©The Author(s) 2025.
World J Gastrointest Surg. Nov 27, 2025; 17(11): 112124
Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.112124
Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.112124
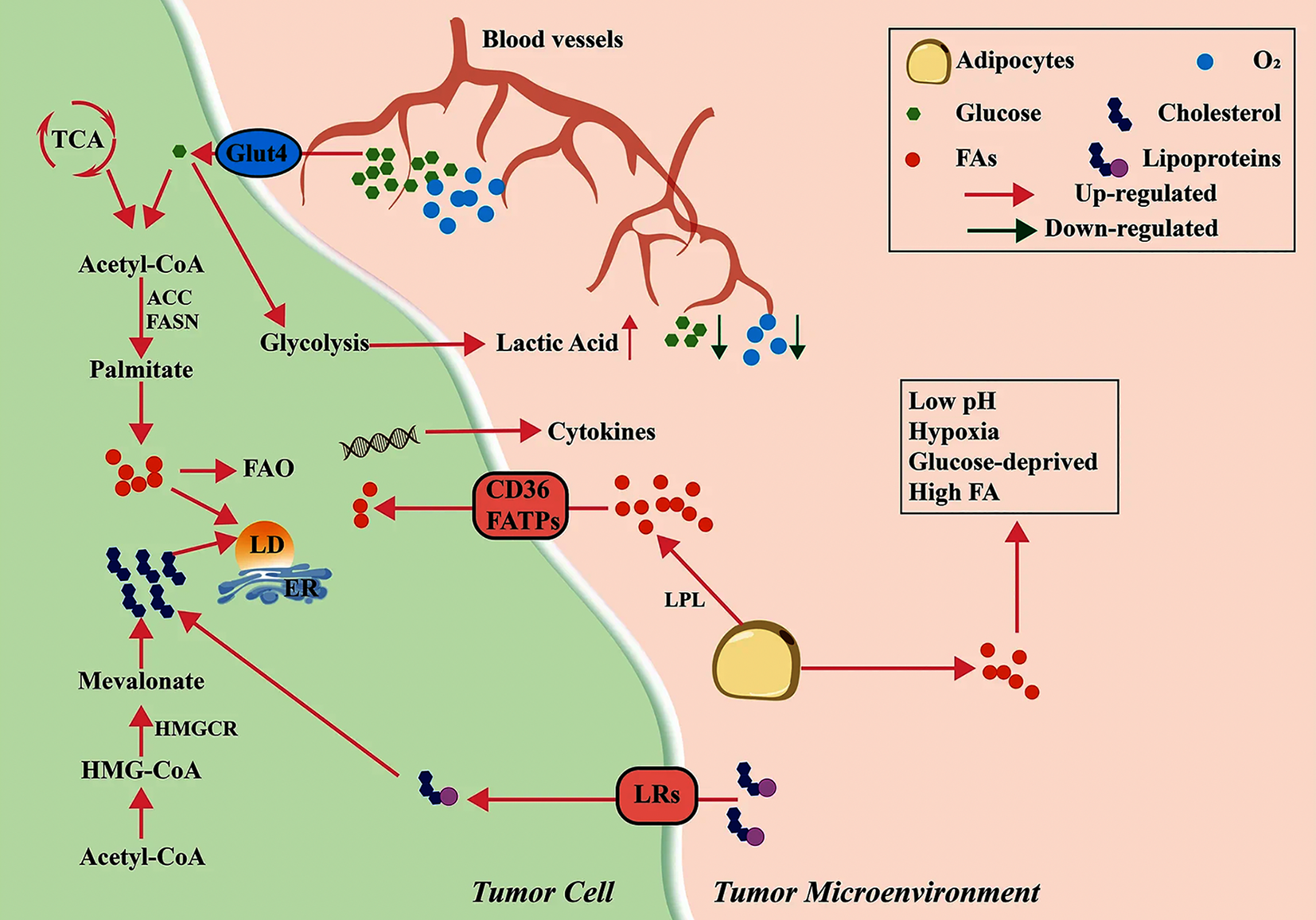
Figure 1 Metabolic reprogramming in the tumor microenvironment[3].
In the tumor microenvironment, disorganized blood vessels deliver glucose and oxygen, mostly taken up by tumor cells, leading to hypoxia and glucose deprivation. Tumor cells show activated glycolysis, producing more lactic acid but insufficient energy. Lipoprotein lipase from tumor and stromal cells activates adipocytes, inducing lipolysis of triglycerides and fatty acid (FA) secretion; FAs enter cells via CD36 or FA transport proteins. Tumor cells also generate FAs de novo using acetyl-CoA from glucose catabolism, involving acetyl-coA carboxylase and FA synthase. These FAs participate in FA oxidation or other pathways, producing immunosuppressive factors or forming lipid droplets. Additionally, lipoproteins in the microenvironment are transported via lipoprotein receptors and catabolized to cholesterol intracellularly; tumor cells also synthesize cholesterol via the mevalonate pathway. Dysregulated lipid metabolism in tumor cells promotes an acidic, hypoxic, glucose-deprived, and lipid-rich immunosuppressive microenvironment. Citation: Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, Ping Y, Zhang Y. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol 2021; 14: 187. Copyright ©The Author(s) 2021. Published by Biomed Central (Supplementary material). LPL: Lipoprotein lipase; FA: Fatty acid; FATP: Fatty acid transport protein; FAO: Fatty acid oxidation; LD: Lipid droplet; LR: Lipoprotein receptor.
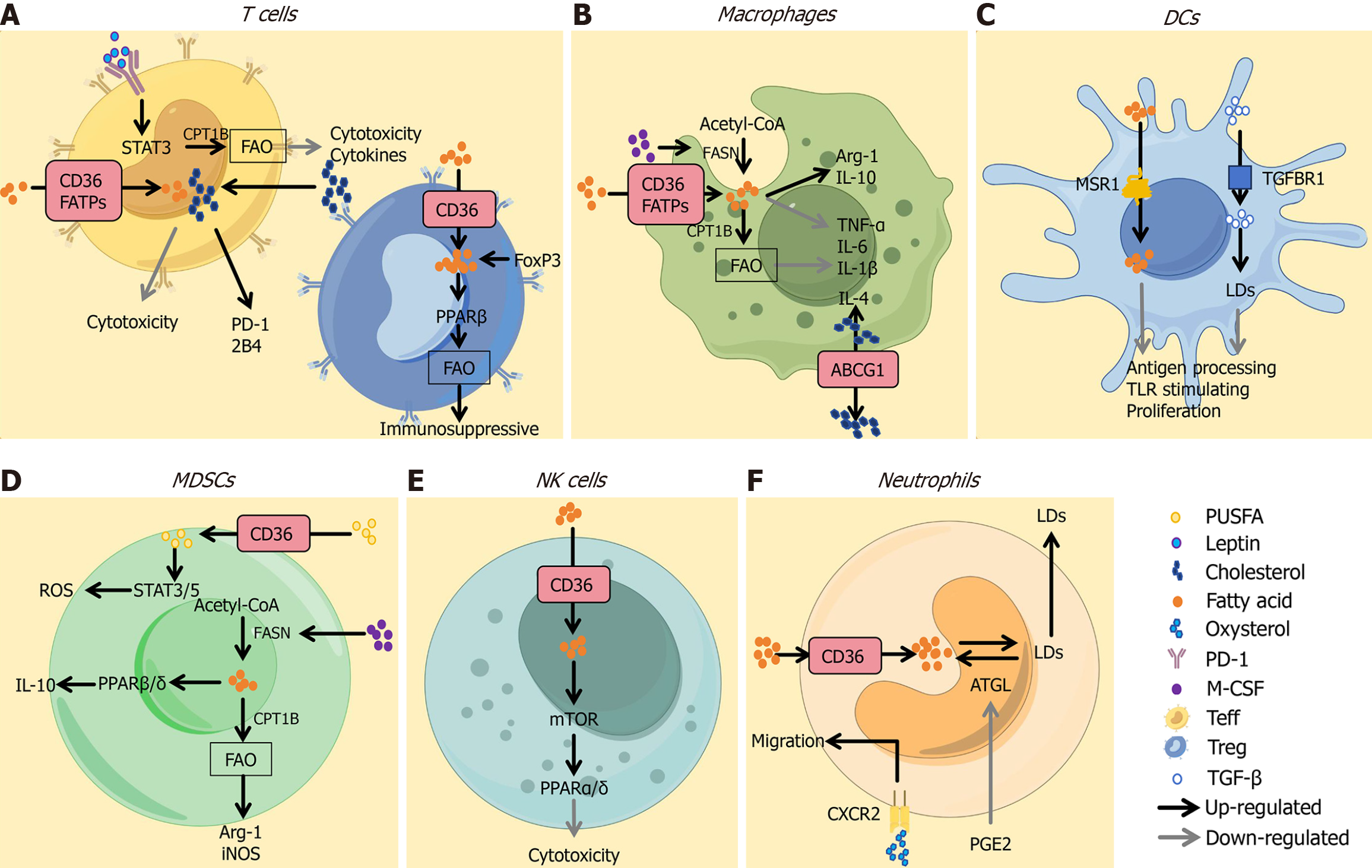
Figure 2 Lipid metabolism in pro-tumor immune responses[3].
A: Fatty acids (FAs) taken up via CD36 or FA transport proteins mediate immuno
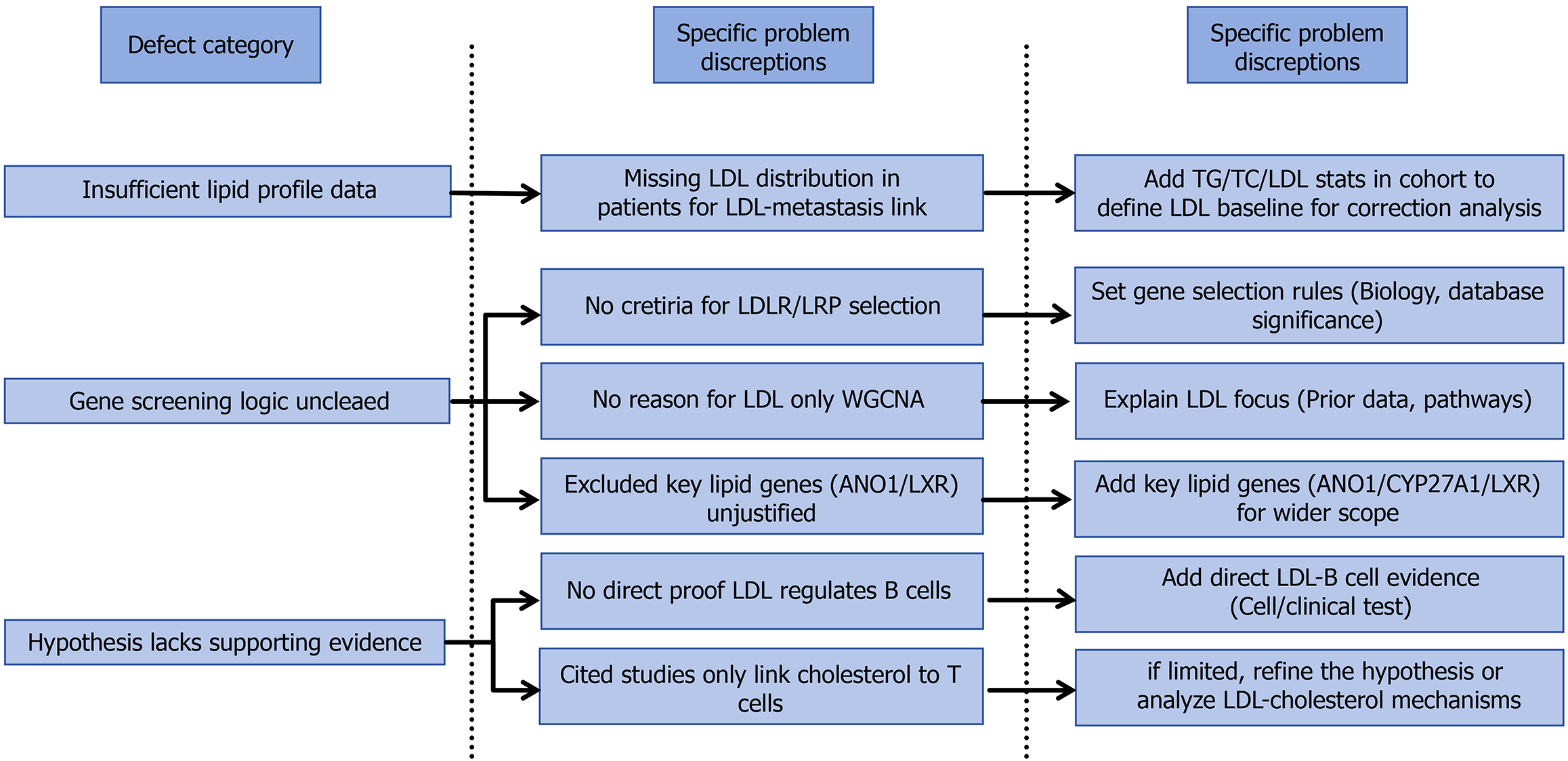
Figure 3 The original study exhibits critical limitations across three core dimensions: Data completeness, methodological logic, and rigor of the evidence chain.
By supplementing baseline data on lipid parameters in the patient cohort, clarifying the rationale for gene screening while expanding the analytical scope, and strengthening the association between hypotheses and supporting evidence, the reliability and scientific validity of the study’s conclusions can be significantly enhanced. This would enable a more accurate reflection of the complex interplay between lipid metabolism and immune regulation in esophageal cancer. LDL: Low-density lipoprotein; TG: Triglyceride; TC: Total cholesterol.
- Citation: Cui X, Liang Z. Concerns regarding lipid metabolism, immune regulation, and methodology in a study on esophageal cancer lymph node metastasis. World J Gastrointest Surg 2025; 17(11): 112124
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/112124.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.112124