Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.109053
Revised: June 24, 2025
Accepted: August 8, 2025
Published online: September 15, 2025
Processing time: 136 Days and 1.8 Hours
The management of diabetic ketoacidosis can be challenging in high-risk patients. Recent studies have reported a significant increase in diabetic ketoacidosis hospi
Core Tip: Diabetic ketoacidosis is the most common life-threatening acute complication of uncontrolled hyperglycemia. The concurrent presence of co-morbid conditions in patients with diabetic ketoacidosis can further worsen the clinical scenario. While standard guidelines exist for the treatment of diabetic ketoacidosis, the management of these high-risk patients requires careful modifications from the standard treatment protocol. In this review, we summarize the management principles of diabetic keto
- Citation: Ray S, Palui R. Managing diabetic ketoacidosis in special conditions: Difficulties and dilemmas. World J Diabetes 2025; 16(9): 109053
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/109053.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.109053
Diabetic ketoacidosis (DKA) is an acute hyperglycemic emergency with significant morbidity and mortality risk[1]. Although the risk of DKA is higher in patients with type 1 diabetes (T1D), a significant risk of DKA also remains for patients with type 2 diabetes (T2D)[2]. There has been a recent increase in hospital admission rate for DKA worldwide[3,4]. The mortality rate of DKA has significantly decreased in recent times; however, the duration of hospital stay and the cost of therapy have increased[5]. The risk of complications like cerebral edema and the overall mortality rate are higher in resource-limited developing countries than developed countries[6]. The basic prin
Diabetes remains one of the major factors underlying the development of CKD. However, the occurrence of DKA is usually rare in advanced CKD[10]. The risk of DKA in CKD patients is paradoxically lower because of improved glycemic control due to lack of renal insulin clearance, reduced renal neoglucogenesis and reduced insulin resistance in CKD patients[11]. From the pathophysiological point of view, the presentation of DKA in patients with CKD differs from patients with normal renal function. Hypovolemia and electrolyte depletion are usually not seen in DKA with advanced CKD due to lack of glucosuria and osmotic diuresis. In contrast, volume overload is more commonly seen in CKD patients due to increased water intake secondary to stimulation of thirst center due to plasma hypertonicity. As the expected osmotic diuresis is impaired in these patients, excess water accumulates to increase intravascular and interstitial volume[12]. Moreover, higher glucose level, hyperkalemia, hyponatremia and hyperphosphatemia are seen in CKD patients with DKA[13]. Higher complication rates, including increased hypoglycemic events, longer length of hospital stay, higher re-admission rates and increased cost of treatment are more common in end stage renal disease (ESRD) patients with DKA compared to patients with better renal functions[10,14].
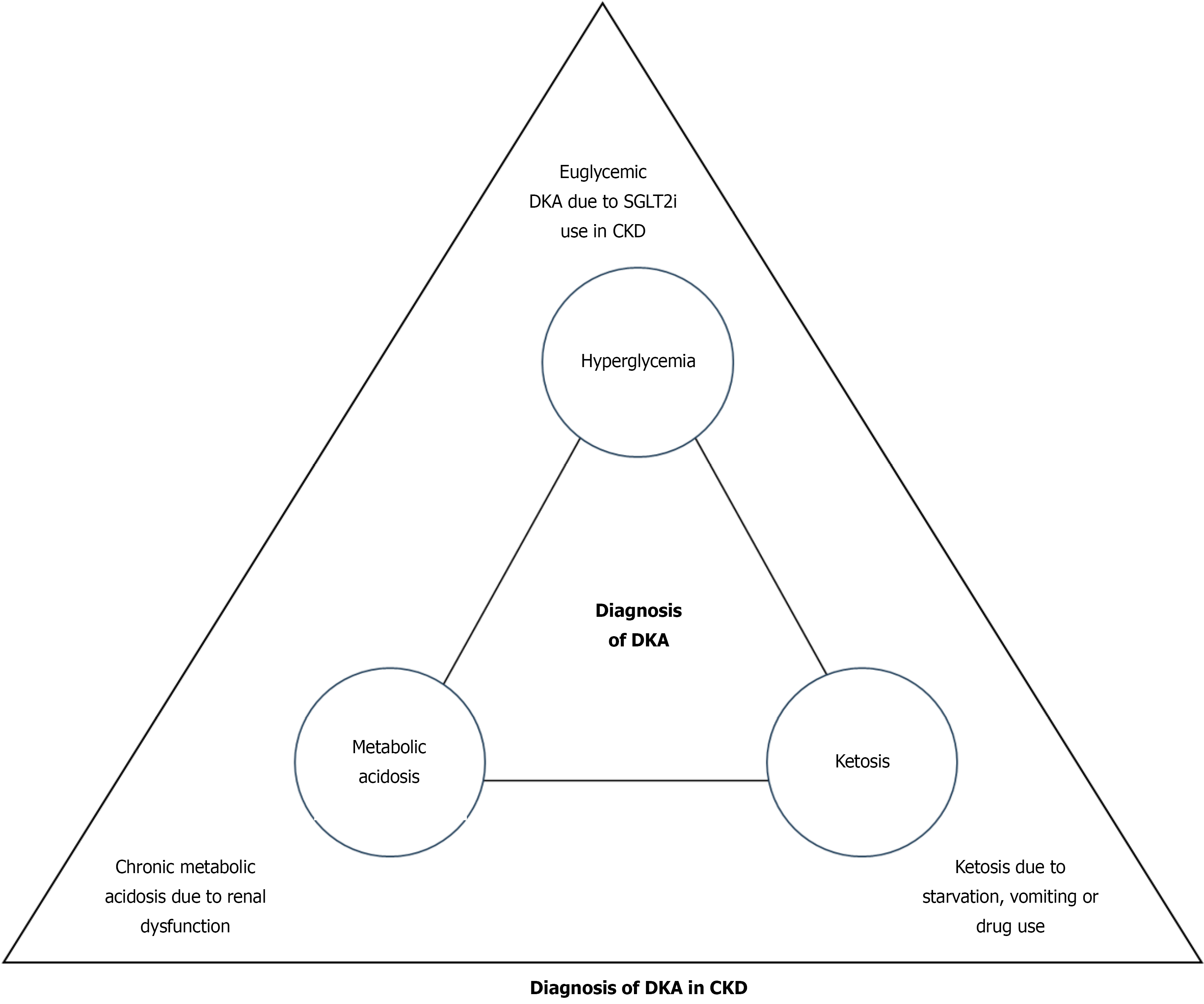
Diagnostic challenges: The diagnosis of DKA patients with CKD is difficult and tricky due to various reasons. Clinically, the diagnosis of DKA is often missed or delayed in CKD patients. The classical features of DKA, like dehydration, are not present in renal dysfunction, and other symptoms like nausea, vomiting and acidotic breathing may be attributed to renal failure itself. Moreover, the laboratory diagnosis of DKA in renal dysfunction is difficult. DKA is diagnosed as a triad of hyperglycemia, high anion-gap metabolic acidosis and ketonemia[7]. Due to the widespread use of sodium-glucose cotransporter-2 inhibitors (SGLT-2i) in CKD, hyperglycemia may be masked, and patients can remain euglycemic, as discussed later. As CKD itself is a state of chronic high anion gap metabolic acidosis, diagnosis of DKA is challenging in this condition. The diagnosis of DKA in CKD patients is usually considered if the anion gap is more than 20 mEq/L[15]. Thus, measurement of ketones, preferably serum β-hydroxybutyrate, is an important tool to establish the laboratory diagnosis of DKA in renal disease. However, ketosis can also be observed in renal dysfunction secondary to the effect of drugs, like metformin-induced suppression of neoglucogenesis, or following starvation due to loss of appetite or vomiting in CKD[12]. Moreover, lower serum β-hydroxybutyrate levels have been reported in ESRD patients with DKA compared to patients with mild to moderate renal dysfunction[16]. Irrespective of these conflicting findings, ketosis is considered to be present if serum β-hydroxybutyrate is more than 3 mmol/L[17]. Because of these overlapping factors, diagnosis of DKA in the background of renal dysfunction is always challenging (Figure 1).
Management principles: The management strategy of DKA in CKD requires selective modifications from the standard treatment protocol[7]. As volume overload rather than volume depletion is more common, initial fluid replacement is not required in euvolemic CKD patients, specifically in ESRD or in patients on dialysis[7]. The volume status should be evaluated at initial assessment, and if found hypovolemic, a small aliquot of 250 mL intravenous fluid (normal saline or dextrose) should be given slowly. The patient should be reassessed and a repeat bolus of a similar amount should be given only if hypovolemia persists. In addition to clinical examination, assessment of volume status should be guided by hemodynamic monitoring (central venous pressure) or imaging techniques. DKA patients with advanced kidney disease can even present with severe hypervolemia leading to pulmonary edema requiring hemodialysis[17]. Insulin replacement should be done by fixed rate intravenous insulin infusion (FRIII) at an initial hourly rate of 0.1 units/kg. However, in case of patients with ESRD or if rate of fall of plasma glucose is more than 3 mmol/L/hour or when plasma glucose falls below 18 mmol/L, a reduced hourly FRIII rate of 0.05 units/kg should be used[7,17]. Routine supplementation of potassium is not recommended in CKD patients, as hyperkalemia is more common due to reduced renal function and metabolic acidosis. Potassium should be measured frequently, and cardiac monitoring is necessary if potassium is more than 5.5 mmol/L. In cases of severe hyperkalemia (> 6.5 mmol/L), dialysis may even be required along with standard treatment. Potassium replacement should be given only if serum potassium remains below 3.5 mmol/L[7,17]. Bicarbonate therapy is usually not recommended in DKA and usually needed only in cases of very severe metabolic acidosis (pH < 6.9)[8,18]. As bicarbonate generation is already compromised in advanced CKD or ESRD patients with pre-existing chronic metabolic acidosis, bicarbonate therapy may be considered when pH is less than 7.2 along with nephrology consultation[17]. Renal replacement therapies may be needed in DKA patients with advanced CKD in cases of severe hyperkalemia, volume overload or persistent acidosis[12].
Management of DKA along with co-existing cardiac aliments like HF or acute coronary syndrome (ACS) is difficult and medically challenging. Every 1% increase in glycosylated hemoglobin increases the risk of HF by 10%[19]. On the other hand, HF was associated with increased risk of hospital mortality and prolonged duration of hospital stay in patients with DKA[20]. Similarly, there is bidirectional relationship between DKA and ACS. Both conditions can trigger each other, often creating a perfect example of ‘chicken and egg conundrum’. Increased troponin levels are also indicators of poor prognosis in patients with DKA[21]. There is a lack of specific guidelines related to the management of DKA patients with a concomitant acute cardiac event.
Diagnostic challenges: The diagnosis of new onset DM in patients with HF can be missed, as the classical symptoms of DM like polyuria and polydipsia can be attributed to the use of diuretics. Although DKA per se can cause elevation of troponin level, all DKA patients with elevated troponin levels should be thoroughly evaluated to diagnose any possible concomitant acute coronary event[22].
Management principles: The concomitant presence of both DKA and HF/ACS can be life threatening; thus, patients should always be admitted into High-Dependency Unit or Critical Care Units[7]. Treating DKA with HF is clinically challenging because it requires the balance between two fundamentally opposing management protocols. On one hand, aggressive fluid replacement is needed in DKA, while on the other hand, fluid restriction with diuretics is the main treatment for HF. These patients usually have peripheral edema (signs of volume overload) along with paradoxical intravascular volume depletion. Thus, assessment of volume status is very difficult and requires combinations of continuous clinical (jugular venous pressure, peripheral edema, pulmonary congestion) and advanced hemodynamic monitoring (central venous pressure monitoring, inferior vena cava size and collapsibility, arterial blood pressure)[23]. Initial bolus fluid replacement as per the standard DKA management protocol can be detrimental in this setting and may further decompensate HF. Thus, cautious and slow replacement of minimal fluid bolus (250-500 mL; up to 1-1.5 L over 1-2 hours as per clinical setting) with frequent hemodynamic monitoring is suggested for patients with hypovolemia[9,23]. Normal urine output may be because of osmotic diuresis and does not guarantee achievement of adequate hydration status. Persistent hypotension even after initial fluid replacement can be due to HF per se and may require administration of vasoactive agents[7,23]. If the patient is already hypervolemic and requires dextrose infusion as plasma glucose falls below 14 mmol/L, concentrated infusion (25% dextrose) instead of standard dextrose infusion should be used. Common precipitant causes of both disorders, like infections, diarrhea, stresses-like trauma, and major surgery, should be thoroughly investigated and managed accordingly[23]. Depending upon the clinical situation, discontinuation or modifications of chronic medications like glucagon-like peptide-1 receptor agonists, SGLT-2i, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics may also be needed[22].
Acute pancreatitis is a critical condition that mostly presents with acute abdominal pain. Several articles had reported the co-occurrence of acute pancreatitis and DKA[24-26]. However, this concurrent presentation can be another example of ‘chicken and egg conundrum’, and it is very difficult to determine whether DKA leads to acute pancreatitis or vice versa[24,25,27]. Severe hypertriglyceridemia is the common factor that is primarily present along with these two disorders, and together they are referred to in the literature as the ‘Enigmatic triangle’[28,29].
Diagnostic challenges: Both DKA and acute pancreatis can have overlapping symptoms of abdominal pain and vomiting. Though increased serum amylase and lipase are typical markers of acute pancreatitis, both markers can also be elevated in DKA[30]. Because of these overlapping features, the concurrent presentation of these two life threatening conditions can lead to a diagnostic dilemma and clinical suspicion is needed for timely diagnosis.
Management principles: Increased mortality rate, higher risk of multi-organ failure and prolonged duration of hospital stay have been reported in these patients[28]. Proper management of this patients should include the early initiation of fluid replacement, intravenous insulin infusion, treatment of electrolyte imbalance along with concurrent management of acute pancreatitis. Delay in the initiation of management of dehydration and metabolic acidosis due to missed diagnosis can lead to poor clinical outcome[31]. Use of intravenous insulin helps resolve DKA, and it is also effective in rapidly reducing serum triglyceride levels[32,33].
The key points for management of DKA in these above-mentioned co-morbid conditions are summarized in Table 1.
| Disease | Key management principles |
| DKA with chronic kidney disease | Routine bolus fluid replacement is not needed in euvolemic patients |
| In patients with hypovolemia, small aliquot of 250 mL i.v. fluid should be given slowly | |
| In severe hypervolemia with refractory pulmonary oedema, hemodialysis may be needed | |
| Lower dose of fixed rate intravenous insulin infusion (0.05 units/kg/hour) is preferred in ESRD patients | |
| Routine supplementation of potassium is not recommended | |
| In case of severe hyperkalemia (> 6.5 mmol/L), hemodialysis may even be required | |
| Bicarbonate therapy can rarely be required in pre-existing chronic metabolic acidosis (pH < 7.2) after nephrology consultation | |
| DKA with heart failure | Strict advanced hemodynamic monitoring is needed for assessment of volume status |
| Cautious and slow replacement of minimal fluid bolus (250-500 mL) at a time, only if required | |
| Concentrated dextrose infusion may be needed (25%dextrose) in patients with hypervolemia | |
| Persistent hypotension can be due to HF and will require administration of vasoactive agents | |
| Treatment of precipitating factors including any cardiac events | |
| Modifications of chronic medications (discontinuation SGLT2i) | |
| DKA with acute pancreatitis | Early initiation of fluid replacement and intravenous insulin infusion |
| Early diagnosis and prompt initiation of management of acute pancreatitis | |
| Use of intravenous insulin infusion will also help reduce concomitant hypertriglyceridemia, if any |
DKA during pregnancy is considered an obstetrical emergency. However, there are limited robust epidemiological data, and incidence ranging from 0.5% to 10% of pregnancies complicated by pre-existing diabetes[34]. The altered metabolic environment of pregnancy means that DKA can develop more rapidly in less severe cases of hyperglycemia than in non-pregnant women. DKA is associated with high rates of pregnancy loss with considerable maternal and neonatal morbidity. Women from a lower socioeconomic status present with DKA more frequently with poor glycemic control prior to and during pregnancy[35]. Counseling for all women with diabetes at risk of adverse outcomes pregnancy complicated by DKA should be available, and clinicians should have a high index of suspicion for DKA in all pregnant women who present with symptoms that could be attributed to this condition.
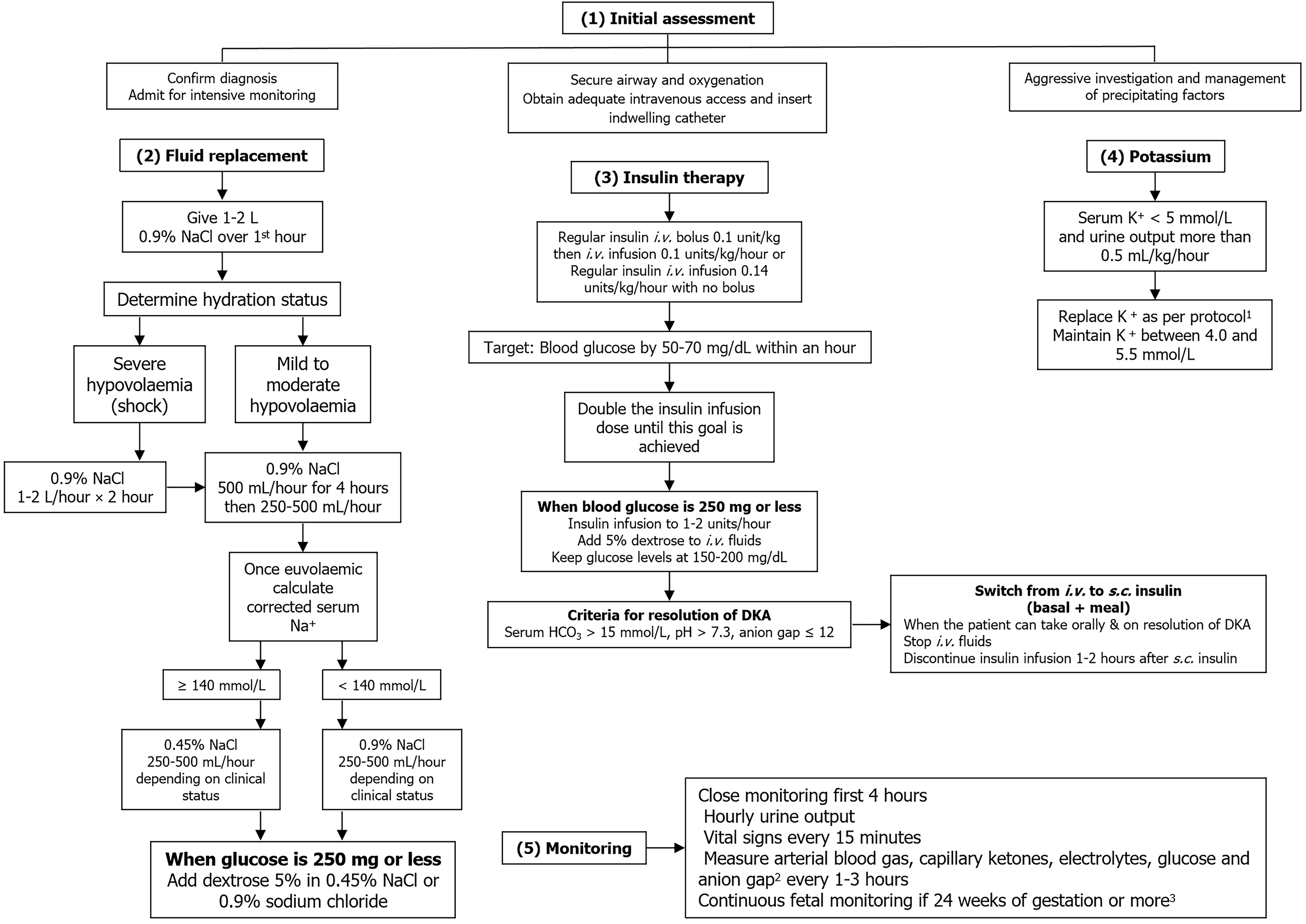
DKA during pregnancy primarily occurs in women with TID, but patients with T2D or gestational diabetes are sometimes affected. DKA in pregnancy should be managed as a medical and obstetric emergency in an intensive care facility by a professional team experienced in dealing with similar cases. Management of DKA in pregnancy is similar to that for nonpregnant individuals[13]. Management incorporates several aspects that should be performed simultaneously. The management algorithm of DKA during pregnancy is outlined in Figure 2. There are not any available prospective studies on the use of bicarbonate in DKA in pregnancy, and it is not recommended because there is no demonstrable benefit and it may in fact be harmful to the patient and the fetus[13]. Hyperglycemia following steroid administration should be managed by increasing basal and prandial insulin doses (typically by 50%) or by adding a variable rate of intravenous insulin infusion so as not to induce DKA[36]. The clinical status of the patient should be monitored with a regular assessment of hydration, blood pressure, mental status, fluid intake and urine output along with laboratory parameters, and the fluid and electrolyte therapy should be adjusted accordingly. Blood glucose levels should be monitored hourly until they are stable, whereas blood urea nitrogen, creatinine, blood ketones, serum electrolytes and venous pH should be measured every 2-4 hours, depending on disease severity and clinical response[37]. Monitoring the fetal heart rate is recommended for gestational age of 24 weeks or above.
For the unborn child, DKA during labor or delivery poses a potentially life-threatening condition[38]. Both endocrine and obstetric expertise should be involved in the management of DKA during labor. During labor and delivery, preventive strategies include adequate administration of carbohydrate and insulin during labor, as well as early detection of possible precipitating factors, including infection and dehydration[39]. For patients with an insulin pump, it can be safely stopped for up to 60 minutes but must be restarted immediately post-operatively to prevent DKA[36]. A recent observational study reported that pregnant women with T1D who were already on automated insulin delivery can continue safely using it during labor and delivery, attaining more time in range without severe hypoglycemia or DKA[40]. DKA during pregnancy is not an indication for emergency delivery, which should be decided after an evaluation of the clinical condition of the mother, treatment response, gestational age and fetal status[13].
DKA in older adults is often confounded by various clinical conditions that may mask disease pathology and lead to a delay in DKA diagnosis and management. Insulin noncompliance and comorbidities are the most common precipitants for DKA in older patients[41]. Elderly patients often present atypically with predominant neurologic symptoms, and gastrointestinal symptoms may be less prominent. Among older adults, the presence of both comorbidities and severe precipitating factors like sepsis, myocardial infarction and stroke are more frequently observed[41]. A large retrospective study from India highlights that DKA is common in older adults[42]. Older adults have an atypical presentation of DKA and usually have precipitation with infections and non-infectious disorders. In older adults, SGLT2i increased the probability of DKA and could result in missed DKA diagnosis[43]. Clinicians need to have a low threshold for DKA diagnosis in this patient group because they may be relatively asymptomatic in the early stages[44,45].
In the elderly, the rate and volume of fluid replacement may need to be modified. Balanced crystalloid solutions have been suggested as an alternative owing to a lower likelihood to cause hyperchloremic metabolic acidosis. However, large volume administration of crystalloid fluids that are more hypotonic than normal saline may increase the risk of cerebral edema, particularly in elderly individuals[46]. Osmotic diuresis exacerbates dehydration and eventual decrease in renal function. It is more noticeable in older adults and ultimately leads to reduced metabolism of several drugs including insulin. In elderly patients, DKA is often complicated by sepsis, atrial fibrillation, nonketotic hyperosmolar states, acute kidney injury (AKI), dementia and polypharmacy[43]. Older patients with DKA are at increased risk for in-hospital mortality and prolonged hospital stay, long-term mortality and greater chances of recurrent DKA[41,43]. Therefore, not only treatment of metabolic abnormalities of DKA, but also careful attention to comorbidities and complications are necessary in this population.
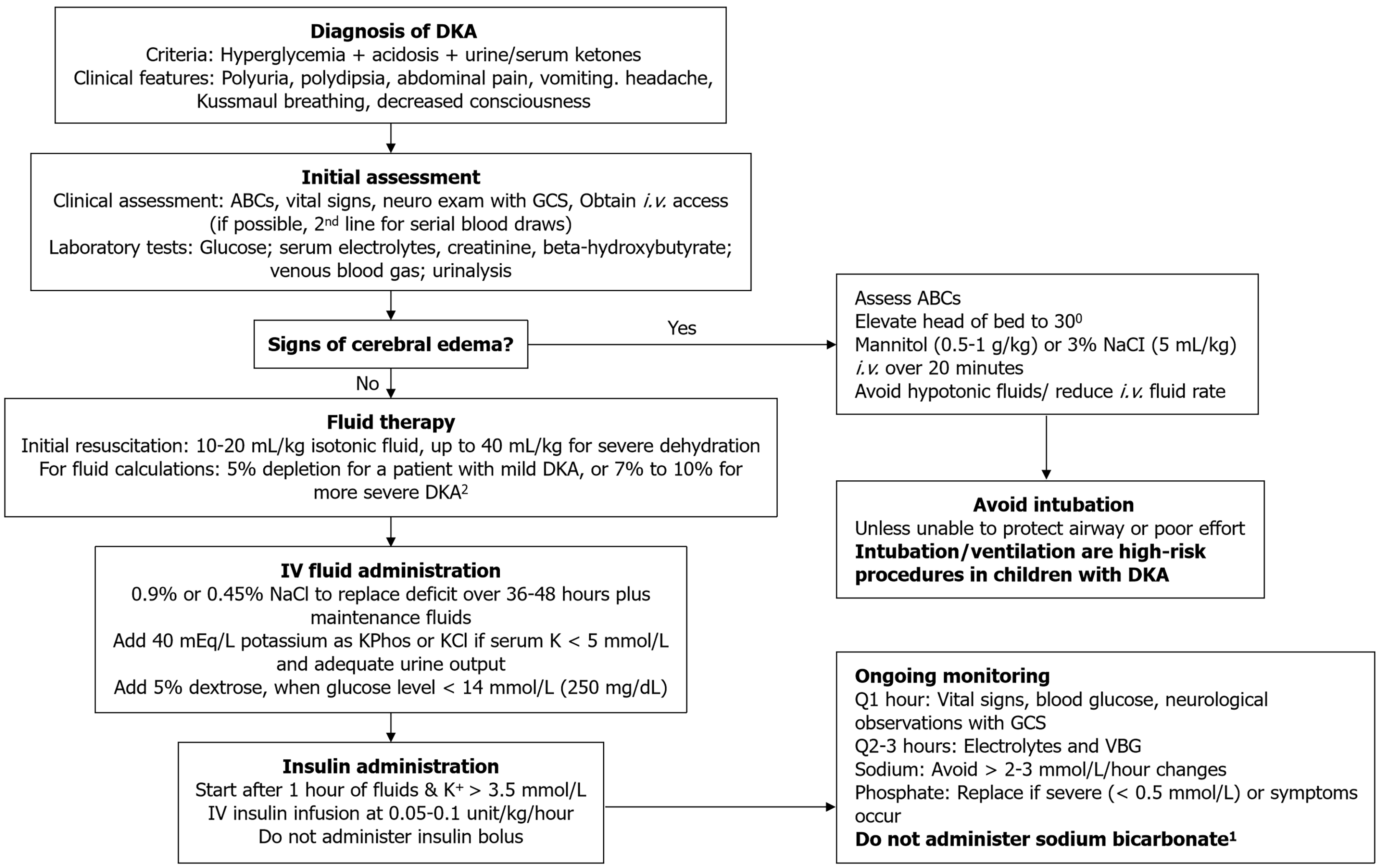
In children, several neurological complications may occur in DKA, with cerebral injury that may lead to cerebral edema being the most common. Children without overt neurological symptoms during DKA treatment may have subtle evidence of brain injury, particularly memory deficits, after recovery from DKA[47]. Therefore, efforts should be made to ensure minimization of neurological damage while treating pediatric DKA. Resuscitation with intravenous (i.v.) fluid and administration of insulin are the mainstays of DKA treatment. An algorithm for managing DKA in children is provided in Figure 3.
Fluid and electrolyte therapy: When the difference in body weight measured at the presentation of DKA and at discharge is compared, most children had 5%-10% weight loss[48]. Initial rehydration with 10 mL/kg normal saline bolus is indicated. Those with Glasgow Coma Scale scores of 14 or 15 who have persistent tachycardia or signs of impaired perfusion may receive an additional normal saline bolus immediately after the first bolus is completed. Following bolus administration, further i.v. fluids should be continued to replete the fluid deficit remaining and deliver ongoing maintenance. The latest National Institute for Health and Care Excellence guideline advocates the use of the Holliday-Segar formula for calculation of maintenance fluids[49]. This formula is the basis of the calculation of maintenance fluid applied in the PECARN FLUID trial. It considers both insensible loss and urinary losses based on the child's weight[50]. Intravenous fluids are continued until resolution of acidosis and when the patient can tolerate oral feeding and is transitioned from i.v. to subcutaneous insulin. In the PECARN FLUID trial, 0.45% sodium chloride (NaCl) was compared with 0.9% NaCl, but no difference was detected in the occurrence of clinically apparent cerebral edema or AKI; nevertheless, hyperchloremic acidosis was more prevalent among patients receiving 0.9% NaCl[50]. There is limited evidence for the use of other crystalloids in childhood DKA management, and more research is necessary on the use of crystalloid fluids in the management of DKA in children[51,52].
In DKA, children have a total body potassium deficit of around 3-6 mEq/L attributable to various factors[53]. The presence of insulin deficiency and metabolic acidosis before the initiation of DKA treatment leads to an extracellular shift of potassium, and consequently, potassium levels are rarely low at presentation. Nevertheless, upon insulin initiation, there is a shift of potassium into the intracellular space, leading to a drop in serum potassium. Contemporary guidelines recommend early initiation of maintenance fluids with potassium, unless the patient is anuric or exhibits hyperkalemia. Potassium chloride may be used along with potassium phosphate. Potassium administration solely as potassium chloride may lead to hyperchloremic metabolic acidosis.
Insulin infusion: The International Society for Paediatric and Adolescent Diabetes (ISPAD) recommends starting insulin infusions at least 1 hour after starting fluid replacement therapy. Insulin should be deferred in children with potassium levels < 3.5 mmol/L, and potassium bolus be given promptly. In children with moderate to severe DKA (defined by pH < 7.2), regular insulin should be administered in the form of continuous intravenous infusion (0.05-0.1 units/kg/hour)[54,55]. Insulin boluses should be avoided, as they can increase the risk of cerebral edema and can aggravate hypokalemia. The insulin infusion should be continued until the resolution of acidosis.
Hypoglycemia prevention: When blood glucose levels reach below 250 mg/dL (14 mmol/L), dextrose should be added to the intravenous fluids without changing the insulin dose. A “two-bag” fluid system can help titrate the patient’s blood glucose by changing the rates of two types of fluid. One bag includes 0.9% or 0.45% saline with 10% dextrose, and the other contains 0.9% or 0.45% saline but no dextrose[56]. For prevention of hypoglycemia, the dextrose-containing fluid is infused when blood glucose level reaches below 250 mg/dL and adjusted to keep blood glucose levels between 150 and 250 mg/dL. To maintain the total fluid infusion rate constant, the rate of the fluid without dextrose is reduced accordingly. Alternatively, the dextrose content of the fluid can be steadily increased by changing the amount of dextrose as the patient’s blood glucose level falls. If the blood glucose level continues to decrease despite the infusion of 10% dextrose, the insulin infusion dose may be reduced to 0.05 units/kg/hour.
A significant proportion of children hospitalized with DKA experience recurrent episodes[57]. Consequently, it becomes important to educate patients suffering these events to identify triggers and prevent recurrence. Insulin omission, either inadvertently or deliberately, is the cause in most cases. It can be prevented by comprehensive programs that provide education, psychosocial evaluation and treatment under adult supervision[55].
A comparison of major pediatric DKA management guidelines from different countries is provided in Table 2[55,58,59].
| Management aspects | ISPAD Guidelines | BSPED (United Kingdom) | CPS (Canada) |
| Fluid therapy (initial bolus) | Recommends 0.45% or 0.9% NaCl or balanced salt solutions. Infuse fluids (10 mL/kg) over 30-60 minutes for those not in shock; may repeat1 | Infuse 10 mL/kg bolus over 60 minutes to all patients managed with i.v. fluids (not in shock) | Administer 10-20 mL/kg (to a maximum 1000 mL) of isotonic fluid with 0.9% NaCl or a balanced crystalloid for all patients over 20-30 minutes |
| Insulin administration | Continuous insulin infusion at 0.05-0.1 unit/kg/hour starting 1 hour after fluids initiated (0.05 units/kg/hour for mild DKA) | Rate of 0.1 units/kg/hour | Rate of 0.05-0.1 units/kg/hour similar to ISPAD but recommends decreasing to 0.025 units/kg/hour if BG falls rapidly or as a bridge to s.c. insulin |
| Electrolyte management | Start replacing potassium after initial volume expansion and concurrent with starting insulin therapy2; The starting potassium concentration in the infusate should be 40 mmol/L | Use 0.9% NaCl with 20 mmol KCl in 500 mL (or 40 mmol in a L) | Supplemental potassium of at least 40 mmol/L should be added to i.v. fluids when potassium is < 5 mmol/L and after recent urine output is documented |
| Glucose introduction | Suggests adding 5% dextrose before glucose levels fall to 17 mmol/L if dropping rapidly or at 14-17 mmol/L | Use glucose-containing fluids once plasma glucose drops to < 14 mmol/L | Dextrose (usually 5%) should be added when glucose level is between 15 and 17 mmol/L. |
Controversies in DKA management: Fluid therapy in the management of childhood DKA has been controversial because of the rare but dreaded complication of cerebral edema. Over the decades, the frequency of cerebral edema has remained stable despite changes in DKA management protocols[53]. Early studies demonstrated a connection between faster rates of fluid infusion and poor neurologic outcomes, prompting treatment algorithms cantered on slow fluid infusion. Kuppermann et al[50] reported a randomized controlled trial (RCT) in over 1200 children comparing rapid vs slow fluid infusion with either 0.9% NaCl or 0.45% NaCl. The authors concluded that neither the rehydration rate nor fluid composition impacted the rates of development of clinically apparent cerebral edema or occurrence of adverse neurological outcomes. Being the largest study on this issue in children to date, it provides strong evidence that the range of fluid rates and volume administered in the PECARN study protocol can be safely used in DKA management. However, its relevance in resource-poor settings with more severe disease remains uncertain.
Another frequently held belief is that bicarbonate has a role in DKA and should be used to buffer severe acidosis. Nevertheless, there are not any studies that demonstrate a benefit of bicarbonate use in children. A multi-center case-control study reported that children who received bicarbonate therapy were more likely to develop cerebral edema[60]. However, the relationship between the two are unclear[18]. Current guidelines advocate against the routine use of bicarbonate.
Plasma phosphate levels decrease after the initiation of DKA treatment, and this is aggravated by insulin, which promotes entry of phosphate into cells. Older studies on the utility of phosphate replacement in these patient failed to show any beneficial effect; therefore, the routine of phosphate replacement is not recommended[61,62]. However, ISPAD recognized that these studies had limited pediatric populations and thus were not adequately powered. Owing to the risk for hypophosphatemia, particularly in severe DKA, and the potentially detrimental effects if significantly low (plasma phosphate < 1 mg/dL), ISPAD recommends phosphate replacement if available[55]. However, phosphate administration may induce hypocalcemia, and careful monitoring of serum calcium should be performed.
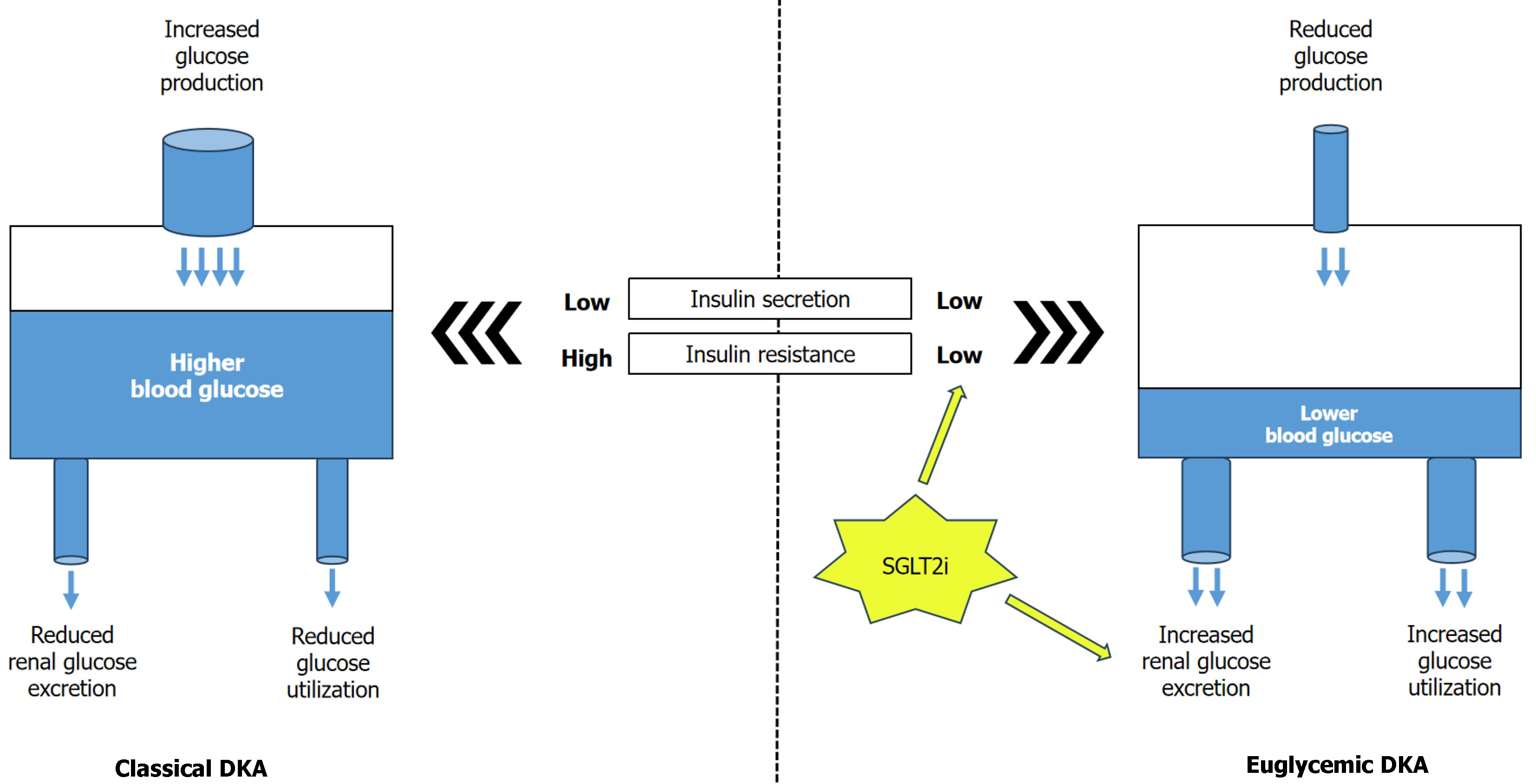
A subgroup of patients with DKA may have normal blood glucose, termed euDKA, which is characterized by relative euglycemia (blood glucose < 250 mg/dL) with metabolic acidosis (serum bicarbonate < 18 mEq/L and pH < 7.3) and ketosis[63-65]. The recognition and incidence of euDKA have recently increased, specifically with the use of SGLT2i in insulin deficient patients with long-standing T2D, T1D, or latent autoimmune diabetes[66-68]. Approximately 2.6%-7% of DKA admissions are euglycemic, and SGLT-2i is associated with an increased risk for DKA, particularly with euDKA[69-72]. Very recently, a retrospective study identified 510 adults; in nearly 11% of these cases, DKA was euglycemic[73]. The euglycemic incidence of DKA remarkably increased over the course of the study, from 7% of patients with DKA in 2018 to 16% in 2023. SGLT-2i use before DKA admission was significantly more common in patients with euDKA than with traditional DKA (44% vs 3%). Notably, individuals with euDKA had a considerably longer time to DKA diagnosis and longer duration of DKA compared with hyperglycemic DKA. However, this phenomenon can develop in the absence of diabetes in settings like pregnancy, restricted carbohydrate intake or starvation, or defects in gluconeogenesis (alcohol misuse or chronic liver disease) or glycogen storage diseases and pancreatitis[63]. In euDKA, insulin deficiency and insulin resistance are often milder, thereby limiting the surge in blood glucose levels. Therefore, glucose overproduction and underutilization are less prevalent than in traditional DKA. The difference in the pathophysiology of hyperglycemic DKA vs euDKA is represented in Figure 4.
Patients with diabetes or with other conditions that predispose them to euDKA and their physicians should be made aware about the triggering factors and how to avoid them. They should be able to promptly recognize signs and symptoms of ketoacidosis and be instructed that ketoacidosis can occur at any glycemic level. Administration of insulin just prior to arrival to the hospital can lead to blood glucose < 200 mg/dL in the presence of DKA. People with diabetes who use interstitial glucose sensors may fail to recognize incipient euDKA by misleading glucose values within or close to target range. To diminish the risk of SGLT-2i-associated euDKA, international position statement[66] recommends: (1) Discontinuation of SGLT-2i at least 24 hours before elective surgery, planned invasive procedures, or expected strenuous physical activity; (2) Immediately stopping for emergency surgery or any severe stressful event or any acute illness; (3) Stopping insulin or reducing the dose should be avoided; and (4) Avoid alcohol abuse and diet very low in carbohydrate or ketogenic diets. Patients should perform self-testing for ketones, preferably blood ketone if experiencing nausea, vomiting, abdominal discomfort, or fatigue. In the event of elevated ketones, the STICH protocol (STop SGLT-2 inhibitor, Insulin administration, Carbohydrate consumption, Hydration) should be initiated[74]. Management of diabetes in pregnancy, especially in the last trimester, is crucial to prevent the development of DKA/euDKA.
The approach to managing euDKA in individuals with diabetes is similar to that of hyperglycemic[37]. At first, treatment is directed towards volume replacement and restoration of adequate renal perfusion. Initial fluid therapy should be followed by i.v insulin administration by continuous infusion. Because patients in euDKA have blood glucose levels < 250 mg/dL, commencing the insulin infusion at a rate of 0.05-0.1 units/kg/hour may be considered[65]. Dextrose-containing fluids should be started early in these patients to avoid hypoglycemia due to insulin infusion with the target blood glucose levels being 150-200 mg/dL. Dextrose 5% should be administered concurrently with i.v. insulin. Dextrose 10% is recommended if hypoglycemia occurs despite infusion of dextrose 5%. In cases of euDKA in individuals without diabetes, i.v. insulin administration is not required, whereas fluid resuscitation, correction of electrolyte disturbances and dextrose-containing fluid are adequate for acidosis resolution[75].
Clinicians should maintain heightened awareness of possible DKA in patients with diabetes who present with acidosis and euglycemia, especially in those who take SGLT-2i. This atypical presentation may pose a diagnostic dilemma and resultant delay in clinical recognition, diagnosis and management of patients may lead to disease progression and potential serious adverse outcomes.
DKA and hyperosmolar hyperglycemic state (HHS) are the two most common life-threatening acute complications of uncontrolled diabetes. Traditionally, HHS and DKA had been considered as two distinct clinical entities. In addition to hyperglycemia, metabolic acidosis and ketosis are characteristic of DKA, whereas in HHS extreme hyperglycemia, hyperosmolarity and severe dehydration are major complications. As both disorders have similar pathophysiological bases, the overlap of DKA and HHS (DKA-HHS) has been seen clinically in up to 30% of cases[76,77]. Multi-organ dysfunctions like acute kidney injury, pancreatitis, rhabdomyolysis, and cardiac arrythmias were commonly seen in this disorder[78]. The mortality rate of DKA-HHS overlap had been reported to be higher than individual entities[77,79]. Thus, early diagnosis and aggressive management is warranted in patients with DKA-HHS overlap. There is no clear guideline regarding the management of DKA-HHS overlap. The diagnosis of DKA-HHS overlap is usually considered if there is presence of all of the following: Marked hypovolemia, ketonemia (> 3 mmol/L), metabolic acidosis (pH < 7.3; bicarbonate < 15-18 mEq/L), severe hyperglycemia (blood glucose > 600 mg/dL) and increased serum osmolarity (> 300 mOsm/kg)[77,80,81]. As the complication rate is higher in this mixed disorder, patients should be ideally managed in critical care settings. Thorough clinical and laboratory assessment, along with identification and management of precipitating causes, should be performed. Replenishment of intravascular volume deficit is the first treatment for patients with DKA-HHS overlap. Like the management protocol of isolated HHS, the target of intravenous fluid therapy is to reach a positive fluid balance of 3-6 L by the first 12 hours of therapy[81]. However, intravenous insulin therapy should not be delayed, and it should be started along with the commencement of intravenous hydration (unlike the HHS management protocol of delaying insulin therapy till correction of hypovolemia). The purpose of early initiation of insulin therapy is to suppress the ketogenesis. Thus, higher rate of FRIII of 0.1 unit/kg/hour, similar to the DKA treatment protocol, is also recommended in DKA-HHS[81]. Higher chance of hyperkalaemia along with increased risk of development of hypokalaemia within 48 hours of admission had been reported in patients with DKA-HHS[77]. Frequent monitoring of serum potassium should be done along with routine supplementation of potassium as per standard DKA protocol. The key points for management of atypical forms of DKA has been summarized in Table 3.
| Disease | Key management principles |
| euDKA | Physicians should have heightened awareness of this nuanced presentation of euDKA |
| To minimize the risk of euDKA associated with SGLT-2 inhibitors, the recommendations from international societies should be followed | |
| To have a low threshold for obtaining ketone levels in diabetic patients with unexplained acidosis, even in absence of significant hyperglycemia | |
| Insulin dose reduction should be achieved by slow, gentle decrements simultaneously to avoid hypoglycemia and sliding toward euDKA | |
| Dextrose containing fluids should be used early in these patients to avoid hypoglycemia due to insulin infusion | |
| In case of euDKA in non-diabetic individuals, insulin infusion is not necessary, whereas fluid replacement and intravenous glucose solution are sufficient for the resolution of acidosis | |
| Diabetic ketoacidosis and hyperosmolar hyperglycemic state overlap | Aggressive hydration is needed in first 12 hours to maintain a positive fluid balance (like management of HHS) |
| Higher dose of fixed rate intravenous insulin infusion (0.1 units/kg/hour) is preferred (like management of DKA) | |
| Early initiation of insulin infusion along with i.v. fluid therapy (like management of DKA) | |
| Frequent monitoring of serum potassium should be done along with routine supplementation of potassium | |
| Identification and management of precipitating causes |
In high-risk patients, the management of DKA can be challenging and special patient related factors should obtain more watchful attention and consideration. Factors such as pregnancy, kidney disease, cardiac illness, older age, and use of SGLT-2i, all influence the approach to treatment and require customized management strategies. Children require specialized treatment and care during a DKA episode due to its long-term neurological and cognitive sequelae. The landmark PECARN study showed a limited role for fluid composition and rate in development of cerebral edema in pediatric DKA. Nevertheless, there is not enough evidence to determine the best fluid therapy for DKA treatment in children and consequently there is an urgent need for more RCTs. Contemporary guidelines usually lack sufficient recommendations about the approach to manage complex patients with specific conditions and comorbidities. Moreover, controversies persist around certain aspects of DKA management, and further investigations are necessary to determine the best management options. Future studies should also prospectively evaluate standard care for atypical presentations like DKA-HHS overlap. Innovations in fluid management and insulin delivery and more personalized management approaches are critical areas for ongoing research, as they could improve patient outcomes, minimize complications, and transform DKA treatment. This article aimed to increase awareness regarding these important issues and urge upon additional studies, standardization, and exploring the promising role of artificial intelligence in early diagnosis and protocol adherence to optimize care for patients with DKA in special conditions.
| 1. | Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 2. | Ooi E, Nash K, Rengarajan L, Melson E, Thomas L, Johnson A, Zhou D, Wallett L, Ghosh S, Narendran P, Kempegowda P. Clinical and biochemical profile of 786 sequential episodes of diabetic ketoacidosis in adults with type 1 and type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2021;9:e002451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | McCoy RG, Herrin J, Galindo RJ, Swarna KS, Umpierrez GE, Golden SH, O'Connor PJ. Rates of Hypoglycemic and Hyperglycemic Emergencies Among U.S. Adults With Diabetes, 2011-2020. Diabetes Care. 2023;46:e69-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in Diabetic Ketoacidosis Hospitalizations and In-Hospital Mortality - United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2018;67:362-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (40)] |
| 5. | Dhatariya KK, Skedgel C, Fordham R. The cost of treating diabetic ketoacidosis in the UK: a national survey of hospital resource use. Diabet Med. 2017;34:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Poovazhagi V. Risk factors for mortality in children with diabetic keto acidosis from developing countries. World J Diabetes. 2014;5:932-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 7. | Dhatariya KK; Joint British Diabetes Societies for Inpatient Care. The management of diabetic ketoacidosis in adults-An updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet Med. 2022;39:e14788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 8. | Umpierrez GE, Davis GM, ElSayed NA, Fadini GP, Galindo RJ, Hirsch IB, Klonoff DC, McCoy RG, Misra S, Gabbay RA, Bannuru RR, Dhatariya KK. Hyperglycemic Crises in Adults With Diabetes: A Consensus Report. Diabetes Care. 2024;47:1257-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 128] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 9. | El-Remessy AB. Diabetic Ketoacidosis Management: Updates and Challenges for Specific Patient Population. Endocrines. 2022;3:801-812. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Hurtado CR, Lemor A, Vallejo F, Lopez K, Garcia R, Mathew J, Galindo RJ. Causes And Predictors For 30-Day Re-Admissions In Adult Patients With Diabetic Ketoacidosis In The United States: A Nationwide Analysis, 2010-2014. Endocr Pract. 2019;25:242-253. [PubMed] [DOI] [Full Text] |
| 11. | Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Al Sadhan A, ElHassan E, Altheaby A, Al Saleh Y, Farooqui M. Diabetic Ketoacidosis in Patients with End-stage Kidney Disease: A Review. Oman Med J. 2021;36:e241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Eledrisi MS, Beshyah SA, Malik RA. Management of diabetic ketoacidosis in special populations. Diabetes Res Clin Pract. 2021;174:108744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Galindo RJ, Pasquel FJ, Fayfman M, Tsegka K, Dhruv N, Cardona S, Wang H, Vellanki P, Umpierrez GE. Clinical characteristics and outcomes of patients with end-stage renal disease hospitalized with diabetes ketoacidosis. BMJ Open Diabetes Res Care. 2020;8:e000763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Seddik AA, Bashier A, Alhadari AK, AlAlawi F, Alnour HH, Bin Hussain AA, Frankel A, Railey MJ. Challenges in management of diabetic ketoacidosis in hemodialysis patients, case presentation and review of literature. Diabetes Metab Syndr. 2019;13:2481-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Galindo RJ, Pasquel FJ, Vellanki P, Zambrano C, Albury B, Perez-Guzman C, Ziduo Z, Umpierrez GE. Biochemical Parameters of Diabetes Ketoacidosis in Patients with End-stage Kidney Disease and Preserved Renal Function. J Clin Endocrinol Metab. 2021;106:e2673-e2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Stathi D, Dhatariya KK, Mustafa OG. Management of diabetes-related hyperglycaemic emergencies in advanced chronic kidney disease: Review of the literature and recommendations. Diabet Med. 2025;42:e15405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Chua HR, Schneider A, Bellomo R. Bicarbonate in diabetic ketoacidosis - a systematic review. Ann Intensive Care. 2011;1:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 510] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | Agarwal MA, Jain N, Podila PSB, Varadarajan V, Patel B, Shah M, Garg L, Khouzam RN, Ibebuogu U, Reed GL, Dagogo-Jack S. Association of history of heart failure with hospital outcomes of hyperglycemic crises: Analysis from a University hospital and national cohort. J Diabetes Complications. 2020;34:107466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Al-Mallah M, Zuberi O, Arida M, Kim HE. Positive troponin in diabetic ketoacidosis without evident acute coronary syndrome predicts adverse cardiac events. Clin Cardiol. 2008;31:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Barski L, Golbets E, Jotkowitz A, Schwarzfuchs D. Management of diabetic ketoacidosis. Eur J Intern Med. 2023;117:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Philip L, Poole R. Double trouble: managing diabetic emergencies in patients with heart failure. Pract Diabetes. 2018;35:139-143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Ma LP, Liu X, Cui BC, Liu Y, Wang C, Zhao B. Diabetic Ketoacidosis With Acute Pancreatitis in Patients With Type 2 Diabetes in the Emergency Department: A Retrospective Study. Front Med (Lausanne). 2022;9:813083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol. 2000;95:2795-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Attar BM, Hinami K, Jaiswal P, Yap JE, Jaiswal R, Devani K, Simons-Linares CR, Demetria MV. Concurrent Diabetic Ketoacidosis in Hypertriglyceridemia-Induced Pancreatitis: How Does It Affect the Clinical Course and Severity Scores? Pancreas. 2017;46:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Li L, Li L. Risk factors for diabetic ketoacidosis in acute pancreatitis patients with type 2 diabetes. BMC Gastroenterol. 2023;23:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Simons-Linares CR, Jang S, Sanaka M, Bhatt A, Lopez R, Vargo J, Stevens T, Chahal P. The triad of diabetes ketoacidosis, hypertriglyceridemia and acute pancreatitis. How does it affect mortality and morbidity?: A 10-year analysis of the National Inpatient Sample. Medicine (Baltimore). 2019;98:e14378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Nair S, Pitchumoni CS. Diabetic ketoacidosis, hyperlipidemia, and acute pancreatitis: the enigmatic triangle. Am J Gastroenterol. 1997;92:1560-1561. [PubMed] |
| 30. | Rizvi AA. Serum amylase and lipase in diabetic ketoacidosis. Diabetes Care. 2003;26:3193-3194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Yuan S, Liao J, Cai R, Xiong Y, Zhan H, Zheng Z. Acute pancreatitis concomitant with diabetic ketoacidosis: a cohort from South China. J Int Med Res. 2020;48:300060520912128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Bak LB. Diabetic Ketoacidosis Related to Hypertriglyceridemia-Induced Pancreatitis: A Case Report. AACN Adv Crit Care. 2023;34:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Sabina M, Rigdon A, Tsai J. Diagnostic and Therapeutic Ambiguities in Diabetic Ketoacidosis With Overlapping Acute Pancreatitis and Hypertriglyceridemia. Cureus. 2024;16:e57508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 201: Pregestational Diabetes Mellitus. Obstet Gynecol. 2018;132:e228-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 35. | Dhanasekaran M, Mohan S, Erickson D, Shah P, Szymanski L, Adrian V, Egan AM. Diabetic Ketoacidosis in Pregnancy: Clinical Risk Factors, Presentation, and Outcomes. J Clin Endocrinol Metab. 2022;107:3137-3143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Dashora U, Levy N, Dhatariya K, Willer N, Castro E, Murphy HR; Joint British Diabetes Societies In Patient group. Managing hyperglycaemia during antenatal steroid administration, labour and birth in pregnant women with diabetes - an updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet Med. 2022;39:e14744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1261] [Article Influence: 74.2] [Reference Citation Analysis (4)] |
| 38. | Lauenborg J, Mathiesen E, Ovesen P, Westergaard JG, Ekbom P, Mølsted-Pedersen L, Damm P. Audit on stillbirths in women with pregestational type 1 diabetes. Diabetes Care. 2003;26:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Ringholm L, Søholm JC, Pedersen BW, Clausen TD, Damm P, Mathiesen ER. Glucose Control During Labour and Delivery in Type 1 Diabetes - An Update on Current Evidence. Curr Diab Rep. 2024;25:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Beunen K, Gillard P, Van Wilder N, Ballaux D, Vanhaverbeke G, Taes Y, Aers XP, Nobels F, Van Huffel L, Marlier J, Lee D, Cuypers J, Preumont V, Siegelaar SE, Painter RC, Laenen A, Mathieu C, Benhalima K. Advanced Hybrid Closed-Loop Therapy Compared With Standard Insulin Therapy Intrapartum and Early Postpartum in Women With Type 1 Diabetes: A Secondary Observational Analysis From the CRISTAL Randomized Controlled Trial. Diabetes Care. 2024;47:2002-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Schwarzfuchs D, Rabaev E, Sagy I, Zimhony-Nissim N, Lipnitzki I, Musa H, Jotkowitz A, Brandstaetter E, Barski L. Clinical and Epidemiological Characteristics of Diabetic Ketoacidosis in Older Adults. J Am Geriatr Soc. 2020;68:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Pannu AK, Kiran R, Kumar A, Sharda SC, Bhatia M, Saroch A, Dutta P, Sharma N. Comparative study of diabetic ketoacidosis in the elderly and non-elderly patients: A nine-year experience from an academic hospital in North India. Diabetes Metab Syndr. 2023;17:102903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Sehgal V, Ulmer B. Clinical Conundrums in the Management of Diabetic Ketoacidosis in the Elderly. J Transl Int Med. 2019;7:10-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. 2019;63:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 45. | Pfützner A, Klonoff D, Heinemann L, Ejskjaer N, Pickup J. Euglycemic ketosis in patients with type 2 diabetes on SGLT2-inhibitor therapy-an emerging problem and solutions offered by diabetes technology. Endocrine. 2017;56:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Gershkovich B, English SW, Doyle MA, Menon K, McIntyre L. Choice of crystalloid fluid in the treatment of hyperglycemic emergencies: a systematic review protocol. Syst Rev. 2019;8:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Ghetti S, Lee JK, Sims CE, Demaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 48. | Sottosanti M, Morrison GC, Singh RN, Sharma AP, Fraser DD, Alawi K, Seabrook JA, Kornecki A. Dehydration in children with diabetic ketoacidosis: a prospective study. Arch Dis Child. 2012;97:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Evidence reviews for fluid therapy for the management of diabetic ketoacidosis: Diabetes (type 1 and type 2) in children and young people: diagnosis and management: Evidence review A. London: National Institute for Health and Care Excellence (NICE); 2020 Dec- . [PubMed] |
| 50. | Kuppermann N, Ghetti S, Schunk JE, Stoner MJ, Rewers A, McManemy JK, Myers SR, Nigrovic LE, Garro A, Brown KM, Quayle KS, Trainor JL, Tzimenatos L, Bennett JE, DePiero AD, Kwok MY, Perry CS 3rd, Olsen CS, Casper TC, Dean JM, Glaser NS; PECARN DKA FLUID Study Group. Clinical Trial of Fluid Infusion Rates for Pediatric Diabetic Ketoacidosis. N Engl J Med. 2018;378:2275-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 51. | Yung M, Letton G, Keeley S. Controlled trial of Hartmann's solution versus 0.9% saline for diabetic ketoacidosis. J Paediatr Child Health. 2017;53:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Williams V, Jayashree M, Nallasamy K, Dayal D, Rawat A. 0.9% saline versus Plasma-Lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial. Crit Care. 2020;24:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 53. | Agwu JC, Ng SM. Fluid and electrolyte therapy in childhood diabetic ketoacidosis management: A rationale for new national guideline. Diabet Med. 2021;38:e14595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Libman I, Haynes A, Lyons S, Pradeep P, Rwagasor E, Tung JY, Jefferies CA, Oram RA, Dabelea D, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2022: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2022;23:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 55. | Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, Sperling MA, Codner E. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19 Suppl 27:155-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 434] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 56. | Tzimenatos L, Nigrovic LE. Managing Diabetic Ketoacidosis in Children. Ann Emerg Med. 2021;78:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Mays JA, Jackson KL, Derby TA, Behrens JJ, Goel S, Molitch ME, Kho AN, Wallia A. An Evaluation of Recurrent Diabetic Ketoacidosis, Fragmentation of Care, and Mortality Across Chicago, Illinois. Diabetes Care. 2016;39:1671-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 58. | Gripp KE, Trottier ED, Thakore S, Sniderman J, Lawrence S. Current recommendations for management of paediatric diabetic ketoacidosis. Paediatr Child Health. 2023;28:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Rugg-Gunn CE, Deakin M, Hawcutt DB. Update and harmonisation of guidance for the management of diabetic ketoacidosis in children and young people in the UK. BMJ Paediatr Open. 2021;5:e001079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, Kaufman F, Quayle K, Roback M, Malley R, Kuppermann N; Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 471] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 61. | Fisher JN, Kitabchi AE. A randomized study of phosphate therapy in the treatment of diabetic ketoacidosis. J Clin Endocrinol Metab. 1983;57:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 121] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 62. | Wilson HK, Keuer SP, Lea AS, Boyd AE 3rd, Eknoyan G. Phosphate therapy in diabetic ketoacidosis. Arch Intern Med. 1982;142:517-520. [PubMed] |
| 63. | Bonora BM, Avogaro A, Fadini GP. Euglycemic Ketoacidosis. Curr Diab Rep. 2020;20:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Jenkins D, Close CF, Krentz AJ, Nattrass M, Wright AD. Euglycaemic diabetic ketoacidosis: does it exist? Acta Diabetol. 1993;30:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Modi A, Agrawal A, Morgan F. Euglycemic Diabetic Ketoacidosis: A Review. Curr Diabetes Rev. 2017;13:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (5)] |
| 66. | Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G, LeRoith D, Umpierrez GE, Weir MR. American Association Of Clinical Endocrinologists And American College Of Endocrinology Position Statement On The Association Of Sglt-2 Inhibitors And Diabetic Ketoacidosis. Endocr Pract. 2016;22:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 67. | Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, Bogle A, Iqbal N, List J, Griffen SC. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 68. | Douros A, Lix LM, Fralick M, Dell'Aniello S, Shah BR, Ronksley PE, Tremblay É, Hu N, Alessi-Severini S, Fisher A, Bugden SC, Ernst P, Filion KB; Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis : A Multicenter Cohort Study. Ann Intern Med. 2020;173:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 69. | Goldenberg RM, Berard LD, Cheng AYY, Gilbert JD, Verma S, Woo VC, Yale JF. SGLT2 Inhibitor-associated Diabetic Ketoacidosis: Clinical Review and Recommendations for Prevention and Diagnosis. Clin Ther. 2016;38:2654-2664.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 70. | Liu J, Li L, Li S, Wang Y, Qin X, Deng K, Liu Y, Zou K, Sun X. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 71. | Long B, Lentz S, Koyfman A, Gottlieb M. Euglycemic diabetic ketoacidosis: Etiologies, evaluation, and management. Am J Emerg Med. 2021;44:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 72. | Rosenstock J, Ferrannini E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care. 2015;38:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 455] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 73. | Schutte B, Savage K, Merwin M, Morris M, Geringer R, Dilsaver DB, Plambeck RW, Jagan N. Euglycemic diabetic ketoacidosis: Rising incidence, diagnostic delays, and the impact of SGLT2 inhibitors in hospitalized patients. J Hosp Med. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Garg SK, Peters AL, Buse JB, Danne T. Strategy for Mitigating DKA Risk in Patients with Type 1 Diabetes on Adjunctive Treatment with SGLT Inhibitors: A STICH Protocol. Diabetes Technol Ther. 2018;20:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 75. | Le Neveu F, Hywel B, Harvey JN. Euglycaemic ketoacidosis in patients with and without diabetes. Pract Diabetes. 2013;30:167-171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | MacIsaac RJ, Lee LY, McNeil KJ, Tsalamandris C, Jerums G. Influence of age on the presentation and outcome of acidotic and hyperosmolar diabetic emergencies. Intern Med J. 2002;32:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Pasquel FJ, Tsegka K, Wang H, Cardona S, Galindo RJ, Fayfman M, Davis G, Vellanki P, Migdal A, Gujral U, Narayan KMV, Umpierrez GE. Clinical Outcomes in Patients With Isolated or Combined Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State: A Retrospective, Hospital-Based Cohort Study. Diabetes Care. 2020;43:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 78. | Brar PC, Tell S, Mehta S, Franklin B. Hyperosmolar diabetic ketoacidosis-- review of literature and the shifting paradigm in evaluation and management. Diabetes Metab Syndr. 2021;15:102313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 79. | Dhatariya K, Mustafa O, Stathi D. Hyperglycemic Crises. 2025 Jun 10. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. [PubMed] |
| 80. | Hassan EM, Mushtaq H, Mahmoud EE, Chhibber S, Saleem S, Issa A, Nitesh J, Jama AB, Khedr A, Boike S, Mir M, Attallah N, Surani S, Khan SA. Overlap of diabetic ketoacidosis and hyperosmolar hyperglycemic state. World J Clin Cases. 2022;10:11702-11711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (6)] |
| 81. | Mustafa OG, Haq M, Dashora U, Castro E, Dhatariya KK; Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Management of Hyperosmolar Hyperglycaemic State (HHS) in Adults: An updated guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Diabet Med. 2023;40:e15005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/