Published online Jul 15, 2017. doi: 10.4239/wjd.v8.i7.351
Peer-review started: September 14, 2016
First decision: November 14, 2016
Revised: December 16, 2016
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: July 15, 2017
Processing time: 293 Days and 7 Hours
To estimate the prevalence of diabetes in the rural population of Tessekere (Senegal) and investigate associated risk factors.
Data from a 2015 survey of 500 individuals age 20 and over representative of the population of the municipality of Tessekere were used. Sociodemographic characteristics, health related variables, capillary whole blood glucose, and weight and height measurements of individuals were collected during face-to-face interviews. Statistical analyses used were bivariate tests and binary logistic regressions.
The percentage of individuals having impaired fasting glucose (IFG) is 6.6%. Those with fasting blood glucose (FBG) levels ≥ 126 mg/dL and/or currently being treated for diabetes is 4.2%. Only mean body mass index (BMI) is significantly higher among diabetic individuals and among those having FBG levels ≥ 110 mg/dL. After adjustment for sex, age, educational level, BMI and hypertension, only BMI is associated with diabetes.
Prevalence of diabetes and IFG in our study correspond to the high range of rural sub-Saharan Africa prevalence. Diabetes is thus becoming a pressing public health concern, even in rural areas. But the risk factors identified in Tessekere suggest that the diabetes epidemic is still in the early stages, such that concerted action would make it possible to contain the devastating impact of this chronic condition.
Core tip: Our study is one of the first, to our knowledge, to estimate the prevalence of diabetes in a rural Senegalese area. In the Tessekere municipality, diabetes prevalence is 4.2%, and that of impaired fasting glucose is 6.6%, corresponding to the high range of prevalence observed in rural sub-Saharan Africa. In our population study, emerging risk factors such as depression and material well-being (identified mainly in developed countries) are not associated with diabetes, indicating that this epidemic is in the early stages in this region.
- Citation: Duboz P, Boëtsch G, Gueye L, Macia E. Type 2 diabetes in a Senegalese rural area. World J Diabetes 2017; 8(7): 351-357
- URL: https://www.wjgnet.com/1948-9358/full/v8/i7/351.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i7.351
The World Health Organization[1] estimates that globally, high blood glucose is the third highest risk factor for premature mortality. According to the International Diabetes Federation[2], some 415 million people worldwide are estimated to have diabetes and about 75% live in low- and middle-income countries. The prevalence of diabetes is increasing rapidly, and it is expected that by 2040 there will be 34.2 million adults in sub-Saharan Africa living with diabetes, more than double the number in 2015[2]. This increasing rate of diabetes mellitus is an additional burden to a region that continues to bear the brunt of communicable diseases such as tuberculosis and malaria[3]. Moreover, Africa Region has the highest proportion of undiagnosed diabetes; over two-thirds (66.7%) of people with diabetes are unaware they have the disease[2]. The diabetic condition is usually only diagnosed once patients are overtly symptomatic or present complications[4], which leads to increased risks of serious and fatal consequences associated with the progression of the disease.
Diabetes was virtually non-existent in West African populations about three decades ago[5,6]. But today, an estimated 4% of urban West African adults have diabetes[7], and this figure is higher in some countries: 7.7% in Ghana[8], 4.2% in Kenya[9], 4% and 7.7% in rural and urban Guinea respectively[10], and 8.8% in Nigeria[11] for example. Among non-modifiable risk factors, age is one of the most important, making the ageing of the sub-Saharan population a major determinant of the global rise in diabetes on the continent[12]. Gender trends, another non-modifiable risk factor, are not clear in Africa[6,7,12]. Genetic susceptibility, family history of diabetes and intrauterine influence are also classified as non-modifiable risk factors for diabetes[12].
Urbanization is known as a major modifiable risk factor for diabetes: Abubakari et al[7] have shown that individuals living in urban areas were over five times more likely to have diabetes than their rural counterparts. The higher diabetes prevalence in urban compared to rural settings is attributable to nutritional and lifestyle changes[12]. Urbanization is then associated with physical inactivity and adiposity, another modifiable risk factors for diabetes. Indeed, several studies have reported the independent association of higher adiposity with diabetes in sub-Saharan Africa[6,9,10,12-14]. Finally, Peer et al[12] have also mentioned that psychosocial stress or depressive syndrome might be considered a potential risk factor for diabetes.
Whereas Senegal is in the top five sub-Saharan African countries in terms of advanced nutritional transition status and dietary composition - which indicates increased risk for non-communicable diseases[15] - few studies have attempted to describe national trends in diabetes. In 1960, Payet et al[16] estimated the prevalence of diabetes in Dakar to be 1.1%. In 2015, the International Diabetes Federation reckoned the prevalence to be 1.8%[2]. In 2009 in Dakar, 17.8% of the population had a fasting blood glucose (FBG) level ≥ 110 mg/dL[17]. Information about diabetes prevalence in Senegalese rural areas is scarce. Consequently, the aim of this study is to estimate the prevalence of impaired fasting glucose (IFG) and diabetes in the rural population of Tessekere municipality (Senegal) and to investigate associated risk factors. Tessekere is a rural area populated mainly by nomadic pastoralists, whose culture and economy revolve around cattle. Our two hypotheses are therefore that the intense physical activity, low-fat diets and traditional way of life characterizing Ferlo’s Fulani population would protect them from diabetes, leading to a low prevalence of this disease, and that considering the type of population, the diabetes epidemic is likely to be in the early stages. Emerging risk factors such as depression or stress, identified mainly in developed countries[18,19], will probably not be associated with diabetes in our population.
In order to carry out this study, a comprehensive survey was conducted from February to August 2015 in the municipality of Tessekere (Ferlo region, northern Senegal). In 2014, according to Senegal’s National Agency for Statistics and Demography (ANSD), a total of 8999 individuals aged 20 and over were living in Tessekere municipality[20]. The population sample selected for this study comprised 500 individuals aged 20 and over. The sample was constructed using the combined quota method (cross-section by age and gender) to strive for representativeness of the population of Tessekere aged 20 and over. Data from the ANSD dating from the last census (2013) were used. The quota variables used were gender (male/female) and age (20-29/30-39/40-49/50 and over).
Eight trained investigators (PhD students in Sociology, Medicine and Pharmacy) started out from different points each day to interview individuals in Wolof or Haalpulaar in each camp. Investigators had a certain number of individuals to interview to meet the quotas. Only one person was selected as a respondent in each home. Investigators went to the house, inquired about the inhabitants and then chose the first person they saw who met the characteristics needed for the quotas. In-person interviews were conducted. They ranged from 30 to 45 min, depending on respondent availability and desire to talk. As the objectives of this study include analysis with BMI, pregnant women were withdrawn from the sample, resulting in a sample of 496 individuals.
The socioeconomic and demographic variables collected were age (20-29/30-39/40-49/50 and over), gender (male/female) and educational level - defined in accordance with the educational system in Senegal - (0/1-5/6-9/10-12/over 12 years of school).
The following question was used as an indicator of economic conditions: “Given your household income, do you feel you … (1) live well? (2) live okay? (3) live okay, but you have to be careful? and (4) have difficulty making ends meet”? This question, taken directly from Razafindrakoto and Roubaud’s study[21], has demonstrated validity and relevance in eight African capitals, including Dakar, to measure economic conditions in the context of subjective well-being. For the analyses, the answers were coded from 1 (poor) to 4 (prosperous). The Mini International Neuropsychiatric Interview (MINI) was used to diagnose major depressive episodes at the time of the interview[22,23].
The biological health variables collected were dysglycemia, blood pressure (BP), body mass index (BMI), waist circumference (WC) and waist-to-hip ratio (WHR). For dysglycemia, subjects were examined during the morning after fasting since the previous evening meal. The day before the investigation, subjects were informed of the need to have nothing to drink or eat in order to measure capillary whole blood glucose. Capillary whole blood (glucose) was obtained from a finger prick and was immediately analyzed using a Hemocue blood glucose analyzer®. The participants were then divided into three categories according to international standards[24]: Fasting plasma glucose < 110 mg/dL; IFG: Fasting plasma glucose levels between 110 and 125 mg/dL; those with diabetes, who had either been previously diagnosed diabetics or had capillary whole blood glucose value greater than or equal to 126 mg/dL.
In order to measure BP, we used an OMRON M5-I digital automatic blood pressure monitor (OMRON®,
s’Hertogenbosch, The Netherlands). Measurements were taken on the upper right arm using an appropriate sized cuff while the participant was sitting and had rested for 5 min. Three readings were taken during the interview. The first was discarded, and the mean of the last two readings were used in the analysis. Hypertension (HTA) was defined as a systolic BP ≥ 140 mmHg (SBP) and/or a diastolic BP ≥ 90 mmHg (DBP) or reported treatment for hypertension.
Finally, overweight was defined as 25 ≤ BMI < 30; obesity corresponded to a BMI of ≥ 30; underweight to a BMI of < 18.5. WC of ≥ 102 cm in men and of ≥ 88 cm in women was considered central obesity. Lastly, a WHR of > 0.9 in men and a WHR of > 0.8 in women were considered central obesity[25].
Files compiled on the basis of the 496 questionnaires were processed and coded in Excel (2013). We used χ2 tests to measure the presence, strength, and independence of statistical association of sociodemographic and biological variables and diabetes. We also carried out binary logistic regression analyses to estimate the risk factors for diabetes. All analyses were performed using SPSS software, version 20. A two-sided P-value of less than 0.05 was considered significant.
In our sample, more than two-thirds of the participants were aged < 40 years. Almost three-quarters had not attended school and the majority was not depressive. Proportions of men and women were balanced, as the proportions of people with good and poor material well-being. Most individuals were not hypertensive and not obese by WC, whereas 40% of individuals had central obesity measured by WHR. Prevalence of diabetes and IFG in our sample was 4.2% (95%CI: 2.44%-5.96%) and 6.6% (95%CI: 4.42%-8.78%) respectively.
Table 1 shows that in Tessekere, only mean BMI is significantly higher among diabetic individuals and among those having FBG ≥ 110 mg/dL. Furthermore, IFG is significantly more prevalent in individuals aged 50 and over and in individuals with central obesity (by WC and WHR).
| Variables | Categories | < 126 mg/dL | ≥ 126 mg/dL | Test | < 110 mg/dL | ≥ 110 mg/dL | Test | Total |
| Total | 475 | 21 | 463 | 33 | 496 | |||
| Sex | Men | 233 | 8 | χ2 = 0.967; | 228 | 13 | χ2 = 1.196; | 241 |
| Women | 242 | 13 | P = 0.326 | 235 | 20 | P = 0.274 | 255 | |
| Education | 0 | 358 | 15 | χ2 = 0.204; | 350 | 23 | χ2 = 3.044; | 373 |
| level | 1-5 | 82 | 4 | P = 0.903 | 81 | 5 | P = 0.218 | 86 |
| 6-9 | 18 | 0 | 16 | 2 | 18 | |||
| 10-12 | 11 | 2 | 10 | 3 | 13 | |||
| > 12 | 6 | 0 | 6 | 0 | 6 | |||
| Age | 20-29 | 193 | 7 | χ2 = 3.026; | 189 | 11 | χ2 = 11.893; | 200 |
| bracket | 30-39 | 112 | 3 | P = 0.553 | 111 | 4 | P = 0.018 | 115 |
| 40-49 | 73 | 4 | 72 | 5 | 77 | |||
| ≥ 50 | 97 | 7 | 91 | 13 | 104 | |||
| Material | Well | 71 | 1 | χ2 = 5.554; | 69 | 3 | χ2 = 2.351; | 72 |
| well-being | Okay | 145 | 11 | P = 0.135 | 142 | 14 | P = 0.503 | 156 |
| Okay but careful | 203 | 6 | 197 | 12 | 209 | |||
| Difficulties | 56 | 3 | 55 | 4 | 59 | |||
| MINI | Not depressive | 438 | 20 | χ2 = 0.261; | 426 | 32 | χ2 = 1.072; | 458 |
| Depressive | 37 | 1 | P = 0.610 | 37 | 1 | P = 0.301 | 38 | |
| HTA | HTA - | 327 | 13 | χ2 = 0.449; | 319 | 21 | χ2 = 0.396; | 340 |
| HTA + | 148 | 8 | P = 0.503 | 144 | 12 | P = 0.529 | 156 | |
| WC | Non obese | 421 | 16 | χ2 = 2.970; | 412 | 25 | χ2 = 5.143; | 437 |
| Obese | 54 | 5 | P = 0.085 | 51 | 8 | P = 0.023 | 59 | |
| WHR | Non obese | 271 | 8 | χ2 = 2.937; | 266 | 13 | χ2 = 4.082; | 279 |
| Obese | 204 | 13 | P = 0.087 | 197 | 20 | P = 0.043 | 217 | |
| BMI | Mean | 20.8396 | 23.8 | t = 3.303; P = 0.001 | 20.8 | 23.1 | t = 3.155; P = 0.002 | 20.9 |
| SBP | Mean | 125.3 | 127.8 | t = 0.400; P = 0.693 | 125.2 | 127.5 | t = 0.470; P = 0.641 | 125.4 |
| DBP | Mean | 82.1 | 80.6 | t = 0.579; P = 0.563 | 82.1 | 81.5 | t = 0.301; P = 0.763 | 82.1 |
The previously identified relationship between FBG, sociodemographic and biological health variables was tested by binary logistic regression. The results of the binary logistic regression are presented in Table 2.
| Variables | Categories | IFG | Diabetes | ||||||||
| P | Odds ratios | IC for OR (95%) | P | Odds ratios | IC for OR (95%) | ||||||
| Sex (men) | Women | 0.744 | 1.153 | 0.49 | - | 2.716 | 0.805 | 1.141 | 0.4 | - | 3.254 |
| Age bracket (≥ 50 yr)1 | < 50 yr | 0.0271 | 2.731 | 1.12 | - | 6.663 | 0.228 | 2.006 | 0.647 | - | 6.216 |
| Education level (≥ 1 yr)1 | 0 yr | 0.157 | 1.832 | 0.792 | - | 4.236 | 0.41 | 1.55 | 0.546 | - | 4.396 |
| HTA (HTA-) | HTA + | 0.449 | 0.717 | 0.304 | - | 1.695 | 0.673 | 0.797 | 0.277 | - | 2.289 |
| BMI (continuous) | 0.049a | 1.095 | 1 | - | 1.198 | 0.018a | 1.142 | 1.023 | - | 1.274 | |
| WHR (obese) | Not obese | 0.334 | 1.532 | 0.645 | - | 3.635 | 0.396 | 1.577 | 0.551 | - | 4.508 |
| WC (obese) | Not obese | 0.927 | 1.053 | 0.348 | - | 3.181 | 0.688 | 0.756 | 0.193 | - | 2.966 |
Results show that actually only BMI was associated with FBG ≥ 126 mg/dL. Indeed, increased BMI is associated with increased risks of diabetes (Table 2). Furthermore, age and BMI were independently associated with IFG. Variables concerning central obesity (WC and WHR) were no longer associated with FBG after adjustment for age, sex and education level.
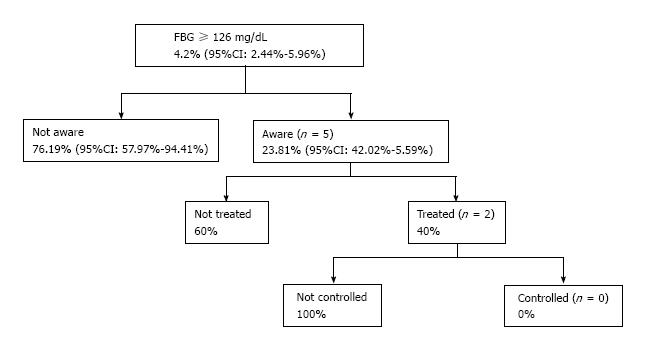
Finally, among individuals with FBG ≥ 126 mg/dL, 5 (23.8%) were aware of their diabetic condition, 2 (9.5%) of the diabetics were treated, and 0 diabetic individuals under treatment had controlled FBG (i.e., FBG < 126 mg/dL) (Figure 1).
The present study is to our knowledge one of the first to evaluate the prevalence of diabetes and IFG in a rural area in Senegal. The prevalence of diabetes in our sample is 4.2%, and that of IFG, 6.6%, corresponding to the high range of prevalence observed in rural sub-Saharan Africa, ranging from 0.8% to 6% for diabetes[26,27]. Our results thus confirm those obtained in Guinea[10], Nigeria[28] and Mali[29], suggesting that Fulani populations have a high prevalence of diabetes. The reasons for this high prevalence remain unclear. In the Tessekere municipality, Barral et al[30] showed that in 1981, a decreased consumption of cereals and an increased consumption of sugar (with tea, which was introduced recently at that time) and fat was observed since the 1950s, inducing a physiological modification that may have an impact on diabetes or hypertension. Indeed, following the severe droughts in the Sahelian region in the 1970s, local products (millet, leaves, milk, cowpea, etc.), which provided food security were replaced by imported foodstuffs (rice, peanut oil, pasta, bread, etc.) and by small ruminant holdings (sheep, goats) to compensate for economic losses. It is more than likely that these rapid changes in diet have gradually led to an increase in diabetes over the past 30 years.
The risk factors identified in our population do not differ from those of other countries[12]. Age and BMI are significant predictors of FBG ≥ 110 mg/dL, but it is noteworthy that only BMI, and not age, is a significant predictor of diabetes in Tessekere. Indeed, it might be associated with two facts. First, in our study, the Fulani population presents a very high proportion of undiagnosed diabetes (76.19%). This result is in line with the results generally obtained in developing countries, where generally half to three-quarters of all cases are undetected[2,12]. But it also means that the condition is usually only diagnosed once patients are overtly symptomatic or present complications[4]. Consequently, the mortality associated with diabetes must be higher among older age groups, which explains the lack of correlation between age and the prevalence of diabetes. Secondly, even if Senegal is one of the five African countries with the most advanced nutrition transition[15], the Fulani population is very isolated (health centers, roads and stores located more than 5 km from camps, without motorized vehicles[31]), one of the poorest in Senegal, and seems to be just at the beginning of demographic, epidemiological and nutritional transitions. These characteristics could explain the lack of relationship between diabetes and age, as the diabetes epidemic appears to be nascent in our population. The same cause could also explain the lack of association between IFG or diabetes and central obesity. Indeed, whereas central obesity[14], depression[18] and material well-being[19] are generally identified risk factors in populations having long experienced diabetes, the recentness of the diabetes epidemic in our population may explain the absence of these risk factors.
Finally, the 4.2% prevalence of diabetes found in our study is comparable to the 4.6% observed in rural Senegal by Seck et al[32] but is far below the 8.5% to 12.9% prevalence reported for all other parts of the world[2]. In addition, the prevalence of IFG in our Tessekere sample is significantly lower than the 17.9%[17] or the 10.3%[10] observed respectively in Dakar and in urban Ghana with the same criteria and methods, which make direct comparison possible. These results are in line with the majority of the results concerning the urban-rural distribution of diabetes in sub-Saharan Africa[9,12]. Currently, 38% of the population of sub-Saharan Africa live in urban areas. But this proportion is predicted to increase to 45% by 2030, with a demographic inflection point to be attained by 2040: More urban than rural residents[33]. Due to inherent growth in urban districts and massive migration from rural areas[6], diabetes will certainly rise in the next few years in Senegal, where a focus on actions to optimize lifestyle management is critically important, given that obtaining drugs to treat diabetes is challenging due to cost and availability[34,35].
Our study has some limitations. First, as in many surveys, our FBG levels were based on capillary blood measurements, which may have underestimated the prevalence rates[36]: The 2006 WHO/IDF update states that glycaemic values on venous and capillary plasma are identical but that plasma measures are 11% higher than whole blood measures. Second, HbA1c measurements are missing, but would have been important to accurately diagnose diabetes mellitus in our sample. Furthermore, the small absolute number of diabetic subjects makes assessment of the relationship to other variables examined difficult, and it is possible that only gross differences, such as the relation between diabetes and BMI will be found. Third, the small absolute number of diabetic subjects with known, treated and controlled diabetes made it impossible to perform trend analysis of age/sex subgroups. Finally, diagnosis of diabetes should not be based on a single abnormal result in an asymptomatic subject, so that future studies on the area should repeat the capillary whole blood glucose test for every subject.
The prevalence of diabetes in the rural area of Tessekere (Senegal) is 4.2%, and of particular concern is the high burden of undiagnosed and uncontrolled diabetes. If the nutritional transition is to develop in this part of the country, diabetes and its complications are certain to become a major health issue within several years. The challenge of diabetes in rural areas of sub-Saharan Africa is to provide accessible, affordable and optimal care for the management of the disease. It is then possible that collaboration with traditional healers would be appropriate and respectful of the populations’ cultural values, and could represent a first step toward an integrative approach combining biomedical knowledge and traditional medicine.
The authors wish to thank all the people of Tessekere municipality who took the time to answer their questions. We also thank the Senegalese chief of staff interviewers who participated in this study: Amadoune Gueye and Babacar Kane.
The World Health Organization estimates that globally, high blood glucose is the third highest risk factor for premature mortality. The increased rising rate of diabetes mellitus is an additional burden to sub-Saharan Africa that continues to bear the brunt of communicable diseases such as tuberculosis and malaria. Moreover, Africa Region has the highest proportion of undiagnosed diabetes; over two-thirds (66.7%) of people with diabetes are unaware they have the disease. Whereas Senegal is in the top five sub-Saharan African countries in terms of advanced nutritional transition status and dietary composition - which indicates increased risk for non-communicable diseases - few studies have attempted to describe national trends in diabetes and no information exists about diabetes prevalence in rural Senegalese areas.
Diabetes was virtually non-existent in West African populations about three decades ago. But today, it is estimated that 4% of urban West African adults have diabetes, and this figure is higher in some countries: 7.7% in Ghana, 4.2% in Kenya, 4% and 7.7% in rural and urban Guinea respectively, 8.8% in Nigeria for example. Identified risk factors for diabetes in sub-Saharan Africa are age, family history of diabetes and intrauterine influence. Urbanization is known as a major modifiable risk factor for diabetes, attributable to nutritional and lifestyle changes and physical inactivity. Finally, in developed countries, psychosocial stress or depressive syndrome might be considered potential risk factors for diabetes.
The study is the first, to our knowledge, to estimate the prevalence of diabetes in a rural Senegalese area. In Tessekere municipality, diabetes prevalence is 4.2%, and that of impaired fasting glucose, 6.6%, corresponding to the high range of prevalence observed in rural sub-Saharan Africa. In the population study, emerging risk factors such as depression and material well-being (identified mainly in developed countries) are not associated with diabetes, indicating that this epidemic is in the early stages in this region.
It seems necessary to study the determinants of the high prevalence of diabetes observed in Fulani in sub-Saharan Africa. The study also casts doubt on the relationship between emerging factors for diabetes such as depression and material well-being and stages of the nutritional transition.
The introduction provides sufficient background and includes all relevant references. The research design is appropriate. The methods are adequately described. The results are clearly presented. The conclusions are supported by the results.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Senegal
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Duvvuru NR, Kushiyama A, Liao KF, Masaki T, Nishio K, Qi L, Romani A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization 2009; . |
| 2. | International Diabetes Federation. Diabetes Atlas. 7th edition. Available from: http://www.diabetesatlas.org/. |
| 3. | de-Graft Aikins A, Unwin N, Agyemang C, Allotey P, Campbell C, Arhinful D. Tackling Africa’s chronic disease burden: from the local to the global. Global Health. 2010;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Tuei VC, Maiyoh GK, Ha CE. Type 2 diabetes mellitus and obesity in sub-Saharan Africa. Diabetes Metab Res Rev. 2010;26:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Sobngwi E, Mauvais-Jarvis F, Vexiau P, Mbanya JC, Gautier JF. Diabetes in Africans. Part 1: epidemiology and clinical specificities. Diabetes Metab. 2001;27:628-634. [PubMed] |
| 6. | Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (16)] |
| 7. | Abubakari AR, Lauder W, Jones MC, Kirk A, Agyemang C, Bhopal RS. Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health. 2009;123:602-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Cook-Huynh M, Ansong D, Steckelberg RC, Boakye I, Seligman K, Appiah L, Kumar N, Amuasi JH. Prevalence of hypertension and diabetes mellitus in adults from a rural community in Ghana. Ethn Dis. 2012;22:347-352. [PubMed] |
| 9. | Christensen DL, Friis H, Mwaniki DL, Kilonzo B, Tetens I, Boit MK, Omondi B, Kaduka L, Borch-Johnsen K. Prevalence of glucose intolerance and associated risk factors in rural and urban populations of different ethnic groups in Kenya. Diabetes Res Clin Pract. 2009;84:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Baldé NM, Diallo I, Baldé MD, Barry IS, Kaba L, Diallo MM, Kaké A, Camara A, Bah D, Barry MM. Diabetes and impaired fasting glucose in rural and urban populations in Futa Jallon (Guinea): prevalence and associated risk factors. Diabetes Metab. 2007;33:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Enang OE, Otu AA, Essien OE, Okpara H, Fasanmade OA, Ohwovoriole AE, Searle J. Prevalence of dysglycemia in Calabar: a cross-sectional observational study among residents of Calabar, Nigeria. BMJ Open Diabetes Res Care. 2014;2:e000032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Peer N, Kengne AP, Motala AA, Mbanya JC. Diabetes in the Africa Region: an update. Diabetes Res Clin Pract. 2014;103:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Amoah AG, Owusu SK, Adjei S. Diabetes in Ghana: a community based prevalence study in Greater Accra. Diabetes Res Clin Pract. 2002;56:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Bakari AG, Onyemelukwe GC. Indices of obesity among type 2 diabetic Hausa-Fulani Nigerians. Inter J of Diabetes and Meta. 2004;13:28. |
| 15. | Abrahams Z, McHiza Z, Steyn NP. Diet and mortality rates in Sub-Saharan Africa: stages in the nutrition transition. BMC Public Health. 2011;11:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Payet M, Sankalé M, Pene P. Les principaux aspects du diabète sucré en milieu africain à Dakar. Presse Med. 1960;68:2054. |
| 17. | Duboz P, Chapuis-Lucciani N, Boëtsch G, Gueye L. Prevalence of diabetes and associated risk factors in a Senegalese urban (Dakar) population. Diabetes Metab. 2012;38:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Pouwer F, Kupper N, Adriaanse MC. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med. 2010;9:112-118. [PubMed] |
| 19. | Chida Y, Hamer M. An association of adverse psychosocial factors with diabetes mellitus: a meta-analytic review of longitudinal cohort studies. Diabetologia. 2008;51:2168-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Senegal’s national statistical office. General census of Populations, Dwellings, Agriculture and Breeding Report (RGPHAE, 2013). Dakar: UNFPA/USAID 2015; . |
| 21. | Razafindrakoto M, Roubaud F. Les déterminants du bien-être individuel en Afrique francophone: le poids des institutions. Afr Contemp. 2007;4:191-223. [DOI] [Full Text] |
| 22. | Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33; quiz 34-57. [PubMed] |
| 23. | American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 4th ed (DSM-IV). Washington, DC: American Psychiatric Association 1994; . |
| 24. | World Health Organization/International Diabetes Federation. Report of a WHO/IDF consultation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva: WHO/IDF 2006; . |
| 25. | Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord. 2001;25:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Kengne AP, Echouffo-Tcheugui JB, Sobngwi E, Mbanya JC. New insights on diabetes mellitus and obesity in Africa-part 1: prevalence, pathogenesis and comorbidities. Heart. 2013;99:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 28. | Nyenwe EA, Odia OJ, Ihekwaba AE, Ojule A, Babatunde S. Type 2 diabetes in adult Nigerians: a study of its prevalence and risk factors in Port Harcourt, Nigeria. Diabetes Res Clin Pract. 2003;62:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Fisch A, Pichard E, Prazuck T, Leblanc H, Sidibe Y, Brücker G. Prevalence and risk factors of diabetes mellitus in the rural region of Mali (West Africa): a practical approach. Diabetologia. 1987;30:859-862. [PubMed] |
| 30. | Barral H, Bénéfice E, Boudet G, Denis JP, Wispelaere GD, Diaite I, Noël J. Systèmes de production d’élevage au Sénégal dans la région du Ferlo: synthèse de fin d’études d’une équipe de recherches pluridisciplinaire. Dakar: Ministère de la Recherche et de l’Industrie, GERDAT-ORSTOM 1983; . |
| 31. | Senegal’s National Statistical Office. Geographic disparities in access to basic social services in Senegal. Dakar: Ministery of Economic and Financial Affairs 2011; . |
| 32. | Seck SM, Dia DG, Doupa D, Diop-Dia A, Thiam I, Ndong M, Gueye L. Diabetes Burden in Urban and Rural Senegalese Populations: A Cross-Sectional Study in 2012. Int J Endocrinol. 2015;2015:163641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2014 Revision, CD-ROM Edition, 2014. |
| 34. | Dièye AM, Sarr A, Diop SN, Ndiaye M, Sy GY, Diarra M, Rajraji Gaffary I, Ndiaye Sy A, Faye B. Medicinal plants and the treatment of diabetes in Senegal: survey with patients. Fundam Clin Pharmacol. 2008;22:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | BeLue R, Diaw M, Ndao F, Okoror T, Degboe A, Abiero B. A cultural lens to understanding daily experiences with type 2 diabetes self-management among clinic patients in M’bour, Senegal. Int Q Community Health Educ. 2013;33:329-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Colagiuri S, Sandbaek A, Carstensen B, Christensen J, Glumer C, Lauritzen T, Borch-Johnsen K. Comparability of venous and capillary glucose measurements in blood. Diabet Med. 2003;20:953-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |