Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.110515
Revised: July 8, 2025
Accepted: August 26, 2025
Published online: September 15, 2025
Processing time: 95 Days and 18.9 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) is featured by the accumulation of excessive fat in the liver. It is caused by many factors, such as overweight, obesity, diabetes, and high plasma levels of sugar, cholesterol, and triglycerides. MASLD is commonly associated with type 2 diabetes (T2D), which is characterized by a pathophysiological deficiency of insulin secretion due to impaired function of pancreatic β cells and insulin resistance. T2D has become a global pandemic that influences more than 21.7 million people worldwide. Pre-clinical and clinical studies have been performed to investigate molecular cro
Core Tip: Macrophages are an important cellular component that regulates metabolic disorders, including metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes (T2D). Macrophage-derived cytokines, chemokines, and metabolic products contribute to metabolic inflammation and insulin resistance, immune cell infiltration, and tissue injury. The phenotype and function of macrophages are important factors for evaluating the efficacy of MASLD and T2D treatments. Clinical trials are ongoing to dissect the roles of macrophages and test macrophage-targeted therapies in MASLD, T2D, and other related metabolic disorders.
- Citation: Zhang CY, Liu S, Yang M. Macrophage and inflammation in diabetes and metabolic dysfunction-associated steatotic liver disease: From mechanisms to therapeutic strategies. World J Diabetes 2025; 16(9): 110515
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/110515.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.110515
Metabolic dysfunction-associated steatotic liver disease (MASLD), (previously known as non-alcoholic fatty liver disease or NAFLD) is characterized by the accumulation of excessive fat in the liver[1]. Many factors, such as overweight, obesity, diabetes, and high levels of plasma sugar, cholesterol, and triglycerides, contribute to MASLD development. Metabolic dysfunction-associated steatohepatitis (MASH) is an advanced stage of MASLD with the progression of liver inflammation, cell death, and various liver fibrosis. The progression of MASLD or MASH could increase the risk of liver cirrhosis and primary liver cancer, with a predominant type of hepatocellular carcinoma[2]. MASLD is a rapidly growing health concern, affecting more than a quarter of the global population[3]. Type 2 diabetes (T2D) is closely associated with MASLD, and approximately 60%-70% of T2D patients have MASLD[4]. T2D is characterized by the pathophysiological deficiency of insulin secretion due to impaired function of the pancreatic islet β-cells and the occurrence of insulin resistance. T2D has become a global pandemic and influences more than 21.7 million people worldwide[5]. Given the high co-occurrence of MASLD and T2D, it is necessary to investigate the underlying molecular and cellular crosslinks between MASLD and T2D[6], which can provide the foundation for developing therapeutic targets and treatment strategies for MASLD and T2D.
Macrophages play a crucial role in host health and disease through immune surveillance[7]. They are responsible for detecting the pathogen-related molecules or damage-related molecules and clearing pathogens and dead cell components by phagocytosis[8]. Tissue resident macrophages maintain local tissue homeostasis[9]. Accumulating data show that macrophages, such as adipose tissue macrophages and liver-resident macrophages consisting of Kupffer cells and monocyte-derived macrophages, function as important mediators in the cellular mechanisms in both MASLD and T2D[10,11]. Meanwhile, inflammation plays a pivotal role in both MASLD and T2D. Notably, macrophage-mediated inflammation is essential in metabolic disorders[12,13]. Therefore, in this review, we focus on the function of macrophages and inflammation and summarize the cellular and molecular mechanisms mediating the crosslinking between MASLD and T2D. In addition, the related therapeutic targets, treatment strategies, and clinical trials are summarized.
Macrophages are immune cells that play essential roles in host immune defense through sensing, engulfing, and digesting pathogens or dead cell components. In addition, macrophages mediate inflammation by secreting molecules, such as cytokines and chemokines, and maintain tissue homeostasis by regulating tissue remodeling and repair[14]. Macrophages also play a pivotal role in metabolic regulation. Upon different stimulation, macrophages can be polarized into two different phenotypes, M1 and M2 polarization, which possess distinct functionality[15]. Classically activated M1 macrophages show proinflammatory function due to the production of proinflammatory molecules such as interleukin (IL)-6, IL-12, and tumor necrosis factor (TNF). In contrast, alternatively active M2 macrophages own anti-inflammatory function, which is characterized by the release of anti-inflammatory molecules, such as IL-10 and transforming growth factor beta (TGF-β)[8]. The ratio or balance of M1 and M2 is critical in maintaining tissue homeostasis and inflammatory status[16].
Tissue-resident macrophages refer to the macrophages that are specifically located in different tissues, characterized by their heterogeneity depending on the tissue types in which they reside. Liver-resident macrophages, consisting of Kupffer cells and monocyte-derived macrophages, and adipose tissue macrophages, play a pivotal role in the progression of MASLD and T2D[17,18]. In the following sections, we will dissect the roles of these macrophages.
Kupffer cells and monocyte-derived macrophages are cellular inflammatory regulators in MASLD and T2D, which reciprocally interact with each other to impact disease progression[19,20]. A proinflammatory condition increases the risk of T2D development and progression in patients. Mechanistically, Kupffer cells and monocyte-derived macrophages are differentiated into M1 macrophages to exacerbate an inflammatory response via the secretion of cytokines and che
Kupffer cells are originally derived from erythromyeloid progenitors of the yolk sac[23]. They can be polarized into M1 phenotype by cytokines, such as IL-1β, IL-6, IL-12, interferon-gamma (IFN-γ), TNF-α, and lipopolysaccharide (LPS). These activated Kupffer cells can subsequently trigger the proinflammatory response to induce tissue inflammation and damage[24,25]. A recent study revealed that CCAAT/enhancer binding protein beta (C/EBPβ) was overexpressed in Kupffer cells in a high-fat and high-cholesterol diet-induced mouse MASLD model. C/EBPβ is a transcription factor that contributes to inflammation, metabolic dysfunction, and immune dysregulation. Metabolic abnormality induced the upregulation of C/EBPβ in the liver tissues of MASLD subjects. The elevated level of C/EBPβ further increased the expression of vascular cell adhesion protein 1 in Kupffer cells, which exacerbated hepatic immune cell infiltration and liver inflammation[26]. Interestingly, C/EBPβ is known to contribute to the pathological progression of T2D, which is associated with pancreatic β-cell dysfunction and insulin secretion reduction[27]. Meanwhile, C/EBPβ also plays a significant role in the peroxisome proliferator-activated receptors signaling pathway, a well-known pathway involved in both MASLD and T2D[28].
Blood circulating monocytes, which originated from hematopoietic stem cells, can differentiate into M1 macrophages upon the cytokine stimulation of granulocyte-macrophage colony-stimulating factor along with IFN-γ and LPS[29,30]. This process results in the formation of monocyte-derived M1 macrophages, which can secrete lots of proinflammatory cytokines, including IL-1α, IL-1β, IL-6, IL-12, TNF-α, IL-23, CXCL9, CXCL10, CXCL11, CXCL16 and CCL5. Overexpression of these cytokines and chemokines can cause inflammation and tissue damage[31,32].
Monocyte-derived macrophages also play an essential role in MASLD and T2D. A recent study focused on the Glycoprotein non-metastatic melanoma protein b (GPNMB), encoded by the gene Gpnmb. GPNMB is a lipid-associated macrophage marker[33,34]. It is a transmembrane glycoprotein, which is predominantly expressed in hepatic monocyte-derived macrophages and white adipose tissues[35]. Numerous studies have demonstrated that there is an upregulation of Gpnmb expression in MASLD livers[36]. A recent study using a myeloid-specific Gpnmb-knockout mouse model discovered that knockout of Gpnmb maintained the population of Kupffer cells and reduced liver steatosis and fibrosis. This study also revealed that myeloid-specific knockout of Gpnmb directed monocyte differentiation towards a monocyte-derived macrophage subset rather than lipid-associated macrophages, which can exacerbate the severity of MASLD[33,37]. In a fructose-palmitate-cholesterol diet-induced mouse MASLD model, knockdown of Gpnmb showed a protective effect by ameliorating liver steatosis and hepatitis. The underlying mechanism is that knockdown of Gpnmb increased the expression levels of activin A to protect against liver injury in MASLD[36]. Interestingly, T2D patients also exhibit elevated levels of serum GPNMB, which are associated with the progression of insulin resistance[35,38]. Another study showed that an increased ratio of monocytes to apolipoprotein A1 predicted a higher risk of MASLD in patients with T2D[39]. This further highlights the monocyte-derived macrophages in the correlation and connection between MASLD and T2D.
Liver resident macrophages, including both Kupffer cells and monocyte-derived macrophages, are considered to function as a great contributor in linking MASLD and T2D. In disease conditions, MASLD and T2D, the liver resident ma
| Mediators | Sources | Function in T2D | Function in MASLD |
| IL-1α | Kupffer cells and recruited monocyte-derived macrophages | Promote inflammation and worsen insulin resistance and metabolic syndrome | Contribute to hepatic steatosis, inflammatory cell recruitment, fibrosis progression, and systemic insulin resistance |
| IL-1β | Adipose tissue macrophages and Kupffer cells | Promote β-cell inflammation and ER stress, induce β-cell apoptosis, and impair glucose-stimulated insulin secretion | Exacerbate liver inflammation and MASLD development and promote the progression from steatosis to steatohepatitis |
| IL-6 | Adipose tissue macrophages, Kupffer cells | Contribute to systemic insulin resistance and modulate adipocyte metabolism | Promote inflammation in MASLD and hepatic acute-phase response |
| IL-12 | Adipose tissue macrophages and Kupffer cells | Reduce glucose infusion rates and impair insulin sensitivity | Boost hepatic inflammation and insulin resistance in MASLD |
| IL-23 | Adipose tissue macrophages and Kupffer cells | Contribute to IL-17/IL-23 axis-mediated inflammatory responses and induce metabolic stress | Promote pro-inflammatory responses mediated by IL-17/IL-23 axis and induce metabolic stress |
| TNF-α | Adipose tissue macrophages and Kupffer cells | Promote insulin resistance via insulin receptor substrate 1 serine phosphorylation, exacerbate β-cell dysfunction and apoptosis, and induce lipolysis | Promotes hepatocyte apoptosis, fibrosis, and inflammation in MASLD, and contribute to meta-inflammation and systemic inflammation |
| IL-10 | M2 macrophages (alternatively activated) | Promote tissue repair and protect against insulin resistance | Promote tissue repair and reduce inflammatory progression and fibrosis in MASLD |
| CCL2 (MCP-1) | Macrophages, adipose tissue macrophages | Promote recruitment of circulating monocytes to adipose tissue, promote macrophage infiltration and inflammation, and exacerbate insulin resistance | Recruit circulating monocytes to adipose tissue, perpetuate macrophage infiltration and inflammation, promote hepatic macrophage accumulation and fibrosis |
| CCL5 | Macrophages | Induce chronic inflammation in T2D | Promote MASLD and liver fibrosis progression |
| CXCL9 | Adipose tissue macrophages and Kupffer cells | Induce chronic inflammation in adipose tissue, stimulate inflammatory cytokine production, exacerbate insulin resistance, and induce tissue damage | Exacerbate liver inflammation and fibrosis, and augment systemic insulin resistance |
| CXCL10 | Adipose tissue macrophages, Kupffer cells, and monocyte-derived macrophages | Promote metabolic inflammation and impaired insulin signaling, contribute to systemic and local inflammation, and exacerbate insulin resistance | Promote M1 macrophage polarization and recruitment, boost chronic inflammation in MASLD, and exacerbate insulin resistance |
| CXCL11 | Adipose tissue macrophages, adipose tissue macrophages, Kupffer cells, and monocyte-derived macrophages | Exacerbate insulin resistance and influence systemic metabolism | Boost inflammatory and fibrotic progression in MASLD/MASH and promote insulin resistance and metabolic dysregulation |
| CXCL16 | Adipose tissue macrophages, Kupffer cells, and recruited monocyte-derived macrophages | Induce chronic inflammation and metabolic dysfunction and contribute to insulin resistance in T2D | Increase macrophage infiltration, stimulate inflammatory cytokine secretion (e.g., TNF-α), influence hepatocyte metabolism and stellate cell collagen production, and increase steatohepatitis severity and fibrosis progression |
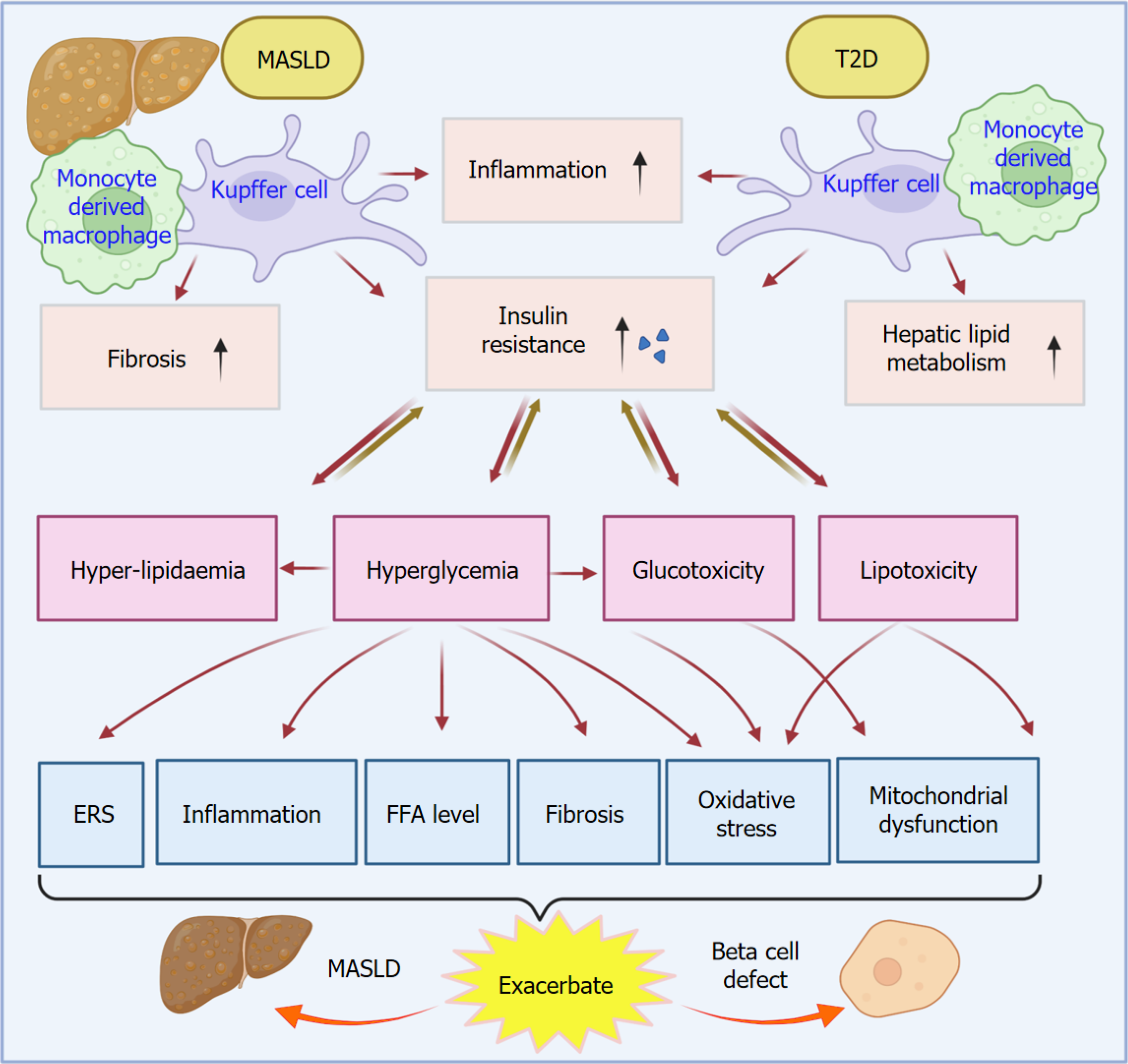
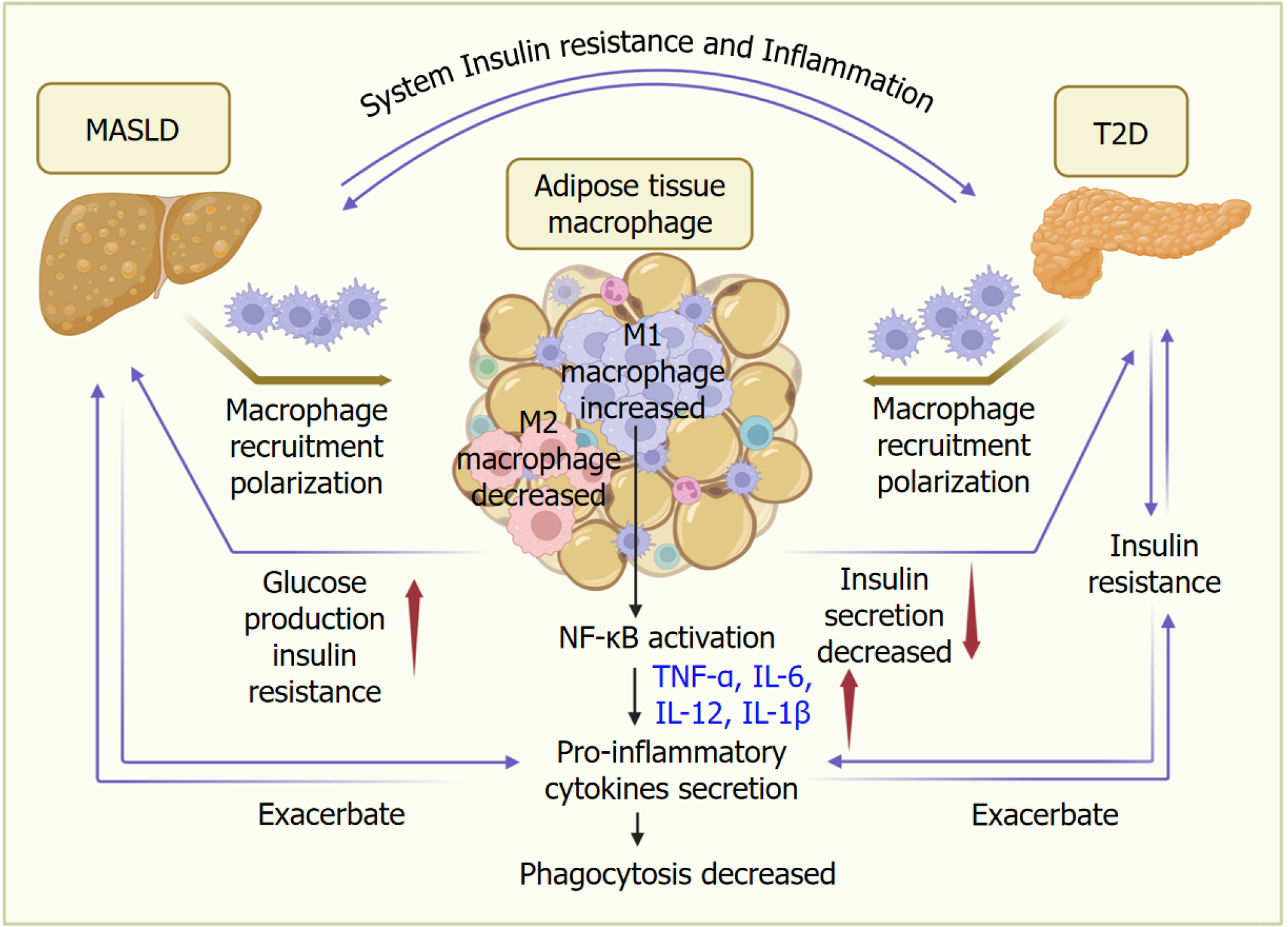
In MASLD or T2D, a large number of macrophages infiltrate into adipose tissues, followed by their polarization[50-52], with an increase of M1 macrophages and a decrease of M2 macrophages (Figure 2). The activation of nuclear factor kappa-light-chain-enhancer of activated B cells of M1 macrophages promotes the secretion of proinflammatory cytokines, such as TNF-α, IL-6, IL-12, and IL-1β, consequently decreasing the phagocytosis function[53]. Proinflammatory en
Adipose tissue macrophage serves a critical role in the linkage between MASLD and T2D. The underlying mechanisms of this crosstalk include inflammation, insulin resistance, insulin tissue impairment, fibrogenesis, and metabolic dysfunction[11,57]. In MASLD, fat accumulation causes chronic inflammation in adipose tissue[58]. Since macrophages are the most abundant immune cells in inflamed adipose tissues, and they predominantly differentiate into proinflammatory M1 type, these macrophages serve as a great contributor to tissue inflammation and dysregulation of the insulin-related signaling pathways[59]. These conditions can promote insulin resistance to induce the development of T2D. Insulin tissue impairment can further induce fat metabolic dysregulation, FFA production, and lipotoxicity, which confer an unfavorable condition for both MASLD and T2D[60,61].
A recent clinical trial performed in participants diagnosed with both MASLD and T2D demonstrated the synergistic therapeutic effect by combining the insulin-sensitizer pioglitazone and glucose control medicine empagliflozin. Em
Polarized adipose tissue M1 macrophages contribute to fibrogenesis in adipose tissues. In MASLD and/or T2D, polarized adipose tissue M1 macrophages induced by proinflammatory response can secrete pro-fibrotic cytokines, such as TNF-α, TGF-β1, IL-1β, IL-6, IL-13, and platelet-derived growth factor, to induce the activation of fibroblasts and consequently cause tissue damage and fibrosis[63]. Activated fibroblasts produce more collagen to induce the accumulation of extracellular matrix (ECM) components and fibrosis[64,65]. On the other hand, the reduced collagen degradation of macrophages also contributes to the failure of ECM clearance and ECM deposition. Moreover, preadipocytes interact with proinflammatory M1 macrophages to increase the formation of ECM, including collagen 1 and fibronectin. Fi
It has been reported that in patients with MASLD, the degree of hepatic fibrosis was correlated with the amount of proinflammatory adipose tissue macrophages, and the progression of liver fibrosis could be ameliorated by modulating adipose tissue macrophages in a MASH model. In T2D, treatment with bromocriptine, an inhibitor of CREBZF in macrophages, improved adipose tissue macrophage activation, alleviated systemic insulin resistance, and attenuated the symptoms of T2D. The associated mechanism was contributed by the crosstalk between adipose tissue macrophages and adipocytes[67]. The deficiency of CREBZF in macrophages reduced macrophage infiltration in adipose tissues, hy
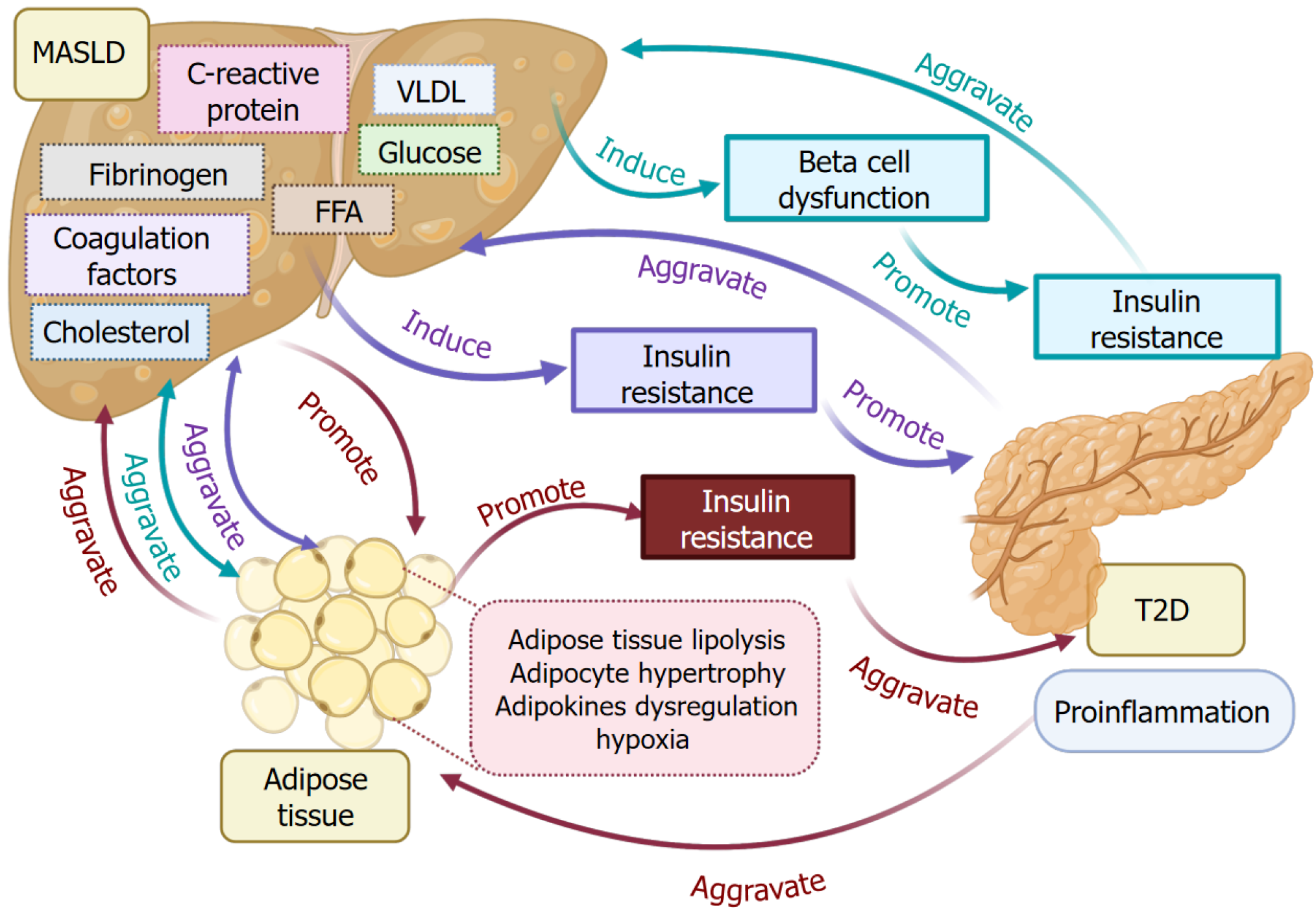
MASLD pathogenesis is featured by elevated levels of many factors, such as glucose, FFA, very-low-density lipoprotein (VLDL), cholesterol, C-reactive protein, coagulation factors, and fibrinogen (Figure 3). Those factors result from MASLD and reciprocally contribute to the exacerbation of MASLD and T2D pathogenesis[69-71]. Overexpression or an increase in these factors can induce β-cell dysfunction and insulin resistance, promoting the progression of T2D. The insulin resistance in T2D further aggravates the development of MASLD. MASLD interacts with T2D through adipose tissues. Adipose tissue metabolism dysfunction in MASLD causes adipose tissue proinflammation and lipolysis, adipocyte hypertrophy, adipokine dysregulation, and hypoxia[72-74]. Those mentioned processes resulting from MASLD and T2D also contribute to their progression. Consequently, those processes further promote insulin resistance and aggravate T2D and inflammation.
Glucose uptake from blood and storage as glycogen are essential physiological processes for maintaining a normal blood glucose level in healthy conditions. In this physiological process, insulin plays a pivotal role in sensing and signaling to initiate the process. However, in MASLD and T2D, the insulin resistance could decrease this process, resulting in an increased level of blood glucose, or hyperglycemia. Meanwhile, glucose and fructose can be metabolized into fatty acids through the de novo lipogenesis (DNL) pathway, followed by the incorporation of triglycerides[75,76]. Triglycerides are the main components of body fat. Notably, in MASLD and T2D, this process continues to promote excessive fat production and accumulation, which in turn worsens insulin resistance. Glucose production is controlled by insulin in healthy conditions. However, in pathological conditions, such as MASLD and T2D, insulin resistance can impair insulin suppression function, causing an increase in glucose production, known as glucogenesis. Glucogenesis can induce an increased level of glucose production and an elevated level of blood glucose, which leads to the aggravation of hyperglycemia[77,78].
Glucose dysregulation, its associated insulin resistance, and lipid dysregulation contribute to the flow of carbohydrate disruption via the pathway of glycolysis, DNL, and gluconeogenesis. Moreover, mitochondrial dysfunction and fatty acid oxidation induce overexpression of reactive oxygen species (ROS) and oxidative stress, which ultimately cause tissue injury. It has been reported that glucose-lowering drugs, such as sodium-glucose cotransporter-2 inhibitors, displayed beneficial treatment effects on decreasing hepatic fat, alleviating histological features, and alleviating liver cirrhosis in patients with MASLD and T2D[79,80].
Imbalance of FFA metabolism is highly associated with the development of MASLD. In both MASLD and T2D, an increased level of FFA contributes to the metabolic dysregulation and oxidative stress, consequently inducing β-cell dysfunction to promote the progression of T2D and MASLD[81,82]. Increased levels of FFA can cause lipotoxicity, consequently leading to oxidative stress, ER stress, inflammation, autophagy, and lipid apoptosis. All those factors contribute to the progression of MASLD[47,83].
Adipose tissue-released FFAs, originating from adipose tissue lipolysis, are taken by the liver, and then involved in the processes of lipogenesis, DNL, lipolysis, VLDL-related triglyceride secretion, and fatty acid β-oxidation[84,85]. An increase of FFA uptake, lipogenesis, and DNL, and a decrease of lipolysis, VLDL-related triglyceride secretion, and β-oxidation, can induce FFA metabolism imbalance and lead to the development of MASLD. Moreover, higher levels of FFAs worsen the MASLD and T2D by elevating insulin resistance.
According to a study conducted on enrolled patients, including a large population of the MASLD group and the non-MASLD group, the results showed that a higher level of FFAs was significantly associated with insulin resistance, prediabetes, and T2D in the group of MASLD, but not in the non-MASLD group. This result provides insight into the value of managing the level of FFAs in MASLD patients to monitor the risk of T2D development[60].
VLDL is responsible for transporting triglycerides from the liver to different tissues through the vascular system. Upon the dislocation of triglycerides by enzymes, VLDL converts into low-density lipoprotein, which is considered unfavorable cholesterol. A high level of VLDL is a contributing factor to the development of metabolic syndrome and fatty liver[86]. In MASLD, an increased level of VLDL secretion in the liver, as well as in blood bloodstream, contributes to insulin resistance and dyslipidemia[87]. In T2D patients, an increased level of VLDL and triglycerides results from VLDL metabolic dysregulation and insulin resistance[88]. According to a recently published report, the excess triglycerides in VLDL or triglyceride-rich VLDL could be a useful biomarker for predicting MASLD in patients with T2D[89]. Another study reported that the ratio of triglyceride to high-density lipoprotein cholesterol can be applied to predict T2D in patients with MASLD[69].
The accumulation of cholesterol influences mitochondrial function. Mitochondria are responsible for the tricarboxylic acid cycle, fatty acid oxidation, energy metabolism, and thermogenesis. In addition, adipose tissue produces lipids, adipokines, cytokines, and chemokines induce oxidative stress, ER stress in MASLD livers, causing mitochondrial dysfunction and subsequently triggering ROS production. This process can induce mitochondrial oxidative damage, thereby resulting in severe metabolic dysfunction that otherwise could be well-maintained by mitochondria under normal conditions[90,91]. A recent study focused on targeting mitochondrial energy metabolism using HU6, a mitochondrial uncoupler, to treat individuals with MASLD and high body mass index (BMI) (28-45 kg/m2). The clinical trial results showed that at least a 30% reduction in liver fat was observed when compared to baseline on day 61 in patients with MASLD and a high BMI[92]. Another study showed that salsalate, a non-steroidal anti-inflammatory drug, uncoupled mitochondria to demonstrate the function in alleviating metabolic disorders such as MASLD through adenosine 5’-monophosphate-activated protein kinase (AMPK) signaling pathway[93]. Thus, targeting mitochondria to manage the MASLD and T2D has gained extensive attention[94].
C-reactive protein is produced in the liver and is responsible for inflammation. An increased level of C-reactive protein is a common feature in both MASLD and T2D[95]. In hepatocytes, leptin regulates lipid metabolism to maintain energy balance and metabolic health. However, an increased C-reactive protein can disturb leptin regulatory function to cause lipid accumulation. This process leads to ROS production, followed by mitochondrial DNA production[96]. On the other hand, the released mitochondrial DNA triggered the activation of Toll-like receptors in the Kupffer cells and promoted the secretion of IL-6 and IL-1β. Meanwhile, accumulated lipids interacted with FFA receptors on the surface of Kupffer cells and induced ROS production, which further facilitated the release of inflammatory cytokines. This process was mediated by the activation of nuclear factor kappa-B signaling pathway via the mitogen-activated protein kinase/extracellular signal-regulated kinase and AMPK/mammalian target of rapamycin (AMPK/mTOR) signaling pathways[97-99].
Fibrinogen, coagulation factor I, is produced by the liver and distributed to the bloodstream[100]. Upon the injury, it can be converted into fibrin and is responsible for stopping bleeding. In both MASLD and T2D patients, the level of fibrinogen is increased[101,102]. The elevated level of fibrinogen can induce the excessive production of fibrin in the liver and adipose tissues, which worsens the condition of inflammation and injury. Fibrinogens contribute to platelet aggregation, which can lead to a higher risk of thromboembolism[103]. According to a cross-sectional study performed in T2D patients, plasma fibrinogen level was correlated with increased hemoglobin A1c level, as well as associated with a decreased glycemic status[104].
In addition to fibrinogen, the imbalance of other coagulation factors such as antihemophilic factor (factor VIII), prothrombin (factor II), and thromboplastin (factor III) could cause the impairment of fibrinolysis and prothrombotic imbalance in both MASLD and T2D. This process could induce a hypercoagulable environment[105,106].
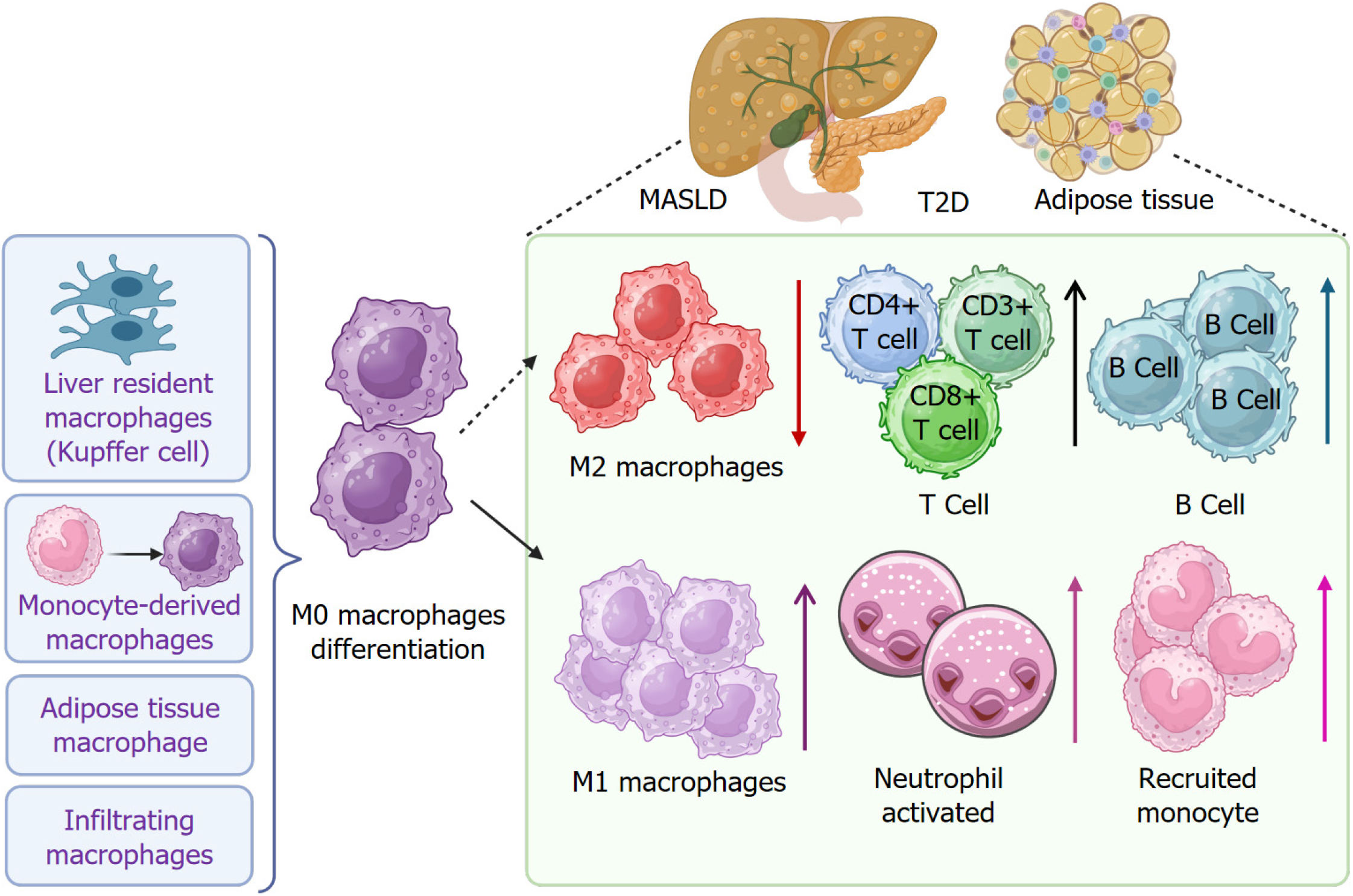
Immune cell infiltration serves a critical role in the progression of metabolic disorders in MASLD and T2D[107-109], as well as in the adipose tissues (Figure 4). Macrophage recruitment and polarization are activated in response to many factors, as elaborated in earlier sections. Elevated M1 macrophage polarization occurs in Kupffer cells, monocyte-derived macrophages, and adipose tissues, which contribute to the proinflammatory response by releasing cytokines, chemokines, and enzymes[110-112]. This process induces and aggravates inflammation. Meanwhile, a decrease in M2 macrophage number further weakens the anti-inflammatory response. In addition to macrophages, B-cell and T-cell infiltration is increased, such as cluster of differentiation (CD) 4+ T cells and CD8+ T cells[113-115]. Neutrophil activation and monocyte recruitment are also increased[109,116-118].
According to currently published reports related to immune cell infiltration in MASLD and T2D, researchers demonstrate that insulin-related immune cell infiltration increases the risk of fatty liver disease, MASH, and liver fibrosis in T2D patients. The underlying mechanism is attributed to the CD4+ and CD8+ T cell senescence and exhaustion. Furthermore, in diet-induced and chemical-induced mouse MASH models, an increased expression of senescence and exhaustion markers was also detected in liver-infiltrated CD4+ and CD8+ T cells, which emphasizes the roles of T cells in liver disease progression[119-121]. These studies shed light on the importance of immune cell infiltration-oriented therapy on MASLD and T2D management.
Tripartite motif containing 38 can regulate M2 macrophage polarization by interacting with heat shock protein family A member 5 to suppress LPS-induced macrophage activation and decrease hepatic steatosis[122]. Treatment of exogenous prostaglandin E2 significantly decreased messenger RNA and protein levels of TNF-α in primary Kupffer cells, peritoneal macrophages, and bone-marrow-derived macrophages induced by LPS. Interestingly, depletion of cyclooxygenase 2 in macrophages can abrogate LPS-induced expression levels of TNF-α proteins in primary Kupffer cells and peritoneal macrophages[123]. Knockout of triggering receptor expressed on myeloid cells 2 (Trem2) in mice with a high-fat diet can exacerbate hepatic steatosis and inflammation, whereas myeloid-specific overexpression of Trem2 can suppress the progression of MASLD[124]. In addition, Trem2 was also found to be upregulated in MASLD or MASH-associated macrophages in human patients, which was associated with liver inflammation and steatosis[124]. Myeloid-specific knockout of gene Gpnmb in mice can significantly decrease hepatic steatosis and modestly inhibit liver fibrosis by reducing the formation of lipid-associated macrophages in the liver[33]. Neurogenic locus notch homolog protein-recombination signal binding protein for immunoglobulin kappa J region (Rbpj) signaling pathway regulates monocyte differentiation to macrophages. Rbpj deficiency decreases the differentiation of monocytes to Kupffer cells. In Ly6C-low expressing monocytes, Rbpj deficiency accelerates lipid uptake by upregulating the expression of CD36[125]. Transcriptional factors such as interferon regulatory factor 5 also play important roles in macrophage activation and apoptosis[126].
Similarly, alteration of macrophage function is a key cellular event in T2D. TNF-α-licensed exosome-integrated titanium can promote M2 macrophage polarization by activating autophagy through inhibition of phosphatidylinositol 3-kinase/protein kinase B/mTOR signaling pathway[127]. Bacteroides Fragilis and its extracellular vesicles (EVs) can promote vascular calcification in mice with T2D by promoting macrophage M2 polarization[128]. Mechanistically, double-stranded DNAs derived from EVs induce activation of stimulator of interferon response cyclic guanosinc monophosphate-adenosine 5’-monophosphate interactor 1 to promote the phosphorylation of myocyte enhancer factor 2D and increase the expression of tribbles pseudo kinase 1, resulting in macrophage M2 polarization[128].
Moreover, some genes or proteins play important roles in both MASLD and MASH, as well as T2D. For example, the levels of growth differentiation factor-15 (GDF-15) in plasma are increased in patients with obesity with MASLD, T2D, or both due to the accumulation of macrophages in adipose tissues[129]. Suppression of macrophage GDF-15 expression can reduce its plasma level and exacerbate the progression of obesity[129]. Apoptotic vesicles can reprogram macrophages into an anti-inflammatory phenotype in the livers of mice with T2D. Calreticulin functions on the surface of apoptotic vesicles to regulate macrophage transformation and accumulation[130]. Overall, modulation of macrophage polarization, inflammation, and accumulation during MASLD and T2D can impact disease progression (Table 2).
| Disease | Signaling pathway | Function | Ref. |
| MASLD | TRIM38-HSPA5 axis | Macrophage M2 polarization | Yang et al[122] |
| MASH | LPS-COX-2-PGE2 | Macrophage inflammation | Vahrenbrink et al[123] |
| MASLD | Trem2 | Macrophage pyroptosis and inflammation | Xiang et al[124] |
| MASLD | GPNMB | Retention of liver resident Kupffer cells and rerouting monocyte differentiation to Kupffer cell-resembling macrophages | Wang et al[33] |
| MASLD or MASH | Notch-RBPJ | Monocyte-to-macrophage transition | Guo et al[125] |
| MASLD | IRF5 | Immunosuppressive and anti-apoptotic function | Alzaid et al[126] |
| T2D | PI3K/AKT/mTOR | Macrophage M2 polarization | Yang et al[127] |
| T2D | STING/Mef2D | Macrophage M2 polarization | Chen et al[128] |
| MASH, T2D | GDF-15 | Regulation of obesity | L'homme et al[129] |
| T2D | Calreticulin | Macrophage transformation and accumulation | Zheng et al[130] |
Clinical trials have evaluated different treatments in patients with T2D and MASLD. For example, a phase 2 clinical trial (No. NCT03459079, https://clinicaltrials.gov/, access date: May 30, 2025) has evaluated the effect of a pan-peroxisome proliferator-activated receptors agonist lanifibranor in regulating intrahepatic triglycerides, hepatic glucose production, DNL, hemoglobin A1c, and lipid profiles, as well as hepatic, adipose tissue, and muscle insulin resistance[131]. One phase 1 clinical study (No. NCT05943886) showed that after 5 weeks of treatment, a dual agonist of glucagon-like peptide-1 and fibroblast growth factor 21 significantly reduced hepatic fat and improved glycemic control, insulin resistance, and lipid profiles in patients with MASLD and T2D[124].
In this section, we review some clinical trials that evaluate the effect of treatments on macrophages in patients with MASLD, T2D, and both or their related metabolic disorders (Table 3).
| Clinical trial | Study type or phase | Disease | Treatment and purpose |
| NCT04059068 | Observational | MASLD | To identify macrophage-derived drug targets to reverse liver disease and reduce macrophage-mediated adipose tissue inflammation |
| NCT06950710 | Observational | MASLD and hepatic ischemia-reperfusion injury | To elucidate the role and mechanisms of macrophage polarization in patients with hepatic ischemia-reperfusion injury and MASLD |
| NCT06571474 | Observational | Obesity, MASLD, T2D | To evaluate the role of transcription factor EB in adipose tissue macrophages in regulating adipose tissue and systemic metabolic function |
| NCT06007404 | Observational | T2D, prediabetes, insulin resistance, obesity | To better understand the role of meta-inflammatory monocytes in adolescent insulin resistance by measuring the influence of body composition, lifestyle habits, and diet |
| NCT02498119 | Observational | T2D | To test the role of arachidonic acid metabolites in the lipid droplets of macrophages |
| NCT03836443 | Interventional | MASLD | To determine the effect of cultured hepatocyte supernatant exposed to MASLD patient plasma on human macrophages in vitro |
| NCT03426111 | Interventional | MASH | To evaluate macrophage function in patients treated with endoscopic gastric tubulization and lifestyle modification |
| NCT05681468 | Interventional | T2D, prediabetes, overweight, and obesity | To test the effect of ketogenic diets on immune cell function and inflammation, including macrophages |
| NCT02768935 | Interventional | T2D, cardiovascular complications | To test the infiltration and polarization of macrophages in patients with T2D after myocardial infarction and the role of macrophage miRNAs |
| NCT02330549 | Phase 2 | T2D and suspected MASLD | To test the effect of cenicriviroc (152 mg), CCR2 and CCR5 antagonism, on insulin sensitivity in subjects with prediabetes or T2D and suspected MASLD |
| NCT04521114 | Phase 2 | MASH | To test the effect of anti-human CCR5 monoclonal antibody leronlimab (PRO 140) on MASH |
| NCT03151343 | Phase 3 | T2D, heart disease | To test the effect of SGLT-2 inhibitor on myocardial perfusion, function, and metabolism, as well as adipose tissue macrophage infiltration |
| NCT02285985 | Phase 4 | T2D | To test the effect of DPP-4 inhibitor saxagliptin on the reduction of adipose tissue inflammation and macrophage infiltration |
In conclusion, macrophages play an essential role in health and disease. Liver resident macrophages, such as Kupffer cells and monocyte-derived macrophages, as well as adipose tissue macrophages, play a significant role in metabolic-associated diseases such as MASLD and T2D. The crosstalk between the two diseases confers complications on each other due to the various interplays among them, including metabolic pathways, cellular and molecular pathways. Cellular and molecular mechanisms of macrophage-mediated inflammation and immune cell infiltration contribute to the crosstalk between MASLD and T2D. Functionality of macrophage and inflammation in linking MASLD and T2D includes metabolic inflammation, tissue damage, and insulin resistance. Thus, therapeutic targets on macrophage and inflammation in MASLD and T2D possess extensive attraction for future prognostic and therapeutic purposes, especially for individuals with the co-occurrence of MASLD and T2D.
| 1. | Loomba R, Wong VW. Implications of the new nomenclature of steatotic liver disease and definition of metabolic dysfunction-associated steatotic liver disease. Aliment Pharmacol Ther. 2024;59:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Hagström H, Shang Y, Hegmar H, Nasr P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol Hepatol. 2024;9:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 138] [Reference Citation Analysis (0)] |
| 3. | Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 423] [Article Influence: 211.5] [Reference Citation Analysis (0)] |
| 4. | Qadri S, Yki-Järvinen H. Surveillance of the liver in type 2 diabetes: important but unfeasible? Diabetologia. 2024;67:961-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 5. | Nanda M, Sharma R, Mubarik S, Aashima A, Zhang K. Type-2 Diabetes Mellitus (T2DM): Spatial-temporal Patterns of Incidence, Mortality and Attributable Risk Factors from 1990 to 2019 among 21 World Regions. Endocrine. 2022;77:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Ntikoudi A, Papachristou A, Tsalkitzi A, Margari N, Evangelou E, Vlachou E. Metabolic-Associated Steatotic Liver Disease (MASLD) and Type 2 Diabetes: Mechanisms, Diagnostic Approaches, and Therapeutic Interventions. Diabetology. 2025;6:23. [DOI] [Full Text] |
| 7. | Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, Rao L, Duo Y. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1264] [Reference Citation Analysis (0)] |
| 8. | Zhang C, Yang M, Ericsson AC. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front Immunol. 2021;12:620510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Lazarov T, Juarez-Carreño S, Cox N, Geissmann F. Physiology and diseases of tissue-resident macrophages. Nature. 2023;618:698-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 305] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 10. | Yang M, Liu S, Sui Y, Zhang C. Macrophage metabolism impacts metabolic dysfunction-associated steatotic liver disease and its progression. Immunometabolism. 2024;6. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Chavakis T, Alexaki VI, Ferrante AW Jr. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat Immunol. 2023;24:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 12. | Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol. 2022;18:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 13. | Pellegrini V, La Grotta R, Carreras F, Giuliani A, Sabbatinelli J, Olivieri F, Berra CC, Ceriello A, Prattichizzo F. Inflammatory Trajectory of Type 2 Diabetes: Novel Opportunities for Early and Late Treatment. Cells. 2024;13:1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 14. | Greene CJ, Nguyen JA, Cheung SM, Arnold CR, Balce DR, Wang YT, Soderholm A, McKenna N, Aggarwal D, Campden RI, Ewanchuk BW, Virgin HW, Yates RM. Macrophages disseminate pathogen associated molecular patterns through the direct extracellular release of the soluble content of their phagolysosomes. Nat Commun. 2022;13:3072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 15. | Kadomoto S, Izumi K, Mizokami A. Macrophage Polarity and Disease Control. Int J Mol Sci. 2021;23:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 16. | Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, Striz I. M1/M2 macrophages and their overlaps - myth or reality? Clin Sci (Lond). 2023;137:1067-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 368] [Reference Citation Analysis (0)] |
| 17. | Nobs SP, Kopf M. Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol. 2021;42:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Wu Y, Hirschi KK. Tissue-Resident Macrophage Development and Function. Front Cell Dev Biol. 2020;8:617879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 19. | Blériot C, Barreby E, Dunsmore G, Ballaire R, Chakarov S, Ficht X, De Simone G, Andreata F, Fumagalli V, Guo W, Wan G, Gessain G, Khalilnezhad A, Zhang XM, Ang N, Chen P, Morgantini C, Azzimato V, Kong WT, Liu Z, Pai R, Lum J, Shihui F, Low I, Xu C, Malleret B, Kairi MFM, Balachander A, Cexus O, Larbi A, Lee B, Newell EW, Ng LG, Phoo WW, Sobota RM, Sharma A, Howland SW, Chen J, Bajenoff M, Yvan-Charvet L, Venteclef N, Iannacone M, Aouadi M, Ginhoux F. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity. 2021;54:2101-2116.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 20. | Han M, Geng J, Zhang S, Rao J, Zhu Y, Xu S, Wang F, Ma F, Zhou M, Zhou H. Invariant natural killer T cells drive hepatic homeostasis in nonalcoholic fatty liver disease via sustained IL-10 expression in CD170(+) Kupffer cells. Eur J Immunol. 2023;53:e2350474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Yao JM, Ying HZ, Zhang HH, Qiu FS, Wu JQ, Yu CH. Exosomal RBP4 potentiated hepatic lipid accumulation and inflammation in high-fat-diet-fed mice by promoting M1 polarization of Kupffer cells. Free Radic Biol Med. 2023;195:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Dong T, Hu G, Fan Z, Wang H, Gao Y, Wang S, Xu H, Yaffe MB, Vander Heiden MG, Lv G, Chen J. Activation of GPR3-β-arrestin2-PKM2 pathway in Kupffer cells stimulates glycolysis and inhibits obesity and liver pathogenesis. Nat Commun. 2024;15:807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Habash SA, Takahashi N, Eltalkhawy YM, Abdelnaser RA, Ogata-Aoki H, Okada S, Takizawa H, Usuki S, Etoh K, Hino S, Morino-Koga S, Ogawa M, Suzu S. Macrophages with different origins proliferate ex vivo and do not lose their core intrinsic features. iScience. 2025;28:112635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 25. | Lokhonina A, Elchaninov A, Fatkhudinov T, Makarov A, Arutyunyan I, Grinberg M, Glinkina V, Surovtsev V, Bolshakova G, Goldshtein D, Sukhikh G. Activated Macrophages of Monocytic Origin Predominantly Express Proinflammatory Cytokine Genes, Whereas Kupffer Cells Predominantly Express Anti-Inflammatory Cytokine Genes. Biomed Res Int. 2019;2019:3912142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Lin SZ, Xie Y, Cheng YQ, Xue R, Su YS, Liu M, Chen YW, Fan JG. C/EBPβ-VCAM1 axis in Kupffer cells promotes hepatic inflammation in MASLD. JHEP Rep. 2025;7:101418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Takai T, Matsuda T, Matsuura Y, Inoue K, Suzuki E, Kanno A, Kimura-Koyanagi M, Asahara SI, Hatano N, Ogawa W, Kido Y. Casein kinase 2 phosphorylates and stabilizes C/EBPβ in pancreatic β cells. Biochem Biophys Res Commun. 2018;497:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Guru B, Tamrakar AK, Manjula SN, Prashantha Kumar BR. Novel dual PPARα/γ agonists protect against liver steatosis and improve insulin sensitivity while avoiding side effects. Eur J Pharmacol. 2022;935:175322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 29. | Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 501] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 30. | Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012;188:5752-5765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 414] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 31. | Cavaillon JM. Cytokines and macrophages. Biomed Pharmacother. 1994;48:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 302] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Xuan W, Qu Q, Zheng B, Xiong S, Fan GH. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol. 2015;97:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Wang J, Wang H, Yang W, Zhao D, Liu D, Tang L, Chen XP. GPNMB regulates the differentiation and transformation of monocyte-derived macrophages during MASLD. Int Immunopharmacol. 2025;154:114554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. J Immunol. 2007;178:6557-6566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Gong XM, Li YF, Luo J, Wang JQ, Wei J, Wang JQ, Xiao T, Xie C, Hong J, Ning G, Shi XJ, Li BL, Qi W, Song BL. Gpnmb secreted from liver promotes lipogenesis in white adipose tissue and aggravates obesity and insulin resistance. Nat Metab. 2019;1:570-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Liu H, Yerevanian A, Westerhoff M, Hastings MH, Guerra JRB, Zhao M, Svensson KJ, Cai B, Soukas AA, Rosenzweig A. Roles of Activin A and Gpnmb in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Diabetes. 2024;73:260-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Katayama A, Nakatsuka A, Eguchi J, Murakami K, Teshigawara S, Kanzaki M, Nunoue T, Hida K, Wada N, Yasunaka T, Ikeda F, Takaki A, Yamamoto K, Kiyonari H, Makino H, Wada J. Beneficial impact of Gpnmb and its significance as a biomarker in nonalcoholic steatohepatitis. Sci Rep. 2015;5:16920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 2001;276:8125-8134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Wang W, Huang L, Qiu XP, Tu M, Guo XL. Monocytes to Apolipoprotein A1 ratio is associated with metabolic dysfunction-associated fatty liver disease in type 2 diabetes mellitus. Sci Rep. 2024;14:31396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Wu Q, Yang Y, Lin S, Geller DA, Yan Y. The microenvironment in the development of MASLD-MASH-HCC and associated therapeutic in MASH-HCC. Front Immunol. 2025;16:1569915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 41. | Demir S, Nawroth PP, Herzig S, Ekim Üstünel B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv Sci (Weinh). 2021;8:e2100275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 42. | Pan X, Kaminga AC, Wen SW, Liu A. Chemokines in Prediabetes and Type 2 Diabetes: A Meta-Analysis. Front Immunol. 2021;12:622438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | Yousri NA, Suhre K, Yassin E, Al-Shakaki A, Robay A, Elshafei M, Chidiac O, Hunt SC, Crystal RG, Fakhro KA. Metabolic and Metabo-Clinical Signatures of Type 2 Diabetes, Obesity, Retinopathy, and Dyslipidemia. Diabetes. 2022;71:184-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 44. | Fu J, Cui Q, Yang B, Hou Y, Wang H, Xu Y, Wang D, Zhang Q, Pi J. The impairment of glucose-stimulated insulin secretion in pancreatic β-cells caused by prolonged glucotoxicity and lipotoxicity is associated with elevated adaptive antioxidant response. Food Chem Toxicol. 2017;100:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | El-Assaad W, Joly E, Barbeau A, Sladek R, Buteau J, Maestre I, Pepin E, Zhao S, Iglesias J, Roche E, Prentki M. Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 ss-cells. Endocrinology. 2010;151:3061-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 47. | Vilas-Boas EA, Almeida DC, Roma LP, Ortis F, Carpinelli AR. Lipotoxicity and β-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress. Cells. 2021;10:3328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 48. | Szpigel A, Hainault I, Carlier A, Venteclef N, Batto AF, Hajduch E, Bernard C, Ktorza A, Gautier JF, Ferré P, Bourron O, Foufelle F. Lipid environment induces ER stress, TXNIP expression and inflammation in immune cells of individuals with type 2 diabetes. Diabetologia. 2018;61:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 49. | Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE((-/-)) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int J Mol Sci. 2021;22:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 50. | Fujisaka S. The role of adipose tissue M1/M2 macrophages in type 2 diabetes mellitus. Diabetol Int. 2021;12:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 52. | Ni Y, Zhuge F, Ni L, Nagata N, Yamashita T, Mukaida N, Kaneko S, Ota T, Nagashimada M. CX3CL1/CX3CR1 interaction protects against lipotoxicity-induced nonalcoholic steatohepatitis by regulating macrophage migration and M1/M2 status. Metabolism. 2022;136:155272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 53. | Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 725] [Article Influence: 51.8] [Reference Citation Analysis (1)] |
| 54. | Nepomuceno R, Vallerini BF, da Silva RL, Corbi SCT, Bastos AS, Dos Santos RA, Takahashi CS, Orrico SRP, Scarel-Caminaga RM. Systemic expression of genes related to inflammation and lipid metabolism in patients with dyslipidemia, type 2 diabetes mellitus and chronic periodontitis. Diabetes Metab Syndr. 2019;13:2715-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Al-Mansoori L, Al-Jaber H, Prince MS, Elrayess MA. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation. 2022;45:31-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 56. | Bijnen M, Josefs T, Cuijpers I, Maalsen CJ, van de Gaar J, Vroomen M, Wijnands E, Rensen SS, Greve JWM, Hofker MH, Biessen EAL, Stehouwer CDA, Schalkwijk CG, Wouters K. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut. 2018;67:1317-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 57. | Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016-E1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 58. | Chan MM, Daemen S, Beals JW, Terekhova M, Yang BQ, Fu CF, He L, Park AC, Smith GI, Razani B, Byrnes K, Beatty WL, Eckhouse SR, Eagon JC, Ferguson D, Finck BN, Klein S, Artyomov MN, Schilling JD. Steatosis drives monocyte-derived macrophage accumulation in human metabolic dysfunction-associated fatty liver disease. JHEP Rep. 2023;5:100877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 60. | Li F, Ye J, Sun Y, Lin Y, Wu T, Shao C, Ma Q, Liao X, Feng S, Zhong B. Distinct Dose-Dependent Association of Free Fatty Acids with Diabetes Development in Nonalcoholic Fatty Liver Disease Patients. Diabetes Metab J. 2021;45:417-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Kalavalapalli S, Leiva EG, Lomonaco R, Chi X, Shrestha S, Dillard R, Budd J, Romero JP, Li C, Bril F, Samraj G, Pennington J, Townsend P, Orlando F, Shetty S, Mansour L, Silva-Sombra LR, Bedossa P, Malaty J, Barb D, Gurka MJ, Cusi K. Adipose Tissue Insulin Resistance Predicts the Severity of Liver Fibrosis in Patients With Type 2 Diabetes and NAFLD. J Clin Endocrinol Metab. 2023;108:1192-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 62. | Lee M, Hong S, Cho Y, Rhee H, Yu MH, Bae J, Lee YH, Lee BW, Kang ES, Cha BS. Synergistic benefit of thiazolidinedione and sodium-glucose cotransporter 2 inhibitor for metabolic dysfunction-associated steatotic liver disease in type 2 diabetes: a 24-week, open-label, randomized controlled trial. BMC Med. 2025;23:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 63. | Ye Q, Liu Y, Zhang G, Deng H, Wang X, Tuo L, Chen C, Pan X, Wu K, Fan J, Pan Q, Wang K, Huang A, Tang N. Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice. Nat Commun. 2023;14:1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 64. | Chen H, Liu P, Pan X, Huang M, Li T, Guo Y, Pang Z, Mohammadtursun N, Yang X. Downregulation of collagen IV deposition and ITGB1-FAK signaling pathway to inhibit adipogenesis: A novel mechanism of swertiamarin in treating type 2 diabetes mellitus. Int J Biol Macromol. 2025;299:140048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Sun K, Li X, Scherer PE. Extracellular Matrix (ECM) and Fibrosis in Adipose Tissue: Overview and Perspectives. Compr Physiol. 2023;13:4387-4407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 66. | Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, Frühbeck G, Catalán V. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol Immunol. 2021;18:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 67. | Martínez-Sánchez C, Bassegoda O, Deng H, Almodóvar X, Ibarzabal A, de Hollanda A, Martínez García de la Torre RA, Blaya D, Ariño S, Jiménez-Esquivel N, Aguilar-Bravo B, Vallverdú J, Montironi C, Osorio-Conles O, Fundora Y, Sánchez Moreno FJ, Gómez-Valadés AG, Aguilar-Corominas L, Soria A, Pose E, Juanola A, Cervera M, Perez M, Hernández-Gea V, Affò S, Swanson KS, Ferrer-Fàbrega J, Balibrea JM, Sancho-Bru P, Vidal J, Ginès P, Smith AM, Graupera I, Coll M. Therapeutic targeting of adipose tissue macrophages ameliorates liver fibrosis in non-alcoholic fatty liver disease. JHEP Rep. 2023;5:100830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 68. | Liu Y, Su W, Liu Z, Hu Z, Shen J, Zheng Z, Ding D, Huang W, Li W, Cai G, Wei S, Li N, Fang X, Li H, Qin J, Zhang H, Xiao Y, Bi Y, Cui A, Zhang C, Li Y. Macrophage CREBZF Orchestrates Inflammatory Response to Potentiate Insulin Resistance and Type 2 Diabetes. Adv Sci (Weinh). 2024;11:e2306685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Bian L, Tang T, Yu Q, Tong X, Hu S, You Y, Zhang S, Wang H, Fu X, Chen J, Zhang X, Wang M, Zhang P. Association between the triglyceride to high-density lipoprotein cholesterol ratio and type 2 diabetes mellitus in non-alcoholic fatty liver disease. Sci Rep. 2024;14:31048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 70. | Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lütjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 71. | Schweiger M, Romauch M, Schreiber R, Grabner GF, Hütter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O, Diwoky C, Doler C, Mayer N, De Cecco W, Breinbauer R, Zimmermann R, Zechner R. Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun. 2017;8:14859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 72. | Zhang F, Zhao S, Yan W, Xia Y, Chen X, Wang W, Zhang J, Gao C, Peng C, Yan F, Zhao H, Lian K, Lee Y, Zhang L, Lau WB, Ma X, Tao L. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine. 2016;13:157-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 73. | Anderson AK, Lambert JM, Montefusco DJ, Tran BN, Roddy P, Holland WL, Cowart LA. Depletion of adipocyte sphingosine kinase 1 leads to cell hypertrophy, impaired lipolysis, and nonalcoholic fatty liver disease. J Lipid Res. 2020;61:1328-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Chen J, Chen J, Fu H, Li Y, Wang L, Luo S, Lu H. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF-2α/PPARα pathway. Am J Physiol Endocrinol Metab. 2019;317:E710-E722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 75. | Imamura F, Fretts AM, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Virtanen JK, Wong K, Bassett JK, Murphy R, Tintle N, Yu CI, Brouwer IA, Chien KL, Chen YY, Wood AC, Del Gobbo LC, Djousse L, Geleijnse JM, Giles GG, de Goede J, Gudnason V, Harris WS, Hodge A, Hu F; InterAct Consortium, Koulman A, Laakso M, Lind L, Lin HJ, McKnight B, Rajaobelina K, Riserus U, Robinson JG, Samieri C, Senn M, Siscovick DS, Soedamah-Muthu SS, Sotoodehnia N, Sun Q, Tsai MY, Tuomainen TP, Uusitupa M, Wagenknecht LE, Wareham NJ, Wu JHY, Micha R, Lemaitre RN, Mozaffarian D, Forouhi NG. Fatty acids in the de novo lipogenesis pathway and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2020;17:e1003102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 76. | Belew GD, Jones JG. De novo lipogenesis in non-alcoholic fatty liver disease: Quantification with stable isotope tracers. Eur J Clin Invest. 2022;52:e13733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Li N, Tan H, Xie A, Li C, Fu X, Xang W, Kirim A, Huang X. Value of the triglyceride glucose index combined with body mass index in identifying non-alcoholic fatty liver disease in patients with type 2 diabetes. BMC Endocr Disord. 2022;22:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Loosen SH, Demir M, Kunstein A, Jördens M, Qvarskhava N, Luedde M, Luedde T, Roderburg C, Kostev K. Variables associated with increased incidence of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9:e002243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Caussy C. Treatment of type 2 diabetes with MASLD: new evidence for personalised medicine. Gut. 2025;74:520-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Mao X, Zhang X, Kam L, Chien N, Lai R, Cheung KS, Yuen MF, Cheung R, Seto WK, Nguyen MH. Synergistic association of sodium-glucose cotransporter-2 inhibitor and metformin on liver and non-liver complications in patients with type 2 diabetes mellitus and metabolic dysfunction-associated steatotic liver disease. Gut. 2024;73:2054-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 81. | Arruda VM, Azevedo GT, Granato MJMG, Matos ACP, Araújo TG, Guerra JFDC. Oxidative Stress and Annexin A2 Differential Expression in Free Fatty Acids-Induced Non-Alcoholic Fatty Liver Disease in HepG2 Cells. Int J Mol Sci. 2024;25:9591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 82. | Shiri H, Fallah H, Abolhassani M, Fooladi S, Ramezani Karim Z, Danesh B, Abbasi-Jorjandi M. Relationship between types and levels of free fatty acids, peripheral insulin resistance, and oxidative stress in T2DM: A case-control study. PLoS One. 2024;19:e0306977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 83. | Cui X, Yao M, Feng Y, Li C, Li Y, Guo D, He S. Exogenous hydrogen sulfide alleviates hepatic endoplasmic reticulum stress via SIRT1/FoxO1/PCSK9 pathway in NAFLD. FASEB J. 2023;37:e23027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Nicholas DA, Proctor EA, Agrawal M, Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L, Jones AR 4th, Raval F, Ip BC, Zhu M, Cacicedo JM, Habib C, Sainz-Rueda N, Persky L, Sullivan PG, Corkey BE, Apovian CM, Kern PA, Lauffenburger DA, Nikolajczyk BS. Fatty Acid Metabolites Combine with Reduced β Oxidation to Activate Th17 Inflammation in Human Type 2 Diabetes. Cell Metab. 2019;30:447-461.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 85. | Yu YY, Feng M, Chen Y, Jia HL, Zhang Q, Tong M, Li YX, Zhao Y, Liu XX, Cao SF, Wang ZK, Li HW, Liu X, Zhang Y. Asprosin-FABP5 Interaction Modulates Mitochondrial Fatty Acid Oxidation through PPARα Contributing to MASLD Development. Adv Sci (Weinh). 2025;12:e2415846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Heebøll S, Risikesan J, Ringgaard S, Kumarathas I, Sandahl TD, Grønbæk H, Søndergaard E, Nielsen S. Impaired Glucagon-Mediated Suppression of VLDL-Triglyceride Secretion in Individuals With Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Diabetes. 2022;71:2402-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Marigorta UM, Millet O, Lu SC, Mato JM. Dysfunctional VLDL metabolism in MASLD. NPJ Metab Health Dis. 2024;2:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 88. | Hiukka A, Ståhlman M, Pettersson C, Levin M, Adiels M, Teneberg S, Leinonen ES, Hultén LM, Wiklund O, Oresic M, Olofsson SO, Taskinen MR, Ekroos K, Borén J. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 2009;58:2018-2026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 89. | Hirano T. Excess Triglycerides in Very Low-Density Lipoprotein (VLDL) Estimated from VLDL-Cholesterol could be a Useful Biomarker of Metabolic Dysfunction Associated Steatotic Liver Disease in Patients with Type 2 Diabetes. J Atheroscler Thromb. 2025;32:253-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 90. | Jiang Y, Gong Q, Gong Y, Zhuo C, Huang J, Tang Q. Vitexin Attenuates Non-alcoholic Fatty Liver Disease Lipid Accumulation in High Fat-Diet Fed Mice by Activating Autophagy and Reducing Endoplasmic Reticulum Stress in Liver. Biol Pharm Bull. 2022;45:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Fan C, Wang G, Chen M, Li Y, Tang X, Dai Y. Therapeutic potential of alkaloid extract from Codonopsis Radix in alleviating hepatic lipid accumulation: insights into mitochondrial energy metabolism and endoplasmic reticulum stress regulation in NAFLD mice. Chin J Nat Med. 2023;21:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 92. | Noureddin M, Khan S, Portell F, Jorkasky D, Dennis J, Khan O, Johansson L, Johansson E, Sanyal AJ. Safety and efficacy of once-daily HU6 versus placebo in people with non-alcoholic fatty liver disease and high BMI: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Gastroenterol Hepatol. 2023;8:1094-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 93. | Li J, Chen C, Zhang W, Bi J, Yang G, Li E. Salsalate reverses metabolic disorders in a mouse model of non-alcoholic fatty liver disease through AMPK activation and caspase-6 activity inhibition. Basic Clin Pharmacol Toxicol. 2021;128:394-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 95. | Ding Z, Wei Y, Peng J, Wang S, Chen G, Sun J. The Potential Role of C-Reactive Protein in Metabolic-Dysfunction-Associated Fatty Liver Disease and Aging. Biomedicines. 2023;11:2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 96. | Zyśk B, Ostrowska L, Smarkusz-Zarzecka J, Witczak-Sawczuk K, Gornowicz A, Bielawska A. Pro-Inflammatory Adipokine and Cytokine Profiles in the Saliva of Obese Patients with Non-Alcoholic Fatty Liver Disease (NAFLD)-A Pilot Study. Int J Mol Sci. 2023;24:2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 97. | Nowatari T, Fukunaga K, Ohkohchi N. Regulation of signal transduction and role of platelets in liver regeneration. Int J Hepatol. 2012;2012:542479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Lima RP, Nunes PIG, Viana AFSC, Oliveira FTB, Silva RAC, Alves APNN, Viana DA, Fonseca SGC, Carvalho AA, Chaves MH, Rao VS, Santos FA. α,β-Amyrin prevents steatosis and insulin resistance in a high-fat diet-induced mouse model of NAFLD via the AMPK-mTORC1-SREBP1 signaling mechanism. Braz J Med Biol Res. 2021;54:e11391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Liao Z, Huang L, Chen J, Chen T, Kong D, Wei Q, Chen Q, Deng B, Li Y, Zhong S, Huang Z. Liraglutide Improves Nonalcoholic Fatty Liver Disease in Diabetic Mice by Activating Autophagy Through AMPK/mTOR Signaling Pathway. Diabetes Metab Syndr Obes. 2024;17:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 101. | Chen CM, Lu CF, Liu WS, Gong ZH, Wang XQ, Xu F, Ji JF, Fang XX. Association between fibrinogen/albumin ratio and arterial stiffness in patients with type 2 diabetes: A cross-sectional study. Front Pharmacol. 2022;13:1120043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 102. | Hu J, Wang H, Li X, Liu Y, Mi Y, Kong H, Xi D, Yan W, Luo X, Ning Q, Wang X. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. 2020;10:9702-9720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 103. | Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Abdelmonem M, Abdulla S, Wasim H, Elawamy H. The correlation between Plasma Fibrinogen Levels and Glycemic Status in type 2 Diabetes Mellitus. Am J Clin Pathol. 2023;160. [DOI] [Full Text] |
| 105. | Kotronen A, Joutsi-Korhonen L, Sevastianova K, Bergholm R, Hakkarainen A, Pietiläinen KH, Lundbom N, Rissanen A, Lassila R, Yki-Järvinen H. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011;31:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | Safdar NZ, Kietsiriroje N, Ajjan RA. The Cellular and Protein Arms of Coagulation in Diabetes: Established and Potential Targets for the Reduction of Thrombotic Risk. Int J Mol Sci. 2023;24:15328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 107. | Waller KJ, Saihi H, Li W, Brindley JH, De Jong A, Syn WK, Bessant C, Alazawi W. Single-cell phenotypes of peripheral blood immune cells in early and late stages of non-alcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:417-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 108. | Diedrich T, Kummer S, Galante A, Drolz A, Schlicker V, Lohse AW, Kluwe J, Eberhard JM, Schulze Zur Wiesch J. Characterization of the immune cell landscape of patients with NAFLD. PLoS One. 2020;15:e0230307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 109. | Song Y, He C, Jiang Y, Yang M, Xu Z, Yuan L, Zhang W, Xu Y. Bulk and single-cell transcriptome analyses of islet tissue unravel gene signatures associated with pyroptosis and immune infiltration in type 2 diabetes. Front Endocrinol (Lausanne). 2023;14:1132194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 110. | Torres-Castro I, Arroyo-Camarena ÚD, Martínez-Reyes CP, Gómez-Arauz AY, Dueñas-Andrade Y, Hernández-Ruiz J, Béjar YL, Zaga-Clavellina V, Morales-Montor J, Terrazas LI, Kzhyshkowska J, Escobedo G. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol Lett. 2016;176:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 111. | Fadini GP, de Kreutzenberg SV, Boscaro E, Albiero M, Cappellari R, Kränkel N, Landmesser U, Toniolo A, Bolego C, Cignarella A, Seeger F, Dimmeler S, Zeiher A, Agostini C, Avogaro A. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia. 2013;56:1856-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 112. | Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol. 2019;10:1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 768] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 113. | Zeng TS, Liu FM, Zhou J, Pan SX, Xia WF, Chen LL. Depletion of Kupffer cells attenuates systemic insulin resistance, inflammation and improves liver autophagy in high-fat diet fed mice. Endocr J. 2015;62:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Zhao C, Lou F, Li X, Ma J, Zhu Z, Li H, Zhai Y, Chen H, Zhang Q, Liu Z, Xiao S. Correlation of CD3+/CD4+, and serum CK-18 fragment levels with glucose and lipid metabolism in elderly type 2 diabetes patients with nonalcoholic fatty liver disease. Am J Transl Res. 2021;13:2546-2554. [PubMed] |
| 115. | Breuer DA, Pacheco MC, Washington MK, Montgomery SA, Hasty AH, Kennedy AJ. CD8(+) T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;318:G211-G224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 116. | Lauszus JS, Eriksen PL, Hansen MM, Eriksen LL, Shawcross DL, Vilstrup H, Thomsen KL, Stoy S. Activation and Functional Priming of Blood Neutrophils in Non-Alcoholic Fatty Liver Disease Increases in Non-Alcoholic Steatohepatitis. Clin Exp Gastroenterol. 2021;14:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 117. | Tsilingiris D, Natsi AM, Gavriilidis E, Antoniadou C, Eleftheriadou I, Anastasiou IA, Tentolouris A, Papadimitriou E, Eftalitsidis E, Kolovos P, Tsironidou V, Giatromanolaki A, Koffa M, Tentolouris N, Skendros P, Ritis K. Interleukin-8/Matrix Metalloproteinase-9 Axis Impairs Wound Healing in Type 2 Diabetes through Neutrophil Extracellular Traps-Fibroblast Crosstalk. Eur J Immunol. 2025;55:e202451664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 118. | Sun G, Wang Y, Yang L, Zhang Z, Zhao Y, Shen Z, Han X, Du X, Jin H, Li C, Wang S, Zhang Z, Zhang D. Rebalancing liver-infiltrating CCR3(+) and CD206(+) monocytes improves diet-induced NAFLD. Cell Rep. 2023;42:112753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 119. | Jia J, Liu R, Wei W, Yu F, Yu X, Shen Y, Chen C, Cai Z, Wang C, Zhao Z, Wang D, Yang L, Yuan G. Monocyte to High-Density Lipoprotein Cholesterol Ratio at the Nexus of Type 2 Diabetes Mellitus Patients With Metabolic-Associated Fatty Liver Disease. Front Physiol. 2021;12:762242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Sim BC, Kang YE, You SK, Lee SE, Nga HT, Lee HY, Nguyen TL, Moon JS, Tian J, Jang HJ, Lee JE, Yi HS. Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis. Cell Death Dis. 2023;14:618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 121. | Niranjan S, Phillips BE, Giannoukakis N. Uncoupling hepatic insulin resistance - hepatic inflammation to improve insulin sensitivity and to prevent impaired metabolism-associated fatty liver disease in type 2 diabetes. Front Endocrinol (Lausanne). 2023;14:1193373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 122. | Yang H, Jung S, Choi EY. E3 ubiquitin ligase TRIM38 regulates macrophage polarization to reduce hepatic inflammation by interacting with HSPA5. Int Immunopharmacol. 2025;157:114662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 123. | Vahrenbrink M, Coleman CD, Kuipers S, Lurje I, Hammerich L, Kunkel D, Keye J, Dittrich S, Schjeide BM, Hiß R, Müller J, Püschel GP, Henkel J. Dynamic changes in macrophage populations and resulting alterations in Prostaglandin E(2) sensitivity in mice with diet-induced MASH. Cell Commun Signal. 2025;23:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 124. | Xiang L, Wang G, Zhuang Y, Luo L, Yan J, Zhang H, Li X, Xie C, He Q, Peng Y, Chen H, Li Q, Li X, Guo L, Lv G, Ding Y. Safety and efficacy of GLP-1/FGF21 dual agonist HEC88473 in MASLD and T2DM: A randomized, double-blind, placebo-controlled study. J Hepatol. 2025;82:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 125. | Guo W, Li Z, Anagnostopoulos G, Kong WT, Zhang S, Chakarov S, Shin A, Qian J, Zhu Y, Bai W, Cexus O, Nie B, Wang J, Hu X, Blériot C, Liu Z, Shen B, Venteclef N, Su B, Ginhoux F. Notch signaling regulates macrophage-mediated inflammation in metabolic dysfunction-associated steatotic liver disease. Immunity. 2024;57:2310-2327.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 126. | Alzaid F, Lagadec F, Albuquerque M, Ballaire R, Orliaguet L, Hainault I, Blugeon C, Lemoine S, Lehuen A, Saliba DG, Udalova IA, Paradis V, Foufelle F, Venteclef N. IRF5 governs liver macrophage activation that promotes hepatic fibrosis in mice and humans. JCI Insight. 2016;1:e88689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 127. | Yang Y, Wang J, Lin X, Zhang Z, Zhang M, Tang C, Kou X, Deng F. TNF-α-licensed exosome-integrated titaniumaccelerated T2D osseointegration by promoting autophagy-regulated M2 macrophage polarization. Biochem Biophys Res Commun. 2024;727:150316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 128. | Chen C, Liang Z, He Y, Gao Y, Ouyang S, Wang L, Liu J, Cao J. Bacteroides Fragilis Exacerbates T2D Vascular Calcification by Secreting Extracellular Vesicles to Induce M2 Macrophages. Adv Sci (Weinh). 2025;12:e2410495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 129. | L'homme L, Sermikli BP, Haas JT, Fleury S, Quemener S, Guinot V, Barreby E, Esser N, Caiazzo R, Verkindt H, Legendre B, Raverdy V, Cheval L, Paquot N, Piette J, Legrand-Poels S, Aouadi M, Pattou F, Staels B, Dombrowicz D. Adipose tissue macrophage infiltration and hepatocyte stress increase GDF-15 throughout development of obesity to MASH. Nat Commun. 2024;15:7173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 130. | Zheng C, Sui B, Zhang X, Hu J, Chen J, Liu J, Wu D, Ye Q, Xiang L, Qiu X, Liu S, Deng Z, Zhou J, Liu S, Shi S, Jin Y. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles. 2021;10:e12109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 131. | Barb D, Kalavalapalli S, Godinez Leiva E, Bril F, Huot-Marchand P, Dzen L, Rosenberg JT, Junien JL, Broqua P, Rocha AO, Lomonaco R, Abitbol JL, Cooreman MP, Cusi K. Pan-PPAR agonist lanifibranor improves insulin resistance and hepatic steatosis in patients with T2D and MASLD. J Hepatol. 2025;82:979-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/