TO THE EDITOR
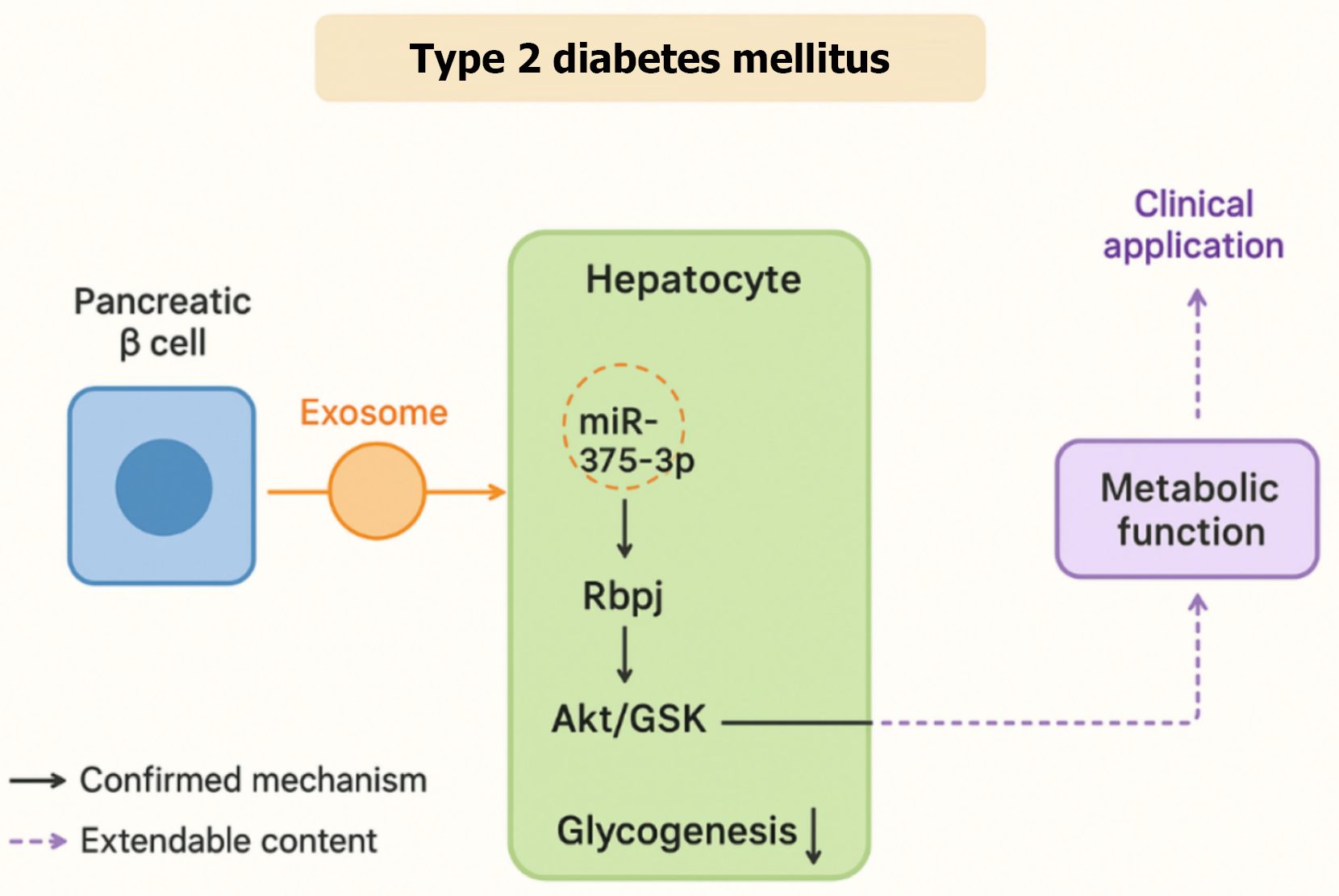
We read with great interest the article by Xu et al[1] entitled “Exosomal transfer of miR-375-3p from pancreatic β cells to hepatocytes impairs hepatic glycogenesis via Rbpj repression”. In this study, the authors demonstrated that pancreatic β cells release exosomes serving as vehicles for miR-375-3p, which are subsequently taken up by hepatocytes. Within hepatocytes, miR-375-3p directly targets and suppresses the Rbpj, leading to inhibition of the AKT/GSK signaling pathway and a reduction in hepatic glycogenesis (Figure 1). This basic research provides crucial insights into exosome-mediated inter-organ communication in diabetes, highlighting the promising potential of exosomal microRNAs (miRNAs) as both biomarkers and therapeutic targets. Inhibition of the AKT/GSK axis by exosomal miR-375-3p not only suppresses hepatic glycogen synthase activation but may also promote compensatory upregulation of gluconeogenic enzymes and lipid oxidation pathways. These alterations could contribute to increased hepatic glucose output and systemic insulin resistance. Further investigation is required to determine whether similar signaling changes occur in adipose tissue or skeletal muscle, thereby broadening the scope of exosome-mediated metabolic regulation.
Figure 1 Exosome-mediated regulation of hepatocyte glycogen metabolism in type 2 diabetes mellitus.
Exosomes secreted from pancreatic β-cells deliver miR-375-3p to hepatocytes, where it targets Rbpj and subsequently modulates the AKT/GSK signaling pathway to regulate glycogenesis. Solid arrows indicate experimentally confirmed mechanisms, while dashed arrows indicate potential extendable pathways, including possible feedback mechanisms to pancreatic β-cells and links to systemic insulin resistance.
Metabolic syndrome, a cluster of abnormalities including insulin resistance, is a central driver of type 2 diabetes[2-4]. Insulin resistance, defined as reduced cellular responsiveness to insulin, can lead to disturbances in glucose metabolism and contribute to the development of type 2 diabetes and related complications[5]. Glass and Olefsky[6] further demonstrated that insulin resistance can significantly alter metabolism in the liver and adipose tissue of patients with type 2 diabetes and atherosclerosis by mediating inflammatory responses. While research on insulin in the metabolic field is extensive, the study by Xu et al[1] introduces an additional layer of complexity, shifting the focus toward exosome-mediated communication between pancreatic β cells and hepatocytes. This perspective enriches our understanding of how exosomes act as signaling vehicles that influence the progression of metabolic diseases.
Exosomes are extracellular vesicles that function as carriers for nucleic acids and proteins, regulating the function of neighboring or distant cells and playing a significant role in intercellular communication[7,8]. Because of their ability to reflect physiological and pathological states, exosomes can serve as windows into organismal homeostasis[9,10]. Although exosomes carrying proteins can regulate diverse biological functions, increasing attention has been directed toward their role in transferring messenger RNA and miRNA[11]. In oncology, for instance, exosomes facilitate cancer progression by remodeling the tumor microenvironment, stimulating angiogenesis, activating fibroblast[12]. He et al[13] found that ovarian cancer-derived exosomes carrying miRNA-205 promote tumor metastasis by driving angiogenesis, while exosomes miR-934 has been implicated in promoting colorectal cancer metastasis through modulation of tumor-associated macrophages[14]. Interestingly, due to their inherent ability to target cancer cells, exosomes can be utilized as delivery vehicles for anticancer drugs, offering novel strategies for cancer therapy[15].
In the metabolic field, exosomes secreted by adipose tissue, liver, skeletal muscle, and pancreatic β cells play important roles in maintaining systemic metabolic homeostasis[16,17]. Yu et al[18] discovered that adipocytes from high-fat diet-fed mice release exosomes carrying miR-27a, which inhibits PPARγ and its obesity-associated downstream genes, further inducing insulin resistance in skeletal muscle. Similarly, exosomal miR-155-5p can stimulate angiogenesis and permeability in human arterial endothelial cells and promote the expression of genes related to angiogenesis, permeability, and inflammation, influencing the development of carotid atherosclerosis[19]. In line with these findings, Xu et al[1] identified miR-375-3p as a key exosomal mediator that suppresses Rbpj in hepatocytes, inhibiting the AKT/GSK signaling pathway and ultimately reducing hepatic glycogen content. This mechanism is reminiscent of the work by Kamalden et al[20] who showed that pancreatic cell–derived exosomal miR-15a targets AKT3 and contributes to the pathogenesis of diabetic retinopathy. Taken together, these studies highlight the dual role of exosomes as both regulators of pathological signaling and promising biomarkers of disease progression.
Within this broader research context, the work of Xu et al[1] assumes particular importance. By identifying a novel pancreatic β-cell-liver axis mediated by exosomal miR-375-3p in the pathogenesis of diabetes, the authors provide evidence that pancreatic β cells influence hepatic glucose metabolism not only through insulin secretion but also via exosome-dependent mechanisms. This finding underscores the importance of cross-organ communication in glucose homeostasis. It is well-established that the relative stability of blood glucose levels relies on the interaction of multiple organs. For example, insulin secreted by pancreatic β cells, in response to peripheral hyperglycemia, stimulates glycolysis by enhancing the expression of hepatic glucokinase genes, while simultaneously suppressing gluconeogenesis by inhibiting the expression of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase genes[21]. Furthermore, compared to insulin-sensitive individuals, patients with insulin resistance exhibit downregulated hepatic insulin clearance[22]. These observations suggest that impaired β-cell-hepatocyte communication is a pivotal step in the transition from insulin resistance to overt type 2 diabetes. A deeper understanding of these mechanisms may open avenues for novel therapeutic strategies that target both pancreatic β cells and hepatocytes simultaneously. Beyond the liver, exosomes released from pancreatic β-cells may also interact with other metabolic organs such as adipose tissue and skeletal muscle. Uptake of these exosomes could modulate insulin signaling and glucose utilization, thereby contributing to systemic insulin resistance. Tissue-specific differences in exosome recognition and uptake may further determine the direction and strength of this inter-organ communication. In addition, the discovery of exosomal miR-375-3p-mediated pancreas-liver communication provides a conceptual framework for understanding similar mechanisms in other metabolic disorders. Aberrant inter-organ exosomal signaling may underlie insulin resistance in obesity and lipid dysregulation in NAFLD, suggesting that modulation of exosome cargo could represent a common therapeutic strategy across metabolic diseases.
In conclusion, the study by Xu et al[1] expands the conceptual framework of diabetes pathogenesis by uncovering an exosome-mediated β-cell-liver communication axis. This work not only provides new insights into the molecular underpinnings of glucotoxicity but also highlights exosomal miRNAs as promising biomarkers and potential therapeutic targets. The notable stability of exosomal miR-375-3p in circulation enhances its potential utility as a serum biomarker for early β-cell dysfunction. Therapeutically, strategies aimed at neutralizing exosomal miR-375-3p or enhancing Rbpj expression in the liver could represent a novel approach to protect hepatic metabolic function in pre-diabetic or diabetic patients, thereby complementing existing insulin-centric treatments. Future studies focusing on clinical validation, longitudinal effects, and translational applications will be essential to determine how these findings can be harnessed to improve metabolic disease management (Figure 1).