Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.107871
Revised: July 13, 2025
Accepted: August 20, 2025
Published online: September 15, 2025
Processing time: 165 Days and 17.1 Hours
Gestational diabetes mellitus (GDM) poses a substantial health risk during preg
To assess the efficacy of MI in preventing and treating GDM, providing evidence-based guidance for clinical practice.
A systematic review was conducted on studies published on the PubMed, Web of Science, and Embase databases from their inception date to July 2024. Twelve studies encompassing 9018 patients were included in the meta-analysis using fixed-effect and random-effects models. Heterogeneity was quantified with I2 statistics and the Cochrane Q test, and study quality was appraised using the A Measurement Tool to Assess Systematic Reviews 2 checklist.
MI significantly reduced GDM incidence [relative risk (RR): 0.37; 95% confidence interval (CI): 0.32-0.42], fasting blood glucose [standard mean differences (SMD): -1.31 mg/dL; 95%CI: -1.83 to
MI demonstrates therapeutic potential in GDM prevention and management, supporting its potential use as a preventive supplement in early pregnancy for high-risk women. Nonetheless, its therapeutic effects in women diagnosed with GDM require further validation.
Core Tip: This meta-analysis reviews evidence on using inositol supplements to prevent and treat gestational diabetes mellitus (GDM). Findings show it significantly lowers GDM rates, fasting blood sugar, and the risk of bad pregnancy outcomes. This suggests inositol is a safe and effective option. Specifically, myo-inositol supplements reduced new GDM cases by 63% and improved glucose tolerance test results. They also decreased chances of preterm birth and pregnancy-induced high blood pressure. While inositol works well for prevention in high-risk women, we need more trials to confirm its treatment benefits for existing GDM. Therefore, clinicians can consider inositol as a preventive measure early in pregnancy for women with risk factors.
- Citation: Wang RT, Feng YQ, Wang MY, Wei YH, Huang YJ, Guo YJ, Liu X, Lei XC, Huang KW, Huang H. Efficacy of inositol supplementation in the prevention and treatment of gestational diabetes mellitus: A meta-analysis. World J Diabetes 2025; 16(9): 107871
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/107871.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.107871
Gestational diabetes mellitus (GDM) is characterized by glucose intolerance that is first identified during pregnancy, with blood glucose levels exceeding the norm but not fulfilling the criteria for overt diabetes[1]. The prevalence of GDM is on the rise, affecting approximately 16.5% of all pregnancies worldwide, and is associated with significant health complications[2]. GDM heightens the risk of maternal complications, including gestational hypertension, preeclampsia, and the need for obstetric interventions such as cesarean sections[3,4]. Furthermore, offspring born to mothers with GDM are at an increased risk for various adverse outcomes later in life, including obesity, metabolic syndrome, type 2 diabetes, and cardiovascular diseases[3,5]. Lifestyle modifications and insulin therapy are recognized as the gold-standard inter
Inositol, a group of nine stereoisomers, plays a pivotal role in cellular signaling pathways, with myo-inositol (MI) and D-chiro-inositol (DCI) being the most biologically relevant. These isomers are interconvertible through the action of specific epimerase enzymes and function as second messengers in insulin signaling cascades, modulating insulin sensitivity and glucose homeostasis through the formation of inositol polyphosphates[9]. Empirical evidence suggests that inositol supplementation may ameliorate insulin resistance, thereby reducing the risk of GDM-related complications such as preeclampsia, preterm birth, macrosomia, and neonatal hypoglycemia[10-12]. However, the efficacy of inositol supplementation in the prevention and treatment of GDM remains controversial, with some randomized controlled trials (RCTs) reporting a significant reduction in the incidence of GDM in inositol-supplemented groups compared to controls[13-17]. In contrast, other studies, such as that of Fraticelli et al[14], have not observed such a protective effect. The differences in study outcomes highlight the necessity for further research involving diverse phenotypic and ethnic cohorts to elucidate the role of inositol in the prevention and treatment of GDM[18]. The current umbrella systematic analysis aims to critically assess the efficacy of inositol supplementation in the context of GDM prevention and treatment, providing a more nuanced understanding of its potential benefits and addressing the existing discrepancies in the literature.
This study adheres to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses framework[19]. All included studies adopted a randomized controlled design. The protocol for this systematic review and meta-analysis has been prospectively registered on the International Prospective Register of Systematic Reviews database (registration number: CRD42024572199), with no deviations from the pre-specified analysis plan.
A comprehensive literature search was conducted across the PubMed, Web of Science, and Embase databases to identify meta-analyses related to the prevention and treatment of GDM with inositol. The search was concluded on July 21, 2024, and was supplemented by a review of the reference lists of eligible studies to identify additional relevant publications. The search strategy incorporated a combination of Medical Subject Headings terms and keywords. The full methodology is detailed in Supplementary material.
The inclusion criteria were as follows: (1) Meta-analyses that evaluate the efficacy of oral inositol supplementation in the context of GDM prevention and management; (2) Studies involving pregnant women, including those diagnosed with GDM and those at risk for GDM or with normal glucose tolerance, excluding only those with a pre-existing history of diabetes; (3) Interventions where the treatment group receives inositol supplementation, with a placebo or no intervention as the control; and (4) Outcome measures including the primary outcome of GDM incidence, and secondary outcomes such as fasting blood glucose, 1-hour and 2-hour oral glucose tolerance tests (OGTT), preterm birth, ma
The methodological rigor and quality of the included meta-analyses were independently assessed by two reviewers (Wang RT and Wang MY) using the A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2) tool[20]. The AMSTAR 2 tool comprises 16 items, which are scored as “yes”, “partially”, “no”, or “not applicable”. In addition, the quality of evidence was categorized into four levels: “Very low”, “low”, “moderate”, and “high”, as displayed in Table 1.
| Ref. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Overall |
| Chan et al[21] | √ | × | √ | √ | √ | √ | √ | √ | × | √ | √ | √ | × | × | × | × | Moderate |
| Li and Shi[22] | √ | × | √ | √ | √ | √ | √ | × | × | √ | √ | √ | √ | √ | × | × | High |
| Greff et al[10] | √ | × | √ | × | √ | √ | √ | √ | × | √ | √ | √ | √ | × | × | × | High |
| Guo et al[23] | √ | × | √ | × | √ | × | × | × | × | √ | × | × | × | × | × | × | Low |
| Li and Fang[24] | √ | × | √ | × | × | × | × | × | × | √ | × | × | × | × | × | × | Low |
| Factor and Corpuz[25] | √ | × | √ | √ | √ | √ | √ | × | √ | √ | √ | √ | √ | × | √ | × | High |
| Liu and Liu[26] | √ | × | × | × | √ | √ | × | √ | × | √ | √ | √ | √ | × | × | × | Moderate |
| Mashayekh-Amiri et al[27] | √ | × | √ | × | √ | √ | × | √ | √ | √ | √ | √ | √ | √ | × | × | High |
| Vitagliano et al[28] | √ | × | × | × | √ | √ | × | √ | √ | √ | √ | × | × | × | × | × | Moderate |
| Wei et al[12] | √ | × | √ | × | × | × | × | √ | × | √ | √ | √ | × | √ | × | × | Low |
| Zhang et al[29] | √ | × | × | × | × | × | × | √ | × | √ | √ | × | × | × | × | × | Low |
| Zheng et al[30] | × | × | √ | × | √ | √ | √ | √ | × | √ | √ | × | × | √ | × | × | Moderate |
To address potential confounding factors, baseline characteristics (age, pre-pregnancy body mass index, and ethnicity) were extracted from the original studies. Group comparability was evaluated using standard mean differences (SMD) for continuous variables and χ2 tests for categorical variables. A threshold of SMD < 0.1 or P > 0.05 indicated a comparable distribution between intervention and control groups. These analyses were performed using STATA version 16.0 (StataCorp, College Station, TX, United States).
The initial screening of titles and abstracts was performed by two reviewers (Wang RT and Feng YQ) based on the predefined eligibility criteria. Full texts of potentially eligible studies were retrieved and independently evaluated by the same reviewers for final inclusion. Discrepancies were resolved through consensus or by consultation with a third reviewer (Huang KW). Data extracted from the included studies comprised publication year, sample size, geographical location of the study, duration of inositol supplementation, SMD, odds ratio (OR), relative risk (RR), and their corresponding 95% confidence interval (CI). Specifically, OR were converted to RR for consistency in data analysis.
Statistical analyses were conducted using STATA version 16.0. A fixed-effect model was employed when the I2 statistic did not exceed 50%, while a random-effects model was utilized when I2 surpassed 50% to account for heterogeneity between studies. This model assumes variability in effect sizes due to differences in study populations, interventions, and outcomes. The I2 statistic was used to quantify heterogeneity, and sensitivity analyses were performed to assess the robustness and stability of the meta-analysis results. All estimates were presented with 95%CI.
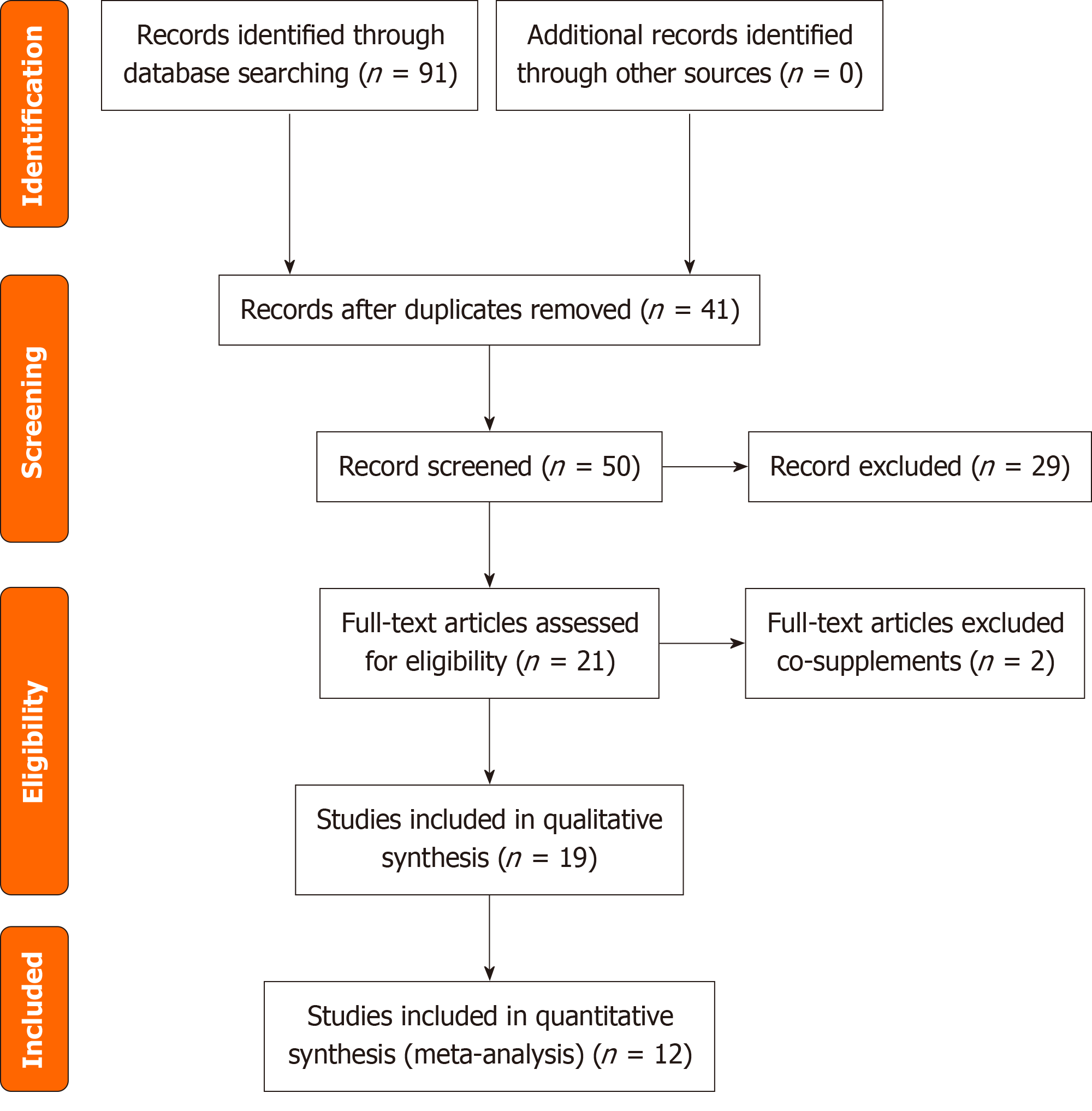
The initial database search yielded a total of 91 studies from PubMed (n = 26), Web of Science (n = 36), and Embase (n = 29), with 41 articles identified as duplicates. The titles and abstracts of the remaining 50 articles were thoroughly screened, and 29 were excluded, encompassing experimental papers, meta-analysis protocols, and related commentaries. The full texts of 21 articles were assessed, and two meta-analyses were excluded due to concerns regarding the authenticity and reliability of the data. Finally, 19 articles meeting the criteria were subjected to qualitative synthesis. In addition, a total of 12 studies were included in the quantitative synthesis, indicating the respective authors and pub
The curated selection of 12 studies comprised a cohort of 9018 female participants. These studies, which met the inclusion criteria, were published between 2015 and 2024. Geographically, the research was distributed across four nations: China (8 studies), Hungary (1 study), the Philippines (1 study), and Iran (1 study). Each included randomized RCTs that utilized inositol as an adjunct to standard and conventional pharmacological interventions for the prevention and treatment of gestational diabetes. Table 2 provides a detailed outline of the key characteristics of the studies incorporated into the meta-analysis. All included studies demonstrated comparable baseline characteristics between groups (age: SMD = 0.08, 95%CI: -0.03 to 0.19; body mass index: SMD = 0.06, 95%CI: -0.05 to 0.17). Detailed comparative data are shown in Table 3.
| Ref. | Participants and case | Study number | Therapy | Intervention time | Outcome | Quality |
| Chan et al[21] | MI and DCI 681 | 2 | 1-MI; 2-DCI | Early pregnancy1 | GDM rate; FPG; 1-hour OGTT; 2-hour OGTT | Yes (allocation, random, incomplete, selective); no (blinding); moderate |
| Li and Shi[22] | MI 1319 | 4 | 1-4 g MI + 400 mg FA; 2-2 g MI + 27.6 mg DCI + 400 μg FA; 3-3 g MI; 4-4 g MI + 400 μg FA | Early pregnancy | GDM rate; FPG; 1-hour OGTT; 2-hour OGTT; PIH; preterm birth; infants with neonatal hypoglycemia | Yes (allocation, random, incomplete); no (blinding, selective); low |
| Greff et al[10] | MI 1357 | 5 | 1-4 g MI + 400 μg FA; 2-1100 mg MI + 27.6 mg DCI; 3-4 g MI + 40 μg FA; 4-4 g MI + 200 μg FA; 5-1100 mg MI + 27.6 mg DCI + 400 μg FA | Early pregnancy | GDM rate; FPG; 1-hour OGTT; 2-hour OGTT; preterm birth; PIH; neonatal hypoglycemia | Yes (allocation, random, incomplete); no (blinding, selective); high |
| Guo et al[23] | MI 686 | 1 | 1-2 g MI + 200 mg FA | Early pregnancy | GDM rate | Yes (allocation, random, incomplete, selective); no (blinding); moderate |
| Li and Fang[24] | MI and DCI 1250 | 3 | 1-2 g MI + 200 μg FA; 2-4 g MI + 400 μg FA; 3-1100 mg MI + 27.6 g DCI + 400 μg FA | From the first prenatal visit to 11 weeks of pregnancy | GDM; 2-hour OGTT; preterm delivery | Yes (allocation, random, incomplete, selective, blinding); high |
| Factor and Corpuz[25] | MI and DCI 586 | 3 | 1-4 g MI + 400 mg FA; 2-2 g MI + 200 μg FA; 3-1100 mg MI + 27.6 mg DCI + 400 μg FA | From pre-pregnancy until delivery | GDM rate; cesarean section; PIH; preterm birth | Yes (allocation, random;); no (blinding, incomplete, selective); low |
| Liu and Liu[26] | MI 344 | 4 | 1-2 g MI + 200 mg FA; 2-4 g MI + 400 μg FA; 3-1100 mg MI + 27.6 g DCI; 4-4 g MI + 200 μg FA | Early pregnancy | GDM rate; 2-hour OGTT; preterm delivery; gestational age at birth; birth weight | Yes (allocation, random, incomplete, selective); no (blinding); moderate |
| Mashayekh-Amiri et al[27] | MI 720 | 1 | 1-2 g MI + 200 μg FA | 12-14 weeks of pregnancy | GDM rate; FPG; 1-hour OGTT; 2-hour OGTT; gestational hypertension; caesarian section; preterm delivery; macrosomia; neonatal hypoglycemia | Yes (allocation, random, selective); no (blinding, incomplete); low |
| Vitagliano et al[28] | MI and DCI 448 | 3 | 1-2 g MI + 200 μg FA; 2-2 g MI + 400 μg FA + 400 mg DCI; 3-1100 mg MI + 27.6 g DCI + 400 μg FA | From 13 to 24 weeks of pregnancy | GDM rate; FPG; 1-hour OGTT; 2-hour OGTT; hypertensive disorders; preterm delivery | Yes (allocation, random, incomplete, selective); no (blinding); moderate |
| Wei et al[12] | MI and DCI 671 | 4 | 1-2 g MI + 200 μg FA; 2-2 g MI + 27.6 mg DCI + 400 μg FA; 3-500 mg DCI + 400 μg FA; 4-0.55-4 g MI + 13.8 mg DCI + 200 μg FA | Early pregnancy | GDM rate; FPG; 1-hour OGTT; 2-hour OGTT | Yes (allocation, random, incomplete); no (blinding, selective); low |
| Zhang et al[29] | MI and DCI 432 | 2 | 1-1100 mg MI + 27.6 g DCI + 400 μg FA;2-2 g MI + 200 μg FA | Early pregnancy | GDM rate; preterm delivery; 2-hour OGTT; gestational age at birth; birth weight; macrosomia | Yes (allocation, random, selective); no (blinding, incomplete); low |
| Zheng et al[30] | MI 524 | 2 | 1-2 g MI + 200 mg FA; 2-4 g MI + 400 mg FA | 12-13 weeks | GDM rate; birth weight; FPG; 1-hour OTGG; 2-hour OTGG | Yes (allocation, random, incomplete, selective); no (blinding); moderate |
| Ref. | Intervention | Control | Age, years (I/C) | ΔAge | P value | BMI, kg/m2 (I/C) | ΔBMI | P value | Ethnicity match | Matched covariates |
| Chan et al[21] | 341 | 340 | 28.3 ± 3.1/28.1 ± 3.4 | +0.2 | 0.32 | 23.8 ± 2.9/23.5 ± 3.1 | +0.3 | 0.41 | Yes | Age, BMI |
| Li and Shi[22] | 659 | 660 | 29.2 ± 4.2/29.0 ± 4.5 | +0.2 | 0.18 | 24.1 ± 3.3/24.3 ± 3.0 | -0.2 | 0.25 | Yes | Age, BMI, ethnicity |
| Greff et al[10] | 679 | 678 | 30.1 ± 4.8/30.3 ± 5.1 | -0.2 | 0.27 | 25.2 ± 3.8/24.9 ± 3.5 | +0.3 | 0.13 | Yes | Age, BMI |
| Guo et al[23] | 343 | 343 | 27.8 ± 3.5/27.6 ± 3.8 | +0.2 | 0.42 | 24.3 ± 2.7/24.5 ± 2.9 | -0.2 | 0.38 | Yes | Age |
| Li and Fang[24] | 625 | 625 | 28.9 ± 4.1/28.7 ± 4.3 | +0.2 | 0.31 | 25.1 ± 3.4/24.8 ± 3.6 | +0.3 | 0.19 | Yes | Age, BMI, parity |
| Factor and Corpuz[25] | 293 | 293 | 31.2 ± 5.3/30.8 ± 5.1 | +0.4 | 0.23 | 28.4 ± 4.2/28.1 ± 4.5 | +0.3 | 0.34 | Yes | BMI, GA |
| Liu and Liu[26] | 172 | 172 | 26.7 ± 3.2/26.9 ± 3.4 | -0.2 | 0.57 | 23.9 ± 2.8/24.2 ± 2.6 | -0.3 | 0.22 | Yes | Age, weight |
| Mashayekh-Amiri et al[27] | 360 | 360 | 29.5 ± 4.4/29.3 ± 4.6 | +0.2 | 0.49 | 27.8 ± 3.9/27.5 ± 4.1 | +0.3 | 0.27 | Yes | Age, BMI, glucose |
| Vitagliano et al[28] | 224 | 224 | 31.6 ± 4.9/31.4 ± 5.2 | +0.2 | 0.65 | 26.7 ± 3.7/26.9 ± 3.8 | -0.2 | 0.51 | Yes | BMI, FHx |
| Wei et al[12] | 336 | 335 | 27.5 ± 3.8/27.8 ± 3.7 | -0.3 | 0.28 | 24.0 ± 3.1/24.3 ± 3.2 | -0.3 | 0.17 | Yes | Ethnicity |
| Zhang et al[29] | 216 | 216 | 28.4 ± 3.9/28.1 ± 4.0 | +0.3 | 0.36 | 23.7 ± 2.8/23.9 ± 2.7 | -0.2 | 0.42 | Yes | Parity, BMI |
| Zheng et al[30] | 262 | 262 | 26.3 ± 3.6/26.5 ± 3.4 | -0.2 | 0.47 | 23.5 ± 2.5/23.8 ± 2.6 | -0.3 | 0.15 | Yes | Pre-BMI |
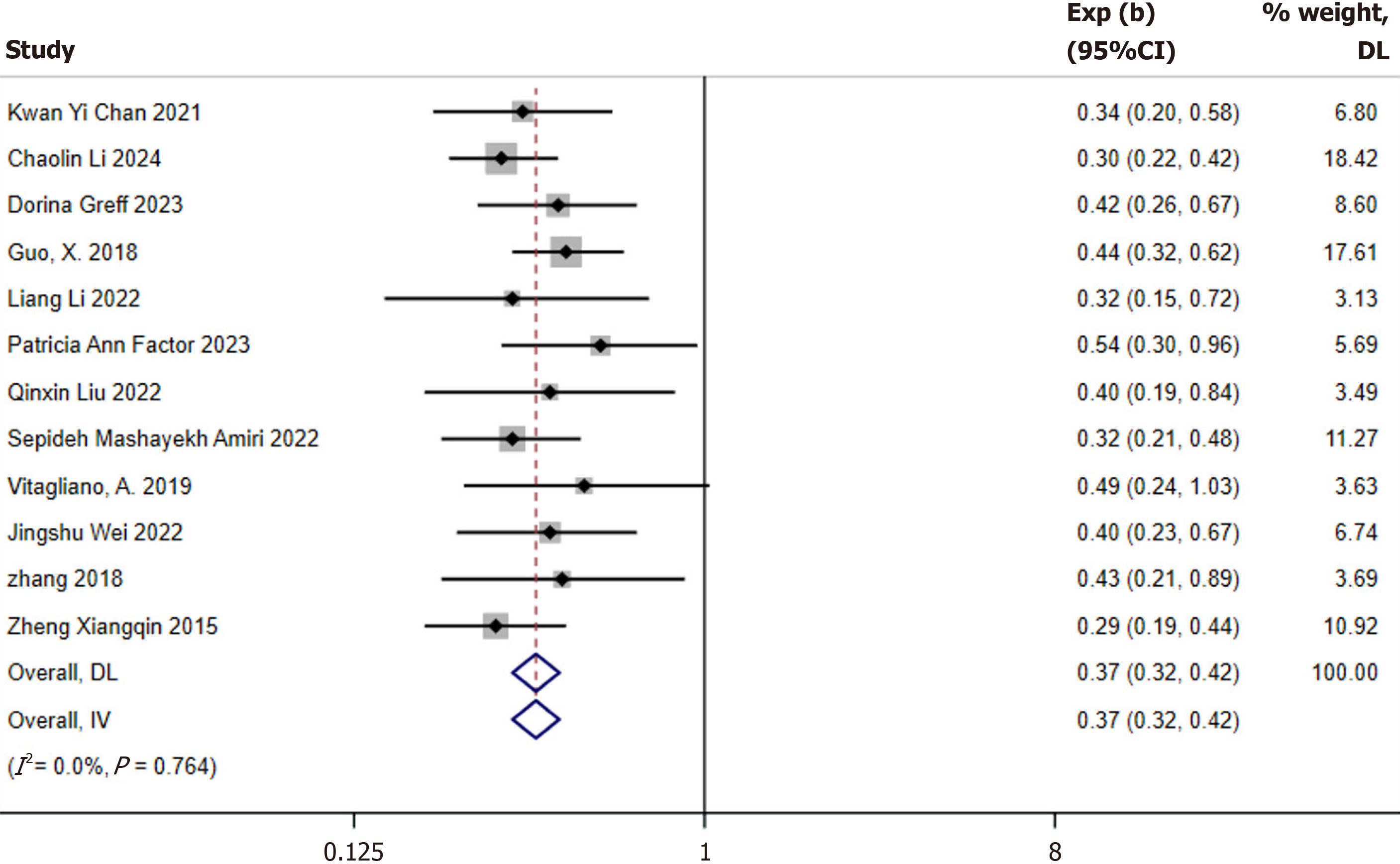
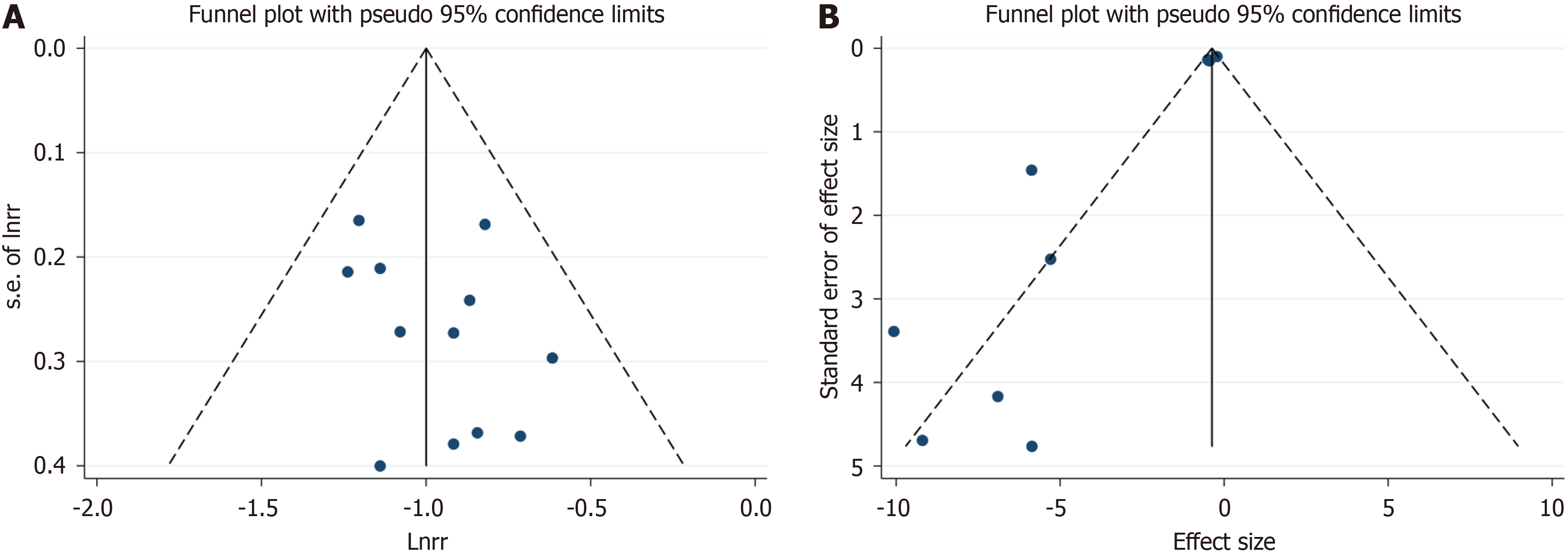
Incidence of GDM: After confirming baseline comparability, a meta-analysis was conducted on the 12 studies reporting the incidence of GDM[10,12,21-30]. Due to the limited number of studies and the inclusion of mixed inositol formulations, no subgroup analysis was performed based on inositol and DCI. A fixed-effects model was applied for the meta-analysis. The results revealed that inositol supplementation significantly reduced the incidence of GDM (RR: 0.37; 95%CI: 0.32-0.42). No significant heterogeneity was observed among the studies (I2 = 0%, P = 0.764; Figure 2). The funnel plot did not indicate the presence of publication bias (Figure 3).
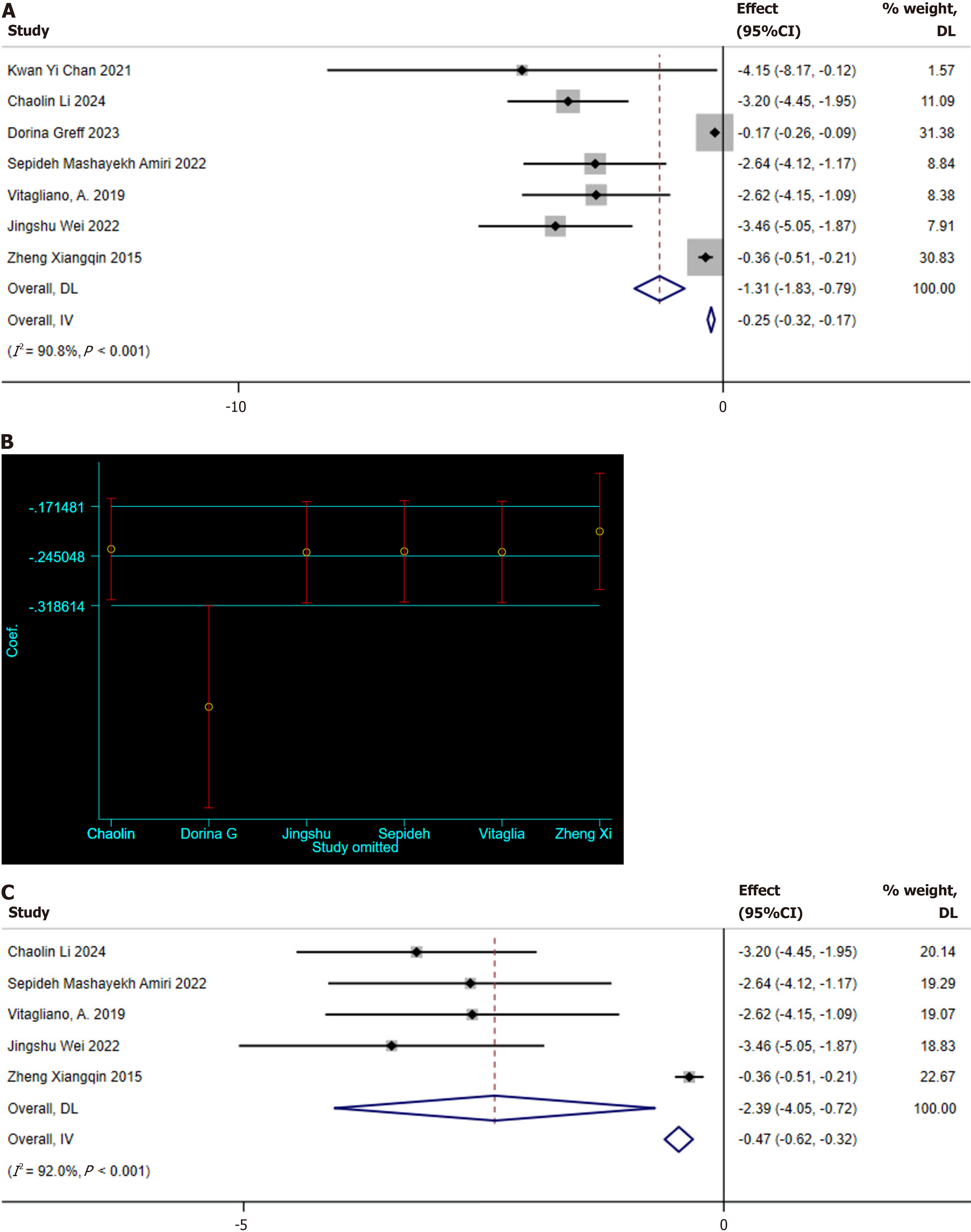
Fasting plasma glucose: Seven studies reporting fasting plasma glucose (FPG) levels were identified[10,12,21,22,27,28,30]. Significant heterogeneity was observed among these studies (I2 = 90.8%, P < 0.001); hence, a random-effects model was used for meta-analysis. The pooled analysis indicated that MI supplementation improved FPG levels in GDM patients (SMD: -1.31 mg/dL, 95%CI: -1.83 to -0.79; Figure 4A). Subsequently, a sensitivity analysis was conducted to explore the sources of heterogeneity, revealing that the study by Greff et al[10] deviated significantly from the others, as shown in Figure 4B. After excluding this study, the sensitivity analysis still showed the positive effect of MI supplementation on FPG levels (SMD: -1.31 mg/dL, 95%CI: -1.83 to -0.79; Figure 4C), but significant heterogeneity was still present (I2 = 92%, P < 0.001).
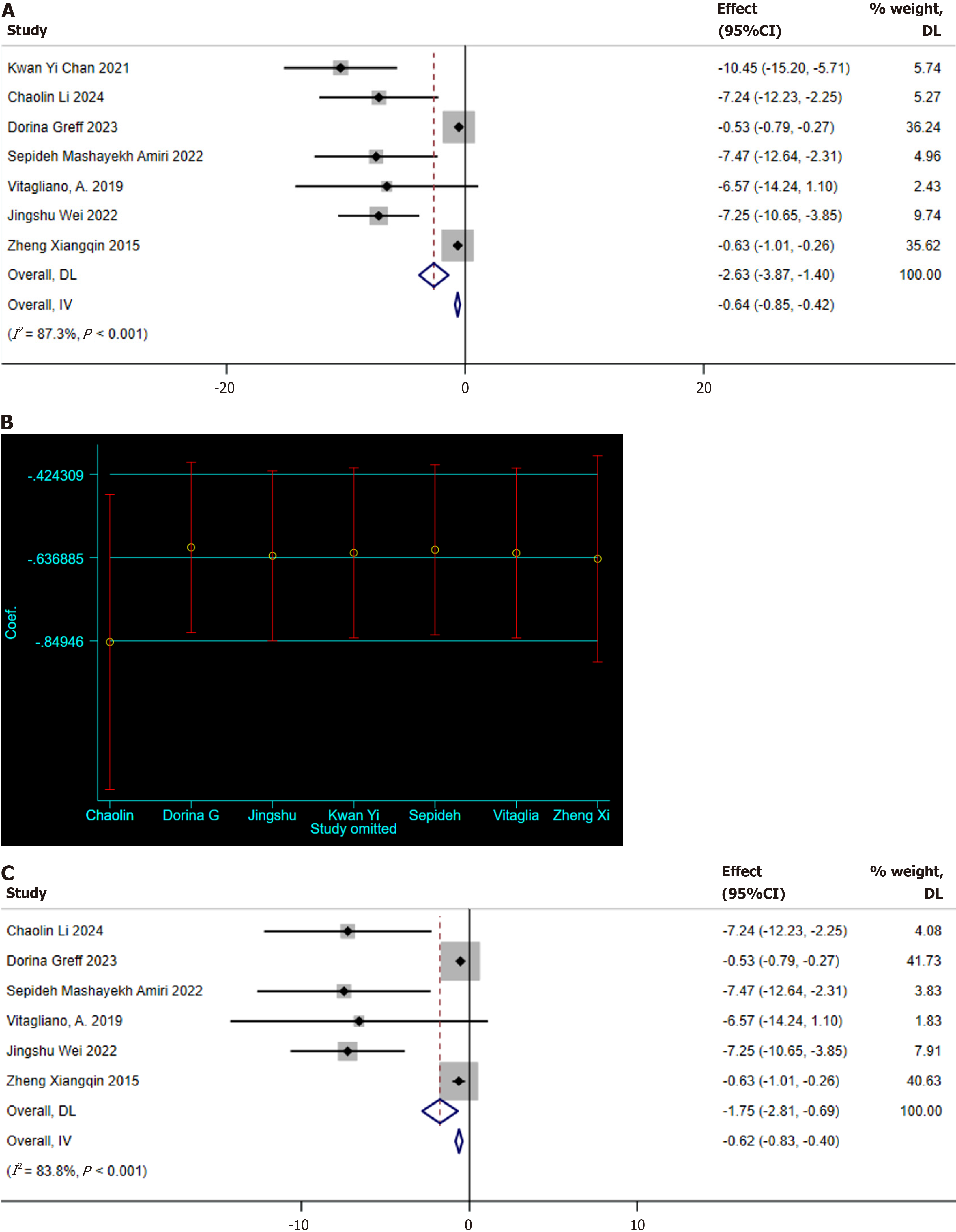
One-hour OGTT: Seven studies reporting on the 1-hour OGTT were identified[10,12,21,22,27,28,30]. Significant heterogeneity was noted among these studies (I2 = 87.3%, P < 0.001), which warranted the use of a random-effects model for meta-analysis. The results indicated that MI supplementation effectively reduces the 1-hour OGTT blood glucose levels in GDM patients (SMD: -2.63 mg/dL; 95%CI: -3.87 to -1.40; Figure 5A). A sensitivity analysis was conducted to determine the potential sources of heterogeneity, revealing that the study by Li and Shi[22] deviated significantly from the others (Figure 5B). After excluding this study, the sensitivity analysis still showed the positive effect of MI supplementation on the 1-hour OGTT blood glucose levels (SMD: -1.75 mg/dL, 95%CI: -2.81 to -0.69; Figure 5C), while significant heterogeneity was still present (I2 = 83.8%, P < 0.001).
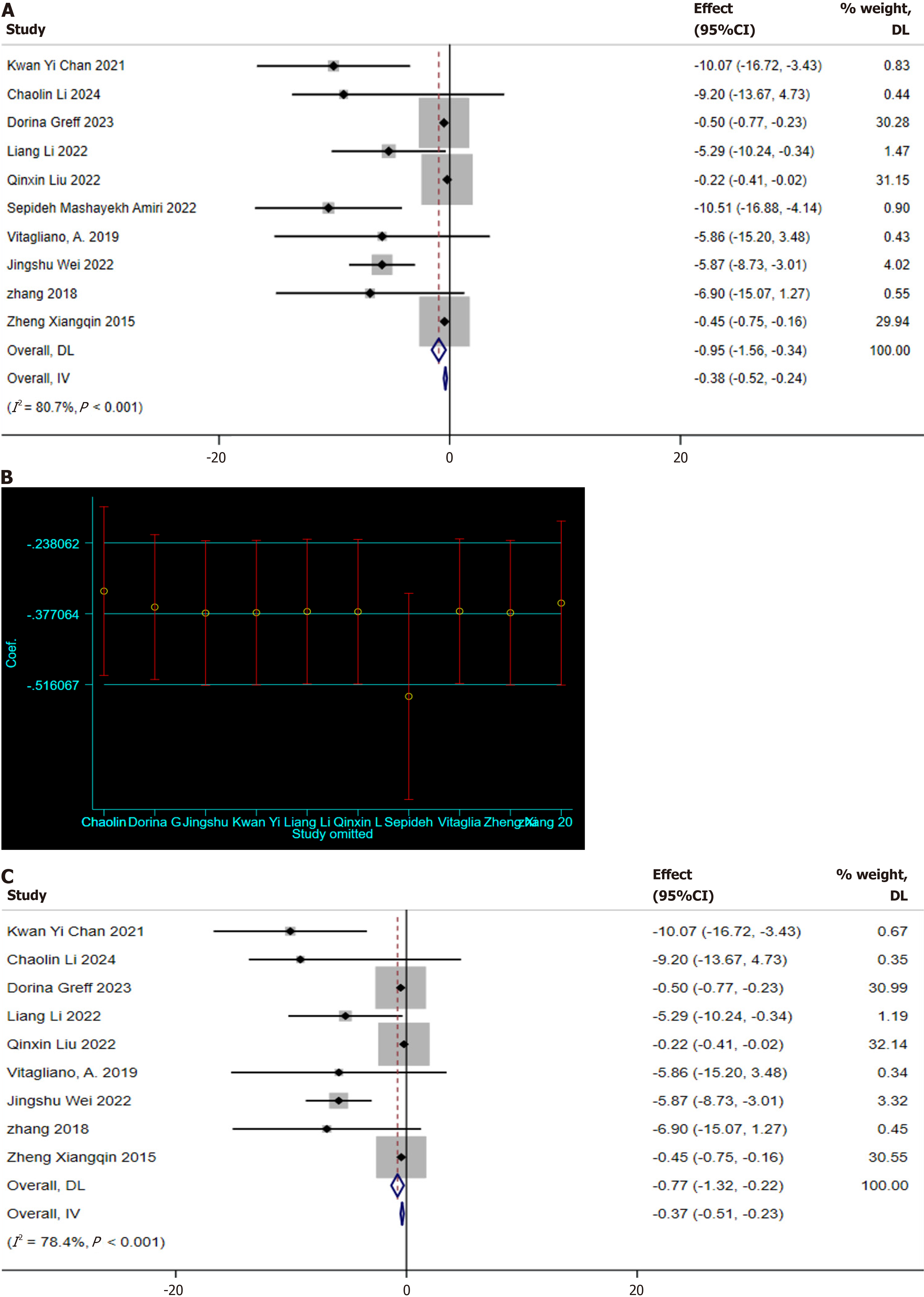
Two-hour OGTT: Ten studies reporting on the 2-hour OGTT were identified[10,12,21,22,24,26-30]. Significant heterogeneity was detected among these studies (I2 = 80.7%, P < 0.001), necessitating the use of a random-effects model for meta-analysis. The results demonstrated that MI supplementation effectively reduces the 2-hour OGTT blood glucose levels in GDM patients (SMD: -0.95 mg/dL; 95%CI: -1.56 to -0.34; Figure 6A). A sensitivity analysis was conducted to explore the sources of heterogeneity, revealing that the study by Mashayekh-Amiri et al[27] deviated significantly from the others (Figure 6B). After excluding this study, the sensitivity analysis still indicated that MI supplementation positively impacted the 2-hour OGTT blood glucose levels (SMD: -0.77 mg/dL, 95%CI: -1.32 to -0.22; Figure 6C). Yet, significant heterogeneity was still observed (I2 = 78.4%, P < 0.001). Meanwhile, the funnel plot shows the presence of publication bias (Figure 3B).
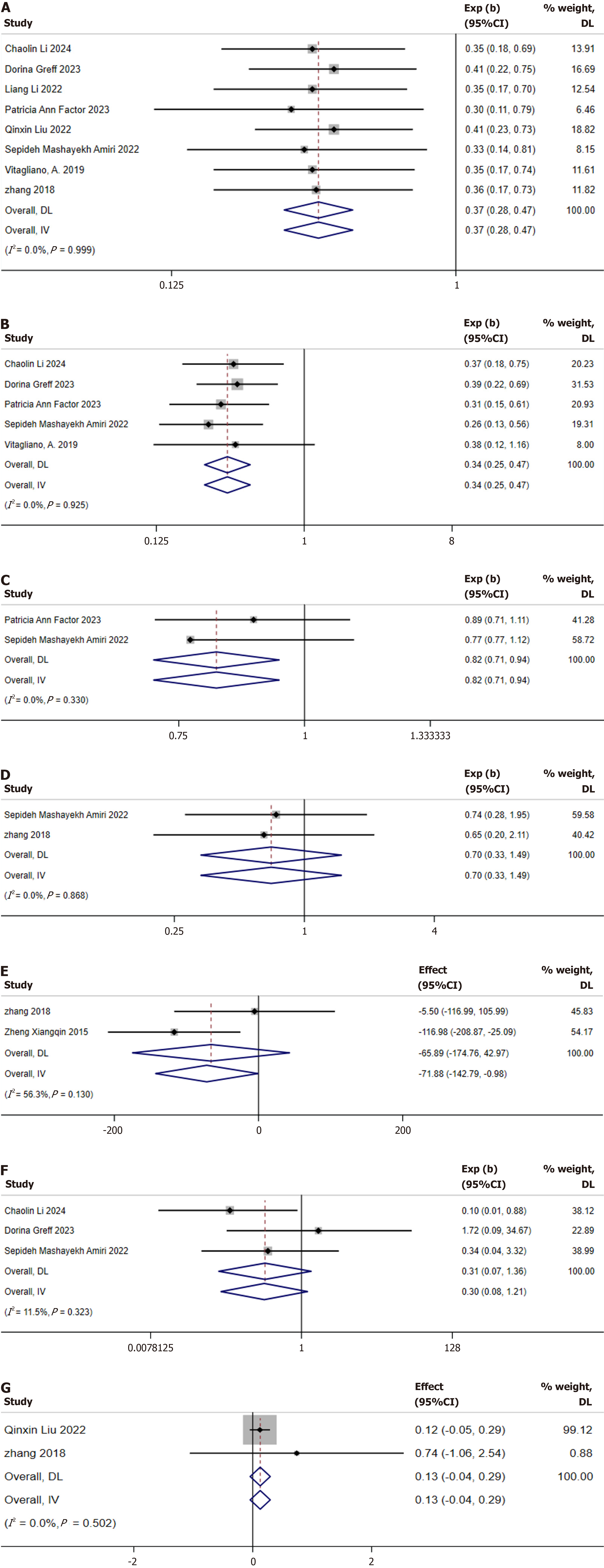
Preterm birth: Eight studies reported the incidence of preterm birth[10,22,23-26,28,29]. No heterogeneity was detected among these studies (I2 = 0.0%, P = 0.999); therefore, a fixed-effects model was utilized for the meta-analysis. The results demonstrated that inositol supplementation significantly reduced the risk of preterm birth in patients with GDM (RR: 0.37; 95%CI: 0.28-0.47; Figure 7A).
PIH: Five studies reported PIH[10,22,25,27,28]. No heterogeneity was detected among these studies (I2 = 0.0%, P = 0.925), which justified the use of a fixed-effects model for the meta-analysis. The results indicated that inositol supplementation significantly reduced the risk of PIH in patients with GDM (RR: 0.34; 95%CI: 0.25-0.47; Figure 7B).
Cesarean section: Only two studies reported the rate of cesarean sections[25,27]. Assessment of heterogeneity indicated no significant differences among the studies (I2 = 0.0%, P = 0.330). Utilizing a fixed-effects model, the results revealed that inositol supplementation has a modest effect on reducing the rate of cesarean sections in patients with GDM (RR: 0.82; 95%CI: 0.71-0.94; Figure 7C). However, further evidence is required to confirm this finding.
Macrosomia: Two studies reported the incidence of macrosomia[27,29]. No heterogeneity was detected among the two studies (I2 = 0.0%, P = 0.868), and a fixed-effects model was employed for the meta-analysis. The results indicated that inositol supplementation did not significantly reduce the incidence of macrosomia in patients with GDM (RR: 0.70; 95%CI: 0.33-1.49; Figure 7D).
Birth weight: Two studies reporting birth weight were identified[29,30]. Heterogeneity assessment showed no significant differences between studies (I2 = 11.5%, P = 0.323), so a fixed effects model was used for meta-analysis. The results showed that MI supplementation was modestly effective in reducing the birth weight of infants born to women with GDM (SMD: -0.25 kg; 95%CI: -0.32 to -0.17; Figure 7E).
Neonatal hypoglycemia: Three studies were found to report the incidence of neonatal hypoglycemia in infants[10,22,27]. Assessment of heterogeneity indicated no significant variability among the studies (I2 = 11.5%, P = 0.323), supporting the use of a fixed-effects model for the meta-analysis. The results indicated that inositol supplementation significantly reduced the incidence of neonatal hypoglycemia in infants born to mothers with GDM (RR: 0.30; 95%CI: 0.08-1.21; Figure 7F).
Gestational age at birth: Two studies reported the gestational age at birth[26,29]. Assessment of heterogeneity indicated no significant variability among the studies (I2 = 0.0%, P = 0.502), which justified the use of a fixed-effects model for the meta-analysis. The results indicated that inositol supplementation exerted no significant effect on gestational age at birth in infants of mothers with GDM (SMD: -0.13 weeks; 95%CI: -0.04 to 0.29; Figure 7G).
At present, the management of obesity and insulin resistance during pregnancy primarily involves diet and lifestyle modifications, with insulin and metformin being the main pharmacological options for controlling GDM[18]. According to current clinical guidelines, metformin is considered a second-line treatment for selected patients who refuse or are not suitable candidates for insulin therapy[31]. Identifying novel and efficacious strategies for the prevention and ma
In this study, inositol supplementation was found to significantly contribute to the management of complications in women with GDM and supports its potential role in preventive and management strategies for diabetes in pregnant women without pre-existing diabetes. However, considerable heterogeneity was observed among the various meta-analyses. The high heterogeneity warrants careful interpretation, particularly as significant variability remained despite the sensitivity analyses. Significant overall effects have been reported in prior meta-analyses, but some controversies persist. The discrepancies in outcomes can be ascribed to variations in treatment dosages and durations, types of analyses employed, quality of meta-analyses conducted, and the sizes of the study populations. In the included meta-analyses, the quality of the studies was evaluated using the AMSTAR 2 checklist. This checklist comprises 16 items that cover various aspects of meta-analysis. Among all included studies, 4 were high-quality meta-analyses, 4 were moderate-quality meta-analyses, and 4 were low-quality meta-analyses. These findings indicate that our meta-umbrella results should be interpreted with caution; thus, additional research is needed to draw conclusive results.
GDM is a multifactorial disease influenced by both genetic and environmental factors, leading to maternal and fetal circulatory disorders and neonatal complications[34]. The current guidelines recommend treatment with insulin and metformin, as they effectively maintain blood glucose concentrations within the normal range[35]. Insulin, in particular, is the gold standard therapy for GDM as it does not cross the placenta, ensuring minimal risk to the fetus[36]. However, the use of insulin therapy in gestational diabetes may increase the risk of fetal growth restriction, macrosomia, neonatal hypoglycemia, congenital malformations, and neonatal hypocalcemia[37]. Furthermore, although the safety of metformin has been established, ongoing research continues to evaluate its long-term effects on maternal and fetal health.
Inositol serves as a pivotal molecule within the insulin signaling cascade, activating protein kinase B by facilitating the conversion of phosphatidylinositol, thereby modulating glucose uptake and glycogen synthesis. Distinct forms of inositol, notably MI and DCI, exert unique effects on glucose metabolism; MI predominantly enhances glucose uptake, whereas DCI is implicated in glycogen storage. Insulin sensitizers, such as inositol, may ameliorate insulin resistance and the associated hyperandrogenic phenotype[38]. A growing body of evidence suggests that inositol exerts therapeutic effects in a variety of conditions, including polycystic ovarian syndrome, GDM, and the prevention of neural tube defects[12,39,40]. In recent years, several studies have investigated the prevention and management of diabetes during pregnancy, indicating that the efficacy of inositol in preventing and treating GDM is comparable to that of metformin[41]. Moreover, inositol, either as a monotherapy or in conjunction with probiotics and a Mediterranean diet, has achieved significant outcomes in the prevention and treatment of GDM[42-44].
Our study has several strengths. To our knowledge, the current study is the first comprehensive analysis exploring the impact of inositol supplementation on GDM. The present article adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, ensuring the transparency and reproducibility of our research. Nevertheless, the limitations of the present study should be acknowledged. Considering the small number of included studies, a certain degree of publication bias was inevitable. Thus, further meta-analytic research should be conducted in this area. The quality of primary studies included in the meta-analysis was mixed, and the quality of the meta-analysis was moderate, highlighting the need for high-quality studies in the future. Additionally, future meta-analyses should separately evaluate MI or DCI, as these two supplements may have different effects. In addition, different treatment dosages should be analyzed. Given that the primary population included in the meta-analysis comprises high-risk individuals for GDM and those diagnosed with GDM, the impact of inositol on other reproductive health conditions in women could not be ascertained. Consequently, future research should focus on exploring other conditions to provide a more comprehensive understanding of the roles of inositol.
In light of our meta-analytical findings, women at a higher risk of GDM, as well as those diagnosed with GDM, may consider inositol supplementation. This includes women with a history of GDM, those who are obese, or individuals with a strong familial predisposition to diabetes. Recommendations stratified by risk status: (1) High-risk women: 1-4 g MI from 1st trimester; and (2) Diagnosed GDM: Maintain standard therapy (lifestyle/insulin). However, the therapeutic application of inositol in diagnosed populations requires further RCTs[45]. Specifically, the optimal time to initiate inositol supplementation is from the early second trimester of pregnancy for high-risk women, such as those with obesity, a history of GDM, or a strong family predisposition to diabetes, as this period corresponds to the peak risk of GDM development. Supplementation should be discontinued upon stabilization of glycemic levels or progression into the third trimester[46,47]. Inositol shows promising potential, particularly among high-risk groups. Still, its broad application across all pregnancies necessitates further research. Future studies should focus on optimizing dosing regimens and identifying the patient populations that are most likely to benefit from this intervention.
This study meticulously examined and consolidated findings from 12 meta-analyses to evaluate the efficacy of inositol supplementation as a preventive and therapeutic intervention for GDM. After accounting for baseline comparability, our results demonstrate a significant correlation between inositol supplementation and a substantial reduction in the incidence of GDM. Moreover, our findings reveal a positive influence on metabolic parameters in women, including FPG, 1-hour OGTT, 2-hour OGTT, preterm birth rates, PIH, neonatal hypoglycemia, and the occurrence of cesarean sections. However, the conclusions of this study should be interpreted in the context of the methodological quality and heterogeneity of the research. The quality of the included meta-analyses was assessed using the AMSTAR 2 tool, revealing significant variation. Therefore, the results should be interpreted with caution, and more high-quality studies may be needed to confirm the findings. Moreover, the significant heterogeneity observed in this study may stem from various factors, including different inositol dosages, treatment durations, study designs, and differences in participant baseline characteristics. The pooled analysis of inositol isomers, while clinically relevant, limits definitive conclusions on differential effects of MI compared to DCI. Despite the relatively balanced baseline characteristics, most studies have not systematically reported dietary patterns.
Nonetheless, our study results provide robust evidence for the potential of inositol as a preventive and therapeutic agent for GDM. As a safe and potentially effective nutritional supplement, the mechanisms underlying the positive effects of inositol on insulin sensitivity and reducing blood glucose levels offer new perspectives for the management of GDM. Furthermore, considering the long-term impacts of GDM on maternal and fetal health, inositol supplementation, as a non-pharmacological intervention, may provide a novel strategy for the prevention of GDM and its complications. All studies-initiated inositol supplementation in the early stages of pregnancy (≤ 16 weeks; Table 2), whereas GDM is typically diagnosed between 24-28 weeks of gestation. This time gap resulted in a reduced GDM incidence of 63% (RR: 0.37; 95%CI: 0.32-0.42), mainly reflecting prevention efficiency. Currently, for treatment purposes, no evidence supports the supplementation after GDM diagnosis. Future trials should be specifically targeted at this population.
Future research should focus on well-designed, adequately powered, multicenter, randomized controlled clinical trials to evaluate the effects of different dosages and types of inositol supplementation on the prevention and treatment of GDM. Additionally, research should address the long-term safety of inositol supplementation, cost-effectiveness analysis, and its generalizability across different ethnicities and geographical regions. Future studies will provide a more comprehensive understanding of the role of inositol in GDM management and offer precise guidance for clinical practice. In summary, this umbrella review of systematic review provides scientific evidence for the potential application of inositol supplementation in the prevention and treatment of GDM, while emphasizing the need for more high-quality research to further validate these findings.
The authors would like to thank Jun-Feng He of the Second Affiliated Hospital of South China University for a helpful discussion on topics related to this work. Dan Zi of the Department of Rehabilitation of the First Affiliated Hospital of Sun Yat-sen University and Tian-Tian Wei of the Qilu Medical College of Shandong University provided key help in data extraction and analysis in the research. Additionally, Ru-Tong Wang would like to express his sincere gratitude to Ying-Qi Feng for her patience, care, and unwavering support.
| 1. | Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, Hivert MF, Immanuel J, Meek C, Oppermann ML, Nolan CJ, Ram U, Schmidt MI, Simmons D, Chivese T, Benhalima K. Epidemiology and management of gestational diabetes. Lancet. 2024;404:175-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 206] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 2. | Biete M, Vasudevan S. Gestational diabetes mellitus: Impacts on fetal neurodevelopment, gut dysbiosis, and the promise of precision medicine. Front Mol Biosci. 2024;11:1420664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Weir TL, Majumder M, Glastras SJ. A systematic review of the effects of maternal obesity on neonatal outcomes in women with gestational diabetes. Obes Rev. 2024;25:e13747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Del Campo-Rota IM, Delgado-Casillas OM, Ibarra A. Cognitive Impairment Induced by Gestational Diabetes: The Role of Oxidative Stress. Arch Med Res. 2024;55:103016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Saucedo R, Ferreira-Hermosillo A, Robledo-Clemente M, Díaz-Velázquez MF, Valencia-Ortega J. Association of DNA Methylation with Infant Birth Weight in Women with Gestational Diabetes. Metabolites. 2024;14:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Plows JF, Reynolds CM, Vickers MH, Baker PN, Stanley JL. Nutritional Supplementation for the Prevention and/or Treatment of Gestational Diabetes Mellitus. Curr Diab Rep. 2019;19:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Rogozińska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One. 2015;10:e0115526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Noventa M, Vitagliano A, Quaranta M, Borgato S, Abdulrahim B, Gizzo S. Preventive and Therapeutic Role of Dietary Inositol Supplementation in Periconceptional Period and During Pregnancy: A Summary of Evidences and Future Applications. Reprod Sci. 2016;23:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | López-Gambero AJ, Sanjuan C, Serrano-Castro PJ, Suárez J, Rodríguez de Fonseca F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines. 2020;8:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Greff D, Váncsa S, Váradi A, Szinte J, Park S, Hegyi P, Nyirády P, Ács N, Horváth EM, Várbíró S. Myoinositols Prevent Gestational Diabetes Mellitus and Related Complications: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2023;15:4224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Watkins OC, Selvam P, Pillai RA, Cracknell-Hazra VKB, Yong HEJ, Sharma N, Cazenave-Gassiot A, Bendt AK, Godfrey KM, Lewis RM, Wenk MR, Chan SY. Myo-inositol moderates maternal BMI and glycemia related variations in in-vitro placental (13)C-DHA-metabolism, altering their relationships with birthweight. Sci Rep. 2022;12:14895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Wei J, Yan J, Yang H. Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022;14:2831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Celentano C, Matarrelli B, Pavone G, Vitacolonna E, Mattei PA, Berghella V, Liberati M. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: a randomized controlled trial. J Matern Fetal Neonatal Med. 2020;33:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Fraticelli F, Celentano C, Zecca IA, Di Vieste G, Pintaudi B, Liberati M, Franzago M, Di Nicola M, Vitacolonna E. Effect of inositol stereoisomers at different dosages in gestational diabetes: an open-label, parallel, randomized controlled trial. Acta Diabetol. 2018;55:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The Prevention of Gestational Diabetes Mellitus With Antenatal Oral Inositol Supplementation: A Randomized Controlled Trial. Diabetes Care. 2017;40:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D'Anna R, Neri I, Facchinetti F. Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. J Matern Fetal Neonatal Med. 2016;29:3234-3237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | D'Anna R, Di Benedetto A, Scilipoti A, Santamaria A, Interdonato ML, Petrella E, Neri I, Pintaudi B, Corrado F, Facchinetti F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obstet Gynecol. 2015;126:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Formoso G, Baldassarre MPA, Ginestra F, Carlucci MA, Bucci I, Consoli A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab Res Rev. 2019;35:e3154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Moher D, Stewart L, Shekelle P. Establishing a new journal for systematic review products. Syst Rev. 2012;1:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 6497] [Article Influence: 721.9] [Reference Citation Analysis (0)] |
| 21. | Chan KY, Wong MMH, Pang SSH, Lo KKH. Dietary supplementation for gestational diabetes prevention and management: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2021;303:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Li C, Shi H. Inositol supplementation for the prevention and treatment of gestational diabetes mellitus: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2024;309:1959-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Guo X, Guo S, Miao Z, Li Z, Zhang H. Myo-inositol lowers the risk of developing gestational diabetic mellitus in pregnancies: A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. J Diabetes Complications. 2018;32:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Li L, Fang J. Myo-inositol supplementation for the prevention of gestational diabetes: A meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2022;273:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Factor PA, Corpuz H. The Efficacy and Safety of Myo-inositol Supplementation for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Pregnant Women: A Systematic Review and Meta-Analysis. J ASEAN Fed Endocr Soc. 2023;38:102-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 26. | Liu Q, Liu Z. The efficacy of myo-inositol supplementation to reduce the incidence of gestational diabetes: a meta-analysis. Gynecol Endocrinol. 2022;38:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Mashayekh-Amiri S, Mohammad-Alizadeh-Charandabi S, Abdolalipour S, Mirghafourvand M. Myo-inositol supplementation for prevention of gestational diabetes mellitus in overweight and obese pregnant women: a systematic review and meta-analysis. Diabetol Metab Syndr. 2022;14:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Vitagliano A, Saccone G, Cosmi E, Visentin S, Dessole F, Ambrosini G, Berghella V. Inositol for the prevention of gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2019;299:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Zhang H, Lv Y, Li Z, Sun L, Guo W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2249-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Zheng X, Liu Z, Zhang Y, Lin Y, Song J, Zheng L, Lin S. Relationship Between Myo-Inositol Supplementary and Gestational Diabetes Mellitus: A Meta-Analysis. Medicine (Baltimore). 2015;94:e1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Infante M, Leoni M, Caprio M, Fabbri A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J Diabetes. 2021;12:916-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (15)] |
| 32. | Sobota-Grzeszyk A, Kuźmicki M, Szamatowicz J. Myoinositol in the Prevention of Gestational Diabetes Mellitus: Is It Sensible? J Diabetes Res. 2019;2019:3915253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Ibrahim I, Bashir M, Singh P, Al Khodor S, Abdullahi H. The Impact of Nutritional Supplementation During Pregnancy on the Incidence of Gestational Diabetes and Glycaemia Control. Front Nutr. 2022;9:867099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Calvo MJ, Parra H, Santeliz R, Bautista J, Luzardo E, Villasmil N, Martínez MS, Chacín M, Cano C, Checa-Ros A, D'Marco L, Bermúdez V, De Sanctis JB. The Placental Role in Gestational Diabetes Mellitus: A Molecular Perspective. touchREV Endocrinol. 2024;20:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 35. | Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;11:CD012037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 1123] [Article Influence: 160.4] [Reference Citation Analysis (1)] |
| 37. | Pantea-Stoian A, Adriana Stoica R, Diana Stefan S. Insulin Therapy in Gestational Diabetes. In: Ray A, editor. Gestational Diabetes Mellitus - An Overview with Some Recent Advances. London: IntechOpen, 2019. [DOI] [Full Text] |
| 38. | Laganà AS, Myers SH, Forte G, Naem A, Krentel H, Allahqoli L, Alkatout I, Unfer V. Inositols in treating polycystic ovary syndrome and non-insulin dependent diabetes mellitus: now and the future. Expert Opin Drug Metab Toxicol. 2024;20:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Jethaliya H, Gajjar N, Patel V, Deshpande S, Patel R. Efficacy of Myo-inositol on Anthropometric, Metabolic, and Endocrine Outcomes in PCOS Patients: a Meta-analysis of Randomized Controlled Trial. Reprod Sci. 2022;29:2282-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Greene ND, Leung KY, Copp AJ. Inositol, neural tube closure and the prevention of neural tube defects. Birth Defects Res. 2017;109:68-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Martis R, Crowther CA, Shepherd E, Alsweiler J, Downie MR, Brown J. Treatments for women with gestational diabetes mellitus: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2018;8:CD012327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 42. | Lan X, Li B, Zhao J, Stanton C, Ross RP, Chen W, Yang B. Probiotic intervention improves metabolic outcomes in gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Clin Nutr. 2024;43:1683-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Dennison RA, Oliver-Williams C, Qi HLJ, Kotecha D, Seed L, Ward RJ, Griffin SJ. The effectiveness of pharmacological and lifestyle interventions to reduce the risk of diabetes and hyperglycaemia following gestational diabetes: A systematic review and meta-analysis. Diabet Med. 2024;41:e15316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Li J, Wang M, Ma S, Jin Z, Yin H, Yang S. Association of gastrointestinal microbiome and obesity with gestational diabetes mellitus-an updated globally based review of the high-quality literatures. Nutr Diabetes. 2024;14:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 45. | Motuhifonua SK, Lin L, Alsweiler J, Crawford TJ, Crowther CA. Antenatal dietary supplementation with myo-inositol for preventing gestational diabetes. Cochrane Database Syst Rev. 2023;2:CD011507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Matarrelli B, Vitacolonna E, D'Angelo M, Pavone G, Mattei PA, Liberati M, Celentano C. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2013;26:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 47. | Gu ZJ, Song QJ, Gu WQ, Zhang GP, Su Y, Tang Y, Wang MF, Guo Y, Wu WM, Chen J. New approaches in the diagnosis and prognosis of gestational diabetes mellitus. Eur Rev Med Pharmacol Sci. 2023;27:10583-10594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/