Published online Jan 15, 2026. doi: 10.4239/wjd.v17.i1.110502
Revised: July 6, 2025
Accepted: November 18, 2025
Published online: January 15, 2026
Processing time: 220 Days and 18.9 Hours
Diabetic kidney disease (DKD) represents a significant challenge for diabetes management and public health as it affects the quality of life and metabolic control of millions of people around the world. Characterized by persistent al
Core Tip: Diabetic kidney disease is a major complication of diabetes and a leading cause of chronic kidney disease. Traditional markers such as albuminuria and estimated glomerular filtration rate often detect damage at a late stage, limiting early intervention. Emerging urinary biomarkers including kidney injury molecule 1, neutrophil gelatinase associated lipocalin, monocyte chemoattractant protein 1, and urinary microRNAs offer earlier and more specific detection of tubular injury, inflammation, and oxidative stress. These biomarkers enhance risk assessment, disease monitoring, and evaluation of treatment response. Incorporating them into clinical practice could enable earlier diagnosis, personalized therapy, and improved outcomes in diabetic kidney disease management.
- Citation: Gembillo G, Soraci L, Messina R, Lo Cicero L, Spadaro G, Cuzzola F, Calderone M, Ricca MF, Di Piazza S, Sudano F, Peritore L, Santoro D. Urinary biomarkers of diabetic kidney disease. World J Diabetes 2026; 17(1): 110502
- URL: https://www.wjgnet.com/1948-9358/full/v17/i1/110502.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i1.110502
Diabetic kidney disease (DKD) constitutes one of the most significant microvascular complications of diabetes mellitus and remains the leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) globally. Epidemiological data indicate that up to 30%-40% of individuals with diabetes will develop DKD during the course of their disease, imposing a substantial burden on healthcare systems worldwide[1]. Despite considerable advancements in glycemic control and renoprotective therapies, the morbidity and mortality associated with DKD continue to escalate, underscoring the imperative need for improved diagnostic and prognostic tools. The current diagnostic framework for DKD predominantly relies upon the assessment of albuminuria and estimated glomerular filtration rate (eGFR), as endorsed by the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in CKD. Elevated urinary albumin excretion, typically quantified via the urine albumin-to-creatinine ratio (uACR), is widely recognized as a marker of glomerular injury and an independent predictor of progressive renal impairment and cardiovascular events. Nonetheless, both albuminuria and eGFR possess inherent limitations. Albuminuria may exhibit substantial biological variability and can be influenced by non-renal factors such as exercise, infection, and hemodynamic changes, thereby reducing its specificity. Furthermore, a considerable proportion of individuals with diabetes exhibit progressive renal decline in the absence of significant albuminuria, a phenotype now recognized as non-albuminuric DKD (NADKD). NADKD may be especially prevalent in patients with type 2 diabetes, and these individuals tend to be older, more often female, and may progress more slowly to ESKD while still being at increased cardiovascular risk. Furthermore, albuminuria may appear only after substantial structural damage has already occurred, making it a relatively late indicator of disease onset. Emerging evidence suggests that albuminuria reflects primarily glomerular injury, while early tubular and interstitial damage, crucial in DKD pathogenesis, may remain undetected. This discrepancy underscores the need to supplement albuminuria with novel urinary biomarkers that can detect earlier, subtler renal injury and provide more comprehensive risk stratification. These biomarkers, reflecting distinct pathogenic processes including tubular injury, interstitial inflammation, oxidative stress, and fibrogenesis, offer the potential to provide a more sensitive and specific means of detecting early kidney damage, monitoring disease progression, and evaluating therapeutic response in DKD. Urinary biomarkers such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), monocyte chemoattractant protein-1 (MCP-1), and urinary microRNA (miRNA) have been investigated extensively and demonstrate promising associations with histopathological alterations and clinical outcomes that often precede overt microalbuminuria.
This review aims to provide a comprehensive synthesis of current evidence regarding urinary biomarkers in DKD, emphasizing their biological underpinnings, clinical applicability, and the challenges that impede their integration into routine clinical practice. Advancing biomarker-guided strategies holds the promise of facilitating earlier diagnosis, more precise risk stratification, and personalized therapeutic interventions, ultimately improving outcomes for patients with DKD. This review also aims to provide a comprehensive synthesis of current evidence regarding urinary biomarkers in DKD, emphasizing their biological underpinnings, clinical applicability, and the challenges that impede their integration into routine clinical practice. Advancing biomarker-guided strategies holds the promise of facilitating earlier diagnosis, more precise risk stratification, and personalized therapeutic interventions, ultimately improving outcomes for patients with DKD.
DKD is a common microvascular complication in both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), alongside diabetic retinopathy and peripheral neuropathy. The pathophysiology is complex and multifactorial, driven by an interplay between genetic susceptibility, environmental influences, and systemic metabolic derangements.
The primary pathogenic trigger is chronic hyperglycemia, which arises from absolute insulin deficiency in T1DM or insulin resistance in T2DM, disrupting normal glucose homeostasis. Sustained high glucose levels set in motion a cascade of pathogenic processes, such as oxidative stress, inflammation, and the activation of pro-fibrotic signalling pathways, which progressively damage renal structures, leading to glomerular sclerosis, tubulointerstitial fibrosis, and ultimately to a decline in kidney function.
Adipose tissue dysfunction further contributes by releasing excess free fatty acids and proinflammatory cytokines, that exacerbate insulin resistance and promote systemic inflammation[2]. Moreover, there is growing evidence that gut dysbiosis is involved in the development of insulin resistance, emphasising the important role of the intestinal microbiota in maintaining metabolic balance[3].
Mitochondrial glucose metabolism, whether under aerobic or anaerobic conditions, inevitably generates reactive oxygen species (ROS) as by-products with a marked imbalance between pro-oxidant and antioxidant systems[4]. This oxidative stress plays a central role in diabetic complications, including DKD, by inducing cellular injury in renal tissue[5].
Several molecular pathways have been identified in the progression of DKD. One key mechanism involves the accumulation of advanced glycation end-products, which primarily interact with their receptor and trigger a cascade of downstream signalling events that promote inflammation, oxidative stress, and structural alterations[6].
Another critical pathway is protein kinase C (PKC), particularly the PKC-α isoform, which is activated in hy
The hexosamine biosynthetic pathway also contributes to diabetic complications, leading to the generation of pro-inflammatory mediators such as tumor necrosis factor (TNF)-α, transforming growth factor-β, and intercellular adhesion molecule-1, which contribute to vascular and glomerular injury. Although this metabolic pathway was originally studied in the context of diabetic retinopathy, its effects also extend to the kidney and other tissues[8].
In addition, the polyol pathway becomes increasingly active under hyperglycemic conditions, with aldose reductase catalysing the reduction of glucose to sorbitol[9], impairing the cell’s antioxidant defences and exacerbating oxidative stress. Aldose reductase, its downstream effects, and the aforementioned pathogenic mechanisms are all associated with the development and progression of various diabetic complications, which often coexist with DKD or exacerbate its severity[10].
Histologically, early DKD is characterized by thickening of the glomerular basement membrane and loss of endothelial cell fenestrations, changes that are primarily due to oxidative stress and inflammatory insults. These initial alterations precede glomerular hypertrophy and are accompanied by a state of hyperfiltration[11] mediated in part by activation of the renin-angiotensin-aldosterone system (RAAS) and its induced vasoconstriction, and further contributes to glomerular injury, inflammation, and fibrosis[12]. Over time, these early structural and molecular changes progress to more severe pathological features, including diffuse mesangial sclerosis, nodular glomerulosclerosis, glomerular microaneurysms, and intimal fibrosis[13].
An emerging concept in the progression of DKD is metabolic memory, which refers to persistent cellular and tissue dysfunction, even after normoglycemia is achieved. This concept highlights the importance of early intervention and may involve epigenetic modifications, such as DNA methylation and histone acetylation, as well as long-lasting inflammatory and oxidative signals[14].
The treatment of DKD remains particularly challenging due to its multifactorial and progressive pathophysiology, which involves a complex interplay of metabolic, hemodynamic, inflammatory, and fibrotic mechanisms[15]. This complexity necessitates a multifaceted therapeutic approach, yet the heterogeneity in disease progression and individual patient response further complicates effective long-term management[16,17]. The main metabolic, hemodynamic, and inflammatory pathways contributing to DKD are summarized in Figure 1.
In this context, the identification of reliable and specific biomarkers for DKD is crucial, as they could enable earlier diagnosis, better risk stratification, and more personalized therapeutic strategies, ultimately improving clinical outcomes and slowing disease progression.
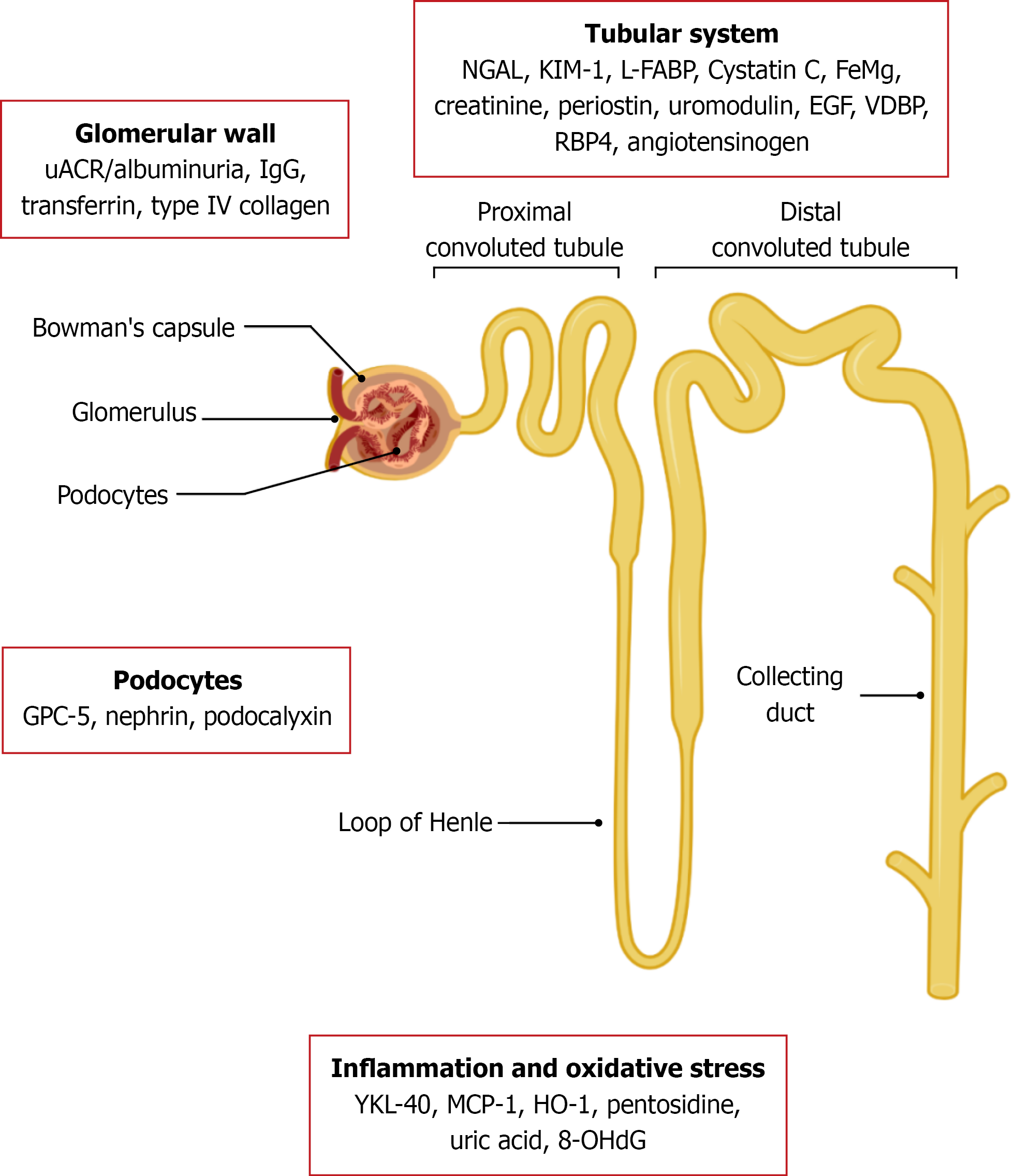
Urinary biomarkers play a pivotal role in understanding the pathogenesis of DKD and offer promising tools for early diagnosis, monitoring progression, and guiding therapeutic interventions. These biomarkers can be categorized based on the renal compartment or pathological process they primarily reflect, such as glomerular damage, tubular injury, inflammation, oxidative stress, or fibrosis (Table 1)[18-30].
| Biomarker | Source | Diagnostic accuracy | Clinical significance and validation status | Prospective | Contras and limitations |
| uACR/albuminuria | Glomerular capillary wall | AUC: 0.522[18]-0.933[19]; sensitivity 77% and specificity 92% for microabuminuria; sensitivity 88% and specificity 90% for macroalbuminuria[20] | Standard early marker for DKD; persistent elevation indicates nephropathy | Widely used; inexpensive, reproducible | Affected by blood pressure, exercise, and infections (risk of false positives). DKD may be normoalbuminuric normal uACR and albuminuria do not always exclude DKD |
| GPC-5 | Podocyte injury | ROC curve of urinary GPC-5/creatinine ratio comparing T2DM without nephropathy and those with nephropathy reveals a sensitivity of 93.3% and a specificity of 80%[21] | Emerging marker of podocyte damage; may reflect early glomerular dysfunction in DKD; more studies needed to validate it as a diagnostic tool of incipient nephropathy | Potential early marker of podocyte injury; non-invasive detection in urine | Limited validation (small sample size studies); clinical utility and standardization not yet established |
| Transferrin | Large protein leakage from the glomerular wall | AUC: 0.846 (95%CI: 0.810-0.882); sensitivity 69.8%, specificity 90.1% (for a cut-off value of 1.15)[22] | Suggests early glomerular barrier compromise before albuminuria. Since significant transferrin levels have been found in normoalbuminuric T2DM patients, it could be suitable as a marker of early-stage DKD; nevertheless, very limited body of research | Earlier than macroalbuminuria; non-invasive | Limited specificity (false positives in primary glomerulonephritis and hypertension in nondiabetic populations; elevated in other proteinuric states) |
| Immunoglobulins (IgG and IgM) | Large protein leakage from the glomerular wall | AUC: 0.894 (95%CI: 0.848-0.930); sensitivity 75.53%, specificity 92.31% (for a cut-off value of 8.56)[23] | Indicates severe dysfunction. Limited body of evidence in T2DM | Indicative of more advanced damage; could be used in combination with transferrin to enhance diagnostic values | May be negative until late stages of disease (due to high molecular weight of IgG). Testing is more invasive on the one hand, and insufficiently standardized on the other |
| Nephrin | Podocyte injury | Not available for DKD | Promising marker for detection of early glomerular injury in multiple studies[24] | Specific to podocyte injury; early detection possible | It does not predict early renal dysfunction in DKD[40]. Requires specialized assays, limited availability |
| Type IV collagen | Matrix remodeling at the glomerular basement membrane | AUC not consistently reported; significantly elevated in early DKD[25]. More sensitive than microalbuminuria but exact percentages not uniformly available | Associated with structural glomerular damage and fibrosis. Validated in large multicentric cohort across DKD stages. Some promising data, but more investigation on larger scale is needed | Reflects chronic structural damage, therefore useful to detect fibrosis | Limited validation (small-to-moderate sample size studies; lack of ROC quantification and cut-offs)[26,30]. Limited utility in early DKD, overlaps with other markers |
| Podocalyxin | Podocyte injury | AUC: 0.86 (95%CI: 0.81-0.91) alone; combined with α2-MG: AUC: 0.88 (95%CI: 0.83-0.93)[27], in another small study, AUC: 0.996[29]. Sensitivity 73.3%, specificity 93.3% at cut-off 43.8 ng/mL[28] | Linked to detachment and loss of podocytes. Validated in cross-sectional and prospective cohorts of 42-200 patients[27]. Might be a highly accurate biomarker of early podocyte injury, but more studies on larger scale are needed | Sensitive for podocyte detachment, useful in active disease | Not widely validated (limited sample size studies)[27,29]; inter-assay variability; requires advanced laboratory methods |
Glomerular biomarkers provide valuable insights into early and progressive damage to the glomerular filtration barrier in DKD. As outlined in Table 1, these markers reflect distinct aspects of glomerular injury, including permeability changes (e.g., albuminuria), podocyte damage (e.g., nephrin, podocalyxin), and structural remodeling (e.g., type IV collagen). High-molecular-weight proteins like transferrin and immunoglobulins G (IgG), typically restricted by an intact glomerular barrier, appear in urine only when that barrier is compromised, indicating more advanced damage. Additionally, markers like ceruloplasmin signal oxidative stress and inflammation within the glomerulus. Collectively, these biomarkers enhance diagnostic and prognostic accuracy beyond albuminuria alone.
UACR: The uACR, is a key indicator of kidney damage, particularly in DKD. A higher ACR, specifically above 30 mg/g, can be an early sign of kidney disease, even with normal eGFR. uACR is commonly used to stratify the risk for progression of CKD and DKD, morbidity and mortality. Schultes et al[31] studied the impact of uACR point-of-care testing (POCT) on the diagnosis and management of DKD. Their study showed that DKD was suspected at visit in 9.9% of the participants based on the actual ACR POCT values, thus ACR POCT increased the detection rate of DKD in patients with diabetes. In 18.5% of the entire study population, ACR POCT led to a change of medication. ACR POCT showed, in this research, a considerable impact on the diagnosis and treatment of DKD.
The diagnosis of DKD is based on some clinical findings, such as albuminuria, which is defined as ACR > 30 mg/g or urinary albumin of 30-300 mg/day. It is classified as the first DKD indicator, a biomarker for the progression of this disease and a cause of GFR reduction. Albumin is a small negatively charged protein synthesized by the liver and it is poorly filtered by glomeruli due to electrostatic repulsion; in fact, in healthy patients, levels of albuminuria should be under 30 mg in a 24-hour urine sample. Albumin urinary excretion is controlled by several hormones, including glucocorticoids and insulin, that are impaired in conditions like diabetes, this dysregulation leads to abnormal albumin metabolism. When the integrity of the glomerular filtration barrier is altered, there are increased levels of albuminuria due to the major pore size and the impaired electrical homeostasis of glomerular basement membrane. It is known that albuminuria is also linked to the megalin and cubilin encoding gene (CUBN): Reduced megalin expression is associated with lower capacity of the kidney tubular epithelial cells to reabsorb albumin filtered by glomeruli[32] and CUBN is involved in the development of albuminuria in European and African populations[33]. Albuminuria is categorized into three levels: A1 < 30 mg/24 hours or < 30 mg/g (normal or mildly increased); A2 30-300 mg/24 hours or 30-300 mg/g (moderately increased); A3 > 300 mg/24 hours or > 300 mg/g (severely increased)[34]. It will gradually worsen and turn into clinically severe albuminuria grade A3 at 10-15 years after diabetes diagnosis. The prevalence of this biomarker is different between patients with type 1 and type 2 diabetes: In T2DM albuminuria A3 ranges from 5% to 48% and A2 occurs in 20% of cases; In T1DM A3 ranges from 8% to 22% and A2 will be found in 13% of cases[34]. The presence of albuminuria A2 in a diabetic patient must be confirmed by two measurements to achieve the diagnosis of DKD, although a significant number of patients with diabetes may present prevailing interstitial damage with no glomerular lesion. Thus, renal function could be impaired without the development of albuminuria. These patients are known as non-albuminuric, affected by NADKD. Their phenotype is characterized by decreased eGFR and albuminuria < 30 mg/24 hours and ACR < 17-30 mg/g. It is possible that this pathological pathway is different from the typical presentation[35]. NADKD has spread among the diabetic population, found in approximately 20%-40% of DKD cases and it presents with a non-typical phenotype: Patients with NADKD tend to have a slower eGFR decline than albuminuric patients and diabetic patients without DKD. They also have a lower risk of progression to ESKD, they are older, often women, with shorter duration of diabetes, lower glycosylated hemoglobin levels and blood pressure[35]. Although there are differences between NADKD and ADKD, a subset of patients might also progress gradually to the end stage without albuminuria. It is fundamental to underline that the occurrence of albuminuria is strictly linked to a major cardiovascular risk. In addition to a reduced eGFR, it increases mortality from all causes, such as endocrinological, metabolic and cardiovascular[36]. Therefore, it is recommended that albuminuric patients are treated promptly with diet, lower blood levels, angiotensin converting enzyme inhibitor/angiotensin receptor blocker, sodium-glucose cotransporter-2 inhibitors, nonsteroidal mineralocorticoid receptor antagonist, or glucagon-like peptide-1 receptor agonist, in order to reduce the glomerular damage and slow the progression of DKD and cardiovascular complications[37]. Albuminuria is linked to an increased risk of developing subclinical atherosclerosis[38] both as a diabetes complication and as an independent risk factor itself. It is also associated with myocardial infarction and heart failure with systolic dysfunction; in fact, it is also correlated with renal and cardiac fibrosis[39].
Glypican-5: Glypican-5 (GPC-5), a podocyte surface heparan sulfate proteoglycan, has emerged as a significant glomerular biomarker in DKD. Elevated urinary GPC-5 levels have been observed in patients with T2DM, particularly those exhibiting early-stage kidney damage. Studies have demonstrated a strong correlation between urinary GPC-5 concentrations and common markers of renal injury, such as albuminuria and eGFR. El-Kafrawy et al[21] demonstrated that urinary GPC-5 concentrations were significantly higher in diabetic patients with renal involvement compared to those without DKD and healthy controls. Furthermore, a 52-week follow-up study indicated that increased urinary GPC-5 levels were associated with a decline in eGFR and an increase in albuminuria, suggesting its potential as an early indicator of renal dysfunction in DKD.
Transferrin: Urinary transferrin has emerged as a promising biomarker for early detection and monitoring of DKD. As a low-molecular-weight glycoprotein (76.5 kDa), transferrin is more readily filtered through the glomerular barrier compared to albumin, making its urinary excretion a sensitive indicator of glomerular injury. Elevated urinary transferrin levels have been observed in diabetic patients, even in the absence of albuminuria. In fact, a recent meta-analysis by Li et al[40] demonstrated that urinary transferrin has a high combined sensitivity and specificity for diagnosing early DKD. Furthermore, longitudinal studies have shown that increased urinary transferrin excretion predicts the development of microalbuminuria in normoalbuminuric diabetic patients. For instance, a study[41] reported that urinary transferrin levels correlated with the progression of biopsy-proven glomerular diffuse lesions and interstitial fibrosis, suggesting its role in monitoring disease progression. Additionally, another study found that urinary transferrin excretion increased significantly with the severity of glomerular lesions in diabetic patients, further supporting its utility as an early biomarker of DKD[42]. These findings underscore the potential of urinary transferrin as a non-invasive biomarker for the early detection and monitoring of DKD, offering a valuable tool for clinicians in managing patients with diabetes at risk of renal complications.
Immunoglobulins: Urinary IgG and immunoglobulin M (IgM) have emerged as significant biomarkers in the early detection and progression monitoring of DKD. Urinary IgG excretion is notably higher in diabetic patients compared to healthy controls, even before the onset of microalbuminuria. This elevation correlates with the progression of glomerular diffuse lesions and can predict the development of microalbuminuria in normoalbuminuric diabetic patients. Furthermore, urinary IgG levels have been associated with the severity of glomerular damage[43]. IgM, being the largest antibody, also appears in urine in diabetic patients, reflecting significant glomerular injury. Increased urinary IgM excretion has been linked to a higher risk of renal failure and is considered an early predictor of DKD progression, independent of albuminuria levels. Notably, urinary IgM excretion is inversely associated with renal survival in diabetic patients, suggesting its potential as a prognostic marker[44]. Importantly, not only total IgG but also its subtypes, such as IgG2 and IgG4, have shown relevance. Gohda et al[45] reported that an elevated IgG2/IgG4 ratio in urine is indicative of altered glomerular size and charge selectivity, which are early indicators of DKD. Another study, further demonstrated that a urinary IgG threshold above 2.45 mg/L was predictive of DKD onset and progression, reinforcing its utility in clinical risk stratification[46]. In support of these findings, Narita et al[47] demonstrated that increased urinary excretions of IgG, along with ceruloplasmin and transferrin, significantly predicted the development of microalbuminuria in patients with type 2 diabetes, suggesting a role for these proteins as early markers of glomerular injury, even before classic albuminuria appears. This predictive value was further reinforced by an earlier study[48], which showed a parallel increase in urinary IgG and other plasma proteins in normoalbuminuric diabetic patients, indicating early glomerular permeability changes independent of albumin excretion. Notably, these abnormalities appear to be reversible with improved glycemic control, as shown by Narita et al[49], who reported that tighter glucose regulation significantly reduced urinary IgG and ceruloplasmin excretion. These studies collectively emphasize that urinary IgG not only reflects existing renal damage but also serves as a dynamic biomarker responsive to therapeutic interventions, underscoring its prognostic and potentially diagnostic utility in early DKD.
Nephrin: Nephrin is a transmembrane protein expressed in glomerular podocytes (180 kDa) and plays a crucial role in maintaining the structural integrity of the glomerular filtration barrier. Its urinary excretion, termed “nephrinuria”, has emerged as an early and sensitive indicator of podocyte injury and is increasingly recognized as a potential biomarker for DKD. Elevated nephrin levels have been detected in the urine of patients across a range of glomerular pathologies, including DKD, lupus nephritis, and preeclampsia, supporting its utility in diverse clinical contexts[50]. Notably, ne
To date, the best validated non-invasive biomarker to detect the onset of diabetes mellitus (DM) is microalbuminuria, but recently it has been shown that many predictive markers are able to detect the presence of the disease even before albuminuria. In addition to the glomerular involvement, there is also a tubular component in the renal complications of diabetes, as demonstrated by the detection of renal tubular proteins and enzymes in the urine. The tubular involvement may precede the glomerular involvement, as these tubular products are already detectable before microalbuminuria[56]. A summary of urinary tubular biomarkers important in the diagnosis of DKD is reported Table 2[57-70].
| Biomarker | Source | Diagnostic accuracy | Clinical significance | Validation status | Prospective | Contras |
| NGAL | Proximal and distal tubular stress injury | AUC: 0.88 (0.84-0.90); sensitivity 0.82, specificity 0.81[57] | Early marker of tubular injury; predictive of nephropathy before albuminuria | Larger-scale, standardized prospective studies are needed to implement uNGAL as a biomarker into routine clinical use | Detects injury before traditional biomarkers; non-invasive and stable in urine | Not specific for DKD; elevated in infections or AKI; limited validation (small sample size studies only, high heterogeneity among studies in terms of results and methodologies) |
| KIM-1 | Proximal tubular injury | AUC: 0.85 (0.82-0.88); sensitivity 0.68, specificity 0.83[58] | Sensitive marker of early tubular injury in DKD; correlates with disease progression | Some promising data, but whether it can be used as a new marker for diagnosing DKD needs further investigation | Highly specific for proximal tubular damage; FDA-qualified for nephrotoxicity | Limited data in T1DM; limited validation (high heterogeneity between studies in terms of results and methodologies) |
| L-FABP | Lipid metabolism and proximal tubular stress | AUC: 0.97 (0.94-1.00); sensitivity 100%, specificity 86.67%[59] | Reflects oxidative and lipid-induced tubular damage | Available research seems to highlight high predictive power even compared to other urinary markers including microalbuminuria, but more studies are necessary in order to validate it as a standard biomarker | Sensitive to oxidative stress and useful for early diagnosis | Affected by diet and comorbidities (false positives); requires special assays; limited validation (small sample size studies only, high heterogeneity among studies in terms of results and methodologies) |
| MCP-1 | Inflammatory chemokine secreted by monocytes and macrophages | AUC: 0.546 (0.453-0.638); sensitivity 67.3%, specificity 50.0%[60] | Correlates with tubulointerstitial inflammation and progression of DKD | May not be sufficient as a standalone diagnostic marker for DKD | Correlates with inflammation and disease activity; useful in prognosis | Moderate sensitivity; poor-to-fair discrimination. Levels can fluctuate widely depending on the extent of kidney damage and the presence of inflammatory stimuli |
| Cystatin C | Proximal tubular function | AUC: 0.807 (0.741-0.873) sensitivity, 70.9%; specificity, 86.3%[61,62] | Indicates proximal tubular dysfunction; used in conjunction with serum cystatin C | Although not specifically approved as a stand-alone biomarker for DKD, it is well recognized as a valuable marker of kidney function and for assessment of overall kidney health, including in the context of DKD | Non-invasive, standardized assays available | Affected by inflammation, corticosteroids, thyroid dysfunction and infections (false positives); not highly specific |
| Creatinine | Muscle metabolism and tubular function | AUC: 0.604-0.8[63] | Used to normalize other urinary biomarkers; reflects kidney filtration but not specific | Limited diagnostic value alone; rarely used; not FDA approved | Readily available, useful for comparative purposes | Not specific or sensitive for kidney damage (it reflects filtration rather than damage); limited diagnostic value alone |
| Fe Mg | Tubular magnesium handling | No data on AUC, sensitivity, or specificity | Early sign of tubular reabsorption impairment | May not be sufficient and specific as a diagnostic biomarker of DKD | Potential early tubular dysfunction marker; measurable in clinical laboratories | Lack of standardization, diagnostic accuracy data, and large-scale validation (limited sample sizes, no established cut-offs) |
| Angiotensinogen | RAAS activity | No data on AUC, sensitivity, or specificity | Reflects intrarenal RAAS activity; elevated in DKD and hypertension | uAGT remains a candidate “theragnostic” biomarker predicting response to RAAS inhibition; further rigorous studies are needed to determine its potential diagnostic role in DKD | Correlates with DKD severity; reflects RAAS dysregulation | Values can be influenced by sex hormones (false positives). Overlap with systemic RAAS activation; requires careful interpretation. Lack of external validation (lack of diagnostic accuracy data, small sample size studies) |
| Periostin | Tubular fibrosis | AUC: 0.78 (0.71-0.86); sensitivity 80.7%, specificity 43.3% (for a cut-off value of 1.01 ng/mg Cr)[64] | Associated with tubular injury and fibrosis; elevated in early DKD | Still sparse, although consistent body of research regarding its role as an early biomarker of DKD | Reflects fibrotic transformation early; may guide interventions | Emerging marker; limited clinical validation and availability (small and cross-sectional studies). Role not clear in early DKD |
| Uromodulin | Thick ascending limb health | Regarding the ability to discriminate between patients with DKD and patients with CKD without diabetes, AUC-ROC for the urinary glycated uromodulin (glcUMOD) resulted in 0.715 (0.597-0.834)[65]; urinary uromodulin with an AUC of 0.81 (sensitivity 80%, specificity 60%) and exosomal UMOD gene expression with an AUC of 0.95 (sensitivity 92%, specificity 84%) are elevated in early DKD[66] | Lower levels associated with increased mortality risk and DKD progression | glcUMOD could represent a novel biomarker for DKD; determination of the clinical value of glcUMOD requires further study | Inverse association with outcomes; not expressed in damage; potentially protective marker | Not a direct damage marker; interpretation depends on clinical context; the limited research has focused on its translational modification (glcUMOD) |
| EGF | Tubular epithelial regeneration | Regarding the association between lower uEGF to creatinine ratio with new-onset eGFR < 60 mL/minute per 1.73 m2: In T2DM normoalbuminuric patients: AUC-ROC: 0.85 (0.81-0.90)[67] | Involved in tubular repair | Low uEGF to creatinine ratio could serve as a biomarker of progressive renal function deterioration in normoalbuminuric patients | Inverse marker (lower levels predict DKD progression); decreases before clinical deterioration | Not routinely measured, lack of sufficient validation |
| VDBP | Vitamin D transport, liver and tubular cells | No data on AUC, sensitivity, or specificity. Significantly elevated in diabetics with normal, micro- and macroalbuminuria compared to controls, in stepwise fashion (SMDs: 1.52, 1.81, and 1.51, respectively; all P < 0.00001)[68] | Elevated in DKD; correlates with albuminuria and tubular injury | uVDBP could be a promising tubular injury biomarker but requires formal accuracy studies on larger scale | Strong correlation with DKD progression; measurable with multiplex assays | Lack of specificity; also elevated in other renal pathologies (false positives); lack of diagnostic accuracy data and established cut-offs |
| RBP4 | Tubular stress, adipocytes | AUC: 0.746 (0.659-0.834), sensitivity 84.6%, specificity 62.5%[69] | Associated with tubular stress and insulin resistance in DKD | Predictive role of urinary RBP4 requires further investigation, with most studies involving circulating/serum RBP4 | Associated with metabolic stress and insulin resistance; useful in early DKD | Limited normative data; influenced by obesity (false positives); it may be complementary in the diagnosis of early DKD |
| YKL-40 | Inflammation and remodeling (monocytes and chondrocytes) | It seems associated with a greater risk of kidney function decline and mortality on a limited male cohort[70] | Elevated in DKD; linked to mortality and inflammation | Serum/plasma YKL-40 predictive power of early DKD has been validated by multiple studies | Rise relevant in DKD | Emerging marker, lacks widespread validation |
NGAL: NGAL, a 25-kDa glycoprotein belonging to the lipocalin family, is covalently bound to gelatinase from human neutrophils and expressed by epithelial cells including those in the kidney. It is notably released by both granulocytes and proximal and distal tubular epithelial cells under ischemic, toxic, or inflammatory stress conditions[71]. In the context of acute kidney injury, NGAL synthesis in the distal and proximal tubule is rapidly and massively upregulated, leading to a quick increase in its urinary concentrations, which often precede the rise in serum creatinine; early elevations in urinary NGAL (uNGAL) are therefore predictive of nephrotoxic and/or ischemic kidney injury[72]. It is worth noting that uNGAL increases not only in conjunction with renal tubular damage, but also in bacterial infections, severe systemic inflammatory responses, and chronic diseases, limiting its specificity[73].
In the context of DKD, tubular damage often precedes glomerular injury, implying the relevance of uNGAL as an emerging biomarker for early detection of the disease, even before the onset of incipient nephropathy[74]. uNGAL could predict the development of albuminuria and may be utilized as a noninvasive indicator for diagnosing, staging, and monitoring the progression of DKD[75].
A recent cross-sectional study by Varatharajan et al[76] proved uNGAL to be significantly elevated in normoalbuminuric diabetic patients when compared to healthy subjects, suggesting this particular biomarker could predict DKD even before to the onset of microalbuminuria, with a sensitivity and specificity of 75.8% and 55%, respectively; these findings are consistent with the results of several previous studies. Most notably, Tang et al[57] formerly conducted a meta-analysis of fourteen observational studies published between 2008 and 2019, indicating NGAL as a preliminary marker of DKD, with reported pooled sensitivity and specificity of 96% and 89%, respectively, in three cohort studies, as compared with 82% sensitivity and 81% specificity in eleven cross-sectional studies. Subsequently, Quang et al[77] found that NGAL had a sensitivity and specificity of 60% and 70%, respectively, in predicting the early stages of DKD, with a cut-off value of 35.2 ng/mL. In addition, Phanish et al[78] described NGAL as a useful biomarker for progressive CKD in nonalbuminuric subjects, suggesting a potentially relevant role of proximal tubular injury and inflammation[79] in the pathophysiology and advancement of DKD. More recently, a systematic review by Karimi et al[80] characterized NGAL as a valuable indicator of initial renal damage in DKD, providing higher sensitivity and specificity compared to proteinuria; furthermore, Kashgary et al[81] conducted a retrospective case-control study corroborating the diagnostic value of NGAL as an early urinary biomarker of DKD, with a sensitivity and specificity of 100% at a cut-off value of 322.5 pg/mL. It is worth noting that the vast majority of available research seems to confirm the value of NGAL as a preliminary indicator of DKD. However, a study by Kim et al[82] questioned its early diagnostic usefulness, as urinary concentrations did not to differ significantly between normoalbuminuric patients and healthy controls.
UNGAL could not only serve as an early indicator of disease, but also as a marker of damage progression[83,84], as indicated by the research from Siddiqui et al[85], which showed a positive correlation between uNGAL levels and progressive kidney impairment. In this context, Duan et al[86] highlighted a higher risk of nephrotic-range proteinuria along with unfavorable renal outcomes in T2DM patients with higher concentrations of uNGAL. As stated by Satirapoj et al[87], uNGAL levels significantly correlate with eGFR decline and higher serum creatinine, providing further evidence of the prominent role that tubulointerstitial changes play in progressive DKD. In a post-hoc analysis of the VA NEPHRON-D trial[88], Chen et al[70] observed that higher baseline uNGAL was independently associated with a 10%-40% increase in the risk of all-cause mortality in a cohort of DKD patients, while a large-scale study by Ide et al[89] followed 2685 DKD patients over a 5-year period, proving uNGAL levels to significantly predict eGFR deterioration in albuminuric subjects. Nevertheless, Chou et al[90] on the one hand, and Rotbain Curovic et al[91] on the other failed to determine a negative correlation between uNGAL and kidney function; it is then safe to affirm that additional high-quality, prospective studies involving larger populations are needed to draw definite conclusions and advance our understanding of uNGAL as a predictor of DKD progression.
Most of the research evaluating the diagnostic and prognostic value of NGAL in DKD was conducted in T2DM-affected populations, implying a shortage of validation in T1DM. Interestingly, a recent meta-analysis involving children and adolescents with T1DM demonstrated uNGAL as an accurate and reliable biomarker for early detection of T1DM-related kidney disease in pediatric populations[92]. However, a previous study by Valent Morić et al[93] did not find a significant difference in uNGAL levels between pediatric patients with type 1 diabetes and healthy controls, suggesting that NGAL should be used cautiously and not as the sole marker for DKD in children and adolescents.
Notably, there is a limited body of research examining the association between uNGAL levels and renal histopathological changes assessed by kidney biopsy. A prospective analytical study from Garg et al[94] demonstrated a statistically significant correlation between urinary NGAL concentrations and morphometric indicators of renal injury in DKD patients, specifically the extent of chronic inflammatory cell infiltration and glomerular sclerosis. These results support the potential utility of uNGAL as a biomarker for personalized management of DKD, facilitating treatment stratification according to underlying disease activity and histological severity.
KIM-1: KIM-1, also known as hepatitis A virus cellular receptor 1 or T-cell mucin domain-1, is a type I transmembrane glycoprotein, belonging to the immunoglobulin and T-cell mucin domain family. Under normal physiological conditions, it is minimally expressed in the kidneys. However, upon proximal tubular injury, due to ischemic, toxic, or inflammatory insults, its expression is markedly upregulated on the apical membrane of proximal tubular epithelial cells. The extracellular portion can split and rapidly enter the tubular lumen after renal injury and then be detected in urine. It has been confirmed that the level of KIM-1 in urine is closely related to the level of KIM-1 in tissue and correlated with renal tissue injury. KIM-1 has not only been shown to be an early biomarker of acute kidney injury but also has a potential role in predicting long-term renal outcome.
Several human studies indicate that tissue expression and urinary excretion of the KIM-1 ectodomain are sensitive and specific markers of injury, as well as predictors of outcome[95]. Recent data suggest that KIM-1 expression has a protective effect during early injury, while in chronic disease, prolonged KIM-1 expression may be maladaptive and could represent a target for the therapy of CKD[96]. In DKD, tubular damage often precedes glomerular injury, and urinary KIM-1 (uKIM-1) has emerged as a potential early marker of the disease as well as a prognostic marker; elevated uKIM-1 levels have been independently associated with increased all-cause mortality in patients with CKD and diabetes, even after adjusting for traditional risk factors such as eGFR and albuminuria[97]. This aligns with recent findings from the chronic renal insufficiency cohort study, which identified KIM-1 and chitinase-3-like protein-1 (also known as YKL-40) as predictors of adverse outcomes in DKD, even after multivariate adjustment.
Furthermore, insulin resistance is a known contributor to DKD onset and progression; a study involving normoalbuminuric patients with T2DM demonstrated that higher insulin resistance, assessed by the homeostasis model assessment of insulin resistance, correlates with increased uKIM-1 levels; this biomarker can serve as an early indicator of renal tubular injury associated with insulin resistance, even in the absence of glomerular involvement[98].
A Chinese case-control study of 129 patients (101 patients with diabetes vs 28 healthy controls) showed significantly higher uKIM-1 levels in diabetic patients: [120.0 (98.4-139.9) ng/g creatinine vs 103.1 (86.8-106.2) ng/g creatinine]; However, no differences were found between normoalbuminuric, microalbuminuric and macroalbuminuric subgroups, nor was a statistically significant correlation observed with eGFR or uACR, indicating potential limitations in CKD stage discrimination[99].
El-Ashmawy et al[100] performed a cross-sectional study involving 60 diabetic patients, with serum creatinine higher than 2 mg/dL, and 20 healthy controls. Similar to previous studies, uKIM-1 levels were ten-fold higher in diabetic microalbuminuric patients compared to control and normoalbuminuric diabetic patients; also, KIM-1 positively correlated with creatinine, urea, body mass index, microalbuminuria and duration of disease; it was negatively correlated with eGFR. Similar results were found in another independent diabetic cohort by Aslan et al[101].
Notably, Kin Tekce et al[102] also showed how KIM-1 levels change among the different stages of CKD, with levels being higher in stage II (2962 ± 724) compared to stage III (2168 ± 642) (P = 0.025) and IV (1765 ± 483) (P = 0.021). More importantly, a double-blind, randomized, placebo-controlled trial[103] studied the utility of KIM-1 in the progression of DKD. At baseline, KIM levels were higher in patients with microalbuminuria [143.5 (98-225)] and macroalbuminuria [281 (186-474)] compared to those in normoalbuminuric [97 (64-145)] patients. Subsequently, KIM-1 was statistically significantly associated with kidney outcome only in unadjusted analysis, but lost significance in the fully adjusted model (hazard ratio = 1.4; KIM 95% confidence interval: 1.0 to 1.9; P = 0.07).
A study aimed to highlight the clinical value of urinary biomarkers using Luminex liquid suspension chip technology. 737 patients were recruited: 585 with DM and 152 with DKD. Propensity score matching of demographic and medical characteristics identified a subset of 78 patients (DM = 39, DKD = 39). It was revealed that urinary levels of vitamin D-binding protein, retinol-binding protein 4 and KIM-1 were significantly higher in the DKD group than in the DM group (P < 0.05), while the levels of tissue inhibitor of metalloproteinase-1, TNF receptor (TNFR)-1, TNFR-2, alpha 1-microglobulin (α1-MG), β2-MG, cystatin C (CysC), nephrin and epidermal growth factor were not significantly different between the groups. This underscores the diagnostic value of uKIM-1 and other selected tubular biomarkers over conventional glomerular indicators in distinguishing early DKD[40].
Liver-type fatty acid-binding protein: Liver-type fatty acid-binding protein (L-FABP) is a cytoplasmic low molecular weight (15 kDa) carrier protein predominantly synthesized and expressed in the proximal tubules of the kidney and the liver. It plays a crucial role in the intracellular transport and metabolism of fatty acids. In the context of DKD, urinary L-FABP (uL-FABP) has emerged as a sensitive biomarker reflecting early tubular injury, often preceding detectable changes in albuminuria[59]. The study by Zhang et al[104] reported significantly higher uL-FABP concentrations in individuals with normoalbuminuria compared to healthy controls, suggesting its potential as an early indicator of renal tubular dysfunction in DKD. Furthermore, uL-FABP levels have been shown to correlate negatively with eGFR and positively with the albumin-to-creatinine ratio, highlighting its association with both glomerular and tubular damage. Additionally, a recent study by Chen et al[105] emphasized the role of uL-FABP in reflecting tubulointerstitial injury, independent of albuminuria, and its potential in assessing the effectiveness of therapeutic interventions in DKD. The clinical relevance of uL-FABP is further underscored by its association with cardiovascular outcomes. A long-term observational study by Araki et al[106] found that higher urinary levels of L-FABP were associated with deteriorating renal function and a higher incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy, suggesting its role in predicting future renal and cardiovascular events. In conclusion, uL-FABP serves as a promising biomarker for the early detection and monitoring of DKD. Its ability to reflect tubular injury independent of albuminuria, coupled with its associations with renal function and cardiovascular outcomes, underscores its potential clinical utility in managing patients with diabetes at risk of nephropathy.
MCP-1: MCP-1, or CCL2 is a chemokine that plays a pivotal role in the recruitment of monocytes, macrophages, and other immune cells to sites of inflammation. Studies have demonstrated that MCP-1 contributes to the progression of renal injury by promoting immune cell infiltration and fibrosis in various kidney diseases, including DKD[107]. MCP-1 is upregulated and highly expressed in the glomerular and renal tubular epithelium in diabetes, with an increase in urinary MCP-1 levels correlating with interstitial inflammatory infiltration and renal tissue macrophage accumulation[108]. Serum MCP-1 levels are occasionally elevated in diabetic patients, although this increase does not correlate with albuminuria, kidney macrophage accumulation, or nephropathy development. In contrast, urinary MCP-1 levels closely reflect kidney MCP-1 production and significantly correlate with albuminuria, serum glycated albumin, urine N-acetylglucosaminidase, and kidney cluster of differentiation 68 + macrophages in both human and experimental DKD[107]. On this matter, Titan et al[109] demonstrated that urinary MCP-1, together with retinol-binding protein, independently predicts renal outcomes in macroalbuminuric DKD, underscoring their value in risk stratification beyond conventional clinical markers such as albuminuria and eGFR. Furthermore, another study by Fufaa et al[110] provided evidence that elevated urinary MCP-1 levels are associated with early DKD lesions in T1DM, reinforcing MCP-1’s role as a biomarker of incipient renal damage before overt clinical manifestations. In conclusion, urinary MCP-1 might be a promising biomarker for early detection and prognosis of DKD, reflecting renal inflammation and predicting disease progression beyond traditional markers.
Urinary CysC: CysC is a cysteine proteinase inhibitor; in a healthy condition, this molecule is almost freely filtered by the renal glomeruli, so the urinary CysC (uCysC) is, in a healthy patient, absent and there is no tubular secretion of CysC, even if we consider the differences among the population (e.g., age or muscle mass). This value is affected by thyroid dysfunction, corticosteroids, and inflammation[111]. Zeng et al[112] performed a consecutive cohort study where they highlighted that uCysC was elevated in T2DM and DKD patients before the outbreak of albuminuria and with a normal uACR. Higher uCysC levels resulted from chronic tubular damage, which can be detected earlier using this marker. Thus, uCysC can predict the progression of T2DM patients with DKD. Furthermore, it is fundamental to underline that T2DM patients with rapid DKD progression show significantly increased levels of uCysC compared with the non-rapid progression group and uCysC levels are positively correlated with uACR in both diabetes and prediabetes: UCysC may play a major role in the early diagnosis of DKD in prediabetes[113]. It is important also to evaluate urinary leakage of other proteins [non-albumin protein (NAP)]. Kim et al[114] in a prospective observational study demonstrated that uCysC was increased in patients with macroalbuminuria, independently of serum CysC. In addition, it showed that NAPs, which include α1-MG, β2-MG, IgG, transferrin nephrin, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1, are stronger predictors for renal and tubular damage than uCysC. They might be considered in the prediction of renal impairment, and in normoalbuminuric patients[115].
Urinary creatinine: Urinary creatinine (UCr) is another biomarker that might be associated with tubular damage, a dysfunction that occurs in diabetic patients. Several studies focused on the possibility that UCr is an early predictor of DKD. Cao et al[116], in their retrospective cross-sectional analysis, observed a non-linear relationship between UCr and DKD. In particular, the risk of developing DKD seems to decrease when UCr is below a threshold of 17421 mmol/L. This information is important because it highlights that UCr represents the compensatory function of the kidneys. As soon as UCr is above this level, there is a positive correlation with DKD progression[117]. This indicates that creatinine excretion increases when damage occurs and renal stress is exacerbated. A combined approach, using UCr with uACR and eGFR, might lead to more accurate risk stratification in DKD patients. In addition, Sinkeler et al[118] showed that creatinine excretion ratio was inversely proportional to risk for mortality of diabetic patients, this statement indicates that the creatinine excretion rate can be used as a risk marker in the diabetic population.
Fractional excretion of magnesium: Hypomagnesemia is a frequently observed biochemical abnormality in patients with T2DM and has been associated with poor glycaemic control, insulin resistance, and the progression of microvascular complications[119]. While serum magnesium levels provide a snapshot of systemic magnesium status, they may not fully capture early renal tubular alterations. In this context, the fractional excretion of magnesium (Fe Mg) emerges as a potential marker of tubular dysfunction, offering additional insight into the subclinical stages of DKD. The Fe Mg is an indicator of the excretion process of magnesium through the renal tubules. It is calculated as the percentage of serum Mg excreted through urine and its normal value is less than 4%. Therefore, Fe Mg could be considered a tubular function index: Its value increases in correlation with the chronicity of the tubulointerstitial damage. On this matter, Fe Mg might correlate with the clinical severity of DKD. In fact, it has been shown in the normoalbuminuric stage of T2DM that the mean value of Fe Mg was 4.1% ± 1%, conversely in the albuminuric stage of disease, Fe Mg was increased to 6.6% ± 2%. Moreover, an inverse correlation between Fe Mg and the reduction of peritubular capillary flow has also been demonstrated[120]. Consequently, Fe Mg could be regarded as a potential urinary biomarker of early tubular damage in DKD.
Urinary angiotensinogen: Renal angiotensinogen is predominantly synthesized by proximal tubular epithelial cells and secreted into the tubular lumen. Recent evidence implicates activation of the intrarenal renin-angiotensin system (RAS) in the pathogenesis and progression of renal injury across various models of hypertension and in kidney diseases such as DKD. Studies have demonstrated that, in the early stages of T2DM with normoalbuminuria, urinary angiotensinogen (uAGT) levels are elevated compared to healthy controls and exhibit strong discriminatory power in distinguishing different stages of T2DM from non-diabetic individuals[121]. Consequently, uAGT has been proposed as a sensitive early biomarker of intrarenal RAS activation in DKD. Notably, a multicenter prospective study by Jeon et al[122] provided compelling evidence supporting the clinical utility of uAGT as a surrogate marker for predicting the antiproteinuric effects of angiotensin receptor blockers in patients with overt proteinuria. The study showed that higher baseline uAGT levels, as well as short-term changes in uAGT following angiotensin receptor blocker initiation, were significantly associated with the degree of proteinuria reduction. This finding suggests that uAGT may serve not only as a biomarker of intrarenal RAS activity but also as a predictor of therapeutic responsiveness to RAAS blockade, offering a valuable tool for tailoring treatment strategies in patients with DKD. Moreover, de Alencar Franco Costa et al[123] revealed sex-dependent differences in renal angiotensinogen expression in diabetic models, with female animals showing a heightened intrarenal angiotensinogen response compared to males. These findings point to a potential influence of sex hormones on RAS activation and raise the possibility that uAGT levels could serve as an early marker of DKD with differential diagnostic and prognostic implications between sexes. Altogether, these studies reinforce the role of uAGT as an early, dynamic, and potentially personalized biomarker for monitoring renal involvement and guiding therapy in DKD.
Periostin: Periostin is a matricellular protein primarily localized in connective tissues, especially in the bone. Its expression in the kidney is transient during embryonic development and virtually undetectable in normal adult renal tissue. In DKD, however, periostin expression is markedly upregulated in both glomerular and tubular epithelial cells reflecting its involvement in renal fibrotic processes and tissue remodeling. Urinary periostin levels are significantly elevated in patients with DKD, regardless of albuminuria status compared to healthy controls[121]. This finding highlights the potential of urinary periostin as an early and sensitive biomarker of renal injury. Supporting this, a study by Satirapoj et al[64] demonstrated that urinary periostin excretion was significantly increased in patients with T2DM and correlated positively with the degree of albuminuria and the progressive decline in eGFR. Moreover, their results suggest that periostin may not only reflect ongoing renal damage but could also play a pathophysiological role in the progression of DKD through mechanisms involving extracellular matrix remodeling and fibrosis. Consequently, periostin represents a promising non-invasive biomarker for early detection and monitoring of diabetic renal injury, with potential implications for risk stratification and therapeutic targeting.
Uromodulin: Uromodulin, also known as Tamm-Horsfall protein, is the most abundant protein in normal human urine and is synthesized by the thick ascending limb of the loop of Henle. Recent studies have highlighted its emerging role as a biomarker in various kidney diseases, including DKD and in the progression of CKD. Takata and Isomoto[124] recently highlighted the important roles of uromodulin in maintaining kidney homeostasis, such as regulating ion transport and protecting against inflammation, positioning it as a promising biomarker for CKD. As uromodulin is produced exclusively in the kidney thick ascending limb, its levels in urine and serum closely reflect nephron mass and renal function. Urinary uromodulin, in fact, correlates positively with eGFR and urine volume but decreases with age and diabetes. This suggests that uromodulin reflects tubular function independently from glomerular filtration markers and may increase per functioning nephron under stress. Notably, higher serum uromodulin is linked to slower kidney decline and less albuminuria in high-risk patients, while low levels can identify early kidney injury before creatinine rises. On this matter, Leiherer et al[125] demonstrated that patients with T2DM exhibited significantly lower serum uromodulin levels compared to non-diabetic controls, and that levels were highest in those who remained normoglycemic throughout follow-up. Furthermore, urinary uromodulin concentrations tend to decline in the presence of macroalbuminuria, reinforcing its potential utility in detecting tubular impairment associated with diabetes[126]. This is supported by Chakraborty et al[127] who highlighted altered expression patterns of Tamm-Horsfall protein in diabetic patients with kidney damage, suggesting that reduced uromodulin synthesis or increased urinary loss may reflect underlying tubular injury and dysfunction in diabetes. Moreover, recent findings indicate that glycated forms of urinary uromodulin are present in early-stage DKD, and their levels correlate with disease severity, pointing to potential applications in early diagnosis and disease monitoring[65]. Overall, uromodulin is a valuable biomarker for tubular health, kidney mass, and predicting CKD progression, especially in diabetes-related renal disease.
Considerations on the diagnostic accuracy of the main urinary DKD markers: Early identification of DKD is essential to delay progression toward end-stage renal disease (ESRD). Traditional markers such as uACR/albuminuria and eGFR, although widely used, have notable limitations, mainly their inability to detect subclinical renal injury and their delayed rise in disease course. Many patients, particularly those with type 2 diabetes, may develop significant renal damage without presenting elevated albumin levels, a condition termed NADKD. This diagnostic gap has spurred the search for more sensitive and specific urinary biomarkers that reflect earlier and broader aspects of kidney injury, including tubular stress, podocyte damage, and inflammatory processes.
For early DKD detection, evidence collected has shown that L-FABP and GPC-5 exhibited the highest diagnostic performance. NGAL and KIM-1 also show strong early predictive capability, especially for tubular injury which may precede albuminuria. In detail, L-FABP demonstrated outstanding diagnostic performance, with an area under the curve (AUC) of 0.97, 100% sensitivity, and 86.7% specificity, making it one of the most accurate early indicators of DKD. GPC-5, a marker of podocyte injury, showed a sensitivity of 93.3% and specificity of 80%, and has emerged as a useful tool for identifying glomerular dysfunction before overt albuminuria appears. NGAL and KIM-1 are key tubular injury markers; both are elevated in normoalbuminuric diabetic patients, signaling early damage. NGAL shows pooled sensitivity and specificity above 80%, while KIM-1 displays an AUC of 0.85 and high specificity for proximal tubular damage.
Together, these biomarkers provide a more comprehensive picture of early DKD pathogenesis. Their integration into clinical practice could significantly enhance early diagnosis, allow better risk stratification, and guide personalized therapeutic strategies, particularly in patients not flagged by traditional tests.
Considerations on DKD markers in T1DM and T2DM: The performance and expression of urinary biomarkers can differ significantly between T1DM and T2DM due to distinct pathophysiological mechanisms, rates of disease progression, and comorbidity profiles. For example, the uACR is a standard marker for both types of DKD, but it tends to appear earlier and more consistently in T1DM, reflecting glomerular damage resulting from longstanding hyperglycemia. In contrast, a significant subset of T2DM patients presents with NADKD, making uACR a less reliable early marker in this group.
Another important difference is related to tubular DKD biomarkers (e.g., NGAL, KIM-1, L-FABP), that show better early predictive values in T2DM, especially for NADKD cases were albuminuria is absent; furthermore, T2DM patients often have higher baseline levels of tubular markers (NGAL, KIM-1, L-FABP), possibly due to coexisting hypertension, obesity, and systemic inflammation, which exacerbate tubulointerstitial injury. In T1DM, data are more limited, but recent pediatric and adolescent studies suggest that NGAL and KIM-1 may still offer early detection potential. Similarly, inflammatory markers (YKL-40, MCP-1) tend to be more elevated in T2DM, correlating with systemic inflammation and metabolic syndrome, which are more prevalent in T2DM.
Among the biomarkers associated with a better prediction in T1DM, podocyte injury agents (e.g., nephrin, podocalyxin, GPC-5) are relevant in both types of diabetes but appear more predictive in T1DM, where glomerular lesions are typically more pronounced and occur earlier. GPC-5 and nephrinuria have shown correlations with disease severity in both types, but standardization across populations is still needed.
Oxidative stress is a central pathogenic mechanism in DKD, contributing to endothelial dysfunction, inflammation, and fibrosis in renal tissues. Specifically, ROS induce epithelial-to-mesenchymal transition of renal tubular cells through activation of signalling pathways such as phosphatidylinositol 3-kinase/protein kinase B/glycogen synthase kinase-3β which can be attenuated by nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1 (HO-1) induction, thereby protecting against renal injury in a high-glucose environment[128].
Urinary biomarkers reflecting oxidative damage have gained prominence for their potential in the early diagnosis and monitoring of DKD progression. Among these, HO-1 has been shown to exert renal protective effects by modulating antioxidative responses and inhibiting apoptosis and inflammation, as demonstrated in both animal models and human studies[128,129]. Urinary HO-1 has been identified as a sensitive marker of renal oxidative stress. Li et al[40] demonstrated that urinary HO-1 levels were significantly elevated in patients with early-stage DKD, correlating with increased albuminuria and decreased GFR. This finding underscores the utility of HO-1 as an early biomarker for renal injury in diabetes. Other oxidative stress-related urinary biomarkers include 8-hydroxy-2’-deoxyguanosine (8-OHdG), pentosidine, and uric acid. 8-OHdG, a product of oxidative DNA damage, has been reported to correlate with the severity of DKD and microvascular complications in diabetic patients[105]. Measurement of urinary 8-OHdG by advanced electrochemical biosensors offers enhanced sensitivity and specificity, supporting its clinical utility in oxidative stress assessment[130]. The non-invasive nature of urine sampling and the specificity of these markers for oxidative damage highlight their clinical relevance in identifying patients at risk for DKD progression. Comparative analyses have demonstrated that urinary 8-OHdG levels correlate well with albumin-to-creatinine ratios and can serve as independent predictors of DKD onset, reinforcing their prognostic significance[131,132]. However, variations in detection methods and lack of standardized thresholds currently limit widespread clinical adoption[133].
Nevertheless, further longitudinal studies are necessary to validate their prognostic value and establish standardized thresholds for clinical application.
Since their discovery, an increasing body of evidence is positioning miRNAs as an attractive approach both as therapeutic targets[134] and for their intricate role in various diseases, such as cardiovascular disease[135], metabolic disorders[136], and cancer[137]. With regard to DKD, the dosage of miRNAs in the blood, urine, saliva, or tissues has shown potential as a biomarker for early detection of high-risk individuals, given that miRNAs contribute to the homeostasis of glucose and are involves in different physiological and pathological process[138].
Focusing on miRNAs detectable in the urine of patients, which can differ from the circulating ones and have attracted particular attention due to their non-invasive accessibility, several studies have evaluated their role and presence in DKD[139]. Indeed, a high number of miRNAs are still under investigation for their role in the various manifestation of DKD.
For example, the study by Petrica et al[140] explored the role of miRNA and long noncoding RNA (lncRNA). Specifically, they focused on miR-21 and miR-124, which have been proved to enhance podocyte injury and proximal tubule lesions, as well as miR-29 and miR-93, known for their protective function on podocyte, endothelial injury and renal fibrosis. In their study cohort, consisting of 136 patients with T2DM and 25 healthy controls, the downregulation of lncRNA myocardial infarction-associated transcript and lncRNA taurine-upregulated gene 1 was associated with increased expression of the protective miR-93 and miR-29a, and reduced activity of the pathogenic miR-21 and miR-124.
Another example of the potential of miRNAs can be found in the study by Zang et al[141], which profiled urinary exosomal miRNAs in 87 diabetic patients with and without impaired renal function. Their results showed that urinary miR-21-5p and miR-23b-3p were upregulated in patients with reduced renal function, while miR-30b-5p and miR-125b-5p expression was downregulated.
Moreover, the expression of miRNA can be linked to the levels of microalbuminuria. Indeed, miRNA in the studies by Zhao et al[142] with miR-4534 and Sinha et al[143] with miR-663a could be involved in the manifestation of microalbuminuria. In particular, Sinha et al[143] profiled urine miRNA from 25 patients stratified as non-proteinuric or proteinuric. Their findings showed different expression of miR-155-5p, miR-28-3p, miR-425-5p, and miR-663a in the two groups. Among these, notably, miR-663a downregulation was more pronounced in non-proteinuric than proteinuric DKD subjects, prompting the investigation of pathways and networks regulated by this miRNA with bioinformatics studies. Another relevant study on miRNA and microalbuminuria was carried out by Barutta et al[144]. In this study, miR-130a and miR-145 were significantly increased in DKD patients, while miR-155 and miR-424 were decreased in microalbuminuria patients compared to normoalbuminuric patients.
Another aspect of DKD in which miRNAs seem to be an actor are the levels of blood sugar and glycosylated hemoglobin. A recent study by Han et al[145] showed that miR-27a-3p was related to blood glucose, glycosylated hemoglobin, and cholesterol levels. Moreover, the same study proved that miR-27a-3p and miR-145-5p were positively correlated with albuminuria and serum creatinine and negatively correlated with the eGFR.
With regard to the role of miRNAs as therapeutic agents, different studies explored this opportunity ranging from in silico to in vivo experiments. In the study by Mishra et al[146], the authors tested whether restoring the down expressed miRNAs could attenuate DKD. They based their experiment on the observation that miRNAs, such as miR-24-3p and miR-200c-3p, which decline in renal tissue could contribute to disease progression. To test this, they injected the miRNAs via urinary exosomes in rats with DKD. Their results showed that treated rats had attenuated renal involvement and recovered expression of miRNAs. Notably, the renoprotective effects appeared to be independent of glucose-lowering, as the treatment did not affect the blood glucose levels. Although these findings are currently limited to animal models, detection of the same miRNAs in human patients supports the rationale for further investigation into their potential therapeutic role in human kidney disease. Another study, by Cho et al[147], analyzed urinary miRNAs in 64 patients undergoing treatment with either dipeptidyl peptidase-4 inhibitors or sulfonylureas, and found no significant difference in urinary miRNA expression between the two groups.
Collectively, all these studies support the hypothesis that urinary miRNAs show promise as early non-invasive biomarkers for predicting DKD and consequently miRNA-centred therapies, although still in the early stages of development, offer a potential target for new treatment strategies[148]. However, it is clear that more studies, especially prospective studies with more detailed clinical data are need to completely define the role of miRNAs and their potential as therapeutic targets.
Metabolomics is the study of metabolites, the end products of cellular processes, which include a wide range of substances such as amino acids, sugars, and lipids[149]. Metabolomic analysis typically focuses on metabolites found in biological fluids. The primary fluids studied are blood and urine, but other fluids such as saliva, seminal plasma, and cerebrospinal fluid can also be analyzed[150,151]. The principal technologies used in metabolomics are mass spectrometry (MS) and nuclear magnetic resonance[151]. Metabolomics is an emerging field and represents a promising strategy for the early detection of DKD[152], enabling the identification of DKD even in its initial stages[153].
Amino acids: Amino acids play an important role in glucose homeostasis by regulating the secretion of glucagon and insulin. The assessment of serum amino acid levels plays a key role in identifying potential biomarkers that may aid in early diagnosis. For instance, Wang et al[154] observed an increased risk of developing T2DM in patients with high plasma concentrations of isoleucine, leucine, valine, tyrosine, and phenylalanine. In opposition, Tofte et al[155] analyzed 637 patients with T1DM and found that isoleucine, leucine, and valine were positively associated with eGFR and a lower risk of ESRD. In support of this, Niewczas et al[156] observed progression of DKD in 80 patients in which 40 progressed to ESRD within 8-12 years. They found that higher levels of leucine and valine were associated with a reduced risk of developing ESRD, whereas elevated concentrations of phenylacetylglutamine and p-cresol sulphate were linked to disease progression. Phenylacetylglutamine and p-cresol sulphate are recognized as uremic toxins derived from altered gut microbiota metabolism, which is notably prevalent in CKD[157].
The differences in results across these studies can be explained by the variations in study design, populations, and clinical endpoints. While all three studies explore metabolomic predictors of diabetes and CKD, they employ distinct methodologies and focus on different patient groups. The study by Wang et al[154] was a nested case-control study that enrolled 2422 subjects at least 35 years old with no history of diabetes or cardiovascular disease, who also underwent an oral glucose tolerance test as part of the Framingham heart study. In this population, the authors identified branched-chain and aromatic amino acids as strong predictors of future T2DM, independent of traditional risk factors. In contrast, Niewczas et al[156] and Tofte et al[155] studied 80 patients with T2DM and 637 with T1DM, respectively, at risk of ESRD. Indeed, unlike Wang’s study[154], which focused on predicting diabetes onset in healthy individuals, the studies by Niewczas et al[156] and Tofte et al[155] shift the focus towards patients already affected by diabetes. The presence of diabetes in their cohorts could have already altered their metabolic phenotype. Moreover, their studies focused on renal outcomes (e.g., ERSD and CKD progression), which could also be influenced by the systemic metabolic disturbances typical of CKD pathophysiological mechanisms. Additionally, the sample sizes varied considerably among the studies and may have influenced the statistical power and the ability to detect or generalize metabolomic associations.
Serum analysis provides valuable insights and should not be underestimated; however, the investigation of biomarkers in urine is gaining increasing attention due to its non-invasive nature and potential for early disease detection. Shao et al[158] analyzed the metabolic profiles of 88 patients, 44 with DKD and 44 with only T2DM. They compared biomarker differences between the two groups and identified 61 serum metabolites and 46 urinary metabolites. In the DKD group, the levels of benzoic acid, fumaric acid, erythrose, and L-arabitol were significantly higher than in the T2DM group. Conversely, levels of glycerol 1-octadecanoate, L-glutamic acid/pyroglutamic acid, fructose 6-phosphate, taurine, and L-glutamine were significantly reduced in DKD patients. In addition, DKD patients showed significantly elevated urinary levels of D-glucose, L-valine, L-histidine, sucrose, gluconic acid, glycine, L-asparagine/L-aspartic acid, L-xylonate-2, and oxalic acid compared with T2DM patients. To improve the predictive utility of metabolites to diagnosis DKD, Pena et al[159] compared urine and plasma metabolites. They observed that in T2DM patients with microalbuminuria there was a reduction in plasma histidine and an increase of butenoylcarnitine, and low hexose, glutamine and tyrosine in urine. Thus, these parameters could predict the progression of albuminuria, independent of eGFR and baseline albuminuria.
In a study involving a cohort of 1001 participants at various stages of DKD, Kwan et al[160] reported that elevated urinary levels of 3-hydroxybutyrate, a metabolic intermediate of valine catabolism, were associated with a more rapid decline in eGFR and an increased likelihood of requiring renal replacement therapy. Similarly, Li et al[161] identified a reduction in urinary levels of several metabolites in DKD patients, including aconitic acid, isocitric acid, 4-hydroxybutyrate, uracil, and glycine. Both studies suggest that the decrease in mitochondrial-derived metabolites reflects a broader suppression of mitochondrial activity in the context of DKD.
Tyrosine is an important amino acid biomarker, produced through the hydroxylation of phenylalanine-by-phenylalanine hydroxylase. In patients with DKD, both urinary and serum levels of tyrosine can serve as indicators of disease progression[162]. Zhang et al[163] found that elevated plasma tyrosine levels are associated with an increased risk of developing DKD, while Liu et al[164] reported that lower urinary tyrosine levels correlate with the presence of DKD. In opposition, Tofte et al[165] observed that elevated levels of tyrosine are associated with increased eGFR, and other studies reported the positive association between downstream metabolites of tyrosine and ESKD[166].
Another biomarker involved in DKD is tryptophan, an aromatic amino acid that is altered in this condition[167]. Renal insufficiency leads to an increase in kynurenine, a downstream metabolite of tryptophan, which in turn promotes leukocyte activation, cytokine production, oxidative stress, and inflammation[168]. Reinforcing the potential role of tryptophan as a biomarker, Mogos et al[169] in a case-control study compared 3 types of patients (normoalbuminuric, microalbuminuria and macroalbuminuria) and healthy patients. They reported a reduction in serum tryptophan in patients, while urinary tryptophan was lower in normoalbuminuric patients than in the other groups. Furthermore, they observed a reduction in urinary glycine with the progression of DKD. Van der Kloet et al[170] found that urinary acyl-glycines and intermediates of tryptophan metabolism were positively correlated with albuminuria and associated with the progression of DKD. Similarly, Chou et al[171] observed that tryptophan metabolism is disrupted in patients with T2DM, with lower plasma tryptophan levels being significantly associated with a more rapid decline in eGFR.
Other amino acids investigated as potential biomarkers for DKD include norvaline, an isomer of valine, and aspartate. In a study by Tavares et al[172] both were inversely associated with DKD progression. Similarly, Hirayama et al[173] reported that aspartate levels were inversely correlated with eGFR and positively associated with albuminuria. Furthermore, Mogos et al[169] supported the involvement of various urinary metabolites in DKD progression, particularly urinary aspartate, which may reflect localized kidney damage at specific segments of the nephron.
Lipids: Lipids play a key role in several metabolic processes related to diabetes, including dyslipidaemia and insulin resistance[174]. Numerous studies have confirmed the involvement of fatty acids and their metabolites in the pathogenesis of DKD[175].
In this context Han et al[176] analyzed the plasma of DKD patients and found that non-esterified fatty acids (NEFAs) were elevated in the early stages of DKD, while esterified fatty acids were reduced. NEFAs may contribute to endothelial cell injury and dysfunction[177]. Supporting this, Mäkinen et al[178] reported elevated levels of NEFA species, including monounsaturated fatty acids 16:1 and 18:1, as well as omega-6, -7, and -9 fatty acids, in patients with DKD. In line with these observations, Zhang et al[179] in a case-control study, observed that elevated concentrations of palmitic acid (C16:0), linolelaidic acid (C18:2N6T), linoleic acid (C18:2N6C), and lysophosphatidylcholine (20:4) in serum were associated with DKD onset. Li et al[161] during the analyses of urine from patients with and without DKD reported an increased level of palmitic acid and stearic acid in patients with DKD. Another study[180] of 200 patients 102 T2DM without DKD, 55 with DKD and 43 healthy patients, revealed elevated octanoic acid and decadienoic acid in DKD patients. Another potential biomarker in DKD is sphingomyelins, when present in high concentrations these have the capacity for podocyte injury and destruction of glomeruli slit diaphragm[181]. Additional studies have reported that increased levels of sphingomyelin in DKD patients are linked to the progression of both DKD and CKD, particularly in individuals with hyperglycemia[178,182,183]. In support, the studies by Zhu et al[184] and Liu et al[185] observed elevated concentrations of sphingomyelins in DKD patients. Furthermore, Afshinnia et al[186] observed that higher levels of non-esterified NEFAs in patients with T2DM and preserved kidney function (eGFR > 90 mL/minute/1.73 m2) were associated with a lower risk of experiencing a > 40% decline in eGFR.
Carnitine, another biomarker has the function of transferring long chain fatty acids into the mitochondria for oxidation. In support of this potential role in DKD pathogenesis are the studies by Ng et al[187] and Sirolli et al[188]: The first study demonstrated an increase in nonanoylcarnitine and 2,6 dimethylthetanoylcarnitine in the urine of DKD patients. The second study reported an increase in short chain and medium chain acylcarnitines and dycarboxilic acylcarninte in DKD patients undergoing hemodialysis.
Other types of lipids used as biomarkers in DKD include phospholipids. The two most representative phospholipids are phosphatidylcholine (PC) and phosphatidylethanolamine. In a case-control study, Liu et al[185] reported lower plasma levels of PC metabolites in DKD patients. In the same study, they also observed that elevated plasma levels of very-long-chain ceramides were associated with greater progression of macroalbuminuria in DKD patients. To better understand the underlying pathophysiological mechanisms, Yoshioka et al[189] conducted a metabolomic analysis, which revealed increased urinary levels of lysophosphatidylcholine in these patients. MS analysis further showed that urinary lysophosphatidylcholine contained saturated fatty acids, specifically palmitic acid (16:0) and stearic acid (18:0). In vitro studies further demonstrated that lysophosphatidylcholine promotes the formation of lipid droplets via activation of peroxisome proliferator-activated receptor-δ, leading to upregulation of the lipid droplet-associated protein perilipin 2 and a reduction in autophagic flux, which ultimately resulted in organelle stress and subsequent apoptosis.
Tricarboxylic acid cycle: Mitochondria carry out a wide array of essential cellular functions, with the tricarboxylic acid (TCA) cycle serving as a central pathway for energy production. This cycle plays a pivotal role in integrating the metabolism of glucose, fatty acids, and amino acids. Consequently, disruptions in TCA cycle activity are likely indicative of mitochondrial dysfunction and may contribute to both the onset and progression of DKD[190].
Interest in the TCA cycle has been growing, particularly regarding the role of fumarate. Fumarate is generated from the precursor adenyl succinate via succinate dehydrogenase and is subsequently converted to malate by fumarate hydratase (FH)[191]. Reduced FH expression, and the consequent accumulation of fumarate in the kidney, may contribute to the decline of eGFR[192]. An important contribution to this field was made by Liu et al[193], who demonstrated elevated urinary levels of TCA cycle metabolites, particularly fumarate, in patients with DKD. Their findings suggest that fumarate may play a key role in the pathophysiological mechanisms underlying the progression of CKD.
Alterations in cellular metabolism are fundamental to the development and progression of DKD, although the underlying mechanisms are not always easy to explain. To explore this further, Azushima et al[194] examined energy metabolism in a murine model of DKD. One of the most notable metabolic findings was the increased urinary lactate levels, accompanied by alterations in TCA cycle intermediates. Moreover, urinary lactate levels were correlated with biomarkers of cellular stress and epithelial injury. These findings suggest that increased urinary lactate may serve as a predictive marker of eGFR decline in DKD.
Nucleic acids: One pathophysiology aspect of DKD is oxidative stress and the relative DNA damage. This changes purine and pyrimidine metabolism, and is related to DKD[195]. Ng et al[187] found elevated 3,7-dimethyluric acid, inosine diphosphate, and 2-deoxyuridin in urine. In addition, Xia et al[196] in a case-control study compared serum biomarkers in patients with and without DKD, and observed elevated levels of purines adenosine, inosine, and xanthine in DKD patients. Also, pyrimidine derivates, uracil and their metabolites were altered in the urine of DKD patients[161]. Solini et al[197] observed in DM patients that high levels of pseuduridine, and uracil derivates were associated with eGFR decline and increased urinary albumin-creatinine ratio. Uracil was identified in another study by Sharma et al[198], who analyzed urinary metabolites using gas chromatography-MS. They examined 94 metabolites, of which only 13 were found to be reduced in the urine of patients with T2DM, including uracil, 3-hydroxyisovalerate, glycolic acid, and others. Additionally, the study suggested that these metabolite alterations were related to organic anion transporters (OATs). Specifically, they observed a reduction in OAT-1 and OAT-3 expression in patients with DKD, which could explain the decreased levels of urinary metabolites identified.
Sharma et al[199] observed a significant association between elevated urinary adenine levels and an increased risk of developing ESKD in patients with DKD. Urinary adenine concentrations were particularly elevated in patients at high risk of progression to ESKD. Notably, they reported that the uACR could help identify patients with normal eGFR and normoalbuminuria who are nonetheless at risk of developing ESKD. This finding is mechanistically supported by experimental studies showing that adenine contributes to kidney fibrosis. In a mouse model, adenine was shown to stimulate the expression of extracellular matrix molecules in proximal tubular cells via activation of the mammalian target of rapamycin pathway, thereby promoting renal matrix accumulation.
The study of metabolomics is making significant progress in the early diagnosis of DKD. However, larger and more comprehensive studies are still needed to fully assess the predictive value of these metabolites. Only through further research can we truly understand their clinical relevance and improve the therapeutic management of patients affected by DKD.
Another promising approach for identifying early biomarkers of DKD is proteomics, the large-scale study of proteins, which are key indicators of early pathological changes[200]. Proteomics currently allows for the identification of potential biomarkers for CKD[201] and for DKD. The principal biological samples studied are blood and urine. Blood samples can show protein signals derived from kidney, but also from generic inflammatory processes[202] and another problem for proteomics analysis of blood samples is the necessity to have large volumes of blood. As urine collection is simpler and more non-invasive, urinary proteomics is becoming an important instrument for the early diagnosis of DKD[203].
The proteomics biomarker most studied is the urinary peptide classifier CKD-273. This biomarker includes 273 urinary peptides, of which approximately 75% are collagen fragments including uromodulin, clusterin, albumin, β2-MG, and a1 antitrypsin. A transversal study in 2010[204], showed the importance of CKD-273 with a sensitivity of 85% and specificity of 100% for the diagnosis of CKD. This biomarker was validated in many studies, in particular the study by Pontillo and Mischak[205] where significant differences in urinary peptides between CKD patients and individuals with normal renal function were observed. For this analysis, they used a support vector machine to combine 273 urinary peptides into a biomarker panel known as CKD-273. The main components of CKD-273 include collagen fragments and blood-derived protein fragments involved in inflammation and tissue repair. CKD-273 was more important in identifying the progression risk of CKD and it was tested in the PRIORITY study, in which Tofte et al[206] observed that in patients with elevated CKD-273, a major risk factor in the development of microalbuminuria, the use of spironolactone did not prevent microalbuminuria progression. In contrast, Siwy et al[207] showed a correlation between CKD-273 and eGFR decline, but in high-risk patients treated with linagliptin a slow progression of eGFR was observed. In addition to CKD-273, there are other biomarkers that use collagenous fragments to diagnosis the progression of DKD. Zürbig et al[208] reported a reduction in the α1 type 1 collagen chain in urine 3 to 5 years before the development of macroalbuminuria. Therefore, in DKD patients it is thought that the reduction in urinary collagenous fragments is due to accumulation in the extracellular matrix and increased kidney fibrosis[209].
To investigate the role of inflammation, Niewczas et al[210] used an aptamer-based platform to measure a panel of 194 proteins. The study involved 219 participants with T1DM from the Joslin kidney study, 144 with T2DM also from the Joslin cohort, and 162 individuals with diabetes from the Pima Indian study. This analysis led to the identification of a kidney risk inflammatory signature (KRIS), composed of 17 inflammatory proteins, many of which belong to the TNFR superfamily. KRIS was strongly associated with a 10-year risk of progression to ESKD. Moreover, the presence of KRIS proteins in urine was linked to a rapid decline in eGFR. However, there was no correlation between the urinary levels of KRIS proteins and the expression of their corresponding genes in kidney biopsy samples.
Liao et al[211] examined four groups of participants: 35 healthy individuals, 25 subjects with micro- or macroalbuminuria not related to DKD, 24 patients with T2DM without albuminuria, and 16 T2DM patients with microalbuminuria. Their analysis identified urinary haptoglobin (HPT) as a biomarker associated with DKD. Specifically, they observed that the HPT-to-creatinine ratio (HCR) is a strong independent predictor of eGFR decline, demonstrating greater sensitivity and specificity than the commonly used ACR. Moreover, T2DM patients with elevated urinary HCR were found to be at higher risk of progressing to ESKD. HPT is a sialoglycoprotein that binds free hemoglobin, preventing iron loss through the kidneys and protecting against nephron damage[212]. The HPT-hemoglobin complex is not filtered at the glomerular level; thus, elevated urinary concentrations suggest renal tubular injury or oxidative stress[213]. Several studies[213,214] have confirmed the association between urinary HPT levels and eGFR decline.
In conclusion, proteomics has shown great promise in identifying biomarkers for DKD, offering valuable insights into early disease detection. However, its clinical application still faces challenges in terms of sensitivity, specificity, and standardization. Continued research and technological advancements are essential to refine proteomic tools and improve their predictive accuracy. With further development, proteomics could become a cornerstone in the early diagnosis and personalized management of DKD.
Although urinary biomarkers show considerable potential for improving the early diagnosis and management of DKD, their clinical implementation still faces several challenges that need to be addressed to fully realize their potential. A principal obstacle is the lack of standardized assay methodologies and universally accepted clinical thresholds. Variability in sample collection, processing, and analytical techniques across laboratories can lead to inconsistent results, undermining the reliability and reproducibility needed for clinical decision-making. The effectiveness of urinary biomarkers in DKD also depends on the detection method used, particularly in terms of cost, sensitivity, and clinical utility. Enzyme-linked immunosorbent assay (ELISA) is widely used in clinical practice due to its low cost, ease of use, and ability to quantify specific proteins such as albumin, KIM-1, or NGAL. However, ELISA is limited by its reliance on high-quality antibodies and its restricted multiplexing capability. MS, on the other hand, offers superior sensitivity and the ability to detect a broad spectrum of proteins and metabolites simultaneously. This makes it ideal for biomarker discovery and metabolomic profiling. Despite its advantages, MS remains expensive and technically complex, which restricts its widespread clinical application. The complementary use of both methods may offer a practical and effective approach to biomarker-based diagnosis and monitoring in DKD.
Another obstacle is that many biomarkers have been validated only in small or homogeneous cohorts, making it difficult to generalize findings across diverse populations with varying comorbidities and disease stages. Large-scale, multi-center prospective studies are urgently needed to confirm their diagnostic and prognostic accuracy and to establish robust reference ranges. Furthermore, the clinical utility of these biomarkers must be rigorously evaluated by cost-benefit analyses and outcome-based trials to demonstrate that their use leads to improved patient management and health outcomes beyond what is achievable with current standards like eGFR and albuminuria. Regulatory approval processes and integration into existing healthcare workflows also pose challenges, requiring collaboration between researchers, clinicians, and policymakers to develop guidelines and ensure seamless adoption. Importantly, for urinary biomarkers to become part of routine clinical care, they must be evaluated and endorsed by international clinical guidelines through thorough validation studies, standardization efforts, and consensus from expert panels. Without overcoming these obstacles, the translation of urinary biomarkers from bench to bedside will remain limited despite their promising role in personalized medicine for DKD.
DKD remains a major challenge in both diabetes management and nephrology and calls for more sensitive and specific diagnostic and prognostic tools beyond traditional measures such as albuminuria and eGFR. Urinary biomarkers offer a promising avenue in this context, providing deeper insight into the complex and multifactorial pathophysiology of DKD. Emerging candidates such as KIM-1, NGAL, and metabolomic signatures have shown potential not only for early detection but also for monitoring disease progression and enabling more individualized therapeutic strategies. However, translating these findings into clinical practice presents several challenges. Standardization of biomarker assays across laboratories, cost-benefit analyses to justify widespread adoption, and large-scale, multi-center validation studies remain essential steps to ensure reliability, reproducibility, and clinical utility. Moreover, regulatory hurdles and integration into existing clinical workflows must be addressed. While ongoing research continues to tackle these obstacles, successful clinical translation could ultimately enable earlier diagnosis, better risk stratification, and more effective personalized treatment strategies, offering the potential to significantly reduce the burden of DKD and improve outcomes for patients worldwide.
| 1. | Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, Sadusky T, Tandon N, Tuttle KR, Wanner C, Wilkens KG, Zoungas S, Craig JC, Tunnicliffe DJ, Tonelli MA, Cheung M, Earley A, de Boer IH. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 2. | Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320:C375-C391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 1257] [Article Influence: 209.5] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Zhao J, Qin Y, Yu Z, Zhang Y, Ning X, Sun S. The Specific Alteration of Gut Microbiota in Diabetic Kidney Diseases-A Systematic Review and Meta-Analysis. Front Immunol. 2022;13:908219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Giandalia A, Giuffrida AE, Gembillo G, Cucinotta D, Squadrito G, Santoro D, Russo GT. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int J Mol Sci. 2021;22:5808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Bolignano D, Cernaro V, Gembillo G, Baggetta R, Buemi M, D'Arrigo G. Antioxidant agents for delaying diabetic kidney disease progression: A systematic review and meta-analysis. PLoS One. 2017;12:e0178699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Taguchi K, Fukami K. RAGE signaling regulates the progression of diabetic complications. Front Pharmacol. 2023;14:1128872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 7. | Pan D, Xu L, Guo M. The role of protein kinase C in diabetic microvascular complications. Front Endocrinol (Lausanne). 2022;13:973058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Sinha SK, Nicholas SB. Pathomechanisms of Diabetic Kidney Disease. J Clin Med. 2023;12:7349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 9. | Singh M, Kapoor A, Bhatnagar A. Physiological and Pathological Roles of Aldose Reductase. Metabolites. 2021;11:655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000;77:S3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Muglia L, Di Dio M, Filicetti E, Greco GI, Volpentesta M, Beccacece A, Fabbietti P, Lattanzio F, Corsonello A, Gembillo G, Santoro D, Soraci L. Biomarkers of chronic kidney disease in older individuals: navigating complexity in diagnosis. Front Med (Lausanne). 2024;11:1397160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Afonso LG, Silva-Aguiar RP, Teixeira DE, Alves SAS, Schmaier AH, Pinheiro AAS, Peruchetti DB, Caruso-Neves C. The angiotensin II/type 1 angiotensin II receptor pathway is implicated in the dysfunction of albumin endocytosis in renal proximal tubule epithelial cells induced by high glucose levels. Biochim Biophys Acta Gen Subj. 2024;1868:130684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Santoro D, Torreggiani M, Pellicanò V, Cernaro V, Messina RM, Longhitano E, Siligato R, Gembillo G, Esposito C, Piccoli GB. Kidney Biopsy in Type 2 Diabetic Patients: Critical Reflections on Present Indications and Diagnostic Alternatives. Int J Mol Sci. 2021;22:5425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Dong H, Sun Y, Nie L, Cui A, Zhao P, Leung WK, Wang Q. Metabolic memory: mechanisms and diseases. Signal Transduct Target Ther. 2024;9:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 15. | Gembillo G, Ingrasciotta Y, Crisafulli S, Luxi N, Siligato R, Santoro D, Trifirò G. Kidney Disease in Diabetic Patients: From Pathophysiology to Pharmacological Aspects with a Focus on Therapeutic Inertia. Int J Mol Sci. 2021;22:4824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Esposito P, Picciotto D, Cappadona F, Costigliolo F, Russo E, Macciò L, Viazzi F. Multifaceted relationship between diabetes and kidney diseases: Beyond diabetes. World J Diabetes. 2023;14:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 17. | Gembillo G, Cernaro V, Giuffrida AE, Russo G, Giandalia A, Siligato R, Longhitano E, Santoro D. Gender differences in new hypoglycemic drug effects on renal outcomes: a systematic review. Expert Rev Clin Pharmacol. 2022;15:323-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Chuang L, Lin W, Huang C, Lin C, Tseng T. SUN-156 DNlite-IVD103, a novel urinary test, predicts progressive eGFR decline in type 2 diabetes with microalbuminuria. Kidney Int Rep. 2020;5:S264. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Thiengsusuk A, Youngvises N, Pochairach R, Taha RO, Sirisabhabhorn K, Muhamad N, Meesiri W, Chaijaroenkul W, Na-Bangchang K. Urinary Albumin-to-Creatinine Ratio (uACR) Point-of-Care (POC) Device with Seamless Data Transmission for Monitoring the Progression of Chronic Kidney Disease. Biosensors (Basel). 2025;15:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Ahn CW, Song YD, Kim JH, Lim SK, Choi KH, Kim KR, Lee HC, Huh KB. The validity of random urine specimen albumin measurement as a screening test for diabetic nephropathy. Yonsei Med J. 1999;40:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | El-Kafrawy NA, Talaat AO, Ata MB. Study and Clinical Significance of Urinary Levels of Glypican 5 in Diabetic Patients. Menoufia Med J. 2024;37. [DOI] [Full Text] |
| 22. | Qin Y, Zhang S, Shen X, Zhang S, Wang J, Zuo M, Cui X, Gao Z, Yang J, Zhu H, Chang B. Evaluation of urinary biomarkers for prediction of diabetic kidney disease: a propensity score matching analysis. Ther Adv Endocrinol Metab. 2019;10:2042018819891110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 23. | Zhang D, Ye S, Pan T. The role of serum and urinary biomarkers in the diagnosis of early diabetic nephropathy in patients with type 2 diabetes. PeerJ. 2019;7:e7079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Mesfine BB, Vojisavljevic D, Kapoor R, Watson D, Kandasamy Y, Rudd D. Urinary nephrin-a potential marker of early glomerular injury: a systematic review and meta-analysis. J Nephrol. 2024;37:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Iijima T, Suzuki S, Sekizuka K, Hishiki T, Yagame M, Jinde K, Saotome N, Suzuki D, Sakai H, Tomino Y. Follow-up study on urinary type IV collagen in patients with early stage diabetic nephropathy. J Clin Lab Anal. 1998;12:378-382. [PubMed] [DOI] [Full Text] |
| 26. | Swaminathan SM, Rao IR, Shenoy SV, Prabhu AR, Mohan PB, Rangaswamy D, Bhojaraja MV, Nagri SK, Nagaraju SP. Novel biomarkers for prognosticating diabetic kidney disease progression. Int Urol Nephrol. 2023;55:913-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 27. | Hanna C, Etry HE, Ibrahim M, Khalife L, Bahous SA, Faour WH. Podocyturia an emerging biomarker for kidney injury. BMC Nephrol. 2025;26:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Kostovska I, Trajkovska KT, Cekovska S, Topuzovska S, Kavrakova JB, Spasovski G, Kostovski O, Labudovic D. Role of urinary podocalyxin in early diagnosis of diabetic nephropathy. Rom J Intern Med. 2020;58:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Ghorab AAE, Diab ME, Abdelfattah NR, Elmenshawy WR. Study of Urinary Podocalyxin As an Early Biomarker in Diabetic Nephropathy. Egypt J Hosp Med. 2020;80:627-632. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Salem RM, Todd JN, Sandholm N, Cole JB, Chen WM, Andrews D, Pezzolesi MG, McKeigue PM, Hiraki LT, Qiu C, Nair V, Di Liao C, Cao JJ, Valo E, Onengut-Gumuscu S, Smiles AM, McGurnaghan SJ, Haukka JK, Harjutsalo V, Brennan EP, van Zuydam N, Ahlqvist E, Doyle R, Ahluwalia TS, Lajer M, Hughes MF, Park J, Skupien J, Spiliopoulou A, Liu A, Menon R, Boustany-Kari CM, Kang HM, Nelson RG, Klein R, Klein BE, Lee KE, Gao X, Mauer M, Maestroni S, Caramori ML, de Boer IH, Miller RG, Guo J, Boright AP, Tregouet D, Gyorgy B, Snell-Bergeon JK, Maahs DM, Bull SB, Canty AJ, Palmer CNA, Stechemesser L, Paulweber B, Weitgasser R, Sokolovska J, Rovīte V, Pīrāgs V, Prakapiene E, Radzeviciene L, Verkauskiene R, Panduru NM, Groop LC, McCarthy MI, Gu HF, Möllsten A, Falhammar H, Brismar K, Martin F, Rossing P, Costacou T, Zerbini G, Marre M, Hadjadj S, McKnight AJ, Forsblom C, McKay G, Godson C, Maxwell AP, Kretzler M, Susztak K, Colhoun HM, Krolewski A, Paterson AD, Groop PH, Rich SS, Hirschhorn JN, Florez JC; SUMMIT Consortium, DCCT/EDIC Research Group, GENIE Consortium. Genome-Wide Association Study of Diabetic Kidney Disease Highlights Biology Involved in Glomerular Basement Membrane Collagen. J Am Soc Nephrol. 2019;30:2000-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 31. | Schultes B, Emmerich S, Kistler AD, Mecheri B, Schnell O, Rudofsky G. Impact of Albumin-to-Creatinine Ratio Point-of-Care Testing on the Diagnosis and Management of Diabetic Kidney Disease. J Diabetes Sci Technol. 2023;17:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Mori KP, Yokoi H, Kasahara M, Imamaki H, Ishii A, Kuwabara T, Koga K, Kato Y, Toda N, Ohno S, Kuwahara K, Endo T, Nakao K, Yanagita M, Mukoyama M, Mori K. Increase of Total Nephron Albumin Filtration and Reabsorption in Diabetic Nephropathy. J Am Soc Nephrol. 2017;28:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Böger CA, Chen MH, Tin A, Olden M, Köttgen A, de Boer IH, Fuchsberger C, O'Seaghdha CM, Pattaro C, Teumer A, Liu CT, Glazer NL, Li M, O'Connell JR, Tanaka T, Peralta CA, Kutalik Z, Luan J, Zhao JH, Hwang SJ, Akylbekova E, Kramer H, van der Harst P, Smith AV, Lohman K, de Andrade M, Hayward C, Kollerits B, Tönjes A, Aspelund T, Ingelsson E, Eiriksdottir G, Launer LJ, Harris TB, Shuldiner AR, Mitchell BD, Arking DE, Franceschini N, Boerwinkle E, Egan J, Hernandez D, Reilly M, Townsend RR, Lumley T, Siscovick DS, Psaty BM, Kestenbaum B, Haritunians T, Bergmann S, Vollenweider P, Waeber G, Mooser V, Waterworth D, Johnson AD, Florez JC, Meigs JB, Lu X, Turner ST, Atkinson EJ, Leak TS, Aasarød K, Skorpen F, Syvänen AC, Illig T, Baumert J, Koenig W, Krämer BK, Devuyst O, Mychaleckyj JC, Minelli C, Bakker SJ, Kedenko L, Paulweber B, Coassin S, Endlich K, Kroemer HK, Biffar R, Stracke S, Völzke H, Stumvoll M, Mägi R, Campbell H, Vitart V, Hastie ND, Gudnason V, Kardia SL, Liu Y, Polasek O, Curhan G, Kronenberg F, Prokopenko I, Rudan I, Arnlöv J, Hallan S, Navis G; CKDGen Consortium, Parsa A, Ferrucci L, Coresh J, Shlipak MG, Bull SB, Paterson NJ, Wichmann HE, Wareham NJ, Loos RJ, Rotter JI, Pramstaller PP, Cupples LA, Beckmann JS, Yang Q, Heid IM, Rettig R, Dreisbach AW, Bochud M, Fox CS, Kao WH. CUBN is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22:555-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | García-Carro C, Vergara A, Bermejo S, Azancot MA, Sánchez-Fructuoso AI, Sánchez de la Nieta MD, Agraz I, Soler MJ. How to Assess Diabetic Kidney Disease Progression? From Albuminuria to GFR. J Clin Med. 2021;10:2505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Shi S, Ni L, Gao L, Wu X. Comparison of Nonalbuminuric and Albuminuric Diabetic Kidney Disease Among Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2022;13:871272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Cho S, Huh H, Park S, Lee S, Jung S, Kim M, Lee KN, Paek JH, Park WY, Jin K, Han S, Joo KW, Lim CS, Kim YS, Han K, Kim Y, Kim DK. Impact of albuminuria on the various causes of death in diabetic patients: a nationwide population-based study. Sci Rep. 2023;13:295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 37. | de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45:3075-3090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 481] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 38. | Medina-Urrutia A, Juárez-Rojas JG, Posadas-Sánchez R, Jorge-Galarza E, Cardoso-Saldaña G, Vargas-Alarcón G, Martínez-Alvarado R, Posadas-Romero C. Microalbuminuria and its Association with Subclinical Atherosclerosis in the Mexican Mestizo population: the GEA study. Rev Invest Clin. 2016;68:262-268. [PubMed] |
| 39. | Raja P, Maxwell AP, Brazil DP. The Potential of Albuminuria as a Biomarker of Diabetic Complications. Cardiovasc Drugs Ther. 2021;35:455-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Li X, Zhang X, Wang S, Li Y, Meng C, Wang J, Chang B, Yang J. Simultaneous detection of multiple urinary biomarkers in patients with early-stage diabetic kidney disease using Luminex liquid suspension chip technology. Front Endocrinol (Lausanne). 2024;15:1443573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 41. | Cohen-Bucay A, Viswanathan G. Urinary markers of glomerular injury in diabetic nephropathy. Int J Nephrol. 2012;2012:146987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Kanauchi M, Nishioka H, Hashimoto T, Dohi K. Diagnostic significance of urinary transferrin in diabetic nephropathy. Nihon Jinzo Gakkai Shi. 1995;37:649-654. [PubMed] |
| 43. | Mistry K, Kalia K. Non enzymatic glycosylation of IgG and their urinary excretion in patients with diabetic nephropathy. Indian J Clin Biochem. 2009;24:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Tofik R, Torffvit O, Rippe B, Bakoush O. Urine IgM-excretion as a prognostic marker for progression of type 2 diabetic nephropathy. Diabetes Res Clin Pract. 2012;95:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Gohda T, Walker WH, Wolkow P, Lee JE, Warram JH, Krolewski AS, Niewczas MA. Elevated urinary excretion of immunoglobulins in nonproteinuric patients with type 1 diabetes. Am J Physiol Renal Physiol. 2012;303:F157-F162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Meng C, Chen J, Sun X, Guan S, Zhu H, Qin Y, Wang J, Li Y, Yang J, Chang B. Urine Immunoglobin G Greater Than 2.45 mg/L Has a Correlation with the Onset and Progression of Diabetic Kidney Disease: A Retrospective Cohort Study. J Pers Med. 2023;13:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Narita T, Hosoba M, Kakei M, Ito S. Increased urinary excretions of immunoglobulin g, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetes. Diabetes Care. 2006;29:142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 48. | Narita T, Sasaki H, Hosoba M, Miura T, Yoshioka N, Morii T, Shimotomai T, Koshimura J, Fujita H, Kakei M, Ito S. Parallel increase in urinary excretion rates of immunoglobulin G, ceruloplasmin, transferrin, and orosomucoid in normoalbuminuric type 2 diabetic patients. Diabetes Care. 2004;27:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Narita T, Fujita H, Koshimura J, Meguro H, Kitazato H, Shimotomai T, Kagaya E, Suzuki K, Murata M, Usami A, Ito S. Glycemic control reverses increases in urinary excretions of immunoglobulin G and ceruloplasmin in type 2 diabetic patients with normoalbuminuria. Horm Metab Res. 2001;33:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 50. | Kandasamy Y, Smith R, Lumbers ER, Rudd D. Nephrin - a biomarker of early glomerular injury. Biomark Res. 2014;2:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Pätäri A, Forsblom C, Havana M, Taipale H, Groop PH, Holthöfer H. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes. 2003;52:2969-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Elhammady AM, Elshafie HS, El Fallah AA, Khalil MA. Serum suPAR and Urinary Nephrin as Novel Sensitive and Specific Markers for Diabetic Nephropathy in Patients with Type 2 DM. Egypt J Hosp Med. 2023;91:4373-4379. [DOI] [Full Text] |
| 53. | Ng DP, Tai BC, Tan E, Leong H, Nurbaya S, Lim XL, Chia KS, Wong CS, Lim WY, Holthöfer H. Nephrinuria associates with multiple renal traits in type 2 diabetes. Nephrol Dial Transplant. 2011;26:2508-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Jim B, Ghanta M, Qipo A, Fan Y, Chuang PY, Cohen HW, Abadi M, Thomas DB, He JC. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS One. 2012;7:e36041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 55. | do Nascimento JF, Canani LH, Gerchman F, Rodrigues PG, Joelsons G, dos Santos M, Pereira S, Veronese FV. Messenger RNA levels of podocyte-associated proteins in subjects with different degrees of glucose tolerance with or without nephropathy. BMC Nephrol. 2013;14:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Hong CY, Chia KS. Markers of diabetic nephropathy. J Diabetes Complications. 1998;12:43-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Tang XY, Zhou JB, Luo FQ, Han YP, Zhao W, Diao ZL, Li M, Qi L, Yang JK. Urine NGAL as an early biomarker for diabetic kidney disease: accumulated evidence from observational studies. Ren Fail. 2019;41:446-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Kapoula GV, Kontou PI, Bagos PG. Diagnostic Performance of Biomarkers Urinary KIM-1 and YKL-40 for Early Diabetic Nephropathy, in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2020;10:909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Thi TND, Gia BN, Thi HLL, Thi TNC, Thanh HP. Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J Med Biochem. 2020;39:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Hataem D, Alasady RA, Khudhair SA. Evaluation of Urinary Monocyte Chemoattractant Protein-1and Serum Cystatin C as Markers for Detection of Diabetic Nephropathy in Comparison to Albumin to Creatinine Ratio (ACR). Ann Trop Med Public Health. 2020;23. [DOI] [Full Text] |
| 61. | Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Jeon YK, Kim MR, Huh JE, Mok JY, Song SH, Kim SS, Kim BH, Lee SH, Kim YK, Kim IJ. Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci. 2011;26:258-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 63. | Yang CY, Chen FA, Chen CF, Liu WS, Shih CJ, Ou SM, Yang WC, Lin CC, Yang AH. Diagnostic Accuracy of Urine Protein/Creatinine Ratio Is Influenced by Urine Concentration. PLoS One. 2015;10:e0137460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS One. 2015;10:e0124055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Chang CC, Chen CY, Huang CH, Wu CL, Wu HM, Chiu PF, Kor CT, Chen TH, Chang GD, Kuo CC, Wen HC, Huang CY, Chang CH. Urinary glycated uromodulin in diabetic kidney disease. Clin Sci (Lond). 2017;131:1815-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Barr SI, Bessa SS, Mohamed TM, Abd El-Azeem EM. Exosomal UMOD gene expression and urinary uromodulin level as early noninvasive diagnostic biomarkers for diabetic nephropathy in type 2 diabetic patients. Diabetol Int. 2024;15:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 67. | Betz BB, Jenks SJ, Cronshaw AD, Lamont DJ, Cairns C, Manning JR, Goddard J, Webb DJ, Mullins JJ, Hughes J, McLachlan S, Strachan MWJ, Price JF, Conway BR. Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int. 2016;89:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 68. | Chen H, Ni L, Wu X. Performance of urinary vitamin D-binding protein in diabetic kidney disease: a meta-analysis. Ren Fail. 2023;45:2256415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Abbasi F, Moosaie F, Khaloo P, Dehghani Firouzabadi F, Fatemi Abhari SM, Atainia B, Ardeshir M, Nakhjavani M, Esteghamati A. Neutrophil Gelatinase-Associated Lipocalin and Retinol-Binding Protein-4 as Biomarkers for Diabetic Kidney Disease. Kidney Blood Press Res. 2020;45:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Chen TK, Coca SG, Thiessen-Philbrook HR, Heerspink HJL, Obeid W, Ix JH, Fried LF, Bonventre JV, El-Khoury JM, Shlipak MG, Parikh CR; CKD Biomarkers Consortium (BioCon). Urinary Biomarkers of Tubular Health and Risk for Kidney Function Decline or Mortality in Diabetes. Am J Nephrol. 2022;53:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Marakala V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury - A systematic review. Clin Chim Acta. 2022;536:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 87] [Reference Citation Analysis (0)] |
| 72. | Liu X, Guan Y, Xu S, Li Q, Sun Y, Han R, Jiang C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press Res. 2016;41:680-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, de Cal M, Corradi V, Virzi G, Ronco C. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 74. | Fiseha T, Tamir Z. Urinary Markers of Tubular Injury in Early Diabetic Nephropathy. Int J Nephrol. 2016;2016:4647685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Kaul A, Behera MR, Rai MK, Mishra P, Bhaduaria DS, Yadav S, Agarwal V, Karoli R, Prasad N, Gupta A, Sharma RK. Neutrophil Gelatinase-associated Lipocalin: As a Predictor of Early Diabetic Nephropathy in Type 2 Diabetes Mellitus. Indian J Nephrol. 2018;28:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Varatharajan S, Jain V, Pyati AK, Neeradi C, Reddy KS, Pallavali JR, Pandiyaraj IP, Gaur A. Neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and periostin: Novel urinary biomarkers in diabetic nephropathy. World J Nephrol. 2024;13:98880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 77. | Quang TH, Nguyet MP, Thao DP, Thi MH, Phuong Thi Dam L, Thi HH, Van AP, Luong TC, Tuyet MNT, Duy QD, Nhu BD, Duc TN. Evaluation of Urinary Neutrophil Gelatinase Associated Lipocalin and Kidney Injury Molecule-1 as Diagnostic Markers for Early Nephropathy in Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2020;13:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Phanish MK, Chapman AN, Yates S, Price R, Hendry BM, Roderick PJ, Dockrell MEC. Evaluation of Urinary Biomarkers of Proximal Tubular Injury, Inflammation, and Fibrosis in Patients With Albuminuric and Nonalbuminuric Diabetic Kidney Disease. Kidney Int Rep. 2021;6:1355-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1009-R1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 80. | Karimi F, Moazamfard M, Taghvaeefar R, Sohrabipour S, Dehghani A, Azizi R, Dinarvand N. Early Detection of Diabetic Nephropathy Based on Urinary and Serum Biomarkers: An Updated Systematic Review. Adv Biomed Res. 2024;13:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 81. | Kashgary A, Zaki MES, Elwahab AMA, Abdelsalam M, Nada AM. Study of Neutrophil Gelatinase Associated Lipocalin and Kidney Injury Molecule-1 in Patients with Type 2 Diabetes Mellitus Nephropathy. Clin Lab. 2024;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Kim SS, Song SH, Kim IJ, Yang JY, Lee JG, Kwak IS, Kim YK. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Yang YH, He XJ, Chen SR, Wang L, Li EM, Xu LY. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine. 2009;36:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 85. | Siddiqui K, Joy SS, George TP, Mujammami M, Alfadda AA. Potential Role and Excretion Level of Urinary Transferrin, KIM-1, RBP, MCP-1 and NGAL Markers in Diabetic Nephropathy. Diabetes Metab Syndr Obes. 2020;13:5103-5111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 86. | Duan S, Chen J, Wu L, Nie G, Sun L, Zhang C, Huang Z, Xing C, Zhang B, Yuan Y. Assessment of urinary NGAL for differential diagnosis and progression of diabetic kidney disease. J Diabetes Complications. 2020;34:107665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 87. | Satirapoj B, Aramsaowapak K, Tangwonglert T, Supasyndh O. Novel Tubular Biomarkers Predict Renal Progression in Type 2 Diabetes Mellitus: A Prospective Cohort Study. J Diabetes Res. 2016;2016:3102962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 822] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 89. | Ide H, Iwase M, Ohkuma T, Fujii H, Komorita Y, Oku Y, Higashi T, Yoshinari M, Nakamura U, Kitazono T. Usefulness of urinary tubule injury markers for predicting progression of renal dysfunction in patients with type 2 diabetes and albuminuria: The Fukuoka Diabetes Registry. Diabetes Res Clin Pract. 2022;186:109840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One. 2013;8:e54863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Rotbain Curovic V, Hansen TW, Eickhoff MK, von Scholten BJ, Reinhard H, Jacobsen PK, Persson F, Parving HH, Rossing P. Urinary tubular biomarkers as predictors of kidney function decline, cardiovascular events and mortality in microalbuminuric type 2 diabetic patients. Acta Diabetol. 2018;55:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Gkiourtzis N, Stoimeni A, Michou P, Cheirakis K, Moutafi M, Christakopoulos A, Glava A, Panagopoulou P, Tsigaras G, Galli-Tsinopoulou A, Christoforidis A, Tramma D. The NGAL as a prognostic biomarker of kidney injury in children and adolescents with type 1 diabetes mellitus: A systematic review and meta-analysis. J Diabetes Complications. 2025;39:109002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Valent Morić B, Šamija I, La Grasta Sabolić L, Unić A, Miler M. Is the urinary neutrophil gelatinase-associated lipocalin concentration in children and adolescents with type 1 diabetes mellitus different from that in healthy children? Biochem Med (Zagreb). 2024;34:020709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 94. | Garg P, Shetty M, Krishnamurthy V. Correlation of Urinary Neutrophil Gelatinase with the Histopathological Extent of Kidney Damage in Patients with Diabetic Nephropathy. Saudi J Kidney Dis Transpl. 2023;34:S112-S121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 95. | Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010;16:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 96. | Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014;125:293-9; discussion 299. [PubMed] |
| 97. | Vasquez-Rios G, Katz R, Levitan EB, Cushman M, Parikh CR, Kimmel PL, Bonventre JV, Waikar SS, Schrauben SJ, Greenberg JH, Sarnak MJ, Ix JH, Shlipak MG, Gutierrez OM. Urinary Biomarkers of Kidney Tubule Health and Mortality in Persons with CKD and Diabetes Mellitus. Kidney360. 2023;4:e1257-e1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Oliveira LE, Paniz C, Moresco RN, Carvalho JAM. KIM-1 as a biomarker of kidney tubular damage in normoalbuminuric patients with type 2 diabetes mellitus and insulin resistance. J Bras Nefrol. 2025;47:e20240123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 99. | Fu WJ, Xiong SL, Fang YG, Wen S, Chen ML, Deng RT, Zheng L, Wang SB, Pen LF, Wang Q. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine. 2012;41:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | El-Ashmawy NE, El-Zamarany EA, Khedr NF, Abd El-Fattah AI, Eltoukhy SA. Kidney injury molecule-1 (Kim-1): an early biomarker for nephropathy in type II diabetic patients. Int J Diabetes Dev Ctries. 2015;35:431-438. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Aslan O, Demir M, Koseoglu M. Kidney Injury Molecule Levels in Type 2 Diabetes Mellitus. J Clin Lab Anal. 2016;30:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Kin Tekce B, Tekce H, Aktas G, Sit M. Evaluation of the urinary kidney injury molecule-1 levels in patients with diabetic nephropathy. Clin Invest Med. 2014;37:E377-E383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Waijer SW, Sen T, Arnott C, Neal B, Kosterink JGW, Mahaffey KW, Parikh CR, de Zeeuw D, Perkovic V, Neuen BL, Coca SG, Hansen MK, Gansevoort RT, Heerspink HJL. Association between TNF Receptors and KIM-1 with Kidney Outcomes in Early-Stage Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2022;17:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 104. | Zhang L, Xue S, Wu M, Dong D. Performance of urinary liver-type fatty acid-binding protein in diabetic nephropathy: A meta-analysis. Front Med (Lausanne). 2022;9:914587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Chen Y, Liu X, Shengbu M, Shi Q, Jiaqiu S, Lai X. Biomarkers: New Advances in Diabetic Nephropathy. Nat Prod Commun. 2025;20:1934578X251321758. [DOI] [Full Text] |
| 106. | Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H. Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care. 2013;36:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697-F701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 108. | Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Titan SM, Vieira JM Jr, Dominguez WV, Moreira SR, Pereira AB, Barros RT, Zatz R. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012;26:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Knowler WC, Rovin BH, Wu H, Klein JB, Mifflin TE, Feldman HI, Vasan RS, Kimmel PL, Kusek JW, Mauer M; CKD Biomarkers Consortium and the RASS Investigators. Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transplant. 2015;30:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O'Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 272] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 112. | Zeng XF, Lu DX, Li JM, Tan Y, Li Z, Zhou L, Xi ZQ, Zhang SM, Duan W. Performance of urinary neutrophil gelatinase-associated lipocalin, clusterin, and cystatin C in predicting diabetic kidney disease and diabetic microalbuminuria: a consecutive cohort study. BMC Nephrol. 2017;18:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomark Res. 2015;3:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 114. | Kim SS, Song SH, Kim IJ, Jeon YK, Kim BH, Kwak IS, Lee EK, Kim YK. Urinary cystatin C and tubular proteinuria predict progression of diabetic nephropathy. Diabetes Care. 2013;36:656-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 115. | Rico-Fontalvo J, Aroca-Martínez G, Daza-Arnedo R, Cabrales J, Rodríguez-Yanez T, Cardona-Blanco M, Montejo-Hernández J, Rodelo Barrios D, Patiño-Patiño J, Osorio Rodríguez E. Novel Biomarkers of Diabetic Kidney Disease. Biomolecules. 2023;13:633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 116. | Cao H, Song L, Wang X, Guan H. Non-linear relationship between urinary creatinine and diabetic kidney disease: implications for clinical practice. BMC Nephrol. 2025;26:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 117. | Chung JO, Park SY, Kim BN, Cho DH, Chung DJ, Chung MY. Association of urinary creatinine excretion and body mass index with diabetic retinopathy in patients with type 2 diabetes. Sci Rep. 2024;14:17175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Sinkeler SJ, Kwakernaak AJ, Bakker SJ, Shahinfar S, Esmatjes E, de Zeeuw D, Navis G, Lambers Heerspink HJ. Creatinine excretion rate and mortality in type 2 diabetes and nephropathy. Diabetes Care. 2013;36:1489-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Esmeralda CAC, David PE, Maldonado IC, Ibrahim SNA, David AS, Escorza MAQ, Dealmy DG. Deranged Fractional Excretion of Magnesium and Serum Magnesium Levels in Relation to Retrograde Glycaemic Regulation in Patients with Type 2 Diabetes Mellitus. Curr Diabetes Rev. 2021;17:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Futrakul N, Futrakul P. Biomarker for early renal microvascular and diabetic kidney diseases. Ren Fail. 2017;39:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Satirapoj B. Tubulointerstitial Biomarkers for Diabetic Nephropathy. J Diabetes Res. 2018;2018:2852398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 122. | Jeon J, Kim DH, Jang HR, Lee JE, Huh W, Kim HY, Kim DJ, Kim YG. Urinary angiotensinogen as a surrogate marker predicting the antiproteinuric effects of angiotensin receptor blockers in patients with overt proteinuria: a multicenter prospective study. BMC Nephrol. 2020;21:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 123. | de Alencar Franco Costa D, Todiras M, Campos LA, Cipolla-Neto J, Bader M, Baltatu OC. Sex-dependent differences in renal angiotensinogen as an early marker of diabetic nephropathy. Acta Physiol (Oxf). 2015;213:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 124. | Takata T, Isomoto H. The Versatile Role of Uromodulin in Renal Homeostasis and Its Relevance in Chronic Kidney Disease. Intern Med. 2024;63:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 125. | Leiherer A, Muendlein A, Saely CH, Kinz E, Brandtner EM, Fraunberger P, Drexel H. Serum uromodulin is associated with impaired glucose metabolism. Medicine (Baltimore). 2017;96:e5798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Barr SI, Abd El-Azeem EM, Bessa SS, Mohamed TM. Association of serum uromodulin with diabetic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2024;25:421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 127. | Chakraborty J, Below AA, Solaiman D. Tamm-Horsfall protein in patients with kidney damage and diabetes. Urol Res. 2004;32:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Shin JH, Kim KM, Jeong JU, Shin JM, Kang JH, Bang K, Kim JH. Nrf2-Heme Oxygenase-1 Attenuates High-Glucose-Induced Epithelial-to-Mesenchymal Transition of Renal Tubule Cells by Inhibiting ROS-Mediated PI3K/Akt/GSK-3β Signaling. J Diabetes Res. 2019;2019:2510105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 129. | Chang TT, Chiang CH, Chen C, Lin SC, Lee HJ, Chen JW. Antioxidation and Nrf2-mediated heme oxygenase-1 activation contribute to renal protective effects of hydralazine in diabetic nephropathy. Biomed Pharmacother. 2022;151:113139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 130. | Faria AM, Peixoto EBMI, Adamo CB, Flacker A, Longo E, Mazon T. Controlling parameters and characteristics of electrochemical biosensors for enhanced detection of 8-hydroxy-2'-deoxyguanosine. Sci Rep. 2019;9:7411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Serdar M, Sertoglu E, Uyanik M, Tapan S, Akin K, Bilgi C, Kurt I. Comparison of 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels using mass spectrometer and urine albumin creatinine ratio as a predictor of development of diabetic nephropathy. Free Radic Res. 2012;46:1291-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal. 2004;36:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 728] [Article Influence: 33.1] [Reference Citation Analysis (5)] |
| 134. | Nappi F. Non-Coding RNA-Targeted Therapy: A State-of-the-Art Review. Int J Mol Sci. 2024;25:3630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 135. | Colpaert RMW, Calore M. MicroRNAs in Cardiac Diseases. Cells. 2019;8:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 136. | Mondal S, Rathor R, Singh SN, Suryakumar G. miRNA and leptin signaling in metabolic diseases and at extreme environments. Pharmacol Res Perspect. 2024;12:e1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 137. | Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14:dmm047662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 476] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 138. | Chen J, Luo M, Xing Z, Chen Y, Peng C, Li D. Start small, think big: MicroRNAs in diabetes mellitus and relevant cardiorenal-liver metabolic health spectrum. Metabolism. 2025;165:156153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 139. | Lee CC, Chen CC, Hsu CK, Chen YT, Chen CY, Yang KJ, Hung MJ, Wu IW. Urinary microRNA in Diabetic Kidney Disease: A Literature Review. Medicina (Kaunas). 2023;59:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 140. | Petrica L, Hogea E, Gadalean F, Vlad A, Vlad M, Dumitrascu V, Velciov S, Gluhovschi C, Bob F, Ursoniu S, Jianu DC, Matusz P, Pusztai AM, Motoc A, Cretu OM, Radu D, Milas O, Golea-Secara A, Simulescu A, Mogos-Stefan M, Patruica M, Balint L, Ienciu S, Vlad D, Popescu R. Long noncoding RNAs may impact podocytes and proximal tubule function through modulating miRNAs expression in Early Diabetic Kidney Disease of Type 2 Diabetes Mellitus patients. Int J Med Sci. 2021;18:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 141. | Zang J, Maxwell AP, Simpson DA, McKay GJ. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci Rep. 2019;9:10900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 142. | Zhao Y, Shen A, Guo F, Song Y, Jing N, Ding X, Pan M, Zhang H, Wang J, Wu L, Ma X, Feng L, Qin G. Urinary Exosomal MiRNA-4534 as a Novel Diagnostic Biomarker for Diabetic Kidney Disease. Front Endocrinol (Lausanne). 2020;11:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 143. | Sinha N, Puri V, Kumar V, Nada R, Rastogi A, Jha V, Puri S. Urinary exosomal miRNA-663a shows variable expression in diabetic kidney disease patients with or without proteinuria. Sci Rep. 2023;13:4516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 144. | Barutta F, Tricarico M, Corbelli A, Annaratone L, Pinach S, Grimaldi S, Bruno G, Cimino D, Taverna D, Deregibus MC, Rastaldi MP, Perin PC, Gruden G. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One. 2013;8:e73798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 145. | Han LL, Wang SH, Yao MY, Zhou H. Urinary exosomal microRNA-145-5p and microRNA-27a-3p act as noninvasive diagnostic biomarkers for diabetic kidney disease. World J Diabetes. 2024;15:92-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 146. | Mishra DD, Sahoo B, Maurya PK, Sharma R, Varughese S, Prasad N, Tiwari S. Therapeutic potential of urine exosomes derived from rats with diabetic kidney disease. Front Endocrinol (Lausanne). 2023;14:1157194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 147. | Cho NJ, Kim DY, Kwon SH, Ha TW, Kim HK, Lee MR, Chun SW, Park S, Lee EY, Gil HW. Urinary exosomal microRNA profiling in type 2 diabetes patients taking dipeptidyl peptidase-4 inhibitor compared with sulfonylurea. Kidney Res Clin Pract. 2021;40:383-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 148. | Seyhan AA. Trials and Tribulations of MicroRNA Therapeutics. Int J Mol Sci. 2024;25:1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 209] [Reference Citation Analysis (0)] |
| 149. | Wishart DS. Metabolomics: the principles and potential applications to transplantation. Am J Transplant. 2005;5:2814-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 150. | Mal TK, Tian Y, Patterson AD. Sample Preparation and Data Analysis for NMR-Based Metabolomics. Methods Mol Biol. 2021;2194:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Emwas AH, Roy R, McKay RT, Tenori L, Saccenti E, Gowda GAN, Raftery D, Alahmari F, Jaremko L, Jaremko M, Wishart DS. NMR Spectroscopy for Metabolomics Research. Metabolites. 2019;9:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 690] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 152. | Breit M, Weinberger KM. Metabolic biomarkers for chronic kidney disease. Arch Biochem Biophys. 2016;589:62-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 153. | Du F, Virtue A, Wang H, Yang XF. Metabolomic analyses for atherosclerosis, diabetes, and obesity. Biomark Res. 2013;1:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 154. | Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2618] [Cited by in RCA: 2524] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 155. | Tofte N, Suvitaival T, Trost K, Mattila IM, Theilade S, Winther SA, Ahluwalia TS, Frimodt-Møller M, Legido-Quigley C, Rossing P. Metabolomic Assessment Reveals Alteration in Polyols and Branched Chain Amino Acids Associated With Present and Future Renal Impairment in a Discovery Cohort of 637 Persons With Type 1 Diabetes. Front Endocrinol (Lausanne). 2019;10:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 156. | Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, Karoly ED, Kensicki EM, Berry GT, Bonventre JV, Pennathur S, Meyer TW, Krolewski AS. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int. 2014;85:1214-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 157. | Hung SC, Kuo KL, Wu CC, Tarng DC. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J Am Heart Assoc. 2017;6:e005022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 158. | Shao M, Lu H, Yang M, Liu Y, Yin P, Li G, Wang Y, Chen L, Chen Q, Zhao C, Lu Q, Wu T, Ji G. Serum and urine metabolomics reveal potential biomarkers of T2DM patients with nephropathy. Ann Transl Med. 2020;8:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 159. | Pena MJ, Lambers Heerspink HJ, Hellemons ME, Friedrich T, Dallmann G, Lajer M, Bakker SJ, Gansevoort RT, Rossing P, de Zeeuw D, Roscioni SS. Urine and plasma metabolites predict the development of diabetic nephropathy in individuals with Type 2 diabetes mellitus. Diabet Med. 2014;31:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 160. | Kwan B, Fuhrer T, Zhang J, Darshi M, Van Espen B, Montemayor D, de Boer IH, Dobre M, Hsu CY, Kelly TN, Raj DS, Rao PS, Saraf SL, Scialla J, Waikar SS, Sharma K, Natarajan L; CRIC Study Investigators. Metabolomic Markers of Kidney Function Decline in Patients With Diabetes: Evidence From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2020;76:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 161. | Li L, Wang C, Yang H, Liu S, Lu Y, Fu P, Liu J. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of DKD in T2DM patients. Mol Biosyst. 2017;13:2392-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 162. | Kopple JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. 2007;137:1586S-1590S; discussion 1597S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 163. | Zhang S, Li X, Luo H, Fang ZZ, Ai H. Role of aromatic amino acids in pathogeneses of diabetic nephropathy in Chinese patients with type 2 diabetes. J Diabetes Complications. 2020;34:107667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 164. | Liu Y, Chen X, Liu Y, Chen T, Zhang Q, Zhang H, Zhu Z, Chai Y, Zhang J. Metabolomic study of the protective effect of Gandi capsule for diabetic nephropathy. Chem Biol Interact. 2019;314:108815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 165. | Tofte N, Vogelzangs N, Mook-Kanamori D, Brahimaj A, Nano J, Ahmadizar F, van Dijk KW, Frimodt-Møller M, Arts I, Beulens JWJ, Rutters F, van der Heijden AA, Kavousi M, Stehouwer CDA, Nijpels G, van Greevenbroek MMJ, van der Kallen CJH, Rossing P, Ahluwalia TS, 't Hart LM. Plasma Metabolomics Identifies Markers of Impaired Renal Function: A Meta-analysis of 3089 Persons with Type 2 Diabetes. J Clin Endocrinol Metab. 2020;105:dgaa173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 166. | Niewczas MA, Mathew AV, Croall S, Byun J, Major M, Sabisetti VS, Smiles A, Bonventre JV, Pennathur S, Krolewski AS. Circulating Modified Metabolites and a Risk of ESRD in Patients With Type 1 Diabetes and Chronic Kidney Disease. Diabetes Care. 2017;40:383-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 167. | Colombo M, Valo E, McGurnaghan SJ, Sandholm N, Blackbourn LAK, Dalton RN, Dunger D, Groop PH, McKeigue PM, Forsblom C, Colhoun HM; FinnDiane Study Group and the Scottish Diabetes Research Network (SDRN) Type 1 Bioresource Collaboration. Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia. 2019;62:1616-1627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 168. | Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. 2003;54:175-189. [PubMed] |
| 169. | Mogos M, Socaciu C, Socaciu AI, Vlad A, Gadalean F, Bob F, Milas O, Cretu OM, Suteanu-Simulescu A, Glavan M, Balint L, Ienciu S, Iancu L, Jianu DC, Ursoniu S, Petrica L. Biomarker Profiling with Targeted Metabolomic Analysis of Plasma and Urine Samples in Patients with Type 2 Diabetes Mellitus and Early Diabetic Kidney Disease. J Clin Med. 2024;13:4703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 170. | van der Kloet FM, Tempels FW, Ismail N, van der Heijden R, Kasper PT, Rojas-Cherto M, van Doorn R, Spijksma G, Koek M, van der Greef J, Mäkinen VP, Forsblom C, Holthöfer H, Groop PH, Reijmers TH, Hankemeier T. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study). Metabolomics. 2012;8:109-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 171. | Chou CA, Lin CN, Chiu DT, Chen IW, Chen ST. Tryptophan as a surrogate prognostic marker for diabetic nephropathy. J Diabetes Investig. 2018;9:366-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 172. | Tavares G, Venturini G, Padilha K, Zatz R, Pereira AC, Thadhani RI, Rhee EP, Titan SMO. 1,5-Anhydroglucitol predicts CKD progression in macroalbuminuric diabetic kidney disease: results from non-targeted metabolomics. Metabolomics. 2018;14:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 173. | Hirayama A, Nakashima E, Sugimoto M, Akiyama S, Sato W, Maruyama S, Matsuo S, Tomita M, Yuzawa Y, Soga T. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem. 2012;404:3101-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 174. | Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011;365:1812-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 175. | Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 521] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 176. | Han LD, Xia JF, Liang QL, Wang Y, Wang YM, Hu P, Li P, Luo GA. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography-mass spectrometry and its application in the study of diabetic mellitus and diabetic nephropathy. Anal Chim Acta. 2011;689:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 177. | Larbi A, Grenier A, Frisch F, Douziech N, Fortin C, Carpentier AC, Fülöp T. Acute in vivo elevation of intravascular triacylglycerol lipolysis impairs peripheral T cell activation in humans. Am J Clin Nutr. 2005;82:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 178. | Mäkinen VP, Tynkkynen T, Soininen P, Forsblom C, Peltola T, Kangas AJ, Groop PH, Ala-Korpela M. Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics. 2012;8:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 179. | Zhang H, Zuo JJ, Dong SS, Lan Y, Wu CW, Mao GY, Zheng C. Identification of Potential Serum Metabolic Biomarkers of Diabetic Kidney Disease: A Widely Targeted Metabolomics Study. J Diabetes Res. 2020;2020:3049098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 180. | Ibarra-González I, Cruz-Bautista I, Bello-Chavolla OY, Vela-Amieva M, Pallares-Méndez R, Ruiz de Santiago Y Nevarez D, Salas-Tapia MF, Rosas-Flota X, González-Acevedo M, Palacios-Peñaloza A, Morales-Esponda M, Aguilar-Salinas CA, Del Bosque-Plata L. Optimization of kidney dysfunction prediction in diabetic kidney disease using targeted metabolomics. Acta Diabetol. 2018;55:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 181. | Merscher S, Fornoni A. Podocyte pathology and nephropathy - sphingolipids in glomerular diseases. Front Endocrinol (Lausanne). 2014;5:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 182. | Huang J, Huth C, Covic M, Troll M, Adam J, Zukunft S, Prehn C, Wang L, Nano J, Scheerer MF, Neschen S, Kastenmüller G, Suhre K, Laxy M, Schliess F, Gieger C, Adamski J, Hrabe de Angelis M, Peters A, Wang-Sattler R. Machine Learning Approaches Reveal Metabolic Signatures of Incident Chronic Kidney Disease in Individuals With Prediabetes and Type 2 Diabetes. Diabetes. 2020;69:2756-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 183. | Pongrac Barlovic D, Harjutsalo V, Sandholm N, Forsblom C, Groop PH; FinnDiane Study Group. Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia. 2020;63:1847-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 184. | Zhu C, Liang QL, Hu P, Wang YM, Luo GA. Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta. 2011;85:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 185. | Liu JJ, Ghosh S, Kovalik JP, Ching J, Choi HW, Tavintharan S, Ong CN, Sum CF, Summers SA, Tai ES, Lim SC. Profiling of Plasma Metabolites Suggests Altered Mitochondrial Fuel Usage and Remodeling of Sphingolipid Metabolism in Individuals With Type 2 Diabetes and Kidney Disease. Kidney Int Rep. 2017;2:470-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 186. | Afshinnia F, Nair V, Lin J, Rajendiran TM, Soni T, Byun J, Sharma K, Fort PE, Gardner TW, Looker HC, Nelson RG, Brosius FC, Feldman EL, Michailidis G, Kretzler M, Pennathur S. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight. 2019;4:e130317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 187. | Ng DP, Salim A, Liu Y, Zou L, Xu FG, Huang S, Leong H, Ong CN. A metabolomic study of low estimated GFR in non-proteinuric type 2 diabetes mellitus. Diabetologia. 2012;55:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 188. | Sirolli V, Rossi C, Di Castelnuovo A, Felaco P, Amoroso L, Zucchelli M, Ciavardelli D, Di Ilio C, Sacchetta P, Bernardini S, Arduini A, Bonomini M, Urbani A. Toward personalized hemodialysis by low molecular weight amino-containing compounds: future perspective of patient metabolic fingerprint. Blood Transfus. 2012;10 Suppl 2:s78-s88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 189. | Yoshioka K, Hirakawa Y, Kurano M, Ube Y, Ono Y, Kojima K, Iwama T, Kano K, Hasegawa S, Inoue T, Shimada T, Aoki J, Yatomi Y, Nangaku M, Inagi R. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2022;101:510-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 190. | Wei PZ, Szeto CC. Mitochondrial dysfunction in diabetic kidney disease. Clin Chim Acta. 2019;496:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 191. | You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics Reveals a Key Role for Fumarate in Mediating the Effects of NADPH Oxidase 4 in Diabetic Kidney Disease. J Am Soc Nephrol. 2016;27:466-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 192. | Linehan WM, Rouault TA. Molecular pathways: Fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clin Cancer Res. 2013;19:3345-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 193. | Liu JJ, Liu S, Gurung RL, Ching J, Kovalik JP, Tan TY, Lim SC. Urine Tricarboxylic Acid Cycle Metabolites Predict Progressive Chronic Kidney Disease in Type 2 Diabetes. J Clin Endocrinol Metab. 2018;103:4357-4364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 194. | Azushima K, Kovalik JP, Yamaji T, Ching J, Chng TW, Guo J, Liu JJ, Nguyen M, Sakban RB, George SE, Tan PH, Lim SC, Gurley SB, Coffman TM. Abnormal lactate metabolism is linked to albuminuria and kidney injury in diabetic nephropathy. Kidney Int. 2023;104:1135-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 195. | Sung CC, Hsu YC, Chen CC, Lin YF, Wu CC. Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Oxid Med Cell Longev. 2013;2013:301982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 196. | Xia JF, Hu P, Liang QL, Zou TT, Wang YM, Luo GA. Correlations of creatine and six related pyrimidine metabolites and diabetic nephropathy in Chinese type 2 diabetic patients. Clin Biochem. 2010;43:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 197. | Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E. Prediction of Declining Renal Function and Albuminuria in Patients With Type 2 Diabetes by Metabolomics. J Clin Endocrinol Metab. 2016;101:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 198. | Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 468] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 199. | Sharma K, Zhang G, Hansen J, Bjornstad P, Lee HJ, Menon R, Hejazi L, Liu JJ, Franzone A, Looker HC, Choi BY, Fernandez R, Venkatachalam MA, Kugathasan L, Sridhar VS, Natarajan L, Zhang J, Sharma VS, Kwan B, Waikar SS, Himmelfarb J, Tuttle KR, Kestenbaum B, Fuhrer T, Feldman HI, de Boer IH, Tucci FC, Sedor J, Heerspink HL, Schaub J, Otto EA, Hodgin JB, Kretzler M, Anderton CR, Alexandrov T, Cherney D, Lim SC, Nelson RG, Gelfond J, Iyengar R; Kidney Precision Medicine Project. Endogenous adenine mediates kidney injury in diabetic models and predicts diabetic kidney disease in patients. J Clin Invest. 2023;133:e170341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 200. | Al-Rubeaan K, Siddiqui K, Al-Ghonaim MA, Youssef AM, Al-Sharqawi AH, AlNaqeb D. Assessment of the diagnostic value of different biomarkers in relation to various stages of diabetic nephropathy in type 2 diabetic patients. Sci Rep. 2017;7:2684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 201. | Schlosser P, Grams ME, Rhee EP. Proteomics: Progress and Promise of High-Throughput Proteomics in Chronic Kidney Disease. Mol Cell Proteomics. 2023;22:100550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 202. | Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1180] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 203. | Dubin RF, Rhee EP. Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention. Clin J Am Soc Nephrol. 2020;15:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 204. | Good DM, Zürbig P, Argilés A, Bauer HW, Behrens G, Coon JJ, Dakna M, Decramer S, Delles C, Dominiczak AF, Ehrich JH, Eitner F, Fliser D, Frommberger M, Ganser A, Girolami MA, Golovko I, Gwinner W, Haubitz M, Herget-Rosenthal S, Jankowski J, Jahn H, Jerums G, Julian BA, Kellmann M, Kliem V, Kolch W, Krolewski AS, Luppi M, Massy Z, Melter M, Neusüss C, Novak J, Peter K, Rossing K, Rupprecht H, Schanstra JP, Schiffer E, Stolzenburg JU, Tarnow L, Theodorescu D, Thongboonkerd V, Vanholder R, Weissinger EM, Mischak H, Schmitt-Kopplin P. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424-2437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 205. | Pontillo C, Mischak H. Urinary peptide-based classifier CKD273: towards clinical application in chronic kidney disease. Clin Kidney J. 2017;10:192-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 206. | Tofte N, Lindhardt M, Adamova K, Bakker SJL, Beige J, Beulens JWJ, Birkenfeld AL, Currie G, Delles C, Dimos I, Francová L, Frimodt-Møller M, Girman P, Göke R, Havrdova T, Heerspink HJL, Kooy A, Laverman GD, Mischak H, Navis G, Nijpels G, Noutsou M, Ortiz A, Parvanova A, Persson F, Petrie JR, Ruggenenti PL, Rutters F, Rychlík I, Siwy J, Spasovski G, Speeckaert M, Trillini M, Zürbig P, von der Leyen H, Rossing P; PRIORITY investigators. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 207. | Siwy J, Klein T, Rosler M, von Eynatten M. Urinary Proteomics as a Tool to Identify Kidney Responders to Dipeptidyl Peptidase-4 Inhibition: A Hypothesis-Generating Analysis from the MARLINA-T2D Trial. Proteomics Clin Appl. 2019;13:e1800144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 208. | Zürbig P, Jerums G, Hovind P, Macisaac RJ, Mischak H, Nielsen SE, Panagiotopoulos S, Persson F, Rossing P. Urinary proteomics for early diagnosis in diabetic nephropathy. Diabetes. 2012;61:3304-3313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 209. | Rossing K, Mischak H, Parving HH, Christensen PK, Walden M, Hillmann M, Kaiser T. Impact of diabetic nephropathy and angiotensin II receptor blockade on urinary polypeptide patterns. Kidney Int. 2005;68:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 210. | Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier PJ, Satake E, Simeone CA, Shah H, Qiu C, Looker HC, Fiorina P, Ware CF, Sun JK, Doria A, Kretzler M, Susztak K, Duffin KL, Nelson RG, Krolewski AS. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25:805-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 211. | Liao WL, Chang CT, Chen CC, Lee WJ, Lin SY, Liao HY, Wu CM, Chang YW, Chen CJ, Tsai FJ. Urinary Proteomics for the Early Diagnosis of Diabetic Nephropathy in Taiwanese Patients. J Clin Med. 2018;7:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 212. | Lim YK, Jenner A, Ali AB, Wang Y, Hsu SI, Chong SM, Baumman H, Halliwell B, Lim SK. Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int. 2000;58:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 213. | Bhensdadia NM, Hunt KJ, Lopes-Virella MF, Michael Tucker J, Mataria MR, Alge JL, Neely BA, Janech MG, Arthur JM; Veterans Affairs Diabetes Trial (VADT) study group. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int. 2013;83:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 214. | Liu JJ, Liu S, Wong MD, Gurung RL, Lim SC. Urinary Haptoglobin Predicts Rapid Renal Function Decline in Asians With Type 2 Diabetes and Early Kidney Disease. J Clin Endocrinol Metab. 2016;101:3794-3802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/