Published online Nov 27, 2024. doi: 10.4240/wjgs.v16.i11.3538
Revised: August 29, 2024
Accepted: September 10, 2024
Published online: November 27, 2024
Processing time: 132 Days and 21 Hours
The treatment of benign biliary strictures (BBS) is a challenging clinical problem. At present, there is a lack of ideal models for the study of BBS treatment.
To develop a novel animal model of BBS to simulate studies on the processes and mechanisms in the human condition.
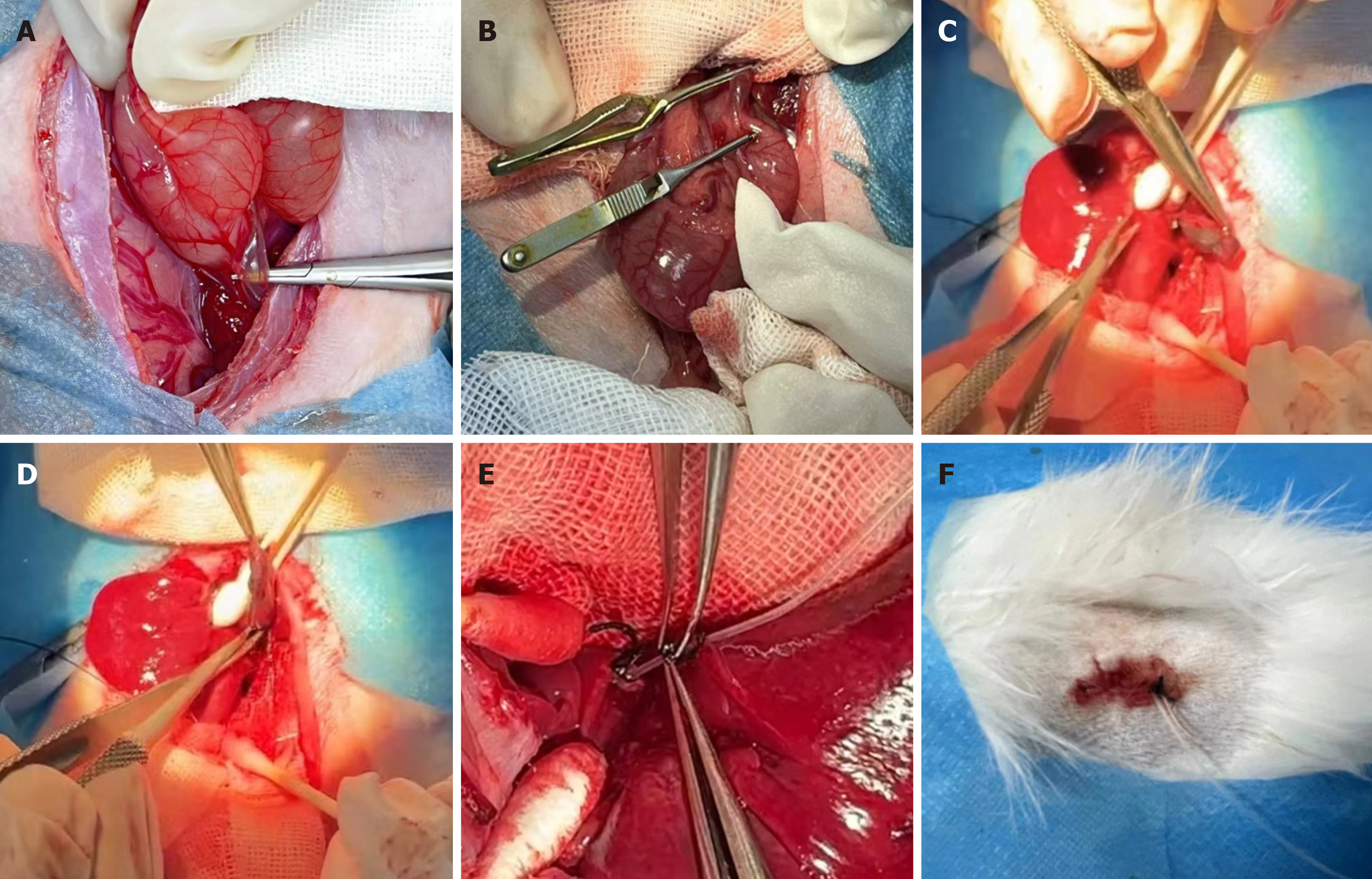
A rabbit model of benign bile duct stricture was established by surgical injury of the bile duct. After removal of the gallbladder, a drainage tube was placed th
Compared with the control group, the model rabbits showed gross jaundice, increased serum bilirubin, and decreased liver function. Cholangiography showed segmental bile duct stenosis in the model rabbits. Pathological staining showed inflammatory cell infiltration and fibrosis in the biliary tract of rabbits in the model group. This was consistent with the clinical manifestations of BBS. This model provided serology, imaging, pathology, and other aspects of BBS.
We have successfully established an animal model of benign stricture of the lower bile duct with repeatable administration, which is consistent with the clinical manifestations of BBS.
Core Tip: A model of benign biliary stricture (BBS) was established and certified by biliary drainage angiography, pathology, and other evaluations. This model could allow better long-term low-dose repeated action of drugs on biliary scars, and provide new ideas for the treatment of BBS. When the efficacy of this model is confirmed, new biological agents can be used to treat BBS in humans through endoscopic retrograde cholangiopancreatography in the future.
- Citation: Sun QY, Cheng YM, Sun YH, Huang J. New rabbit model for benign biliary stricture formation with repeatable administration. World J Gastrointest Surg 2024; 16(11): 3538-3545
- URL: https://www.wjgnet.com/1948-9366/full/v16/i11/3538.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i11.3538
Benign biliary stricture (BBS) is a common clinical problem. Due to the anatomical and physiological characteristics of the biliary tract, bile duct injury and recurrent cholangitis often lead to biliary cicatricial constriction. BBS can cause recurrent biliary tract infection, biliary cirrhosis, and even liver failure[1].
Current treatment techniques only achieve temporary recanalization of the BBS, and the recurrence rate of stenosis is still as high as 40%[2,3]. The lack of an ideal biliary model for research purposes has led to basic and wound healing studies of the biliary tract significantly lagging behind similar studies of the skin and gastrointestinal tract. Currently, there is an urgent need to establish more suitable animal models to evaluate new treatments for BBS.
It is reported that due to small bile ducts in small animals such as mice, it is difficult to establish a model in such animals, since it cannot be observed clearly and has certain clinical limitations[4,5]. Although large animals such as dogs and pigs are convenient for clinical observation, they are expensive, and the above models cannot achieve continuous drug administration[1,6].
In the present study, an animal model of BBS was established by surgically removing the gallbladder and creating a stricture of the lower common bile duct, which could be continuously administered locally, in order to facilitate clinical research.
Ten male New Zealand rabbits (clean grade), weighing 2.50 (± 0.25) kg, were purchased from Danyang Changyi Experimental Animal Breeding Co., Ltd., with the animal use license number: SCXK (Su) 2021-0002. The animals were raised in the incubation area of West Taihu Lake Medical Industry in a standard single cage, with a 12-12 h light-dark cycle, temperature of 17-23 °C, and air relative humidity of 30%-70% and provided with laboratory standard rabbit diet and free access to drinking water. This study was approved by the Ethics Committee of Changzhou University.
The ten New Zealand rabbits were randomly divided into two groups, 5 in healthy control group (group A) and 5 in model group (group B). No surgical procedures were performed in the healthy control group. After 1 wk of adaptive feeding, the rabbits in the model group were fasted for 12 h before surgery, with free access to drinking water. The rabbits were anesthetized with an intramuscular injection of xylazine hydrochloride, and tiletamine hydrochloride and zo
The rabbits were sacrificed after 14 d. Two blood samples, each approximately 2 mL, were taken from the inferior vena cava of each rabbit. The blood samples were immediately centrifuged, and total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and γ-glutamyl transferase (GGT) were measured.
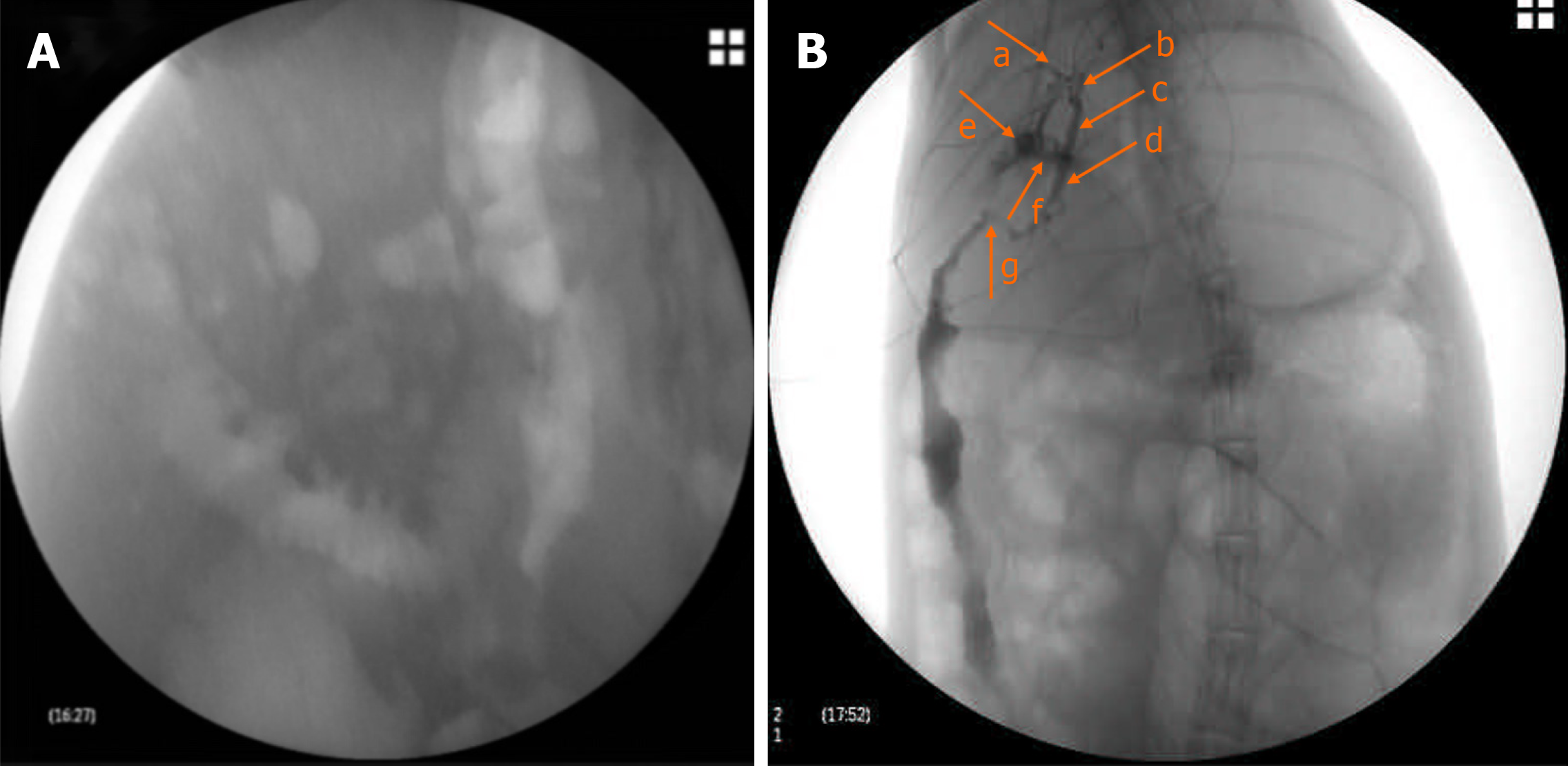
On the fourteenth day after surgery, contrast agent was injected slowly through the drainage tube, and the position of the drainage tube and the biliary stricture were evaluated by X-ray imaging.
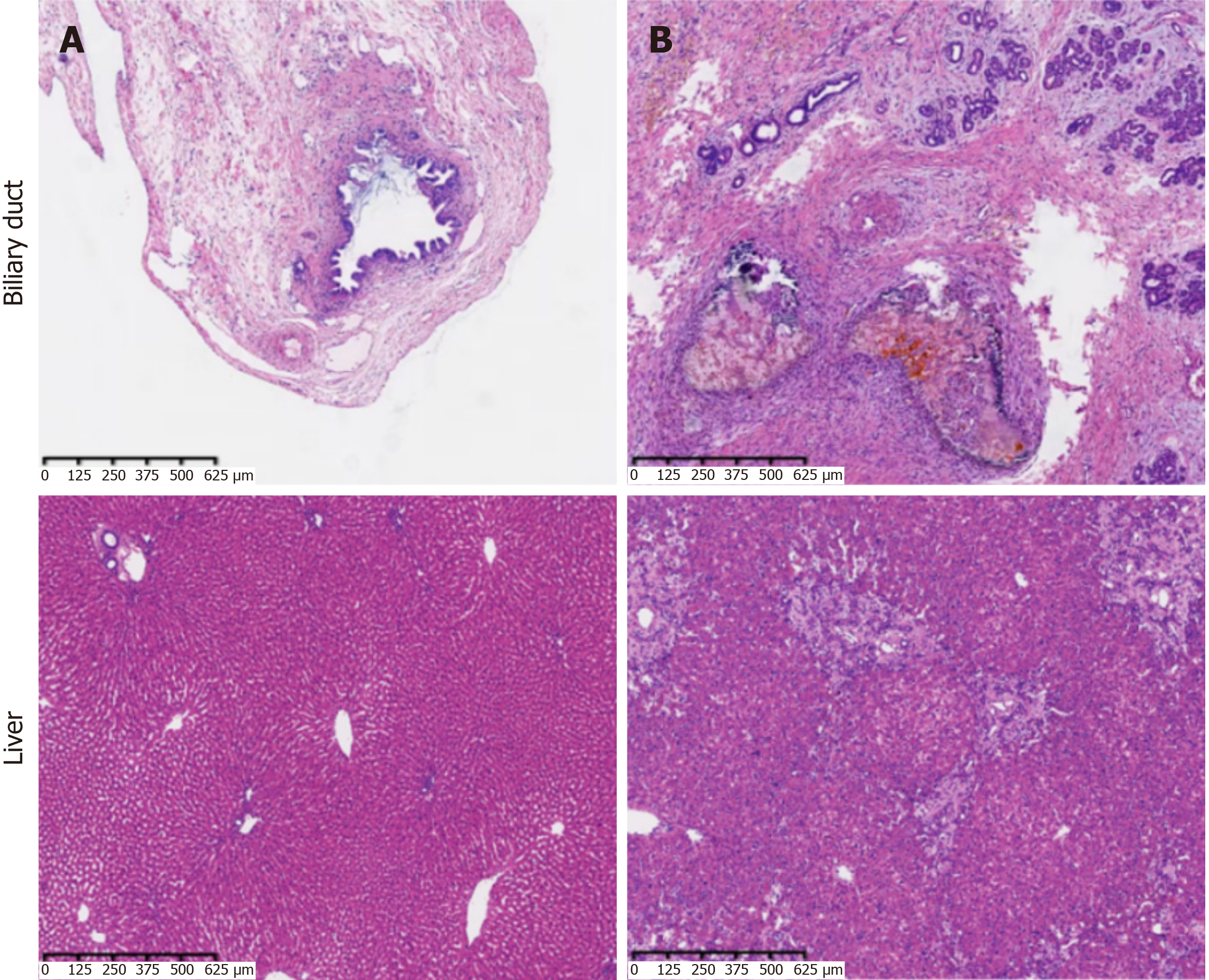
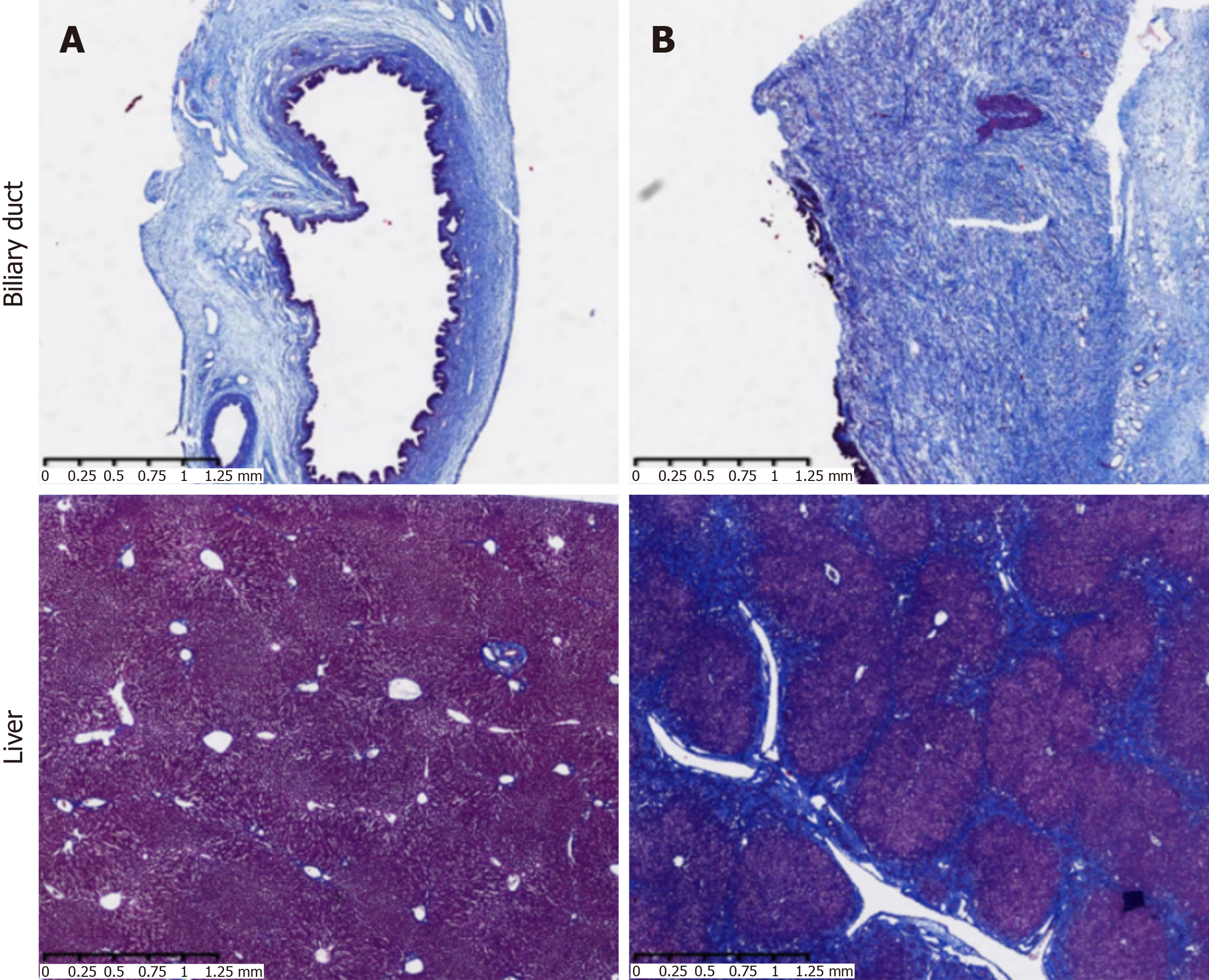
Liver tissues were stained with hematoxylin and eosin (HE) to observe the presence of inflammatory cell infiltration, tissue damage, and scar hyperplasia. Liver tissues were collected for Masson staining to observe tissue structure and the degree of fibrosis.
Bile duct tissues from the rabbits in each group were fixed in 10% formalin fixative solution for 1 d. After fixation, the specimens were embedded in paraffin and serially sectioned with a microtome at a thickness of 5 μm. After deparaffinization, HE staining and Masson staining were performed. The resin was air-dried, and the pathological changes in rabbits' bile ducts were observed under a light microscope. Collagen deposition and fibrosis were used as indicators for assessment.
All data were processed and analyzed using SPSS 19.0 statistical software. The t-test was used for independent sample analysis, and P values < 0.05 were considered statistically significant. GraphPad Prism 9 software was used for mapping, and P < 0.05 was considered statistically significant.
Compared with the healthy control group, the model group showed significantly reduced activity, listlessness, irri
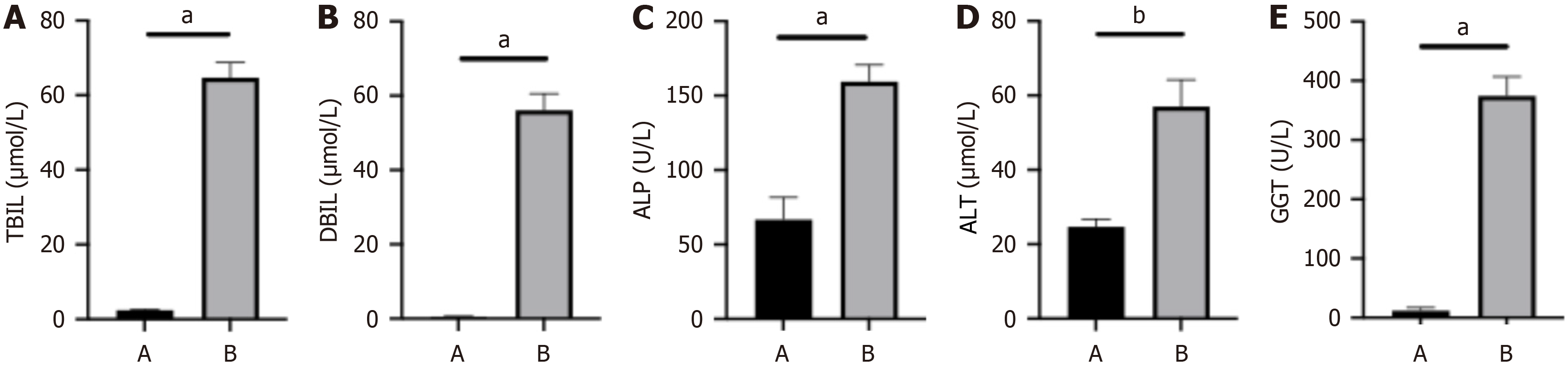
The results of liver function tests showed that the levels of TBIL, DBIL, ALT, ALP, and GGT in the model group were significantly higher than those in the control group (P < 0.0001, P < 0.0001, P < 0.0001, P < 0.005, and P < 0.0001, res
On the fourteenth day after surgery, contrast agent was injected slowly through the drainage tube. X-ray imaging showed that the drainage tube was in place, the upper bile duct was obviously dilated, and the contrast agent was blocked due to the bile duct stricture and slowly entered the duodenum (Figure 3).
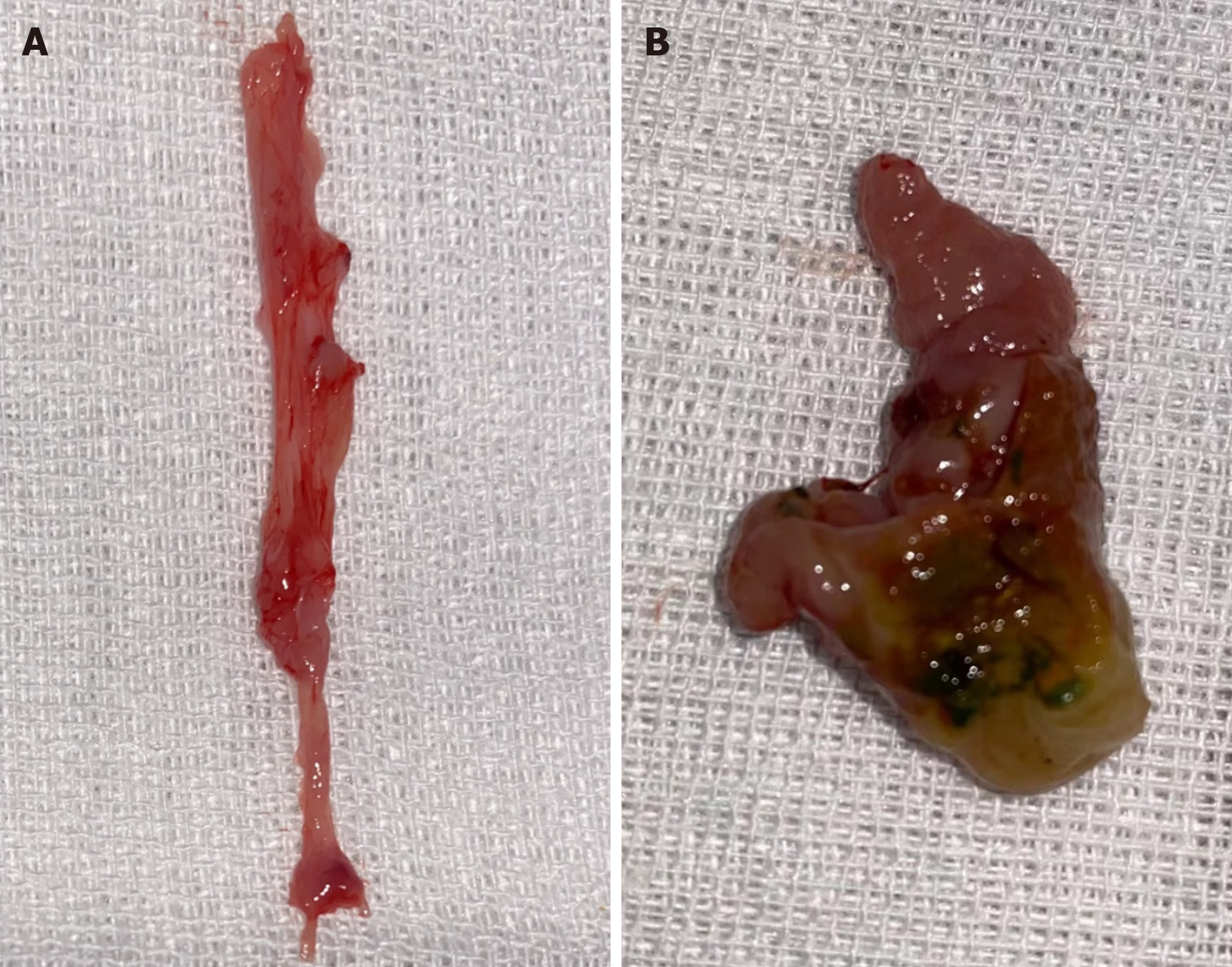
In the model group, bile duct stenosis formed after surgery, and scar hyperplasia at the lesion adhered to the surrounding connective tissue. Black and yellow-green sand-like stones could be seen in the bile duct lumen. Compared with the model group, there were no abnormal changes in the control group (Figure 4).
Bile duct in group A: Bile duct epithelial cells and surrounding cells were arranged neatly, without matrix hyperplasia, inflammatory cell infiltration, or gland expansion.
Bile duct in group B: Bile duct wall thickening, scar hyperplasia, shedding of a large number of bile duct epithelial cells and bleeding, acute inflammation (neutrophil infiltration), and chronic inflammation (massive lymphocyte infiltration and histiocyte phagocytosis) in each layer of tissue were observed, and the injury was serious.
Liver in group A: Normal hepatic lobules and hepatic cords were seen, which were arranged radially around the central vein and were clearly divided. No glandular dilatation was observed.
Liver in group B: The liver cells were disordered, deformed, and necrotic, with intrahepatic cholestasis. A large number of collagen fibers were wrapped in the portal area, and the fibrous septum was obvious. Intrahepatic bile ducts were obviously dilated, significant cholestasis was seen around the area, and a large number of inflammatory cells were observed. The proliferation of small bile ducts was greater, and a small amount of bile duct atresia was observed (Figure 5).
Bile duct in group A: There was no bile duct wall fibrosis.
Bile duct in group B: There was a large amount of collagen deposition in the submucosa, and the bile duct wall was significantly thickened, showing fibrosis.
Liver in group A: No liver fibrosis.
Liver in group B: The fibrous septum was severe, the central vein was absent, and the normal hepatic lobule structure was absent, which was replaced by pseudolobules. The intrahepatic bile duct wall was thickened. In combination with HE staining (hepatocyte necrosis, intrahepatic cholestasis, and extracellular bile plug), the features of secondary cho
Benign bile duct strictures are most often caused by iatrogenic injury, which is more common in direct or indirect bile duct injury during surgery[7-9]. With regard to the treatment of biliary stricture, the lack of an ideal animal biliary model for drug therapy may result in basic and wound healing studies of the biliary tract significantly lagging behind similar studies of the skin and gastrointestinal tract. Biliary strictures require long-term treatment and are difficult to cure; thus, models allowing repeated and continuous administrations are needed.
At present, there are different opinions on the best treatment for BBS both nationally and internationally. Currently, the main treatment methods are endoscopic intervention, surgery, and drug therapy. Surgical treatment requires dilatation of the bile duct and placement of stents, which not only causes secondary injury to the bile duct, but also has a poor postoperative effect and high recurrence rate of stricture[10]. Drug therapy can only be administered systemically at present, with low effective drug concentrations in the bile duct and many side effects. Therefore, new BBS models are needed to facilitate observational studies of long-term topical administration.
In the previous modeling of BBS, Oruç et al[4] and Ker and Wu[5] induced BBS in rats by common bile duct ligation, but the rats had no gallbladder, and their model had certain limitations. Infective peritonitis was prone to occur and the mortality rate was high, which is not conducive to treatment research on BBS. Geng et al[11] performed bile duct incision and suture in dogs, which can be used for the study of bile duct stenosis, but this model cannot be used for the study of drug efficacy. Some studies established porcine models following injury of the porcine bile duct. However, this model involves high equipment demand.
In the present study, we established an animal model by using a vascular clamp at the common bile duct under the microscope to block the lower segment of the bile duct combined with cholecystectomy and inserting a catheter at the stump of the cystic duct. This modeling approach is not only the closest to the cause of BBS production, but also similar to the BBS process[12]. Previous studies have shown that the bile ducts of rats and mice are too thin to perform cholangiography. In addition, conventional non-invasive imaging methods, such as X-ray, computed tomography, and magnetic resonance imaging, cannot clearly display the location of the bile duct, the site of obstruction, the extent of obstruction, and the degree of obstruction, which limits the diagnosis. As rabbits are of moderate size, we used rabbits as models and endoscopic retrograde cholangiopancreatography (ERCP) cholangiography. Not only was the bile duct clearly seen, but this method has the advantages of being effective, non-invasive, convenient, and more ethical. Therefore, our rabbit model of BBS can easily simulate the human condition and can be studied by imaging, pathology, surgery, and other methods.
In previous studies on the drug treatment of BBS models, gavage or intravenous administration was mostly used. The limited opportunity to cross the blood-bile barrier into the site of biliary stricture, poor bioavailability, and the flowing bile environment cause difficulty in local absorption of the drug. Therefore, the efficacy is poor, and slow and repeated administrations are required. This also contributes to the limited effectiveness of conventional medical therapy, which may be addressed by injection of drugs through a biliary drainage tube. We established a new type of BBS drainage tube model in rabbits by means of surgery. The duration of biliary stricture was long, and the model could not only be used for local administration, but also for continuous administration. The timing, mode, and frequency of administration can be modulated by the presence of tubes. This allows better long-term low-dose repeated action of drugs on the biliary scar, and provides new ideas for the treatment of biliary stricture. When the efficacy of this technique is confirmed, new biological agents can be used to treat BBS in humans through ERCP in the future.
In conclusion, we have successfully established a modified rabbit model of BBS. This model can simulate the occurrence and development of the disease, and can be used to evaluate BBS lesions by serology, cholangiography, and pathological examination. The model included an external drainage tube that allowed continuous infusion of drugs for the treatment of biliary strictures, and can be applied in clinical decision-making for new treatment options.
| 1. | Park JS, Jeong S, Kim JM, Park SS, Lee DH. Development of a Swine Benign Biliary Stricture Model Using Endoscopic Biliary Radiofrequency Ablation. J Korean Med Sci. 2016;31:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Parsi MA. Common controversies in management of biliary strictures. World J Gastroenterol. 2017;23:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Altman A, Zangan SM. Benign Biliary Strictures. Semin Intervent Radiol. 2016;33:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Oruç MT, Ozmen MM, Han U. A new technique for inducing and releasing obstructive jaundice in rats. Eur Surg Res. 2009;43:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ker CG, Wu SC. A simple animal model for inducing and releasing surgical jaundice in rats. Gaoxiong Yi Xue Ke Xue Za Zhi. 1992;8:520-524. [PubMed] |
| 6. | Wang Y, Liang Y, Wang W, Jin R, Cai X. Management of electrothermal injury of common bile duct with a degradable biliary stent: an experimental study in a porcine model. J Gastrointest Surg. 2013;17:1760-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Keane MG, Devlin J, Harrison P, Masadeh M, Arain MA, Joshi D. Diagnosis and management of benign biliary strictures post liver transplantation in adults. Transplant Rev (Orlando). 2021;35:100593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (37)] |
| 8. | Im BS, Gwon DI, Chu HH, Kim JH, Ko GY, Yoon HK. Percutaneous Transhepatic Treatment of Benign Bile Duct Strictures Using Retrievable Covered Stents: Long-Term Outcomes in 148 Patients. Korean J Radiol. 2022;23:889-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Eminler AT, Koksal AS, Toka B, Karacaer C, Uslan Mİ, Parlak E. Benign biliary strictures associated with acute biliary pancreatitis. Surg Endosc. 2023;37:2587-2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Liu SH, Chen XF, Xie ZB, Zhou J. EGFR monoclonal antibody panitumumab inhibits chronic proliferative cholangitis by downregulating EGFR. Int J Mol Med. 2019;44:79-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Geng ZM, Yao YM, Liu QG, Niu XJ, Liu XG. Mechanism of benign biliary stricture: a morphological and immunohistochemical study. World J Gastroenterol. 2005;11:293-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Inui H, Kwon AH, Kamiyama Y. Managing bile duct injury during and after laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 1998;5:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/