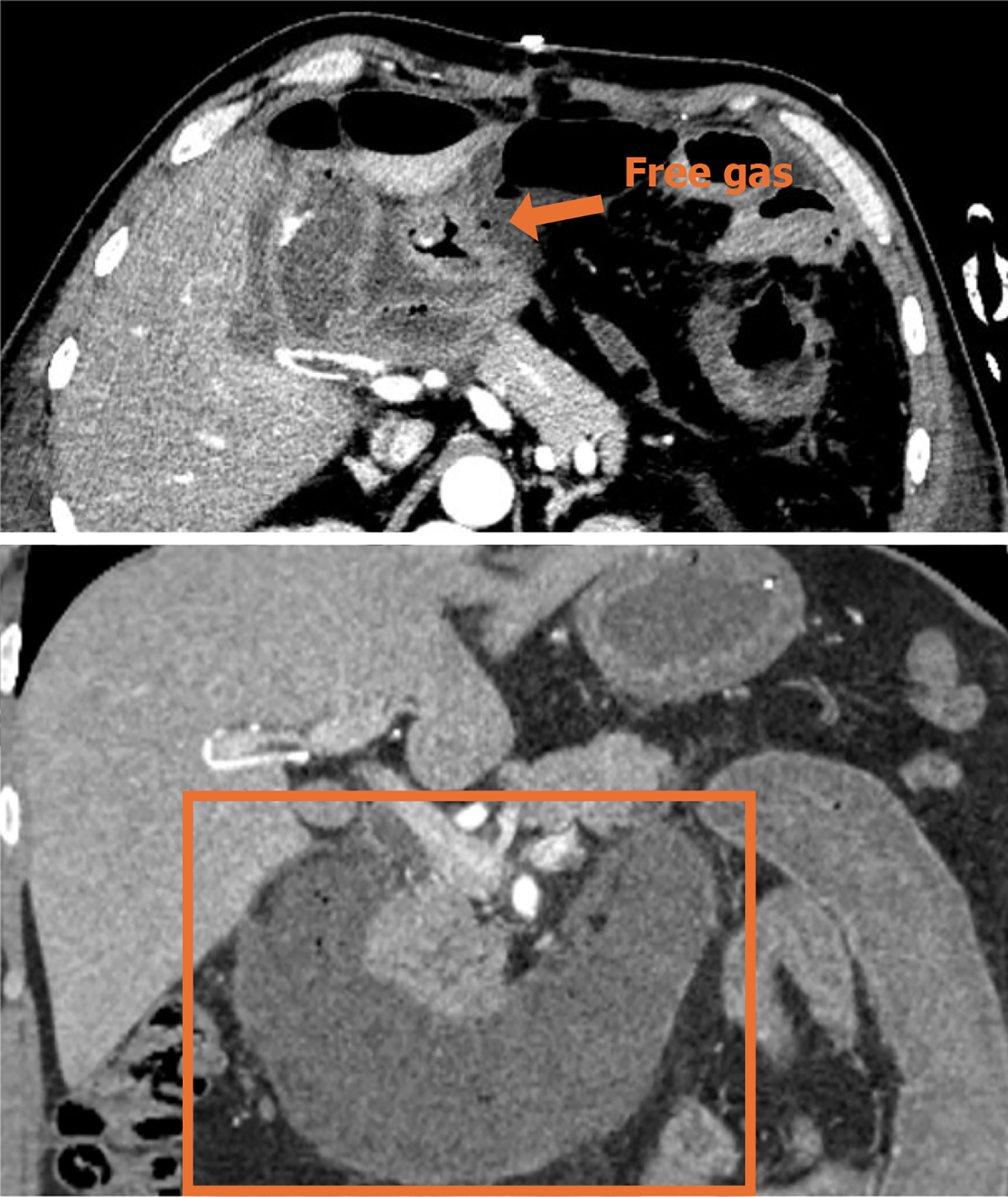
Contrast-enhanced CT (CECT) of the abdominopelvic cavity was performed after admission to the emergency room (Figure 1).
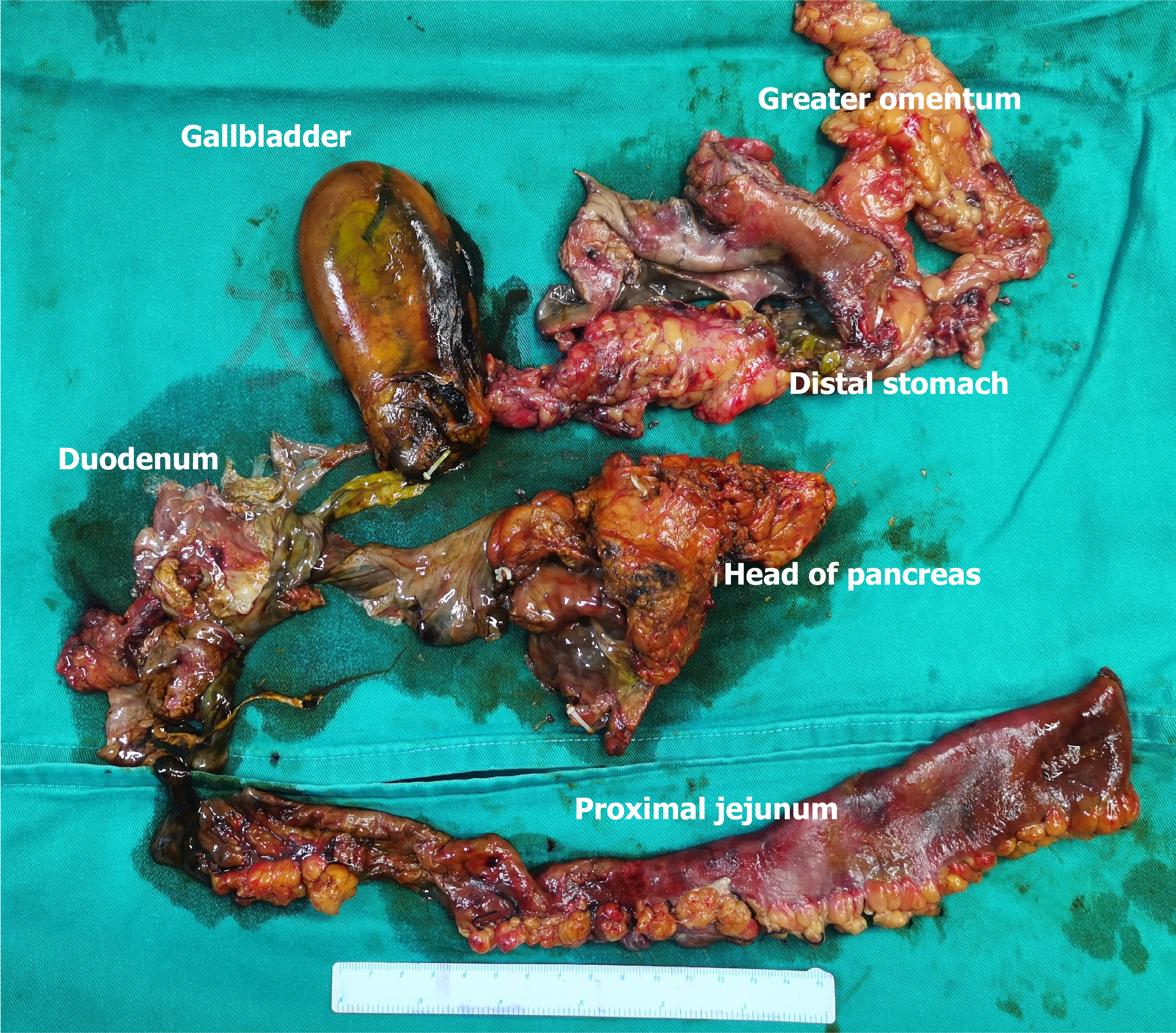
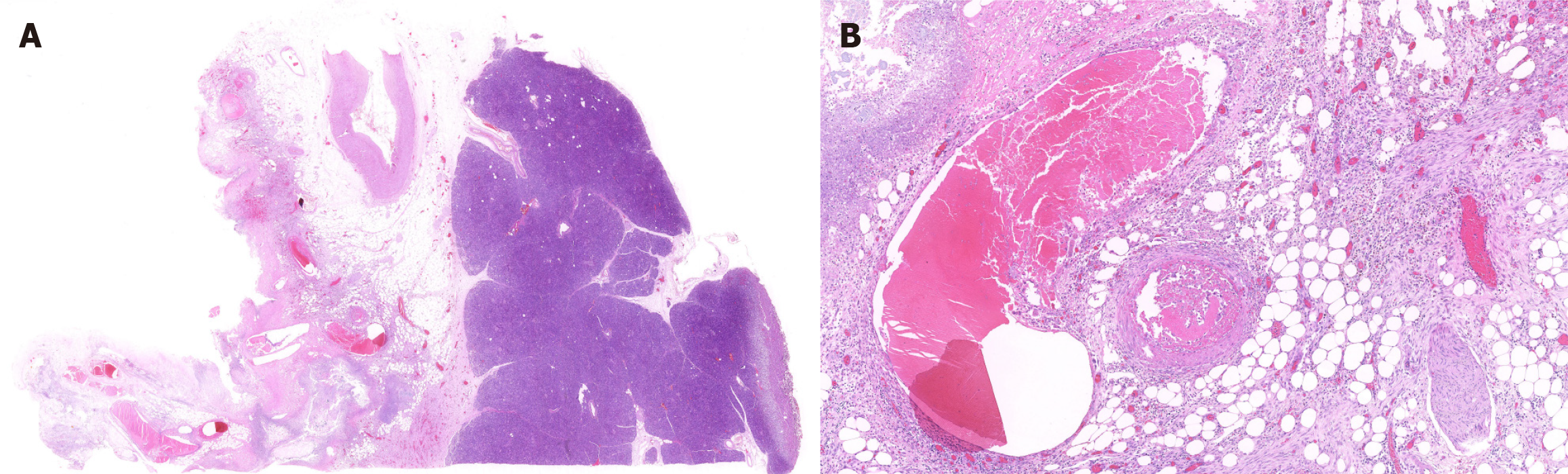
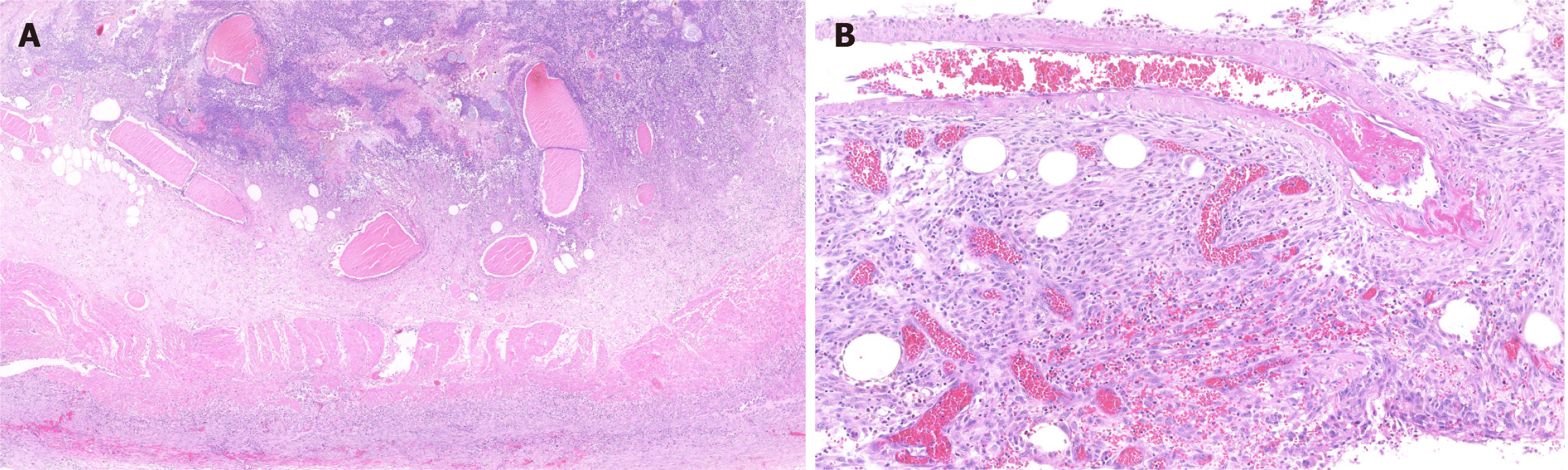
The gross specimen is shown in Figure 2. The pathologic section is shown in Figures 3 and 4. As the amount of the normal pathologic tissue was very small, the pathologist could not satisfactorily explain the extensive necrosis.
Early stage of diagnosis and treatment, the patient was diagnosed with SAP, gastrointestinal intestinal necrosis and perforation. After surgery, patients were successively diagnosed with sepsis, metabolic acidosis, bile leakage, pancreatic fistula, gastrointestinal bleeding, cholangitis, esophageal stricture, moderate anemia, and hypoalbuminemia, etc.
As no obvious symptoms of peritonitis were observed, conservative treatments such as gastrointestinal decompression, acid suppression, enzyme suppression, and antibacterial therapy were first performed. However, instead of improving, the patient’s condition worsened. Therefore, an emergency laparoscopic exploration was performed after 3 days of conservative treatment.
Laparoscopic exploration revealed an ischemic necrosis of the distal stomach. A 3-cm break, covered with pus, in the anterior wall of the greater curvature of the antrum was also seen. The posterior wall of the stomach, where it entered the lesser omental bursa, was found to be necrotic. Intraoperative gastroscopy showed necrosis of the mucosa of the distal gastric lumen. However, the amount of the gastric fluid was too large to be passed through the scope. The gastroscopic image could not be retained. We performed a Kocher maneuver and incised the lateral peritoneal attachments of the duodenum to expose the pancreatic head and duodenum posterior. The necrosis of the duodenum ischemia could be seen along the Kocher incision. The intestinal wall structure had disappeared. The proximal jejunum of the ischemia was approximately 30 cm long. Infected necrotic foci and saponification were noted in the pancreatic head. Retroperitoneal adipose tissue necrosis was also observed around the periphery of the pancreas.
Based on the intraoperative results, the patient’s prognosis was predicted to be extremely poor, with limited treatment measures. The use of a drainage procedure could increase the risk of postoperative sepsis should the drainage become obstructed. On the other hand, if we opt for an EPD, it may lead to a huge surgical trauma and may also cause serious postoperative complications, endangering the life of the patient. However, after consulting with his family, we did decide to perform EPD on the patient.
If biliary-enteric and pancreatico-enteric anastomoses are performed in a contaminated surgical area, the quality of the anastomosis becomes a concern. Therefore, we left biliary and pancreas fistulae intact on the skin in an attempt to decompress the anastomosis and reduce the probability of an anastomotic fistula. We also placed several drainage catheters near the biliary-enteric and pancreatico-enteric anastomoses, as well as subcutaneously, in the pelvis and splenic fossa. During the operation, the patient was hemodynamically unstable because of severe infection and bleeding, with a systolic blood pressure as low as 40 mmHg and a heart rate as low as 30 beats/min. Vital signs were restored after chest compressions and the administration of vasoactive drugs. The patient was reserved for tracheal intubation and sent to the intensive care unit (ICU) for advanced life support. The operation lasted nearly 10 hours, with 1000 mL of intraoperative bleeding.
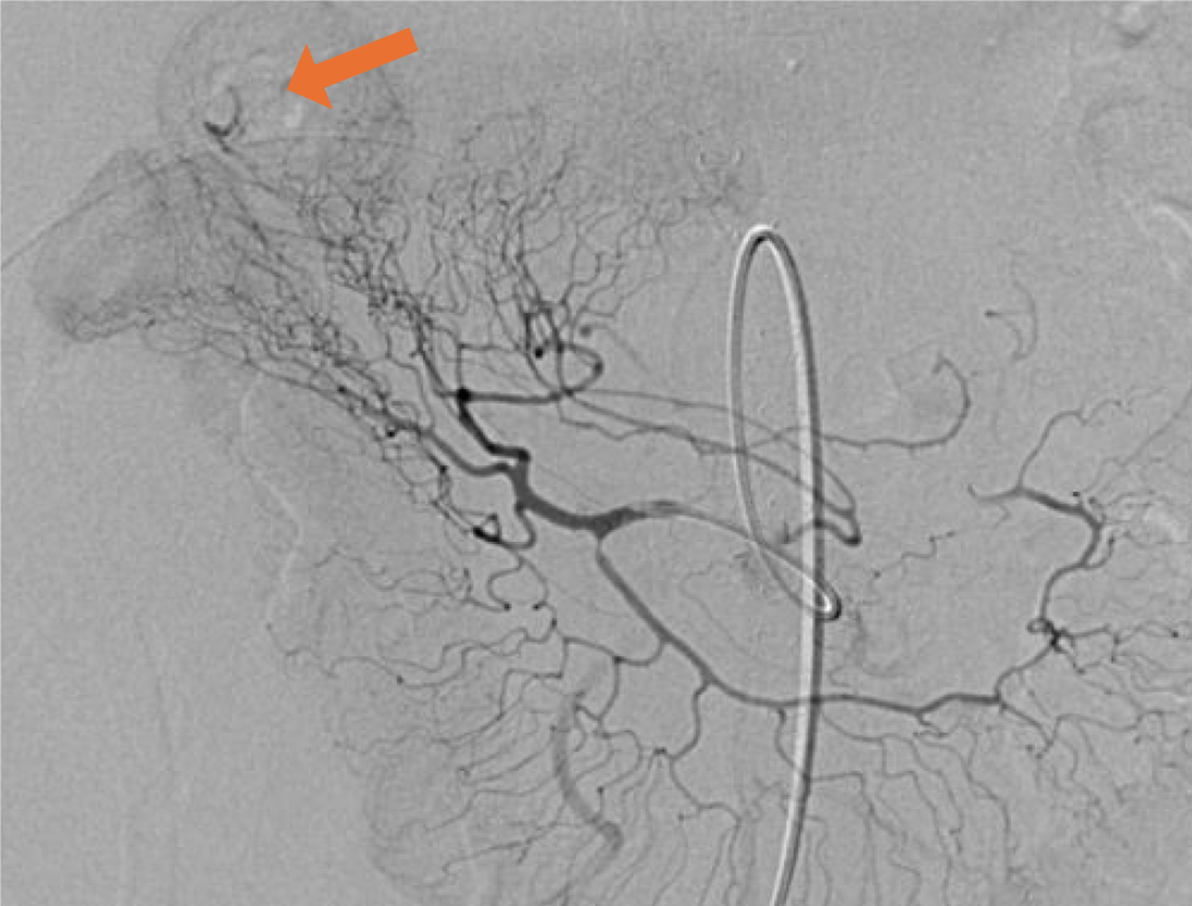
On postoperative day 7, the patient developed bile fistula, pancreatic fistula, and severe complicated infections (multidrug-resistant Acinetobacter baumannii, multidrug-resistant Pseudomonas aeruginosa, Enterobacter cloacae, fungus), gastrointestinal bleeding, incisional infection, acute kidney injury, acute myocardial injury, acute hepatic impairment, diffuse intravascular coagulation, acute respiratory distress syndrome, uncompensated metabolic alkalosis, hyperlactatemia, postoperative delirium, and other complications. He remained in the ICU for nearly 31 days, during which hemofiltration was performed on him. He was also provided with high-level antibiotics, nutritional support, and superselective intra-arterial embolizations twice during his stay in the ICU (Figure 5), after which he was successfully transferred to the general ward for further treatment.
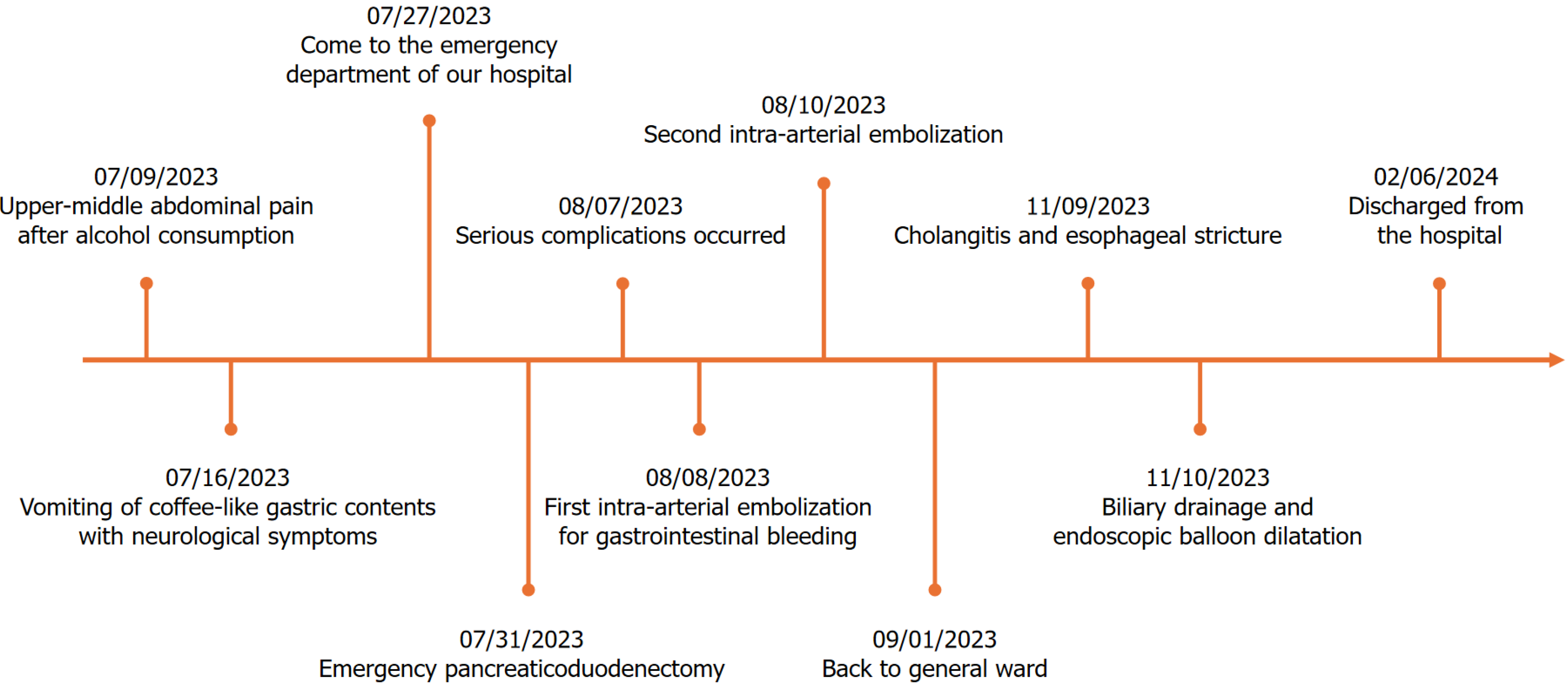
The patient was again subjected to an extended period of treatment after being transferred to the general ward. His anastomotic fistula was healed, and all drainage catheters were successfully removed. The infection at the incision site gradually healed after the drainage site was vacuum-sealed. In addition, the severe compound infection was successfully controlled. The patient gradually transitioned from the jejunal nutritional tube to oral feeding in the fourth month of surgery. However, the patient faced cholangitis and swallowing disorders. In addition, a CT examination showed an obstruction resulting from biliopancreatic collaterals. We proposed to clear this obstruction through the endoscopic placement of drainage catheters. However, the endoscopy showed a full-length segmental narrowing of the esophagus, which could not be passed through by the thinnest endoscopy, and the pathology suggested inflammatory hyperplasia. Consequently, ultrasound-guided percutaneous transhepatic biliary drainage was performed to provide relief from cholangitis and an endoscopic balloon dilatation was performed for esophageal stricture. Once the treatment was successfully completed, the patient was able to eat orally and was discharged from the hospital six months after the surgery. The patient was alive at the time of the submission of this case report (1 year postoperatively). However, he still has to wear a drainage catheter for a long period of time and also undergo regular endoscopic balloon dilatation of the esophagus. Time points corresponding to the diagnostic and therapeutic process is presented in Figure 6. The patient was both fearful and grateful for the entire process, and was satisfied with the quality of medical care and nursing support provided to him.
Gastrointestinal perforation is one of the serious complications of SAP. According to a recent prospective study, gastrointestinal perforation occurs in approximately 16% cases of infected pancreatic necrosis[10]. However, most of the perforations are limited to a specific area only. To the best of our knowledge, this is the largest extent of gastrointestinal necrosis associated with hemorrhagic necrotizing pancreatitis reported to date. The patient was successfully treated with EPD. It is believed that gastrointestinal necrosis occurs in response to inflammation, bacterial infection, and pancreatic enzyme corrosion[10,11]. On the one hand, pancreatitis can directly damage the gastrointestinal tract, while on the other hand, it can promote thrombosis or induce a hypercoagulable state that reduces capillary perfusion, ultimately causing gastrointestinal necrosis[7].
In our case, ischemic necrosis occurred over an extremely extensive area comprising the distal stomach, the full-length duodenum, the proximal 30 cm of jejunum, as well as the retroperitoneal space. Most of the reported gastrointestinal perforations were performed on the second and third segments of the duodenum, in direct contact with the necrotic foci of pancreatitis. The stomach and duodenum possess an extremely rich blood supply. Therefore, it is difficult, but not impossible, to completely infarct the gastric wall. Yu et al[11] reported a case of gastric wall infarction and splenic infarction resulting from SAP. The postoperative pathologic section of the proximal jejunum showed numerous thrombi in the intestinal wall vessels, confirming our hypothesis that it might be a storm of inflammatory factors, resulting from hemorrhagic necrotizing pancreatitis, which ultimately led to disseminated intravascular coagulation.
Our case also differed from the previously reported cases of SAP in that it followed a subacute course. Blood amylase normalized 2 weeks after the onset of pancreatitis. The CECT scan did not suggest a clear necrotic focus. In our patient, the necrosis followed a delayed course, which may have turned the infectious necrosis of the pancreas into a walled-off pancreatic necrosis (WOPN). The WOPN is usually seen more than 4 weeks after the onset of acute pancreatitis. It is usually asymptomatic and can be cured by conservative treatment. However, many patients with WOPN present with pathologic changes in the foci of pancreatic necrosis, such as abdominal pain, biliary compression, gastrointestinal fistulae, thrombosis, and even a vascular event[12]. In our case, we noted a limited necrotic focus with saponification in the head of the pancreas. In addition, we noted that the pancreas of our patient had become hard, brittle, and friable when palpated intraoperatively, which made us suspect that the saponified tissue might have directly compressed the intestinal tract or indirectly formed an embolus to occlude the blood vessels, leading to gastrointestinal necrosis.
We were not surprised by the short-term complications of gastrointestinal necrosis. However, the cause of the esophageal stenosis and biliopancreatic collateral obstruction needs to be further discussed. Since the patient underwent two postoperative superselective arterial embolizations for biliopancreatic collateral bleeding, we suggest that intestinal obstruction may be associated with ischemic enteropathy. We considered several possibilities to explain the etiology of esophageal stenosis, including reflux esophagitis associated with the prolonged application of a jejunal nutritional tube, autoimmune disease, or accidental ingestion of corrosive toxins. In general, strictures resulting from reflux esophagitis tend to be bottom-up, not multistage and scattered. We also considered whether the patient had autoimmune vasculitis that led to extensive intestinal necrosis and esophageal stenosis. However, our test results showed that the patient had normal autoimmune antibodies. Additionally, we confirmed with the patient that he did not ingest any corrosive substances in the early stages of the disease. We also noted that the others who ate with him were asymptomatic. Therefore, his condition most likely resulted from gastroesophageal reflux.
Ischemic necrosis of the gastrointestinal tract is one of the rare surgical indications for SAP. Acute pancreatitis is generally managed through drainage[13]. However, we believe that EPD is more appropriate for extensive gas
Gastrointestinal necrotic perforation, a rare complication of SAP, has a poor prognosis because of its anatomical location in the retroperitoneum. Gastrointestinal necrotic perforation may have an insidious onset in some cases. If imaging suggests that pancreatitis is combined with a poor blood flow in the digestive tract, we should be alert to the occurrence of this complication and strive to drain or surgically treat the necrosis when it is limited. In addition, our case report showed that although EPD has a poor prognosis, it is a life-saving procedure in severe cases of necrotic perforation.
| 1. | Karaisli S, Er A, Örsel A, Kamer E. An extremely rare cause of acute abdomen. Turk J Gastroenterol. 2017;28:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Muñoz Muñoz E, García-Domingo MI, Rodríguez Santiago J, Veloso Veloso E, Marco Molina C. Massive necrosis of the gastrointestinal tract after ingestion of hydrochloric acid. Eur J Surg. 2001;167:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Jabłoński S, Kustalik S, Klejszmit P, Misiak P. Total duodenal necrosis with retroperitoneal perforation in an adolescent with jejunal intussusceptions. J Dig Dis. 2016;17:483-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Champault A, Roudié J, Smadja C. Traumatic duodenal necrosis with peri-ampullary duodenal detachment. J Pediatr Surg. 2004;39:1136-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Takeyama Y, Ueda T, Hori Y, Shinkai M, Ajiki T, Kuroda Y. Duodenal necrosis associated with acute pancreatitis. Pancreas. 2001;22:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Koichopolos J, Keow J, Parfitt J, Yoshy C, Wiseman D, Leslie K. Complete duodenal necrosis associated with non-traumatic duodenal hematoma requiring emergent pancreatico-duodenectomy. Int J Surg Case Rep. 2020;66:53-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Hudgi AR, Coles MJ, Perry I, Vega KJ. Spontaneous duodenal fistulisation from walled-off pancreatic necrosis. BMJ Case Rep. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Sakorafas GH, Tsiotos GG, Sarr MG. Experience with duodenal necrosis. A rare complication of acute necrotizing pancreatitis. Int J Pancreatol. 1999;25:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Popa C, Schlanger D, Chirică M, Zaharie F, Al Hajjar N. Emergency pancreaticoduodenectomy for non-traumatic indications-a systematic review. Langenbecks Arch Surg. 2022;407:3169-3192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Timmerhuis HC, van Dijk SM, Hollemans RA, Umans DS, Sperna Weiland CJ, Besselink MG, Bouwense SAW, Bruno MJ, van Duijvendijk P, van Eijck CHJ, Issa Y, Mieog JSD, Molenaar IQ, Stommel MWJ, Bollen TL, Voermans RP, Verdonk RC, van Santvoort HC; Dutch Pancreatitis Study Group. Perforation and Fistula of the Gastrointestinal Tract in Patients With Necrotizing Pancreatitis: A Nationwide Prospective Cohort. Ann Surg. 2023;278:e284-e292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Yu HM, Myung E, Kim SH, Myung DS, Cho SB, Lee WS, Hur YH, Kim JW, Lee KH, Joo YE. Gastric and splenic infarctions in acute pancreatitis. J Dig Dis. 2016;17:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Rana SS. An overview of walled-off pancreatic necrosis for clinicians. Expert Rev Gastroenterol Hepatol. 2019;13:331-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1095] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/