Published online Jan 15, 2026. doi: 10.4239/wjd.v17.i1.114082
Revised: October 15, 2025
Accepted: November 25, 2025
Published online: January 15, 2026
Processing time: 125 Days and 17.6 Hours
This article discusses a recent article by Zha et al, which explores new pathogenic pathways contributing to diabetic nephropathy in a study conducted on male mice. Diabetic kidney disease outcomes remain suboptimal; it is the leading cause of end-stage kidney disease in the developed world. Previous knowledge about diabetic nephropathy focused on glomerular pathology. Advancing knowledge led to the introduction of new pathogenic concepts beyond glomerulopathy. This work by Zha et al explored an important podocyte pathway and its link to the proximal tubular cells. Podocytes are essential for maintaining glomerular health and preserving body proteins. In a state of hyperglycaemic stress, podocytes show features of internalisation of nephrin, an integral surface protein of the podocytes. The novel pathway uncovered in this experimental study involved crosstalk between the podocytes and the proximal tubular cells, more precisely, the se
Core Tip: This article sheds light on the interesting study by Zha et al. In this experimental study, a novel pathogenic pathway has been described. A novel concept was also demonstrated, namely the crosstalk between the podocytes and the proximal tubular epithelial cells. The crosstalk involves the secretion of interleukin 6 by the proximal tubular epithelial cells, which aggravates nephrin endocytosis in the podocytes using the Rab5 activation pathway. The application of nicotinamide mononucleotide nicotinamide mononucleotide intercepted the pathway. A new horizon for therapeutics could be further explored using the nicotinamide mononucleotide.
- Citation: Abdelaziz TS. Exploring novel pathways and potential therapeutic targets for diabetic nephropathy: The interplay of podocytes and proximal tubular epithelial cells. World J Diabetes 2026; 17(1): 114082
- URL: https://www.wjgnet.com/1948-9358/full/v17/i1/114082.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i1.114082
In 2021, more than 500 million people (10.5% of the population) were estimated to be affected by diabetes mellitus. Diabetic kidney disease remains the most common cause of end-stage kidney disease in the Western world[1,2]. Diabetic nephropathy, or the more recent nomenclature diabetic kidney disease, is the leading cause of end-stage kidney disease in the Western world. Therapeutic options for diabetic nephropathy are still limited.
Podocytes are highly specialized cells that surround the capillary basement membrane of the glomeruli. Podocyte integrity is key to the function of glomeruli. Podocyte injury is now recognized as one of the main pathogenic pathways in diabetic kidney disease[3,4]. What is even more recently revealed is that there is crosstalk between the proximal tubular cells and podocytes. In this way, remote cells (the proximal tubular cells) affect the podocytes’ morphology and function. This recently discovered connection, which is called proximal tubule-podocyte communication, adds to the complexity of diabetic nephropathy pathogenesis. This pathogenesis involves a milieu of immunopathological pathways.
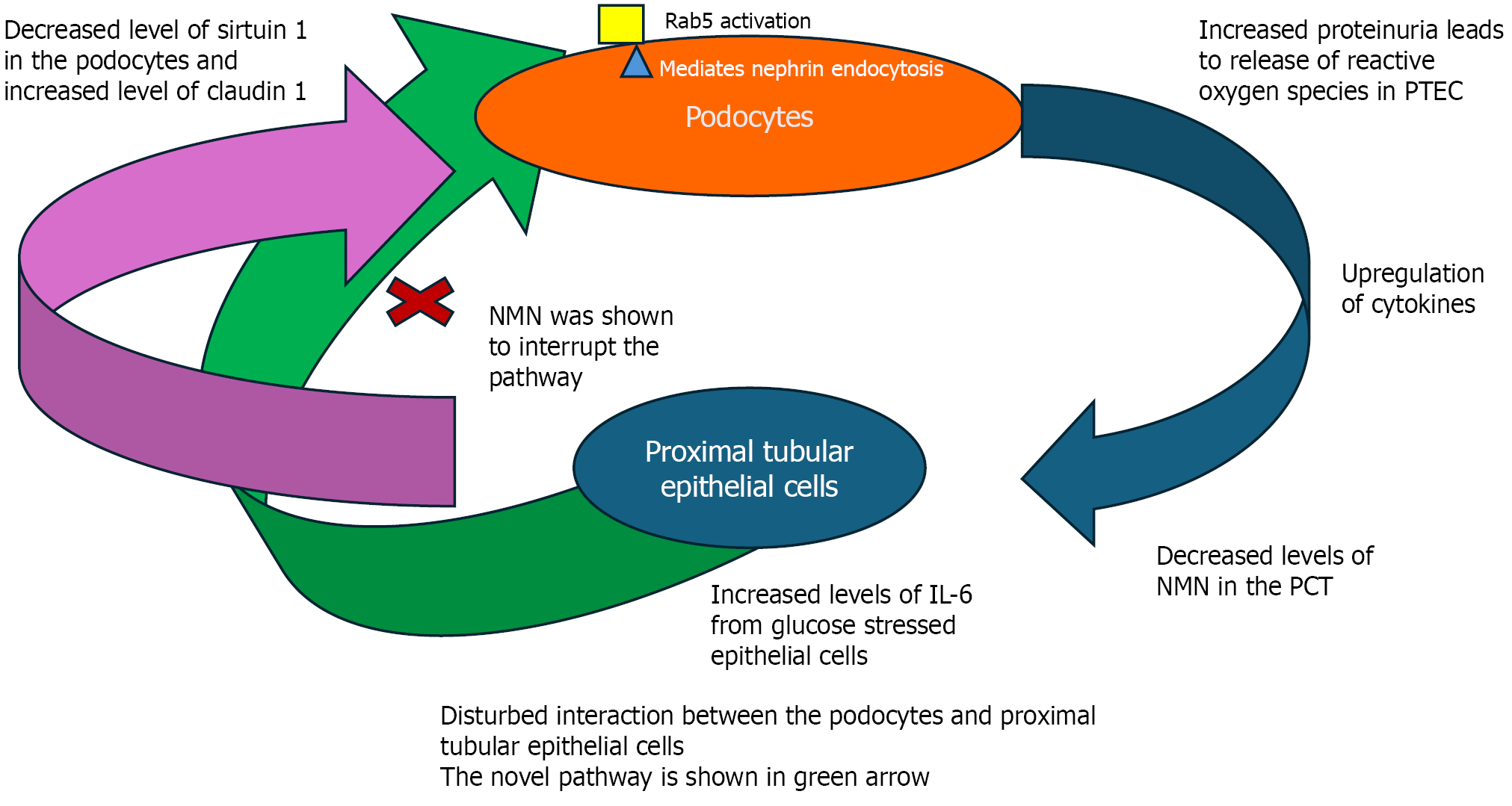
Zha et al[5] have discovered this novel pathway. Recently, interest has been shown in the interaction between podocytes and proximal tubular epithelial cells (PTECs). Although these cells are not adjacent, they affect each other. When podocytes are injured, they leak protein. The leaked protein induces oxidative stress in the PTECs, causing the release of inflammatory chemokines and triggering the signalling pathway Figure 1. This leads to decreased sirtuin 1 and increased expression of claudin on the podocytes, followed by endocytosis of the nephrin into the podocytes. This gives rise to dysmorphology of the podocytes and functional damage and contributes to increased albuminuria. The pathogenic immunoinflammatory pathway is mediated through the interleukin (IL) 6/Rab5 pathway. Rab5 is a small GTPase that significantly controls the process of internalisation of nephrin. This key finding is the first demonstration that under hyperglycaemic conditions, nephrin internalisation into podocytes is mediated through Rab5. In this study, it was possible to block nephrin endocytosis in podocytes, which mitigated the pathologic and morphologic changes in the podocytes.
Another key finding of the study was that increased IL-6 secretion by the stressed PTECs augmented nephrin endocytosis via Rab5. The study then examined the pharmacological effects of blocking IL-6 using neutralising antibodies, which eliminated nephrin endocytosis. Also, nicotinamide mononucleotide (NMN) treatment helped downregulate Rab-mediated nephrin endocytosis.
The gap between the current and the desired treatment for diabetic kidney disease is significant. Currently, the hallmark of treatment for diabetic kidney disease consists of inhibition of the renin angiotensin axis, angiotensin converting enzyme inhibitors, alongside tightening glycaemic control and other risk factors like hypertension and dyslipidaemias[6].
In the contemporary era, sodium-glucose cotransporter 2 inhibitors revolutionised the treatment of diabetic kidney disease for both the albuminuria and non-albuminuria[7]. Despite the above, a mismatch remains between advances in science concerning the pathogenesis of diabetic nephropathy and the desired outcome and treatment.
NMN is a precursor of the nicotinamide adenine dinucleotide (NAD+), which is a vital coenzyme found in eukaryotic cells[8]. Recently, interest has been growing in NAD+ and its association with sirtuins, proteins that affect a variety of metabolic and biochemical processes. Deficient levels of NAD+ have been extensively studied and implicated as a key contributor to the pathophysiological determinants of major diseases, specifically age-related disease and diabetes mellitus[9].
Nicotinamide has shown favourable effects in previous experimental studies, exerting a beneficial effect on glucose uptake in patients with type 2 diabetes mellitus through a favourable effect on the adipose tissues[10]. However, NMN in human studies have failed to show beneficial effects. Currently, a few clinical trials have obtained approval for the use of nicotinamide mononucleotide in the treatment of various diseases.
Although the newly discovered pathogenic pathway is interesting, its blockage by NMN may not provide tangible positive effects, as was the case with previous human clinical studies. This would require more studies in human parti
The explanation of the mismatch between the preclinical studies and studies with human participants may be: (1) Most of the animal studies use higher weight-based doses than studies performed on human subjects (the doses used in mice were about 300 mg/kg while the dose used in humans was 500 mg in total); (2) The difference in metabolism pharmacokinetics between lab animals and humans; (3) The limited number, sample size and short duration of human studies; and (4) The fact that, after oral ingestion, NMN breaks down to two metabolites: Nicotinamide and nicotinamide riboside, which can have unpredictable effects compared to the precursor[13,14].
The above limitations have resulted in the Food and Drug Administration prohibiting the pharmacologic use of NMN in human subjects except for investigative research purposes.
The other potential therapeutic implication is the blockage of IL-6 through neutralising antibodies, as in the current experiment. IL-6 is a key proinflammatory regulatory cytokine with hormone-like action. Diabetes mellitus involves complex immunopathological processes. IL-6 is implicated in the pathogenic process underlying type 1 diabetes and, to a lesser extent, in type 2 diabetes mellitus[15]. Meta-analyses and genome-wide analysis have shown that certain variants of the IL-6 gene are associated with the development of diabetic kidney disease. Interestingly, anecdotal evidence from large cohort studies shows a marked increase in the levels of IL-6 in the serum of patients with diabetic kidney diseases. The levels are proportionate to a more advanced stage, suggesting implication in the pathogenesis.
The effects of IL-6 on the kidneys are complex. IL-6 plays a pivotal role in the initiation phase of inflammation and the resolution phase. Under stressful hyperglycaemic conditions, podocytes secrete IL-6, which leads to hypertrophy and apoptosis. IL-6 promotes mesangial cell proliferation and apoptosis. However, the IL-6 that is produced after the exercise improves glucose uptake, glycaemic control and muscle mass. IL-6 infusion to produce the physiological effects of post-exercise has resulted in the same beneficial effect in healthy volunteers[16].
Interestingly, in patients with type 2 diabetes, IL-6 infusion to produce post-exercise levels has resulted in improved insulin sensitivity, as evidenced by decreased insulin levels[17]. In animal models, blockage of IL-6 has resulted in the mitigation of renal fibrosis. In patients with rheumatoid arthritis and diabetes mellitus, treatment with the drug Toci
Although clinical trials in human participants are still sparse, there are encouraging signals. In one clinical trial, the use of ziltivekimab in patients with moderate to severe chronic kidney disease has resulted in amelioration of atherosclerosis markers[19].
Another study has shown that the Janus kinase 1/Janus kinase 2 inhibitor baricitinib reduced albuminuria in patients with diabetic kidney disease[20]. However, blocking IL-6 has not resulted in benefits for the survival of beta islet cells in the pancreas, as shown in the results from the EXTEND study[21].
The study by Zha et al[5] re-demonstrated the crosstalk between the PTECs and the podocytes, both in vivo and in vitro. Crosstalk means that under hyperglycaemic conditions, injured podocytes can provoke an inflammatory state in PTECs through increased proteinuria. The stressed PTECs secrete IL-6, among other chemokines. This leads to decreased NMN, and both adversely affect the podocytes by increasing nephrin endocytosis, resulting in damage to the cytoskeleton and dysfunction.
The study broadens our understanding of the immunoinflammatory pathogenesis of diabetic kidney disease and factors affecting podocytopathy and its crosstalk with PTECs.
The study has several key findings: One of these key findings was demonstrated for the first time: Rab5-mediated nephrin endocytosis by the podocytes. Rab5 is upregulated by the IL-6 secreted by the stressed PTECs. This interaction and its effect on nephrin were first demonstrated in this study. The increased IL-6 decreases NMN. This results in decreased sirtuin 1 expression and increased claudin-1 expression on the podocytes. As a result of the above changes, the podocyte cytoskeleton is distorted, and the function is impaired. A second key finding is that pharmacological inter
This study reveals a novel pathogenic pathway in diabetic nephropathy. This pathway provides new insight into the crosstalk between PTECs and podocytes, where IL-6 secreted by the PTEC leads to Rab5-mediated nephrin endocytosis in the podocytes. Interestingly, the pathway can be blocked by NMN and IL-6 neutralising antibodies. However, despite these experimental beneficial effects, neither therapeutic option has shown tangible benefits in humans, with various reasons for the lack of clinical efficacy of the two novel pharmacological agents despite preclinical success. Numerous recent advances in knowledge about diabetic nephropathy have been made. Transforming this knowledge into mea
| 1. | Li X, Zhang Y, Xing X, Li M, Liu Y, Xu A, Zhang J. Podocyte injury of diabetic nephropathy: Novel mechanism discovery and therapeutic prospects. Biomed Pharmacother. 2023;168:115670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 2. | Fenta ET, Eshetu HB, Kebede N, Bogale EK, Zewdie A, Kassie TD, Anagaw TF, Mazengia EM, Gelaw SS. Prevalence and predictors of chronic kidney disease among type 2 diabetic patients worldwide, systematic review and meta-analysis. Diabetol Metab Syndr. 2023;15:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 3. | Wang M, Hu J, Yan L, Yang Y, He M, Wu M, Li Q, Gong W, Yang Y, Wang Y, Handy DE, Lu B, Hao C, Wang Q, Li Y, Hu R, Stanton RC, Zhang Z. High glucose-induced ubiquitination of G6PD leads to the injury of podocytes. FASEB J. 2019;33:6296-6310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Tomita I, Kume S, Sugahara S, Osawa N, Yamahara K, Yasuda-Yamahara M, Takeda N, Chin-Kanasaki M, Kaneko T, Mayoux E, Mark M, Yanagita M, Ogita H, Araki SI, Maegawa H. SGLT2 Inhibition Mediates Protection from Diabetic Kidney Disease by Promoting Ketone Body-Induced mTORC1 Inhibition. Cell Metab. 2020;32:404-419.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 5. | Zha DQ, Gao P, Wu XY. Nicotinamide mononucleotide protects against diabetic nephropathy via IL-6/Rab5-mediated crosstalk between proximal tubular epithelial cells and podocytes. World J Diabetes. 2025;16:109782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Patel DM, Bose M, Cooper ME. Glucose and Blood Pressure-Dependent Pathways-The Progression of Diabetic Kidney Disease. Int J Mol Sci. 2020;21:2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4523] [Article Influence: 646.1] [Reference Citation Analysis (0)] |
| 8. | Hong W, Mo F, Zhang Z, Huang M, Wei X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front Cell Dev Biol. 2020;8:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 728] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 10. | Popescu RG, Dinischiotu A, Soare T, Vlase E, Marinescu GC. Nicotinamide Mononucleotide (NMN) Works in Type 2 Diabetes through Unexpected Effects in Adipose Tissue, Not by Mitochondrial Biogenesis. Int J Mol Sci. 2024;25:2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, Yamashita K, Shigaki S, Ono T, Yukioka H, Okano H, Nabeshima YI, Imai SI, Yasui M, Tsubota K, Itoh H. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020;67:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, Sindelar M, Pietka T, Patterson BW, Imai SI, Klein S. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 286] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 13. | Song Q, Zhou X, Xu K, Liu S, Zhu X, Yang J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: an Update. Adv Nutr. 2023;14:1416-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Chen T, Cao H, Dong L, Ji Z, Cao JM. Advance in Understanding the Effect of β-Nicotinamide Mononucleotide on Physiological Functions. Food Sci. 44:382-391. [DOI] [Full Text] |
| 15. | Kreiner FF, Kraaijenhof JM, von Herrath M, Hovingh GKK, von Scholten BJ. Interleukin 6 in diabetes, chronic kidney disease, and cardiovascular disease: mechanisms and therapeutic perspectives. Expert Rev Clin Immunol. 2022;18:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 712] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 17. | Harder-Lauridsen NM, Krogh-Madsen R, Holst JJ, Plomgaard P, Leick L, Pedersen BK, Fischer CP. Effect of IL-6 on the insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2014;306:E769-E778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Ogata A, Morishima A, Hirano T, Hishitani Y, Hagihara K, Shima Y, Narazaki M, Tanaka T. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70:1164-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj D, Libby P, Davidson M; RESCUE Investigators. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:2060-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 496] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 20. | Tuttle KR, Brosius FC 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, Tanaka Y, Haneda M, Liu J, Silk ME, Cardillo TE, Duffin KL, Haas JV, Macias WL, Nunes FP, Janes JM. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33:1950-1959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 21. | Greenbaum CJ, Serti E, Lambert K, Weiner LJ, Kanaparthi S, Lord S, Gitelman SE, Wilson DM, Gaglia JL, Griffin KJ, Russell WE, Raskin P, Moran A, Willi SM, Tsalikian E, DiMeglio LA, Herold KC, Moore WV, Goland R, Harris M, Craig ME, Schatz DA, Baidal DA, Rodriguez H, Utzschneider KM, Nel HJ, Soppe CL, Boyle KD, Cerosaletti K, Keyes-Elstein L, Long SA, Thomas R, McNamara JG, Buckner JH, Sanda S; ITN058AI EXTEND Study Team. IL-6 receptor blockade does not slow β cell loss in new-onset type 1 diabetes. JCI Insight. 2021;6:e150074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/